Static Temperature Guideline for Comparative Testing of Sorption Heat Storage Systems for Building Application
Abstract
1. Introduction
2. Operating Principle
3. Reporting in Literature
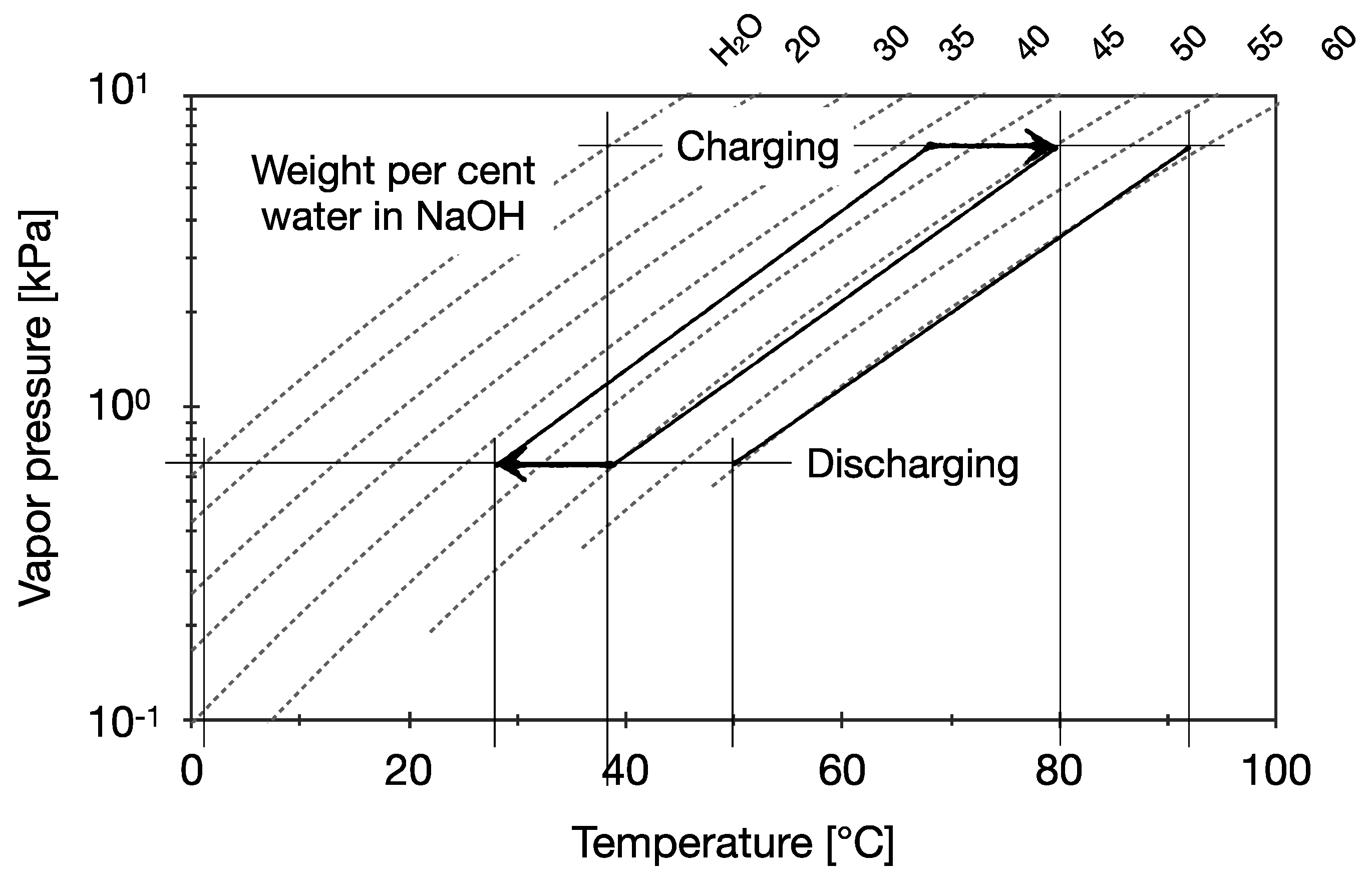
4. Realistic Testing Conditions for the Building Application
5. Discussion and Conclusions
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rathgeber, C.; Hiebler, S.; Lävemann, E.; Dolado, P.; Lazaro, A.; Gasia, J.; de Gracia, A.; Miró, L.; Cabeza, L.F.; König-Haagen, A.; et al. IEA SHC task 42/ECES Annex 29—A simple tool for the economic evaluation of thermal energy storages. Energy Procedia 2016, 91, 197–206. [Google Scholar] [CrossRef][Green Version]
- Zondag, H.A. Sorption heat storage. In Solar Energy Storage; Sørensen, B., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 135–154. [Google Scholar]
- Garg, H.P.; Mullick, S.C.; Bhargava, A.K. Solar Thermal Energy Storage; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Hauer, A. Sorption theory for thermal energy storage. In Thermal Energy Storage for Sustainable Energy Consumption; Paksoy, H.Ö., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 393–408. [Google Scholar]
- Kawasaki, H.; Watanabe, T.; Kanzawa, A. Proposal of a chemical heat pump with paraldehyde depolymerization for cooling system. Appl. Therm. Eng. 1999, 19, 133–143. [Google Scholar] [CrossRef]
- Boman, D.; Hoysall, D.C.; Staedter, M.A.; Goyal, A.; Ponkala, M.J.; Garimella, S. A method for comparison of absorption heat pump working pairs. Int. J. Refrig. 2017, 77, 149–175. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef]
- van Helden, W.; Yamaha, M.; Rathgeber, C.; Hauer, A.; Huaylla, F.; Le Pierrès, N.; Stutz, B.; Mette, B.; Dolado, P.; Lazaro, A.; et al. IEA SHC Task 42 / ECES Annex 29—Working Group B: Applications of Compact Thermal Energy Storage. Energy Procedia 2016, 91, 231–245. [Google Scholar] [CrossRef][Green Version]
- Olsson, J.; Jernqvist, Å.; Aly, G. Thermophysical properties of aqueous NaOHH2O solutions at high concentrations. Int. J. Thermophys. 1997, 18, 779–793. [Google Scholar] [CrossRef]
- Fumey, B.; Weber, R.; Baldini, L. Sorption based long-term thermal energy storage—Process classification and analysis of performance limitations: A review. Renew. Sustain. Energy Rev. 2019, 111, 57–74. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Heintz, J.; Lagre, D.; Luo, L.; Durier, F. Experimental and numerical investigations of a zeolite 13X/water reactor for solar heat storage in buildings. Energy Convers. Manag. 2016, 108, 488–500. [Google Scholar] [CrossRef]
- Weber, R.; Asenbeck, S.; Kerskes, H.; Drück, H. SolSpaces—Testing and Performance Analysis of a Segmented Sorption Store for Solar Thermal Space Heating. Energy Procedia 2016, 91, 250–258. [Google Scholar] [CrossRef]
- Gaeini, M.M.; Javed, M.; Ouwerkerk, H.H.; Zondag, H.H.; Rindt, C.C. Realization of a 4kW thermochemical segmented reactor in household scale for seasonal heat storage. Energy Procedia 2017, 135, 105–114. [Google Scholar] [CrossRef]
- Krese, G.; Koželj, R.; Butala, V.; Stritih, U. Thermochemical seasonal solar energy storage for heating and cooling of buildings. Energy Build. 2018, 164, 239–253. [Google Scholar] [CrossRef]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the artthermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef]
- Palomba, V.; Frazzica, A. Recent advancements in sorption technology for solar thermal energy storage applications. Sol. Energy 2019, 192, 69–105. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Wang, L.W. Wang Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Donkers, P.A.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Mehari, A.; Xu, Z.; Wang, R. Thermal energy storage using absorption cycle and system: A comprehensive review. Energy Convers. Manag. 2020, 206, 112482. [Google Scholar] [CrossRef]
- Liu, H.; N’Tsoukpoe, K.E.; Le Pierrès, N.; Luo, L. Evaluation of a seasonal storage system of solar energy for house heating using different absorption couples. Energy Convers. Manag. 2011, 52, 2427–2436. [Google Scholar] [CrossRef]
- Henninger, S.; Schmidt, F.; Henning, H.-M. Water adsorption characteristics of novel materials for heat transformation applications. Appl. Therm. Eng. 2010, 30, 1692–1702. [Google Scholar] [CrossRef]
- Jeremias, F.; Khutia, A.; Henninger, S.K.; Janiak, C. MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—a promising application. J. Mater. Chem. 2012, 22, 10148–10151. [Google Scholar] [CrossRef]
- Gaeini, M.; Rouws, A.; Salari, J.; Zondag, H.; Rindt, C. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage. Appl. Energy 2018, 212, 1165–1177. [Google Scholar] [CrossRef]
- Courbon, E.; D’Ans, P.; Permyakova, A.; Skrylnyk, O.; Steunou, N.; Degrez, M.; Frère, M. A new composite sorbent based on SrBr2 and silica gel for solar energy storage application with high energy storage density and stability. Appl. Energy 2017, 190, 1184–1194. [Google Scholar] [CrossRef]
- Fröhlich, D.; Pantatosaki, E.; Kolokathis, P.D.; Markey, K.; Reinsch, H.; Baumgartner, M.; van der Veen, M.A.; De Vos, D.E.; Stock, N.; Papadopoulos, G.K.; et al. Water adsorption behaviour of CAU-10-H: A thorough investigation of its structure–property relationships. J. Mater. Chem. A 2016, 4, 11859–11869. [Google Scholar] [CrossRef]
- Tohidi, S.H.; Ziaie, F.; Abdolmaleki, A. Preparation and characterization of SiO2-CaCl2 nanocomposite by the sol-gel method. Int. J. Eng. Trans. B Appl. 2009, 22, 299–305. [Google Scholar]
- Ponomarenko, I.; Glaznev, I.; Gubar, A.; Aristov, Y.; Kirik, S. Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores”. Microporous Mesoporous Mater. 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, H.; Wang, S. Experimental study on composite silica gel supported CaCl2 sorbent for low grade heat storage. Int. J. Therm. Sci. 2006, 45, 804–813. [Google Scholar] [CrossRef]
- Ristić, A.; Maučec, D.; Henninger, S.K.; Kaučič, V. New two-component water sorbent CaCl2-FeKIL2 for solar thermal energy storage. Microporous Mesoporous Mater. 2012, 164, 266–272. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. Enhancing the heat storage density of silica–alumina by addition of hygroscopic salts (CaCl2, Ba(OH)2, and LiNO3). Sol. Energy Mater. Sol. Cells 2015, 140, 351–360. [Google Scholar] [CrossRef]
- Casey, S.P.; Aydin, D.; Riffat, S.; Elvins, J. Salt impregnated desiccant matrices for ‘open’ thermochemical energy storage—Hygrothermal cyclic behaviour and energetic analysis by physical experimentation. Energy Build. 2015, 92, 128–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Zhao, Y.; Li, T.; Riffat, S.; Wajid, N. Development and thermochemical characterizations of vermiculite/SrBr2 composite sorbents for low-temperature heat storage. Energy 2016, 115, 120–128. [Google Scholar] [CrossRef]
- Posern, K.; Linnow, K.; Niermann, M.; Kaps, C.; Steiger, M. Thermochemical investigation of the water uptake behavior of MgSO4 hydrates in host materials with different pore size. Thermochim. Acta 2015, 611, 1–9. [Google Scholar] [CrossRef]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; De Weireld, G.; Steunou, N.; et al. Design of salt–metal organic framework composites for seasonal heat storage applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Stritih, U.; Bombač, A. Description and Analysis of Adsorption Heat Storage Device. Stroj. Vestn. J. Mech. Eng. 2014, 60, 619–628. [Google Scholar] [CrossRef][Green Version]
- Lehmann, C.; Beckert, S.; Gläser, R.; Kolditz, O.; Nagel, T. Assessment of adsorbate density models for numerical simulations of zeolite-based heat storage applications. Appl. Energy 2017, 185, 1965–1970. [Google Scholar] [CrossRef]
- Jänchen, J.; Stach, H.; Hellwig, U. Water sorption in faujasite- and chabazite type zeolites of varying lattice composition for heat storage applications. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, pp. 599–602. [Google Scholar]
- Fumey, B.; Weber, R.; Baldini, L. Liquid sorption heat storage—A proof of concept based on lab measurements with a novel spiral fined heat and mass exchanger design. Appl. Energy 2017, 200, 215–225. [Google Scholar] [CrossRef]
- Johannes, K.; Kuznik, F.; Hubert, J.-L.; Durier, F.; Obrecht, C. Design and characterisation of a high powered energy dense zeolite thermal energy storage system for buildings. Appl. Energy 2015, 159, 80–86. [Google Scholar] [CrossRef]
- Michel, B.; Mazet, N.; Neveu, P. Experimental investigation of an innovative thermo- chemical process operating with a hydrate salt and moist air for thermal storage of solar energy: Global performance. Appl. Energy 2014, 129, 177–186. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Chen, X.; Riffat, S. Novel “open-sorption pipe” reactor for solar thermal energy storage. Energy Convers Manag. 2016, 121, 321–334. [Google Scholar] [CrossRef]
- Nonnen, T.; Beckert, S.; Gleichmann, K.; Brandt, A.; Unger, B.; Kerskes, H.; Mette, B.; Bonk, S.; Badenhop, T.; Salg, F.; et al. A Thermochemical Long-Term Heat Storage System Based on a Salt/Zeolite Composite. Chem. Eng. Technol. 2016, 39, 2427–2434. [Google Scholar] [CrossRef]
- Finck, C.; Henquet, E.; Van Soest, C.; Oversloot, H.; De Jong, A.-J.; Cuypers, R.; Spijker, H.V. Experimental Results of a 3 kWh Thermochemical Heat Storage Module for Space Heating Application. Energy Procedia 2014, 48, 320–326. [Google Scholar] [CrossRef]
- Köll, R.; van Helden, W.; Engel, G.; Wagner, W.; Dang, B.; Jänchen, J.; Kerskes, H.; Badenhop, T.; Herzog, T. Experimental Investigation of a realistic scale seasonal solar sorption storage system for buildings. Sol. Energy 2017, 155, 388–397. [Google Scholar] [CrossRef]
- Palomba, V.; Vasta, S.; Freni, A. Experimental testing of AQSOA FAM Z02/water adsorption system for heat and cold storage. Appl. Therm. Eng. 2017, 124, 967–974. [Google Scholar] [CrossRef]
- Brancato, V.; Gordeeva, L.G.; Sapienza, A.; Palomba, V.; Vasta, S.; Grekova, A.D.; Frazzica, A.; Aristov, Y.I. Experimental characterization of the LiCl/vermiculite composite for sorption heat storage applications. Int. J. Refrig. 2019, 105, 92–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, R.; Li, T.; Nomura, Y. Investigation of a 10 kWh sorption heat storage device for effective utilization of low-grade thermal energy. Energy 2016, 113, 739–747. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, R.; Wang, L.; Roskilly, A. Investigation on an innovative resorption system for seasonal thermal energy storage. Energy Convers. Manag. 2017, 149, 129–139. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Shi, W.; Wang, B.; Li, X. Experimental investigation on charging and discharging performance of absorption thermal energy storage system. Energy Convers. Manag. 2014, 85, 425–434. [Google Scholar] [CrossRef]
- Le Pierrès, N.; Huaylla, F.; Stutz, B.; Perraud, J. Long-term solar heat storage process by absorption with the KCOOH/H2O couple: Experimental investigation. Energy 2017, 141, 1313–1323. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Dawoud, B. Unified Methodology to Identify the Potential Application of Seasonal Sorption Storage Technology. Energies 2020, 13, 1037. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Courbon, E.; D’Ans, P.; Permyakova, A.; Skrylnyk, O.; Steunou, N.; Degrez, M.; Frère, M. Further improvement of the synthesis of silica gel and CaCl2 composites: Enhancement of energy storage density and stability over cycles for solar heat storage coupled with space heating applications. Sol. Energy 2017, 157, 532–541. [Google Scholar] [CrossRef]
- Hauer, A.; Fischer, F.; Rathgeber, C. 4-Temperatures Approach: Testing Thermochemical Heat Storage Materials under Application Conditions. Chem. Ing. Tech. 2021, 93, 618–623. [Google Scholar] [CrossRef]
- EN 14511. Air Conditioners, Liquid Chilling Packages and Heat Pumps with Electrically Driven Compressors for Space Heating and Cooling—Part 2: Test Conditions. Available online: https://www.en-standard.eu/din-en-14511-2-air-conditioners-liquid-chilling-packages-and-heat-pumps-for-space-heating-and-cooling-and-process-chillers-with-electrically-driven-compressors-part-2-test-conditions/ (accessed on 8 May 2021).
- EN 12897. Water Supply—Specification for Indirectly Heated Unvented (Closed) Storage Water Heaters. Available online: https://www.en-standard.eu/din-en-12897-water-supply-specification-for-indirectly-heated-unvented-closed-storage-water-heaters-includes-amendment-2020/ (accessed on 8 May 2021).
- CEN/TR 16355. Recommendations for Prevention of Legionella Growth in Installations inside Buildings Conveying Water for Human Consumption. Available online: https://www.en-standard.eu/une-cen-tr-16355-2014-in-recommendations-for-prevention-of-legionella-growth-in-installations-inside-buildings-conveying-water-for-human-consumption/ (accessed on 8 May 2021).
- Baldini, L.; Fumey, B. Seasonal Energy Flexibility through Integration of Liquid Sorption Storage in Buildings. Energies 2020, 13, 2944. [Google Scholar] [CrossRef]







| Authors | Temperature (Humidity) Conditions | Ref. | |||
|---|---|---|---|---|---|
| Desorption | Condensation | Evaporation | Sorption | ||
| Liu et al. | 45–155 °C | 30 °C | 10 °C | 20 °C | [23] |
| Henninger et al. | 140 °C | 35 °C (5.6 kPa) | 10 °C (1.25 kPa) | 30 °C | [24] |
| Henninger et al. | 95 °C | 35 °C (5.6 kPa) | 10 °C (1.25 kPa) | 40 °C | [24] |
| Jeremias et al. | 140 °C | 35 °C (5.6 kPa) | 35 °C (5.6 kPa) | 40 °C | [25] |
| Gaeini et al. | 150 °C | - | 10 °C (1.25 kPa) | 20 °C | [26] |
| Courbon et al. | 80 °C | 10 °C (1.25 kPa) | 10 °C (1.25 kPa) | 30 °C | [27] |
| Fröhlich et al. | 140 °C | 35 °C (5.6 kPa) | 10 °C (1.25 kPa) | 40 °C | [28] |
| Fröhlich et al. | 120 °C | 10 °C (1.25 kPa) | 10 °C (1.25 kPa) | 20 °C | [28] |
| Tohidi et al. | - | - | 21.4 °C (25 °C 80%RH) | 25 °C | [29] |
| Ponomarenko et al. | - | - | 28 °C (3.7kPa) | 50 °C | [30] |
| Zhu et al. | - | - | 26 °C (30 °C 80%RH) | 30 °C | [31] |
| Ristić et al. | 150 °C | 35 °C (5.6 kPa) | 10 °C (1.25 kPa) | 25 °C | [32] |
| Jabbari-Hichri et al. | 150 °C | 3 °C (0.78 kPa) | 3 °C (0.78 kPa) | 20 °C | [33] |
| Casey et al. | 90 °C | 0% RH | 13 °C (14 °C 95%RH) | 14 °C | [34] |
| Zhang et al. | 110 °C | - | 12–17 °C (20 °C 60–80%RH) | 20 °C | [35] |
| Posern et al. | 130 °C | - | 31 °C (35 °C 80% RH) | 35 °C | [36] |
| Permyakova et al. | 80 °C | 10 °C (1.25 kPa) | 10 °C (1.25 kPa) | 30 °C | [37] |
| Stritihd and Bombac | 95 °C | 22 °C | 18 °C | 22 °C | [38] |
| Lehmann et al. | 180 °C | 7 °C (1.0 kPa) | 7 °C (1.0 kPa) | 20 °C | [39] |
| Jänchen et al. | 450 °C | 4.5 °C (0.85 kPa) | 4.5 °C (0.85 kPa) | 22.5 °C | [40] |
| Donkers et al. | 120 °C | 17.5 °C (2.0 kPa) | 10 °C (1.25 kPa) | 65 °C | [21] |
| Donkers et al. | 100 °C | 17.5 °C (2.0 kPa) | 10 °C (1.25 kPa) | 50 °C | [21] |
| Fumey et al. | 55–65 °C | 20 °C | 20 °C | 28 °C | [41] |
| Johannes et al. | 180 °C | - | 14 °C (20 °C 70%RH) | 20 °C | [42] |
| Tatsidjodoung et al. | 180 °C | - | 14 °C (20 °C 70%RH) | 20 °C | [13] |
| Michel et al. | 82 | 20 °C | 6 °C | 25 °C | [43] |
| Weber et al. | 180 °C | 8 °C (1.0 kPa) | 8 °C (1.0 kPa) | 20 °C | [14] |
| Aydin et al. | 80 °C | −40 °C (0.018 kPa) | 19 °C (20 °C, 2.16 kPa) | 20 °C | [44] |
| Gaeini et al. | 190 °C | - | 8 °C (10 °C, 90%RH) | 10 °C | [15] |
| Nonnen et al. | 180 °C | 8 °C (1.0 kPa) | 13 °C (28 °C, 1.5 kPa) | 28 °C | [45] |
| Finck et al. | 103 °C | 20 °C | 15 °C | 20 °C | [46] |
| Köll et al. | 180 °C | 17 °C | 20 °C | 20 °C | [47] |
| Palomba et al. | 90 °C | 30 °C | 10 °C | 35 °C | [48] |
| Brancato et al. | 90 °C | 30 °C | 12.5 °C | 37 °C | [49] |
| Zhao et al. | 85 °C | 18 °C | 30 °C | 40 °C | [50] |
| Jiang et al. | 150 °C | 15 °C | 15 °C | 30 °C | [51] |
| Zhang et al. | 72 °C | 20 °C | 12 °C | 38 °C | [52] |
| Le Pierrès et al. | 59 °C | 16 °C | 15 °C | 27 °C | [53] |
| Process | Input Temperature (Vapor Pressure *) | Output Temperature |
|---|---|---|
| Desorption | 95 °C (3.0 kPa) | 92 °C |
| Condensation | 30 °C | 35 °C |
| Evaporation | 10 °C (0.87 kPa) | 7 °C |
| Sorption | 30 °C | 35 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumey, B.; Baldini, L. Static Temperature Guideline for Comparative Testing of Sorption Heat Storage Systems for Building Application. Energies 2021, 14, 3754. https://doi.org/10.3390/en14133754
Fumey B, Baldini L. Static Temperature Guideline for Comparative Testing of Sorption Heat Storage Systems for Building Application. Energies. 2021; 14(13):3754. https://doi.org/10.3390/en14133754
Chicago/Turabian StyleFumey, Benjamin, and Luca Baldini. 2021. "Static Temperature Guideline for Comparative Testing of Sorption Heat Storage Systems for Building Application" Energies 14, no. 13: 3754. https://doi.org/10.3390/en14133754
APA StyleFumey, B., & Baldini, L. (2021). Static Temperature Guideline for Comparative Testing of Sorption Heat Storage Systems for Building Application. Energies, 14(13), 3754. https://doi.org/10.3390/en14133754






