Thermal Decomposition of Olive-Mill Byproducts: A TG-FTIR Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Small twigs (OW1), (diameter > 5 mm) separated at the first step of olive-oil production when the olives were cleaned prior to milling;
- Leaves (OW2), separated on sieves before olive cleaning. It also must be taken into consideration that leaves represented 5% of the weight of olives in the oil extraction;
- Solid olive-mill residue from the two-phase decanting method (OW3), which was a mixture of kernels and pulp from the olive fruit.
- Olive-mill wastewater liquid fraction with oil from the three-phase decanting method (OW4).
2.2. Methods
2.2.1. Physico-Chemical Parameters
2.2.2. TG-DTA and TG-FTIR Analyses
2.2.3. Combustion Parameters—Indexes
- Comprehensive combustion index:
- Ignition index:
- Burnout index:
- Flammability index:where Rp is the maximum weight-loss rate (%/min), Rv is the average mass-loss rate (%/min), Ti is the ignition temperature (°C), Tb is the burnout temperature (°C), ti is the ignition time (min), tp is the peak time (min), ∆t1/2 is the time range at half value of Rp (min), and tb is the burnout time (min).
2.2.4. Kinetic Models
3. Results and Discussion
3.1. Physico-Chemical Parameters
3.2. Thermogravimetric Analysis and Combustion Parameters
3.3. Kinetic Models
3.4. FTIR Spectra Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turkish Statistical Institute. 2014. Available online: http://www.turkstat.gov.tr (accessed on 20 April 2021).
- International Olive Oil Council. Available online: http://www.internationaloliveoil.org (accessed on 20 April 2021).
- Anonymous. A snapshot of the Turkish olive oil sector. OLIVAE Off. J. Int. Olive Counc. 2016, 123, 4–5. [Google Scholar]
- Gurkan, N.P. Main actors in the Turkish Olive and Olive Oil Sector: An innovation system framework. OLIVAE Off. J. Int. Olive Counc. 2016, 123, 9–17. [Google Scholar]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey, 2nd ed.; Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef]
- Mizerna, K.; Król, A.; Mróz, A. Environmental assessment of applicability of mineral-organic composite for landfill area rehabilitation. E3S Web Conf. 2017, 19, 2020. [Google Scholar] [CrossRef] [Green Version]
- Wzorek, M. Assessment of leachability of harmful components from alternative fuels prepared with the use of sewage sludge. Ecol. Chem. Eng. S 2012, 19, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Mizerna, K.; Król, A. Directions of development of research methods in the assessment of leaching of heavy metals from mineral waste. E3S Web Conf. 2016, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterization and val-orization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Ducoma, G.; Gautiera, M.; Pietraccinia, M.; Tagutchouc, J.P.; Lebouila, D.; Gourdon, R. Comparative analyses of three olive mill solid residues from different countries and processes for energy recovery by gasification. Renew. Energ. 2020, 145, 180–189. [Google Scholar] [CrossRef]
- Guida, M.Y.; Bouaik, H.; Tabal, A.; Hannioui, A.; Solhy, A.; Barakat, A.; Aboulkas, A.; El Harfi, K. Thermochemical treatment of olive mill solid waste and olive millwastewater Pyrolysis kinetics. J. Therm. Anal. Calorim. 2016, 123, 1657–1666. [Google Scholar] [CrossRef]
- Cliffe, K.R.; Patumsawad, S. Co-combustion of waste from olive oil production with coal in a fluidised bed. Waste Manag. 2012, 21, 49–53. [Google Scholar] [CrossRef]
- Junga, R.; Knauer, W.; Niemiec, P.; Tańczuk, M. Experimental tests of co-combustion of laying hens manure with coal by using thermogravimetric analysis. Renew. Energy 2017, 111, 245–255. [Google Scholar] [CrossRef]
- Junga, R.; Wzorek, M.; Kaszubska, M. Technical and environmental performance of 10 kW understocker boiler during combustion of biomass and conventional fuels. E3S Web Conf. 2017, 19, 1009. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.B.; Calero de Hoces, M.; Garcia, C.M.; Palomino, M.T.; Gálvez, A.R.; Martín-Lara, M.A. Characterization and modeling of pyrolysis of the two-phase olive mill solid waste. Fuel Process. Technol. 2014, 126, 104–111. [Google Scholar] [CrossRef]
- Martin-Lara, M.A.; Ronda, A.; Blazquez, G.; Perez, A.; Calero, M. Pyrolysis kinetics of the lead-impregnated olive stone by non-isothermal thermogravimetry. Process Saf. Environ. 2018, 113, 448–458. [Google Scholar]
- Lajili, M.; Guizani, C.; Escudero Sanz, F.J.; Jeguirim, M. Fast pyrolysis and steam gasification of pellets prepared from olive oil mill residues. Energy 2018, 150, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Mirinda, M.T.; Cabanillas, A.; Rojas, S.; Montero, I.; Ruiz, A. Combined combustion of various phase of olive wastes in conventional combustor. Fuel 2007, 86, 367–372. [Google Scholar] [CrossRef]
- Haykr-Ama, H. Combustion characteristics of different biomass materials. Energy Convers. Manag. 2003, 44, 155–162. [Google Scholar]
- García-Ibañez, P.; Sánchez, M.; Cabanillas, A. Thermogravimetric analysis of olive-oil residue in air atmosphere. Fuel Process. Technol. 2006, 87, 103–107. [Google Scholar] [CrossRef]
- Álvarez, A.; Pizarro, C.; García, R.; Bueno, J.; Lavín, A. Determination of kinetic parameters for biomass combustion. Bioresour. Technol. 2016, 216, 36–43. [Google Scholar]
- Garcia-Maraver, A.; Perez-Jimenez, J.; Serrano-Bernardo, F. Determination and comparison of combustion kinetics parame-ters of agricultural biomass from olive trees. Renew. Energ. 2015, 83, 897–904. [Google Scholar] [CrossRef]
- Yaman, S.; Sahan, M.; Haykiri-Açma, H.; Şeşen, K.; Küçükbayrak, S. Production of fuel briquettes from olive refuse and paper mill waste. Fuel Process. Technol. 2000, 68, 23–31. [Google Scholar] [CrossRef]
- Varol, M.; Atimtay, A.T.; Bay, B.; Olgun, H. Investigation of co-combustion characteristics of low quality lignite coals and bio-mass with thermogravimetric analysis. Thermochim. Acta 2010, 510, 1–2. [Google Scholar] [CrossRef]
- Yilmaz, E.; Wzorek, M.; Akçay, S. Co-pelletization of sewage sludge and agricultural wastes. J. Environ. Manag. 2018, 216, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Yuzbasi, N.S.; Selçuk, N. Fuel air and oxy-fuel combustion characteristics of biomass/lignite blends in TGA-FTIR. Fuel Process. Technol. 2011, 92, 1101–1108. [Google Scholar] [CrossRef]
- Zou, H.; Evrendilek, F.; Liu, J.; Buyukada, M. Combustion behaviors of pileus and stipe parts of lentinus edodes using thermo-gravimetric-mass spectrometry and Fourier transform infrared spectroscopy analyses: Thermal conversion, kinetic, thermodynamic, gas emission and optimization analyses. Bioresour. Technol. 2019, 288, 1481. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, J.; Xie, W.; Kuo, J.; Lu, X.; Chang, K.; Wen, S.; Sun, G.; Cai, H.; et al. Combustion be-haviors of spent mushroom substrate using TG-MS and TG-FTIR. Thermal conversion, kinetic, thermodynamic and emission analyses. Bioresour. Technol. 2018, 266, 389–397. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Evrendilek, F.; Kuo, J.; Buyukada, M. Combustion behaviors of Pteris vittata using thermogravimetric, kinetic, emission and optimization analyses. J. Clean. Prod. 2019, 237, 117772. [Google Scholar] [CrossRef]
- Dhyani, V.; Awasthi, M.K.; Wang, Q.; Kumar, J.; Ren, X.; Zhao, J.; Chen, H.; Wang, M.; Bhaskar, T.; Zhang, T. Effect of composting on the thermal decomposition behavior and kinetic parameters of pig manure-derived solid waste. Bioresour. Technol. 2018, 252, 59–65. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Saidur, R.; Abdelaziz, E.; Demirbas, A.; Hossain, M.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Wzorek, M.; Troniewski, L. Application of sewage sludge as a component of alternative fuel. In Proceedings of the 2nd National Congress of Environmental Engineering 2007, Lublin, Poland, 4–8 September 2005; pp. 311–316. [Google Scholar]
- Wzorek, M. Physical and chemical properties of fuel containing animal waste. WIT Trans. Ecol. Environ. 2008, 109, 69–77. [Google Scholar]
- Demirbas, A.; Pehlivan, E.; Altun, T. Potential evolution of Turkish agricultural residues as bio-gas, bio-char and bio-oil sources. Int. J. Hydrogen Energy 2006, 31, 613–620. [Google Scholar] [CrossRef]
- Yang, Y.B.; Sharifi, V.N.; Swithenbank, J. Effect of air flow rate and fuel moisture on the burning behaviours of biomass and simulated municipal solid wastes in packed beds. Fundam. Mech. Biomass Pyrolysis Oxid. 2004, 83, 1553–1562. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, B.; Yuan, Q.; Cao, H.; Yan, S. Combustion characteristics of cattle manure and pulverized coal co-firing under oxy-fuel atmosphere in non-isothermal and isothermal conditions. BioResources 2018, 13, 6465–6479. [Google Scholar]
- Guo, L.X.; Guo, M.B.; Xu, L.; Wu, H.Z.; Gang, W.X. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim. Acta 2006, 441, 79–83. [Google Scholar]
- Hu, J.; Yan, Y.; Evrendilek, F.; Buyukada, M.; Liu, J. Combustion behaviors of three bamboo residues: Gas emission, kinetic, reaction mechanism and optimization patterns. J. Clean. Prod. 2019, 235, 549–561. [Google Scholar] [CrossRef]
- Ceylan, S.; Topu, Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K. Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA). Bioresour. Technol. 2012, 123, 581–591. [Google Scholar] [CrossRef]
- Ramajo-Escalera, B.; Espina, A.; Garca, J.; Sosa-Arnao, J.; Nebra, S. Model-free kinetics applied to sugarcane bagasse combus-tion. Thermochim. Acta 2006, 448, 111–116. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and PYGC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]


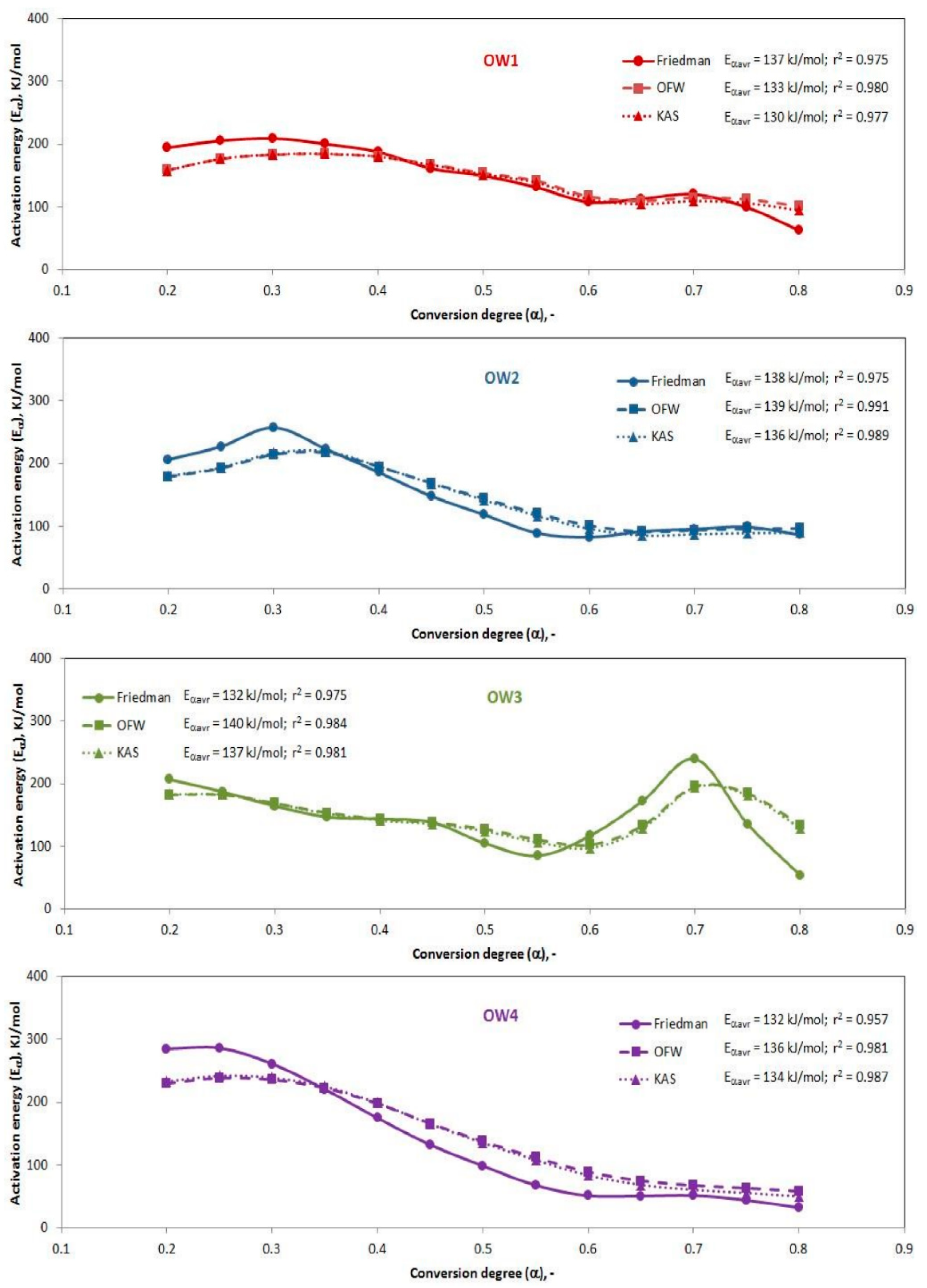
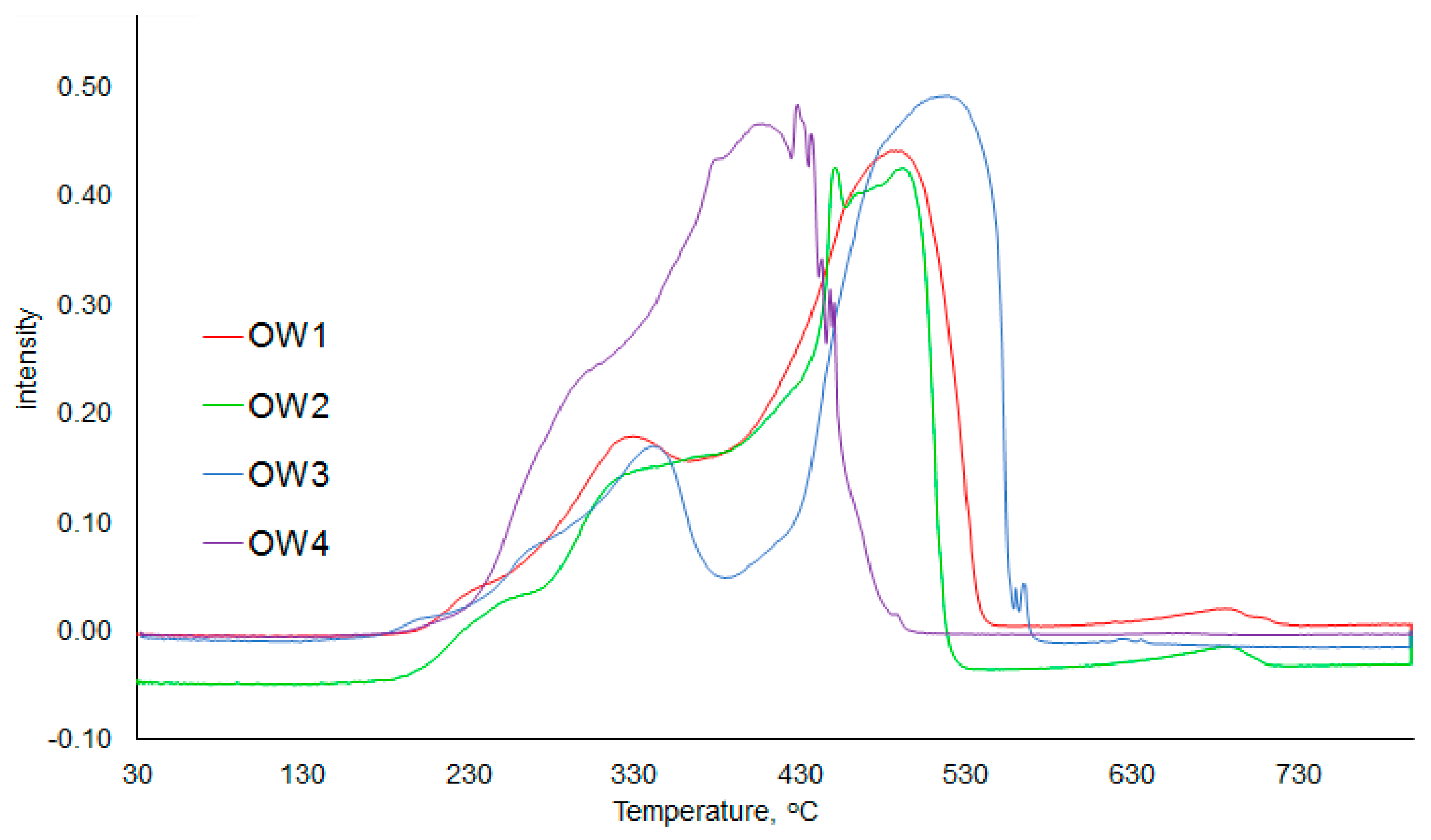


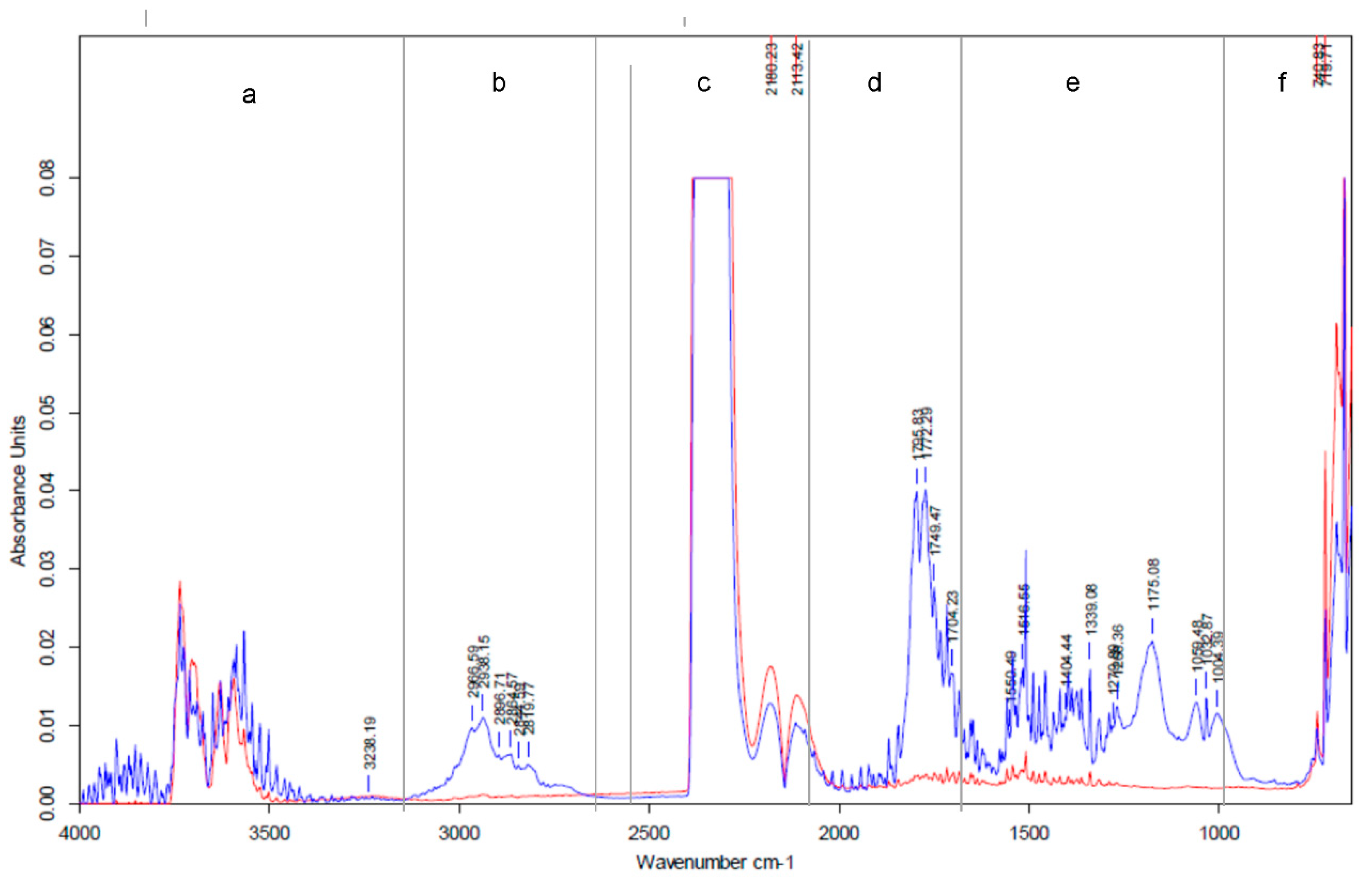
| Samples | Proximate Analysis (wt %) | Ultimate Analysis (wt %) | LHV d.m. MJ/kg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture w.b. | Ash d.m. | Volatile Matter d.m. | Fixed Carbon d.m. | C | H | N | S | O | ||
| d.m. | ||||||||||
| OW1 | 19.16 | 5.10 | 55.32 | 39.58 | 45.11 | 6.34 | 0.89 | 0.08 | 47.58 | 16.49 |
| OW2 | 19.55 | 5.69 | 55.00 | 39.31 | 48.41 | 6.85 | 1.64 | 0.16 | 42.94 | 18.63 |
| OW3 | 53.49 | 3.03 | 51.57 | 45.4 | 51.37 | 6.62 | 0.85 | 0.05 | 41.11 | 19.44 |
| OW4 | 84.24 | 14.19 | 65.66 | 20.15 | 46.23 | 6.12 | 1.06 | 0.17 | 46.42 | 17.37 |
| Sample | Stage IIa °C | Stage IIb °C | Stage IIc °C | Stage III °C |
|---|---|---|---|---|
| 6 K/min | ||||
| OW1 | - | - | 175–367 | 367–520 |
| OW2 | - | 166–267 | 267–371 | 371–506 |
| OW3 | 139–203 | 203–284 | 284–371 | 371–540 |
| OW4 | - | - | 154–347 | 347–476 |
| 10 K/min | ||||
| OW1 | - | - | 177–380 | 380–550 |
| OW2 | - | 168–272 | 272–385 | 385–542 |
| OW3 | 139–205 | 205–292 | 292–378 | 378–570 |
| OW4 | - | - | 157–352 | 352–519 |
| 14 K/min | ||||
| OW1 | - | - | 180–385 | 385–580 |
| OW2 | - | 170–282 | 282–390 | 390–573 |
| OW3 | 141–209 | 209–297 | 297–384 | 384–606 |
| OW4 | - | - | 160–374 | 374–536 |
| Stage IIa | Stage IIb | Stage IIc | Stage IIc | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Ti | Tb | tb | DTGmax | Tmax | DTGmax | Tmax | DTGmax | Tmax | DTGmax | Tmax | S·107 | Di·102 | Db·104 | C·104 |
| °C | °C | min | %/min | °C | %/min | °C | %/min | °C | %/min | °C | %2/(min2K3) | %/min3 | %/min4 | %/(minK2) | |
| 6 K/min | |||||||||||||||
| OW1 | 222 | 520 | 48.3 | - | - | - | - | −2.89 | 303.8 | −2.19 | 454.2 | 0.83 | 0.14 | 0.15 | 0.59 |
| OW2 | 198 | 506 | 49.7 | - | - | −2.07 | 247.9 | −2.60 | 299.9 | −2.27 | 456.2 | 0.98 | 0.13 | 0.15 | 0.66 |
| OW3 | 208 | 540 | 53.8 | −0.89 | 182.0 | −2.53 | 269.7 | −2.92 | 316.3 | −2.24 | 459.9 | 0.95 | 0.13 | 0.13 | 0.67 |
| OW4 | 230 | 476 | 39.8 | - | - | - | - | −4.14 | 270.3 | −2.41 | 382.9 | 1.27 | 0.23 | 0.27 | 0.78 |
| 10 K/min | |||||||||||||||
| OW1 | 224 | 550 | 30.8 | - | - | - | - | −4.50 | 305.7 | −2.79 | 469.1 | 2.07 | 0.52 | 0.89 | 0.90 |
| OW2 | 199 | 542 | 32.3 | - | - | −3.68 | 253.0 | −4.13 | 405.0 | −3.02 | 463.7 | 2.46 | 0.49 | 0.82 | 1.04 |
| OW3 | 209 | 570 | 34.3 | −1.69 | 185.3 | −4.23 | 264.0 | −4.65 | 319.9 | −2.70 | 479.1 | 2.43 | 0.50 | 0.75 | 1.06 |
| OW4 | 234 | 519 | 26.8 | - | - | - | - | −7.15 | 268.7 | −3.45 | 399.2 | 3.32 | 0.91 | 1.59 | 1.31 |
| 14 K/min | |||||||||||||||
| OW1 | 226 | 580 | 23.2 | - | - | - | - | −6.33 | 302.0 | −3.15 | 474.5 | 3.09 | 1.24 | 2.79 | 1.24 |
| OW2 | 206 | 573 | 24.1 | - | - | −5.31 | 259.5 | −5.49 | 303.9 | −3.43 | 469.2 | 4.15 | 1.11 | 2.46 | 1.29 |
| OW3 | 210 | 606 | 26.1 | −2.20 | 188.8 | −5.99 | 268.8 | −6.38 | 325.9 | −2.95 | 482.5 | 4.46 | 1.18 | 2.29 | 1.45 |
| OW4 | 235 | 536 | 19.7 | - | - | - | - | −10.06 | 269.2 | −3.67 | 430.7 | 6.42 | 2.28 | 5.27 | 1.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wzorek, M.; Junga, R.; Yilmaz, E.; Bozhenko, B. Thermal Decomposition of Olive-Mill Byproducts: A TG-FTIR Approach. Energies 2021, 14, 4123. https://doi.org/10.3390/en14144123
Wzorek M, Junga R, Yilmaz E, Bozhenko B. Thermal Decomposition of Olive-Mill Byproducts: A TG-FTIR Approach. Energies. 2021; 14(14):4123. https://doi.org/10.3390/en14144123
Chicago/Turabian StyleWzorek, Małgorzata, Robert Junga, Ersel Yilmaz, and Bohdan Bozhenko. 2021. "Thermal Decomposition of Olive-Mill Byproducts: A TG-FTIR Approach" Energies 14, no. 14: 4123. https://doi.org/10.3390/en14144123
APA StyleWzorek, M., Junga, R., Yilmaz, E., & Bozhenko, B. (2021). Thermal Decomposition of Olive-Mill Byproducts: A TG-FTIR Approach. Energies, 14(14), 4123. https://doi.org/10.3390/en14144123







