A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application
Abstract
:1. Introduction
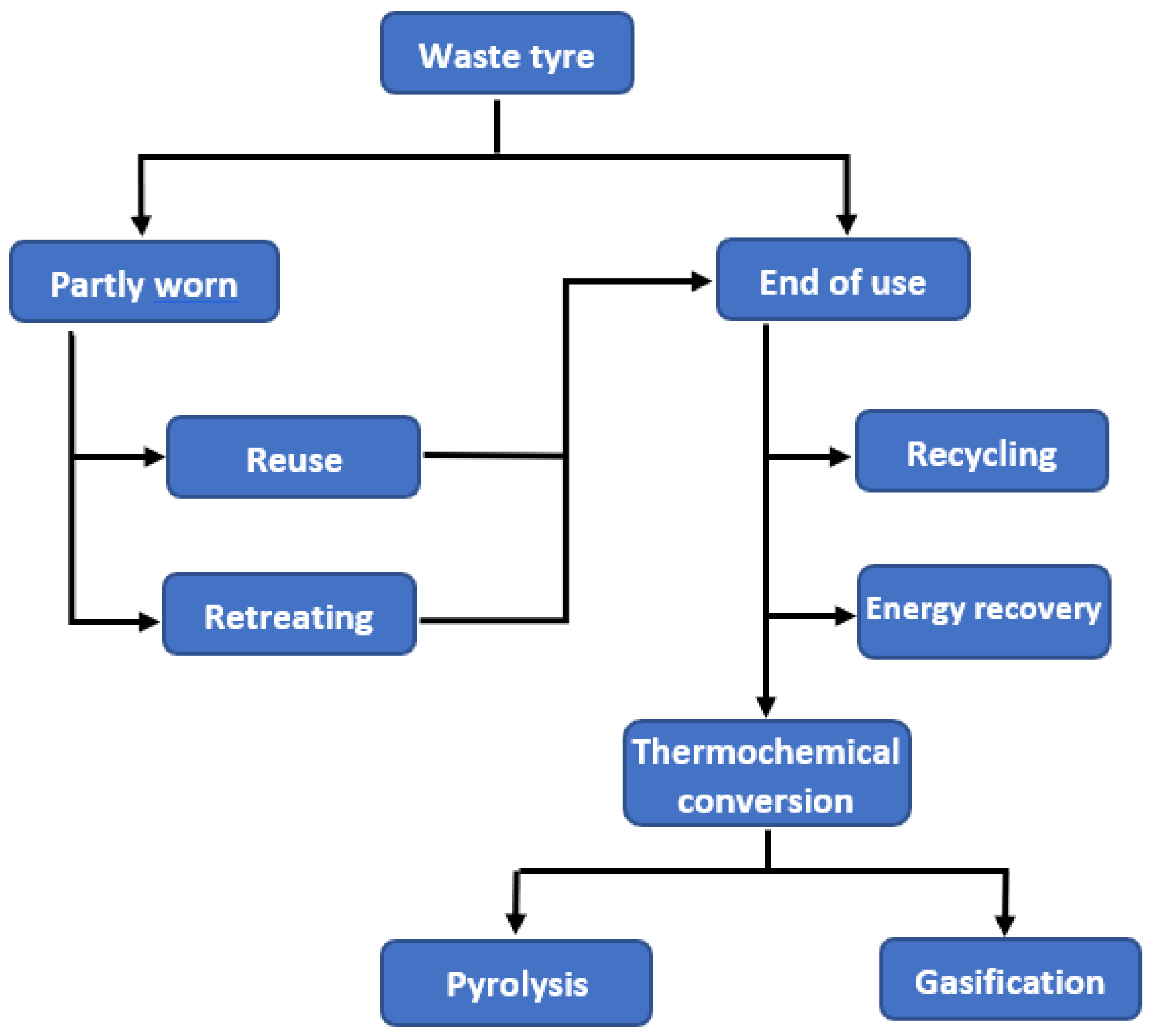
2. Waste Tyre Management Practice
2.1. Reuse and Rethreading
2.2. Recycling
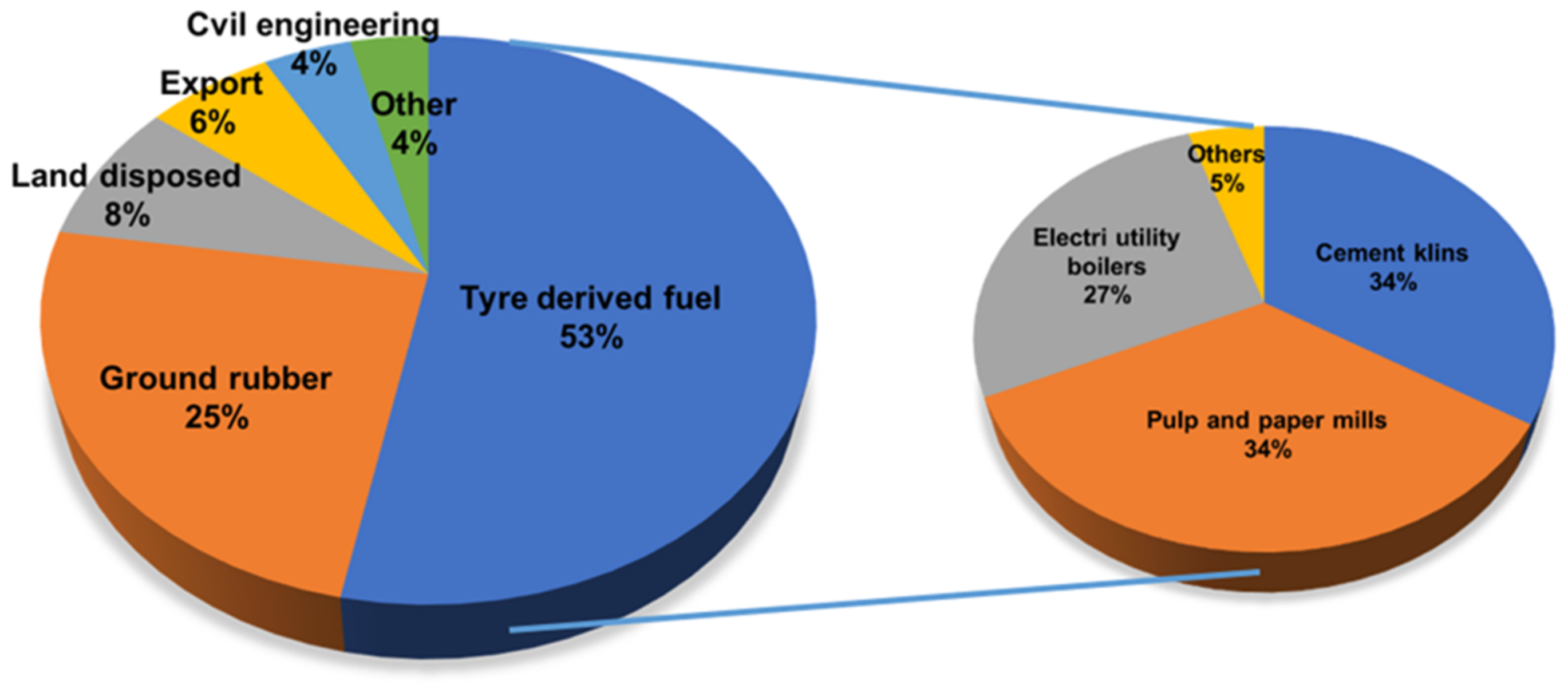
2.3. Energy Recovery
3. Waste Tyre to Fuel Using Thermochemical Conversion
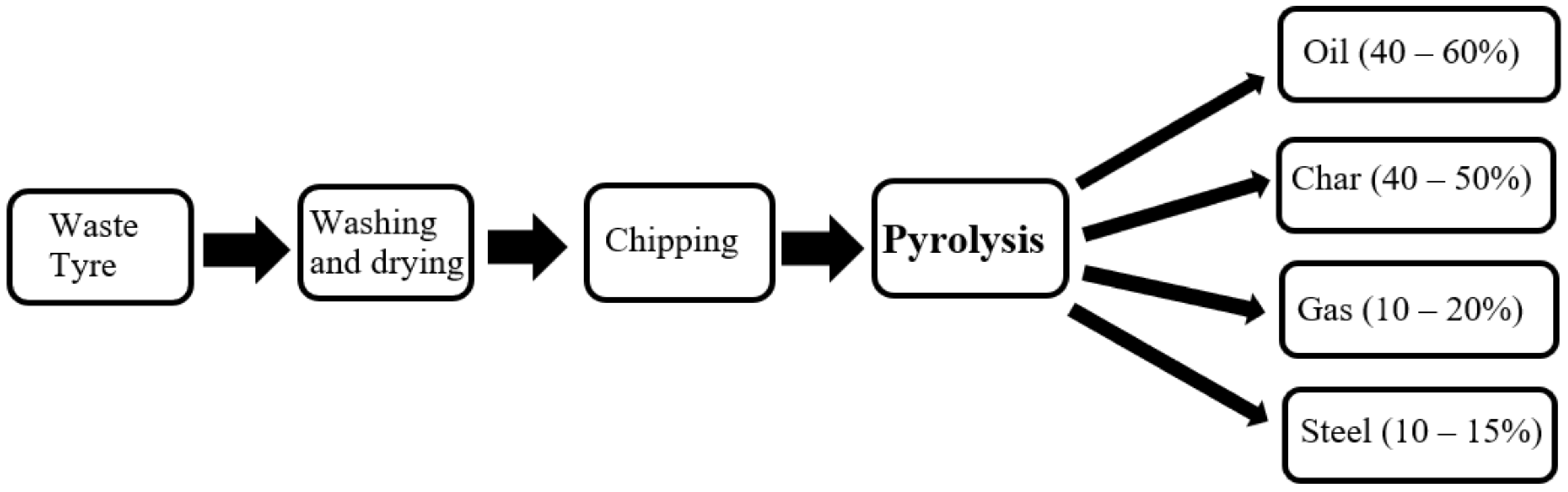
3.1. Waste Tyre Conversion Using Pyrolysis
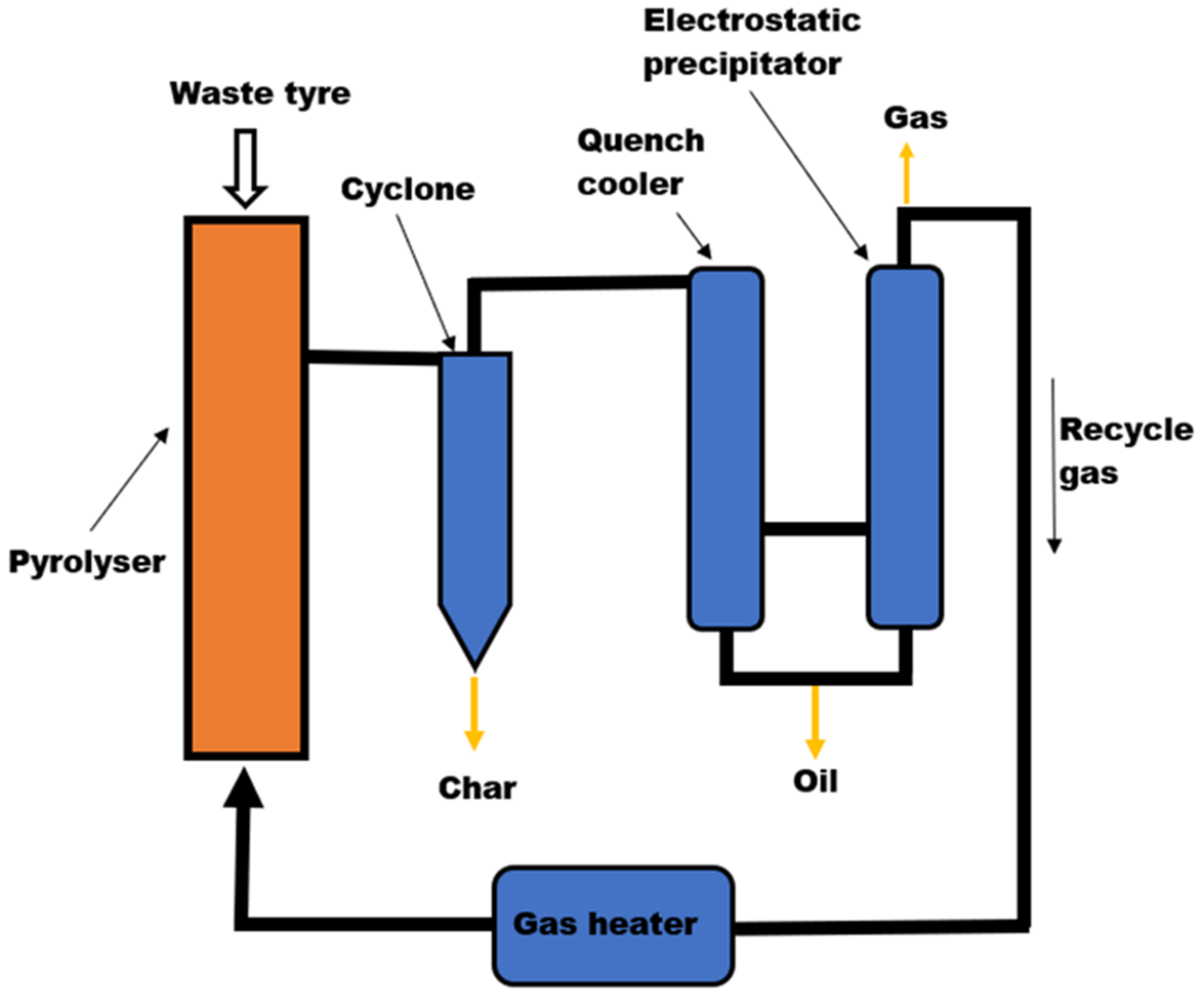
3.1.1. Tyre Pyrolysis Reactors
Fixed Bed Pyrolysis Reactor
Fluidised Bed Reactor
Rotary Kiln Reactor
Screw Bed Reactor
4. Waste Tyre to Oil, Carbon, and Steel
4.1. Oil from Waste Tyres
4.2. Char from Waste Tyres
4.3. Gas from Waste Tyre
4.4. Steel from Waste Tyre
5. Diesel Engine Performance and Exhaust Emission Using Tyre Oil
6. Discussion and Synthesis
- Conduct in-depth energy and economic studies of integrated waste tyre pyrolysis plants over their entire life cycle.
- Recognise the trade-offs between the scale of the waste tyre pyrolysis plant and feedstock, as well as the costs of transportation to a centralised upgrading facility.
- Development of the technology to overcome the limitations of the tyre pyrolysis reactor and process and improve the reliability.
- Identify TPO criteria and quality standards for manufacturers and end-user.
- Improve quality and consistency of TPO through the development of more effective technologies.
- Develop catalyst for TPO upgrading in order to meet vehicle fuel-quality standards.
- Develop deoxygenated catalysts to extract oxygen-containing compounds for pyrolysis processes for oil property improvement.
- Advocacy to develop relevant policy, regulation, and financial incentives for the tyre recyclers, refineries and start-ups who take up the challenges of recycling used tyres to oil.
7. Conclusions
- ⮚
- The product yield and composition of waste tyre pyrolysis depends on reactor type and operating condition. The average production yield of oil, char, syngas, and steel from waste tyre pyrolysis is 40–60%, 40–50%, 10–20%, and 10–15% by weight.
- ⮚
- The pyrolysis temperature range of 450–500 °C is favourable for the high yield of liquid oil, whereas over 600 °C is for gas, and below 400 °C is for solid char production.
- ⮚
- Waste tyre pyrolysis products (oil, gas, and char) contain a high calorific value which is suitable to be directly used as a heat source in boiler, furnace, and other applications.
- ⮚
- The cetane index of WTO is much lower than that of petroleum diesel and biodiesel. The other important properties for automobile engine application, such as density, kinematic viscosity, and aromatic content of TPO, were higher than diesel and biodiesel.
- ⮚
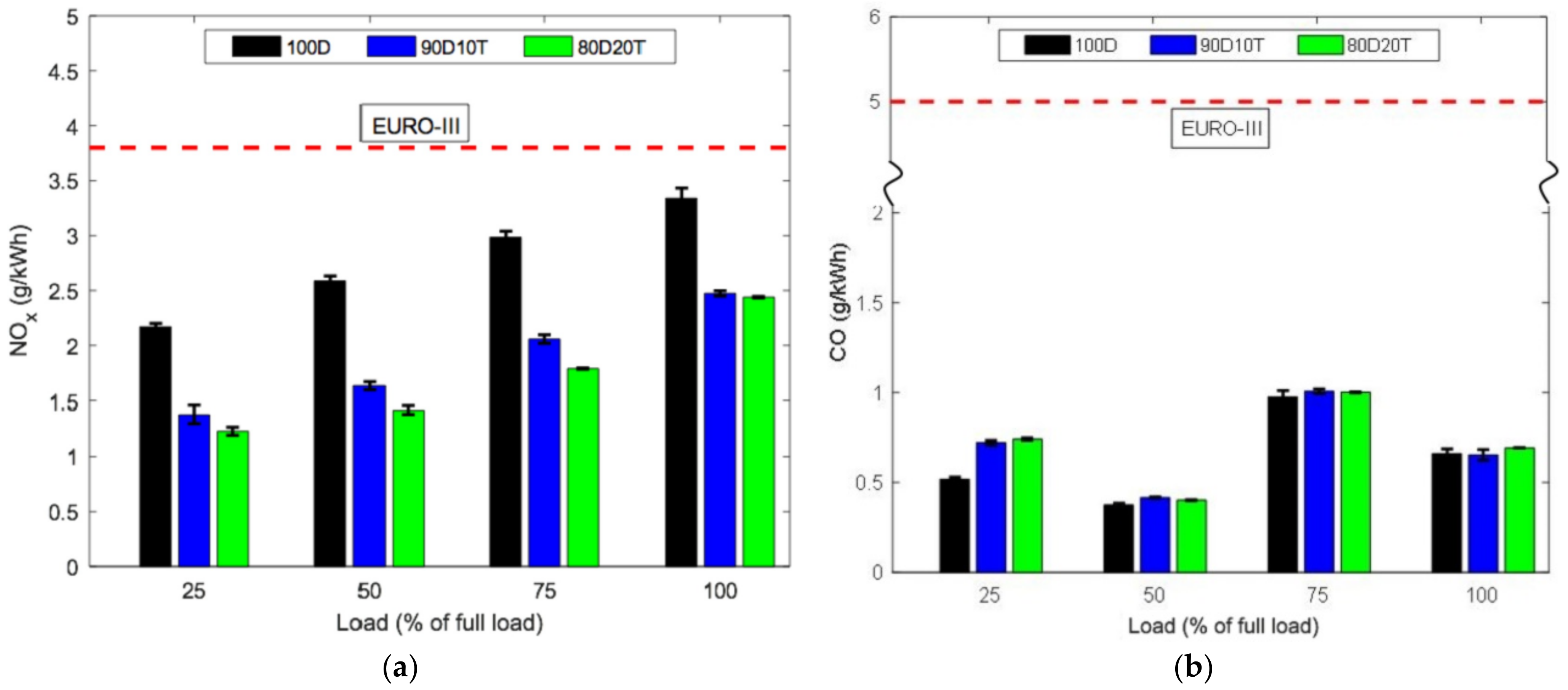
- Many studies have attempted to run diesel with pure TPO and attempted to run diesel with pure TPO and blended with diesel to observe engine combustion performance and exhaust emissions. The summary of these research findings indicated similar power output of engines with TPO and diesel. However, ignition delay and cylinder pressure were found to be higher with TPO due to its high density and viscosity. Incorporated with high content of aromatic compound and combustion variations, TPO shows significantly higher NOx, CO, CO, SOx, and HC emission reported by most of the studies.
- ⮚
- Although pure TPO is not recommended to directly use in commercial automobile engine fuel due to engine durability and exhaust emission issues, a blending below 20% of TPO with diesel can be utilisd.
- ⮚
- The commercialisation and industrial production of waste tyre to commercial automobile liquid fuel requires more in-depth techno-economic assessment and quality improvement of WTO.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uyumaz, A.; Aydoğan, B.; Solmaz, H.; Yılmaz, E.; Yeşim Hopa, D.; Aksoy Bahtli, T.; Solmaz, Ö.; Aksoy, F. Production of waste tyre oil and experimental investigation on combustion, engine performance and exhaust emissions. J. Energy Inst. 2019, 92, 1406–1418. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. A comparative study on the performance, emission and combustion studies of a DI diesel engine using distilled tyre pyrolysis oil–diesel blends. Fuel 2008, 87, 2111–2121. [Google Scholar] [CrossRef]
- Saidur, R.; Jahirul, M.I.; Moutushi, T.Z.; Imtiaz, H.; Masjuki, H.H. Effect of partial substitution of diesel fuel by natural gas on performance parameters of a four-cylinder diesel engine. Proc. Inst. Mech. Eng. Part. A J. Power Energy 2007, 221, 1–10. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Koh, W.; Brown, R.J.; Senadeera, W.; Hara, I.; Moghaddam, L. Biodiesel Production from Non-Edible Beauty Leaf (Calophyllum inophyllum) Oil: Process Optimization Using Response Surface Methodology (RSM). Energies 2014, 7, 5317–5331. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Pourkhesalian, A.M.; Jahirul, M.I.; Stevanovic, S.; Pham, P.X.; Wang, H.; Masri, A.R.; Brown, R.J.; Ristovski, Z.D. Particle emissions from biodiesels with different physical properties and chemical composition. Fuel 2014, 134, 201–208. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Brown, R.J.; Senadeera, W.; Ashwath, N.; Rasul, M.G.; Rahman, M.M.; Hossain, F.M.; Moghaddam, L.; Islam, M.A.; O’Hara, I.M. Physio-chemical assessment of beauty leaf (Calophyllum inophyllum) as second-generation biodiesel feedstock. Energy Rep. 2015, 1, 204–215. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Fazal, M.A.; Jahirul, M.I.; Masjuki, H.H. Compatibility of automotive materials in biodiesel: A review. Fuel 2011, 90, 922–931. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Brown, R.J.; Senadeera, W.; O’Hara, I.M.; Ristovski, Z.D. The Use of Artificial Neural Networks for Identifying Sustainable Biodiesel Feedstocks. Energies 2013, 6, 3764–3806. [Google Scholar] [CrossRef] [Green Version]
- Guan, C.; Cheung, C.S.; Li, X.; Huang, Z. Effects of oxygenated fuels on the particle-phase compounds emitted from a diesel engine. Atmos. Pollut. Res. 2017, 8, 209–220. [Google Scholar] [CrossRef]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Feng, Z.; Zhan, C.; Tang, C.; Yang, K.; Huang, Z. Experimental investigation on spray and atomization characteristics of diesel/gasoline/ethanol blends in high pressure common rail injection system. Energy 2016, 112, 549–561. [Google Scholar] [CrossRef]
- Valentino, G.; Corcione, F.E.; Iannuzzi, S.E.; Serra, S. Experimental study on performance and emissions of a high speed diesel engine fuelled with n-butanol diesel blends under premixed low temperature combustion. Fuel 2012, 92, 295–307. [Google Scholar] [CrossRef]
- Wang, W.-C.; Bai, C.-J.; Lin, C.-T.; Prakash, S. Alternative fuel produced from thermal pyrolysis of waste tires and its use in a DI diesel engine. Appl. Therm. Eng. 2016, 93, 330–338. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Omar, R.; Idris, A. Microwave Pyrolysis for Conversion of Materials to Energy: A Brief Review. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 2104–2122. [Google Scholar] [CrossRef]
- Damodharan, D.; Rajesh Kumar, B.; Gopal, K.; de Poures, M.V.; Sethuramasamyraja, B. Utilization of waste plastic oil in diesel engines: A review. Rev. Environ. Sci. BioTechnol. 2019, 18, 681–697. [Google Scholar] [CrossRef]
- İlkılıç, C.; Aydın, H. Fuel production from waste vehicle tires by catalytic pyrolysis and its application in a diesel engine. Fuel Process. Technol. 2011, 92, 1129–1135. [Google Scholar] [CrossRef]
- Aydın, H.; İlkılıç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 2012, 102, 605–612. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- ETRMA. European Tyre and Rubber Industry Statistics. European Tyre and Rubber Manufacturing Association. Available online: http://www.etrma.org (accessed on 20 December 2014).
- Martínez, J.D.; Rodríguez-Fernández, J.; Sánchez-Valdepeñas, J.; Murillo, R.; García, T. Performance and emissions of an automotive diesel engine using a tire pyrolysis liquid blend. Fuel 2014, 115, 490–499. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Kucinska-Lipka, J.; Janik, H.; Balas, A. Progress in used tyres management in the European Union: A review. Waste Manag. 2012, 32, 1742–1751. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Choi, H.S.; Park, H.C.; Hwang, J.G.; Yoo, H.S.; Lee, B.-K.; Upadhyay, M. Influence of process conditions on product yield of waste tyre pyrolysis—A review. Korean J. Chem. Eng. 2016, 33, 2268–2286. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Zare, A.; Jafari, M.; Bodisco, T.A.; Rainey, T.; Ristovski, Z.D.; Brown, R.J. Diesel engine performance and emissions with fuels derived from waste tyres. Sci. Rep. 2018, 8, 2457. [Google Scholar] [CrossRef] [Green Version]
- Mastral, A.M.; Murillo, R.; Callén, M.S.; García, T. Application of coal conversion technology to tire processing. Fuel Process. Technol. 1999, 60, 231–242. [Google Scholar] [CrossRef]
- Mastral, A.M.; Murillo, R.; Callén, M.S.; García, T.; Snape, C.E. Influence of Process Variables on Oils from Tire Pyrolysis and Hydropyrolysis in a Swept Fixed Bed Reactor. Energy Fuels 2000, 14, 739–744. [Google Scholar] [CrossRef]
- Gent, A.N. Chapter 1—Rubber Elasticity: Basic Concepts and Behavior. In The Science and Technology of Rubber, 4th ed.; Mark, J.E., Erman, B., Roland, C.M., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 1–26. [Google Scholar] [CrossRef]
- Kyari, M.; Cunliffe, A.; Williams, P.T. Characterization of Oils, Gases, and Char in Relation to the Pyrolysis of Different Brands of Scrap Automotive Tires. Energy Fuels 2005, 19, 1165–1173. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wang, C.L. Kinetic study of scrap tyre pyrolysis and combustion. J. Anal. Appl. Pyrolysis 1998, 45, 153–169. [Google Scholar] [CrossRef]
- Lorber, K.E.; Sarc, R.; Aldrian, A. Design and quality assurance for solid recovered fuel. Waste Manag. Res. 2012, 30, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Sarc, R.; Lorber, K.E. Production, quality and quality assurance of Refuse Derived Fuels (RDFs). Waste Manag. 2013, 33, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Sarc, R.; Lorber, K.E.; Pomberger, R.; Rogetzer, M.; Sipple, E.M. Design, quality, and quality assurance of solid recovered fuels for the substitution of fossil feedstock in the cement industry. Waste Manag. Res. 2014, 32, 565–585. [Google Scholar] [CrossRef]
- Dai, X.; Yin, X.; Wu, C.; Zhang, W.; Chen, Y. Pyrolysis of waste tires in a circulating fluidized-bed reactor. Energy 2001, 26, 385–399. [Google Scholar] [CrossRef]
- Banar, M.; Akyıldız, V.; Özkan, A.; Çokaygil, Z.; Onay, Ö. Characterization of pyrolytic oil obtained from pyrolysis of TDF (Tire Derived Fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Rasul, M.G.; Jahirul, M.I. Recent Developments in Biomass Pyrolysis for Bio-Fuel Production: Its Potential for Commercial Applications; Centre for Plant and Water Science, Faculty of Sciences, Engineering and Health, Central Queensland University: Norman Gardens, Australia, 2012; pp. 256–265. [Google Scholar]
- Donatelli, A.; Iovane, P.; Molino, A. High energy syngas production by waste tyres steam gasification in a rotary kiln pilot plant. Experimental and numerical investigations. Fuel 2010, 89, 2721–2728. [Google Scholar] [CrossRef]
- Janajreh, I.; Raza, S.S. Numerical simulation of waste tyres gasification. Waste Manag. Res. 2015, 33, 460–468. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, B.; Duan, P.; Wang, F.; Xu, Y. Hydrothermal conversion of scrap tire to liquid fuel. Chem. Eng. J. 2016, 285, 157–163. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.; Rasul, M.G.; Bhuiya, M.M.K. Energy Recovery from Biomass by Fast Pyrolysis. Procedia Eng. 2014, 90, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Tudu, K.; Murugan, S.; Patel, S.K. Effect of diethyl ether in a DI diesel engine run on a tyre derived fuel-diesel blend. J. Energy Inst. 2016, 89, 525–535. [Google Scholar] [CrossRef]
- Vihar, R.; Seljak, T.; Rodman Oprešnik, S.; Katrašnik, T. Combustion characteristics of tire pyrolysis oil in turbo charged compression ignition engine. Fuel 2015, 150, 226–235. [Google Scholar] [CrossRef]
- Sharma, A.; Murugan, S. Potential for using a tyre pyrolysis oil-biodiesel blend in a diesel engine at different compression ratios. Energy Convers. Manag. 2015, 93, 289–297. [Google Scholar] [CrossRef]
- Zebala, J.; Ciepka, P.; Reza, A.; Janczur, R. Influence of rubber compound and tread pattern of retreaded tyres on vehicle active safety. Forensic Sci. Int. 2007, 167, 173–180. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Y.; Zhao, X.; Sun, J.; Wang, W.; Mao, Y.; Ma, C. Microwave pyrolysis of tire powders: Evolution of yields and composition of products. J. Anal. Appl. Pyrolysis 2017, 123, 152–159. [Google Scholar] [CrossRef]
- Ferrer, G. The economics of tire remanufacturing. Resour. Conserv. Recycl. 1997, 19, 221–255. [Google Scholar] [CrossRef]
- Gieré, R.; Smith, K.; Blackford, M. Chemical composition of fuels and emissions from a coal+tire combustion experiment in a power station. Fuel 2006, 85, 2278–2285. [Google Scholar] [CrossRef]
- Pipilikaki, P.; Katsioti, M.; Papageorgiou, D.; Fragoulis, D.; Chaniotakis, E. Use of tire derived fuel in clinker burning. Cem. Concr. Compos. 2005, 27, 843–847. [Google Scholar] [CrossRef]
- Collins, K.J.; Jensen, A.C.; Mallinson, J.J.; Roenelle, V.; Smith, I.P. Environmental impact assessment of a scrap tyre artificial reef. ICES J. Mar. Sci. 2002, 59, S243–S249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Bridgewater, A.V. Thermal Conversion of Biomass and Waste; Bio-Energy Research Group Aston University: Birmingham, UK, 2001. [Google Scholar]
- Bridgewater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energ. 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Doğan, O.; Çelik, M.B.; Özdalyan, B. The effect of tire derived fuel/diesel fuel blends utilization on diesel engine performance and emissions. Fuel 2012, 95, 340–346. [Google Scholar] [CrossRef]
- White, J.E.; Catall, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrol. 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kothari, R.; Tyagi, V.V. Thermo chemical conversion of biomass—Eco friendly energy routes. Renew. Sustain. Energy Rev. 2012, 16, 1801–1816. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Capareda, S.C. Biomass Energy Conversion. Texas A&M University, USA. Available online: https://www.intechopen.com/ (accessed on 22 November 2013).
- Ceylan, R.; Bredenberg, J.B.s. Hydrogenolysis and hydrocracking of the carbon-oxygen bond. 2. Thermal cleavage of the carbon-oxygen bond in guaiacol. Fuel 1982, 61, 377–382. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Kumaravel, S.T.; Murugesan, A.; Kumaravel, A. Tyre pyrolysis oil as an alternative fuel for diesel engines—A review. Renew. Sustain. Energy Rev. 2016, 60, 1678–1685. [Google Scholar] [CrossRef]
- Miranda, M.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of rubber tyre wastes: A kinetic study. Fuel 2013, 103, 542–552. [Google Scholar] [CrossRef]
- Kar, Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Manag. 2011, 31, 1772–1782. [Google Scholar] [CrossRef]
- Islam, M.R.; Joardder, M.U.H.; Kader, M.; Sarker, M. Valorization of solid tire wastes available in Bangladesh by thermal treatment. In Proceedings of the WasteSafe 2011—2nd International Conference on Solid Waste Management in the Developing Countries, Khulna, Bangladesh, 13–15 February 2011. [Google Scholar]
- Williams, P.T.; Brindle, A.J. Catalytic pyrolysis of tyres: Influence of catalyst temperature. Fuel 2002, 81, 2425–2434. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.; García, T.; Mastral, A. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Williams, P.T. Carbon nanotubes and hydrogen production from the pyrolysis catalysis or catalytic-steam reforming of waste tyres. J. Anal. Appl. Pyrolysis 2016, 122, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Feng, Y. The production of fuel oil and combustible gas by catalytic pyrolysis of waste tire using waste heat of blast-furnace slag. Energy Convers. Manag. 2017, 136, 27–35. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Sangeeta, C.; Jain, A.K. A review of fixed bed gasification systems for biomass. Agric. Eng. Int. 2007, 9, 5. [Google Scholar]
- Altafini, C.R.; Wander, P.R.; Barreto, R.M. Prediction of the working parameters of a wood waste gasifier through an equilibrium model. Energy Convers. Manag. 2003, 44, 2763–2777. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Yin, X.L.; Wu, C.Z. A review on the development and commercialization of biomass gasification technologies in China. Renew. Sustain. Energy Rev. 2004, 8, 565–580. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, R.; Zhang, H. Ex-situ catalytic fast pyrolysis of biomass over HZSM-5 in a two-stage fluidized-bed/fixed-bed combination reactor. Bioresour. Technol. 2017, 243, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, Z.; Ding, K.; Li, M.; Hao, N.; Meng, X.; Ruan, R.; Ragauskas, A.J. Catalytic fast co-pyrolysis of bamboo sawdust and waste tire using a tandem reactor with cascade bubbling fluidized bed and fixed bed system. Energy Convers. Manag. 2019, 180, 60–71. [Google Scholar] [CrossRef]
- Yang, M.; Shao, J.; Yang, H.; Zeng, K.; Wu, Z.; Chen, Y.; Bai, X.; Chen, H. Enhancing the production of light olefins and aromatics from catalytic fast pyrolysis of cellulose in a dual-catalyst fixed bed reactor. Bioresour. Technol. 2019, 273, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Edwin Raj, R.; Robert Kennedy, Z.; Pillai, B.C. Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers. Manag. 2013, 67, 145–151. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Cracking of Scrap Tires Pyrolysis Oil in a Fluidized Bed Reactor under Catalytic Cracking Unit Conditions. Effects of Operating Conditions. Energy Fuels 2019, 33, 3133–3143. [Google Scholar] [CrossRef]
- Kaminsky, W. 8—Fluidized bed pyrolysis of waste polymer composites for oil and gas recovery. In Management, Recycling and Reuse of Waste Composites; Goodship, V., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 192–213. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Jahirul, M.I. Energy recovery from municipal solid waste using pyrolysis technology: A review on current status and developments. Renew. Sustain. Energy Rev. 2021, 145, 111073. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Park, Y.-K.; Ryu, C.; Suh, D.J.; Suh, Y.-W.; Yim, J.-H.; Kim, S.-S. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour. Technol. 2010, 101, S91–S96. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Fantozzi, F.; Colantoni, S.; Bartocci, P.; Desideri, U. Rotary Kiln Slow Pyrolysis for Syngas and Char Production From Biomass and Waste—Part I: Working Envelope of the Reactor. J. Eng. Gas. Turbines Power 2007, 129, 901–907. [Google Scholar] [CrossRef]
- Bortolamasi, M.F.J. Design and sizing of screw feeders. Proceedings of International Congress for Particle Technology (PARTEC), Nuremberg, Germany, 27–29 March 2001. [Google Scholar]
- Badger, P.C.; Fransham, P. Use of mobile fast pyrolysis plants to densify biomass and reduce biomass handling costs—A preliminary assessment. Biomass. Bioenergy 2006, 30, 321–325. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C.; Zhang, Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J. Anal. Appl. Pyrolysis 2009, 85, 334–337. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Heat-treatment of carbon blacks obtained by pyrolysis of used tires. Effect on the surface chemistry, porosity and electrical conductivity. J. Anal. Appl. Pyrolysis 2003, 67, 55–76. [Google Scholar] [CrossRef]
- Islam, M.A.; Rahman, M.M.; Heimann, K.; Nabi, M.N.; Ristovski, Z.D.; Dowell, A.; Thomas, G.; Feng, B.; von Alvensleben, N.; Brown, R.J. Combustion analysis of microalgae methyl ester in a common rail direct injection diesel engine. Fuel 2015, 143, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. The use of tyre pyrolysis oil in diesel engines. Waste Manag. 2008, 28, 2743–2749. [Google Scholar] [CrossRef]
- Hariharan, S.; Murugan, S.; Nagarajan, G. Effect of diethyl ether on Tyre pyrolysis oil fueled diesel engine. Fuel 2013, 104, 109–115. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Brown, J.R.; Senadeera, W.; Ashwath, N.; Laing, C.; Leski-Taylor, J.; Rasul, M.G. Optimisation of Bio-Oil Extraction Process from Beauty Leaf (Calophyllum Inophyllum) Oil Seed as a Second Generation Biodiesel Source. Procedia Eng. 2013, 56, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Conesa, J.A.; Martín-Gullón, I.; Font, R.; Jauhiainen, J. Complete Study of the Pyrolysis and Gasification of Scrap Tires in a Pilot Plant Reactor. Environ. Sci. Technol. 2004, 38, 3189–3194. [Google Scholar] [CrossRef]
- Olazar, M.; Aguado, R.; Arabiourrutia, M.; Lopez, G.; Barona, A.; Bilbao, J. Catalyst effect on the composition of tire pyrolysis products. Energy Fuels 2008, 22, 2909–2916. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Teng, H.; Serio, M.A.; Wojtowicz, M.A.; Bassilakis, R.; Solomon, P.R. Reprocessing of used tires into activated carbon and other products. Ind. Eng. Chem. Res. 1995, 34, 3102–3111. [Google Scholar] [CrossRef]
- Aylón, E.; Murillo, R.; Fernández-Colino, A.; Aranda, A.; García, T.; Callén, M.S.; Mastral, A.M. Emissions from the combustion of gas-phase products at tyre pyrolysis. J. Anal. Appl. Pyrolysis 2007, 79, 210–214. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Yin, X.L.; Zhao, Z.L.; Xu, B.Y.; Chen, Y. Pyrolysis of tire powder: Influence of operation variables on the composition and yields of gaseous product. Fuel Process. Technol. 2002, 79, 141–155. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. Liquefaction of waste tires by pyrolysis for oil and chemicals—A review. J. Anal. Appl. Pyrolysis 2013, 101, 1–16. [Google Scholar] [CrossRef]
- Žvar Baškovič, U.; Vihar, R.; Seljak, T.; Katrašnik, T. Feasibility analysis of 100% tire pyrolysis oil in a common rail Diesel engine. Energy 2017, 137, 980–990. [Google Scholar] [CrossRef] [Green Version]
- Hossain, F.M.; Nabi, M.N.; Rainey, T.J.; Bodisco, T.; Bayley, T.; Randall, D.; Ristovski, Z.; Brown, R.J. Novel biofuels derived from waste tyres and their effects on reducing oxides of nitrogen and particulate matter emissions. J. Clean. Prod. 2020, 242, 118463. [Google Scholar] [CrossRef]
- Vihar, R.; Žvar Baškovič, U.; Seljak, T.; Katrašnik, T. Combustion and emission formation phenomena of tire pyrolysis oil in a common rail Diesel engine. Energy Convers. Manag. 2017, 149, 706–721. [Google Scholar] [CrossRef] [Green Version]
- Pote, R.N.; Patil, R.K. Combustion and emission characteristics analysis of waste tyre pyrolysis oil. SN Appl. Sci. 2019, 1, 294. [Google Scholar] [CrossRef] [Green Version]
- Koc, A.B.; Abdullah, M. Performance of a 4-cylinder diesel engine running on tire oil–biodiesel–diesel blend. Fuel Process. Technol. 2014, 118, 264–269. [Google Scholar] [CrossRef]
- Frigo, S.; Seggiani, M.; Puccini, M.; Vitolo, S. Liquid fuel production from waste tyre pyrolysis and its utilisation in a Diesel engine. Fuel 2014, 116, 399–408. [Google Scholar] [CrossRef]

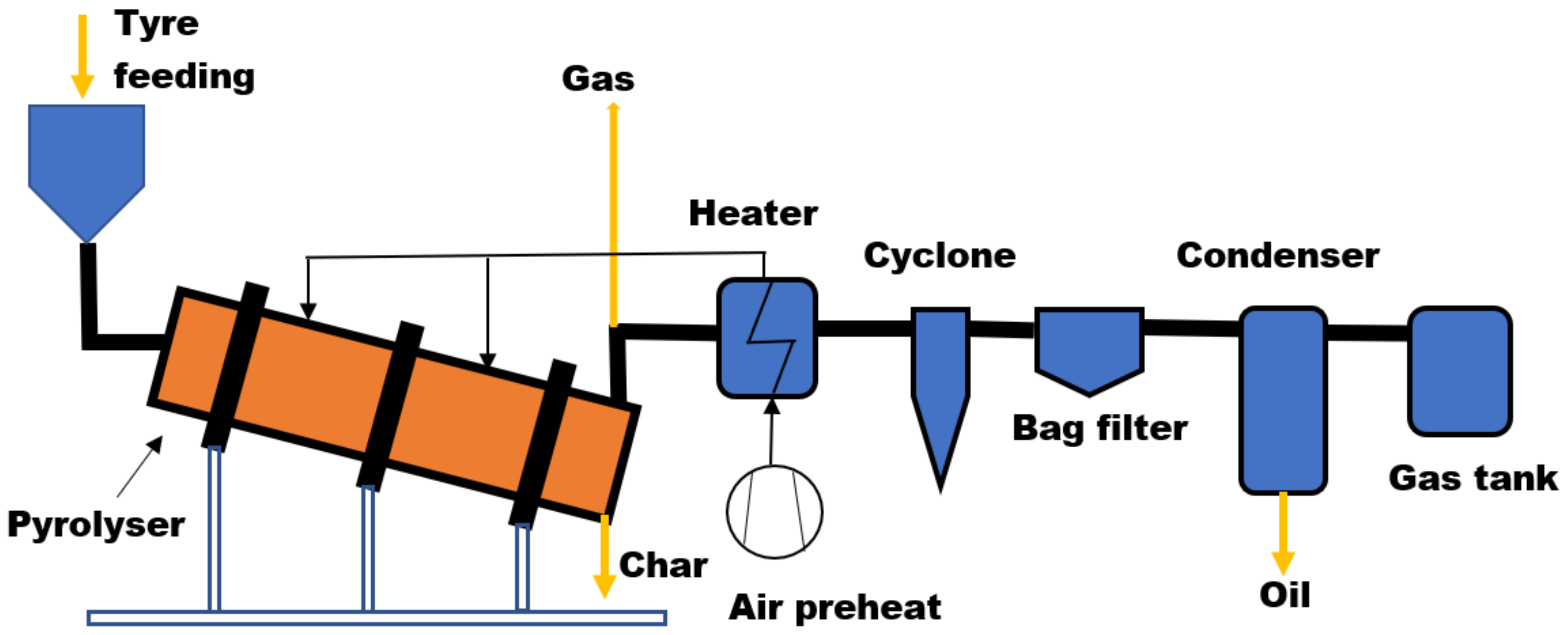
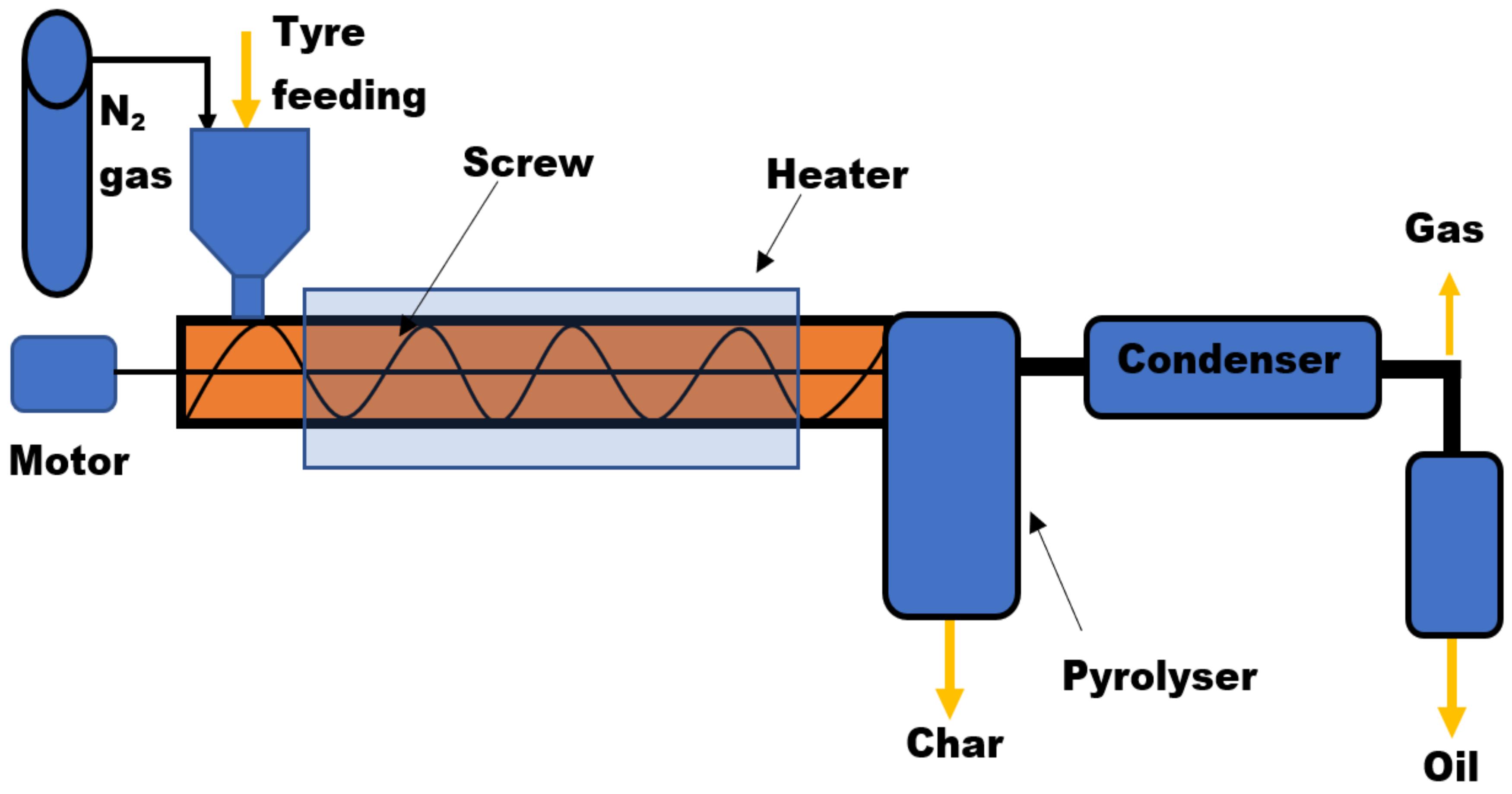
| Reactor Type | Temp. (°C) | Maximum Oil Yield (wt%) | References | ||
|---|---|---|---|---|---|
| Oil | Char | Gas | |||
| Fixed bed, batch | 500 | 40.26 | 47.88 | 11.86 | [17] |
| Fixed bed, batch | 450 | 63 | 30 | 7 | [66] |
| Fixed bed, batch | 500 | 54.12 | 20.22 | 26.40 | [67] |
| Fixed bed, batch | 400 | 38.8 | 34.0 | 27.2 | [34] |
| Fixed bed, batch, | 475 | 55 | 36 | 9 | [68] |
| Fluidised bed | 450 | 55 | 42.5 | 2.5 | [69] |
| Fluidised bed | 450 | 52 | 28 | 15 | [33] |
| Moving screw bed | 600 | 48.4 | 39.9 | 11.7 | [70] |
| Two stages fixed bed | 600 | 22 | 37 | 27.2 | [71] |
| Microwave oven reactor | - | 43 | 45 | 12 | [46] |
| Rotary kiln | 600 | 40.21 | 52.67 | 7.12 | [72] |
| Rotary kiln | 500 | 45.1 | 41.3 | 13.6 | [73] |
| Rotary kiln | 550 | 38.12 | 49.09 | 2.39 | [73] |
| Vacuum | 500 | 56.5 | 33.4 | 10.1 | [74] |
| Vacuum | 550 | 47.1 | 36.9 | 16 | [74] |
| Properties | Units | Diesel | Biodiesel | TPO | References |
|---|---|---|---|---|---|
| Density | kg/L | 0.83 | 0.88 | 0.91–0.96 | [19,43,94] |
| HHV | MJ/kg | 45.6 | 37.2 | 38–42 | [19,43,94] |
| Water content | mg/kg | <30 | -- | 118 | [19,43] |
| Aromatic | % m/m | 26.0 | -- | 39.3 | [19,43,95] |
| Kinematic V. | mm2/s | 2.66 | 4.73 | 3.22–6.3 | [19,43,94] |
| Carbon (C) | wt% | 87.0 | 76.9 | 79.61–88 | [18,19,43,94] |
| Hydrogen (H) | wt% | 13.0 | 12.2 | 9.4–11.73 | [18,19,43,94] |
| Nitrogen (N) | wt% | -- | -- | 0.40–1.05 | [18,19,43,94] |
| Oxygen (O) | wt% | 0 | 10.9 | 0.5–4.62 | [19,43,94,96] |
| Ash content | wt% | 0.01 | 0.02–0.31 | [19,96] | |
| Flash point | °C | 50 | 130 | 20–65 | [19,96,97] |
| Cetane index | 53.2 | 58.6 | 28.6 | [43,94] |
| Properties | Units | Tyre Pyrolysis Char | |||
|---|---|---|---|---|---|
| [98] | [87] | [73] | [99] | ||
| HHV | MJ/kg | 30.8 | 31.5 | 30.7 | 29.3 |
| Water content | mg/kg | 0.37 | 2.35 | 3.57 | |
| Carbon (C) | wt% | 88.19 | 82.17 | 85.31 | 80.3 |
| Hydrogen (H) | wt% | 0.6 | 2.28 | 1.77 | 1.3 |
| Nitrogen (N) | wt% | 0.1 | 0.61 | 0.34 | 0.3 |
| Sulphur (S) | wt% | 1.9 | 2.32 | 2.13 | 2.7 |
| Ash content | wt% | 8.27 | 12.32 | 15.33 | |
| Engine Specification | Test Condition | TPO Fraction | Increase/Decrease vs. Diesel | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power | Torque | BSFC | BTE | Te | ID | CP | CO | Smoke | CO2 | NOx | SO2 | HC | ||||
| 6.8 L, 6C, 4S, CI, CR: 18:1 RP: 162 kW, RS: 2400 rpm | S: 1500–2400 rpm L: 80–140 Nm | 100% |  |  |  |  |  |  |  | [43] | ||||||
| 5.9 L, 6C, 4S, CI, CR: 17.3:1 RP: 162 kW, RS: 2000 rpm | S: 1500 rpm L: 25–100% | 10%, 20% |  |  |  |  |  |  | [106] | |||||||
| 1.6 L, 4C, 4S, CI, CR: 18:1 RP: 66 kW, RS: 4000 rpm | S: 1500 rpm L: 80–140 Nm | 100% |  |  |  |  |  |  |  | [105] | ||||||
| 0.66 L, 1C, 4S, CI, CR: 17.5:1 RP: 4.4 kW, RS: 1500 rpm | S: 1500 rpm L: 1.1–4.4 kW | 40% |  |  |  | [42] | ||||||||||
| 0.40 L, 1C, 4S, CI, CR: 18:1 RP: 7.5 kW, RS: 3600 rpm | S: 1000–1500 rpm L: 1.1–4.4 kW | 5–100% |  |  |  |  |  |  |  |  | [16] | |||||
| 1.6 L, 4C, 4S, CI, CR: 18:1 RP: 66 kW, RS: 4000 rpm | S: 1500–3000 rpm L: 60–100 Nm | 50% |  |  |  |  | [107] | |||||||||
| 0.5 L, 1C, 4S, CI, CR: 17.5:1 RP: 3.7 kW, RS: 1500 rpm | S: 1500 rpm L: 80–140 Nm | 10% |  |  |  |  |  |  |  | [96] | ||||||
| 0.5 L, 1C, 4S, CI, CR: 18.1:1 RP: 3.5 kW, RS: 1500 rpm | S: 1500 rpm L: 0.22–7.4 kg | 10–90% |  |  |  |  |  |  |  |  |  |  | [108] | |||
| 0.4 L, 1C, 4S, CI, CR: 18:1 RP: 5.4 kW, RS: 3000 rpm | S: 1500 rpm L: 3.75–15 Nm | 10% |  |  |  |  |  |  |  |  | [1] | |||||
| 3.3 L, 4C, 4S, CI, CR: 18:1 RP: 60 kW, RS: 2200 rpm | S: 1000–2800 rpm | 5–10% |  |  |  |  |  |  |  | [109] | ||||||
| 0.44 L, 1C, 4S, CI, CR: 20:1 RP: 7.1 kW, RS: 3600 rpm | S: 2000–3000 rpm L: 50–100% | 20–80% |  |  |  |  | [110] | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahirul, M.I.; Hossain, F.M.; Rasul, M.G.; Chowdhury, A.A. A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application. Energies 2021, 14, 3837. https://doi.org/10.3390/en14133837
Jahirul MI, Hossain FM, Rasul MG, Chowdhury AA. A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application. Energies. 2021; 14(13):3837. https://doi.org/10.3390/en14133837
Chicago/Turabian StyleJahirul, Mohammad I., Farhad M. Hossain, Mohammad G. Rasul, and Ashfaque Ahmed Chowdhury. 2021. "A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application" Energies 14, no. 13: 3837. https://doi.org/10.3390/en14133837
APA StyleJahirul, M. I., Hossain, F. M., Rasul, M. G., & Chowdhury, A. A. (2021). A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application. Energies, 14(13), 3837. https://doi.org/10.3390/en14133837








