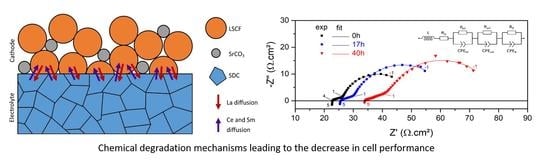
Chemical Degradation of the La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Sm0.2O2−δ Interface during Sintering and Cell Operation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Impedance Measurements
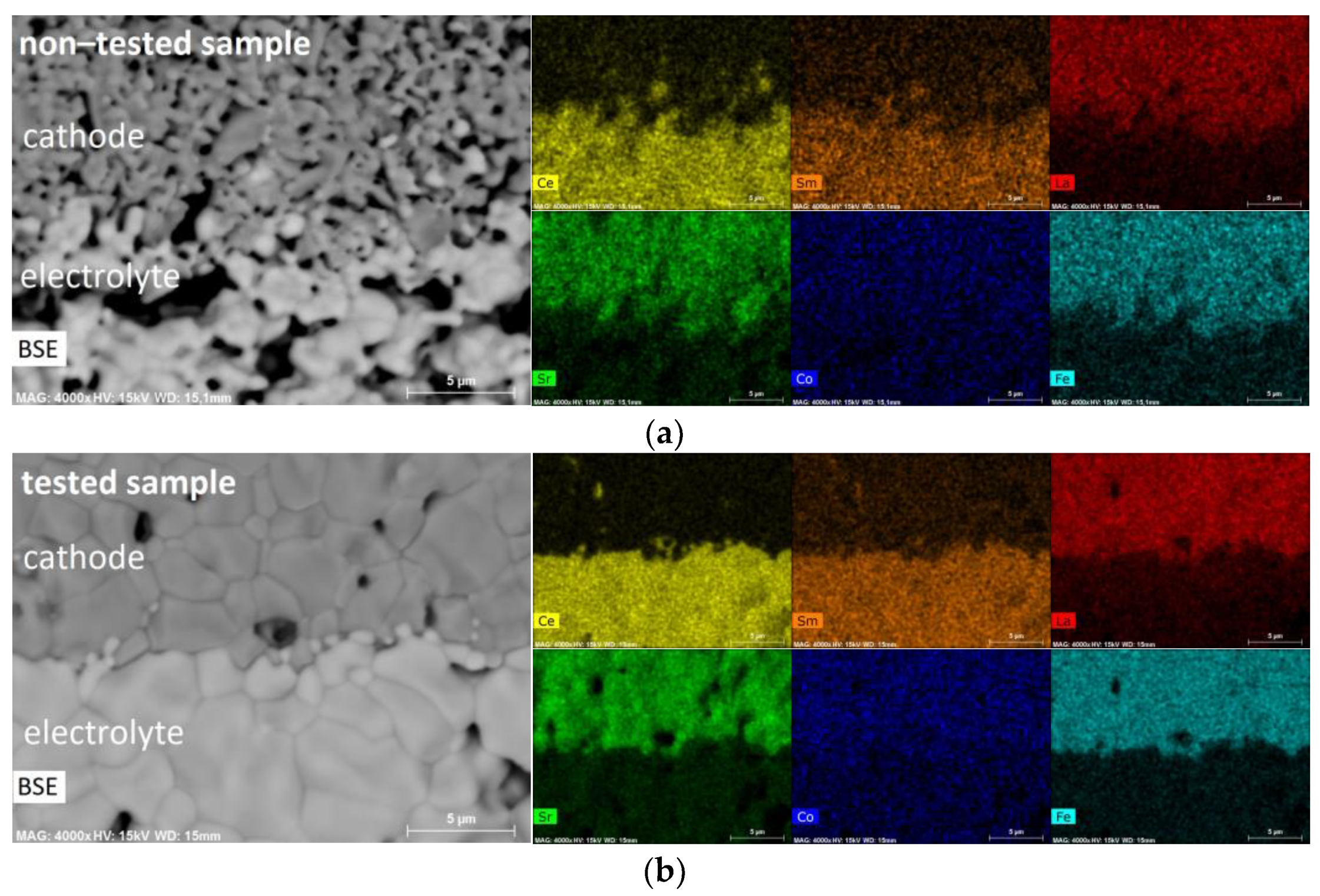
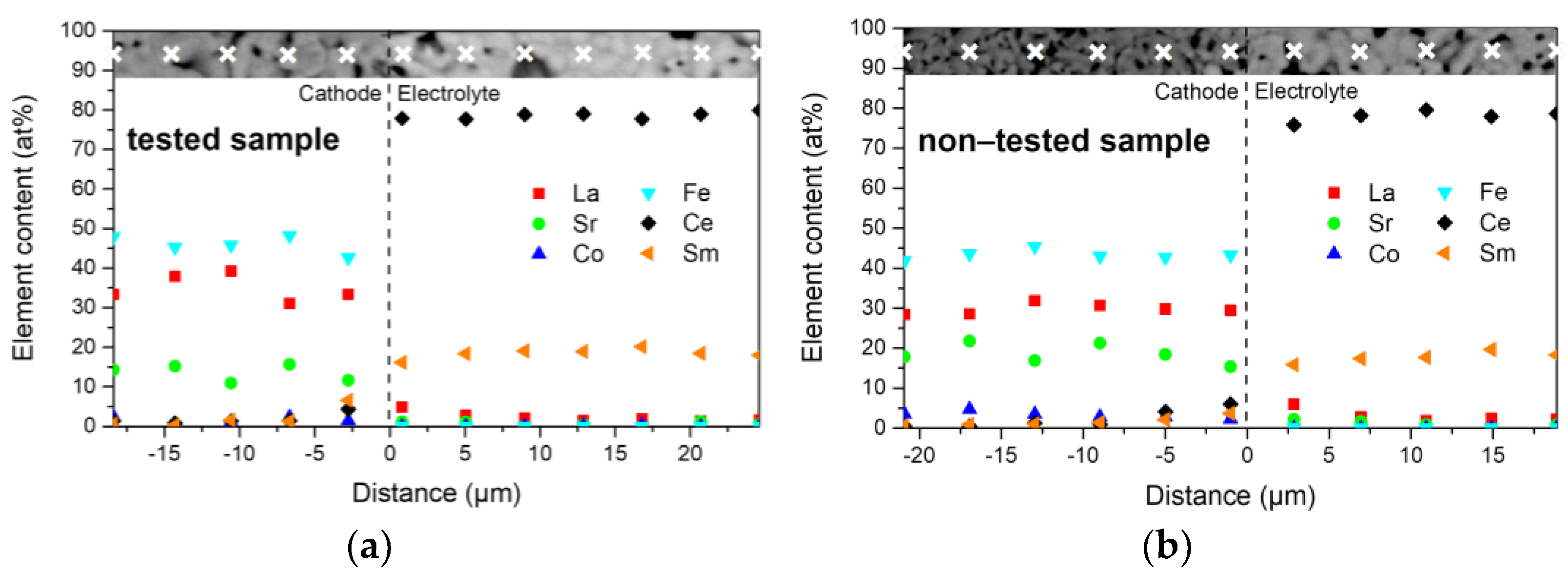
3.2. Microstructural Investigations and Bulk Chemistry Analysis
3.3. Structural and Surface Chemistry Analysis
4. Discussion
5. Conclusions
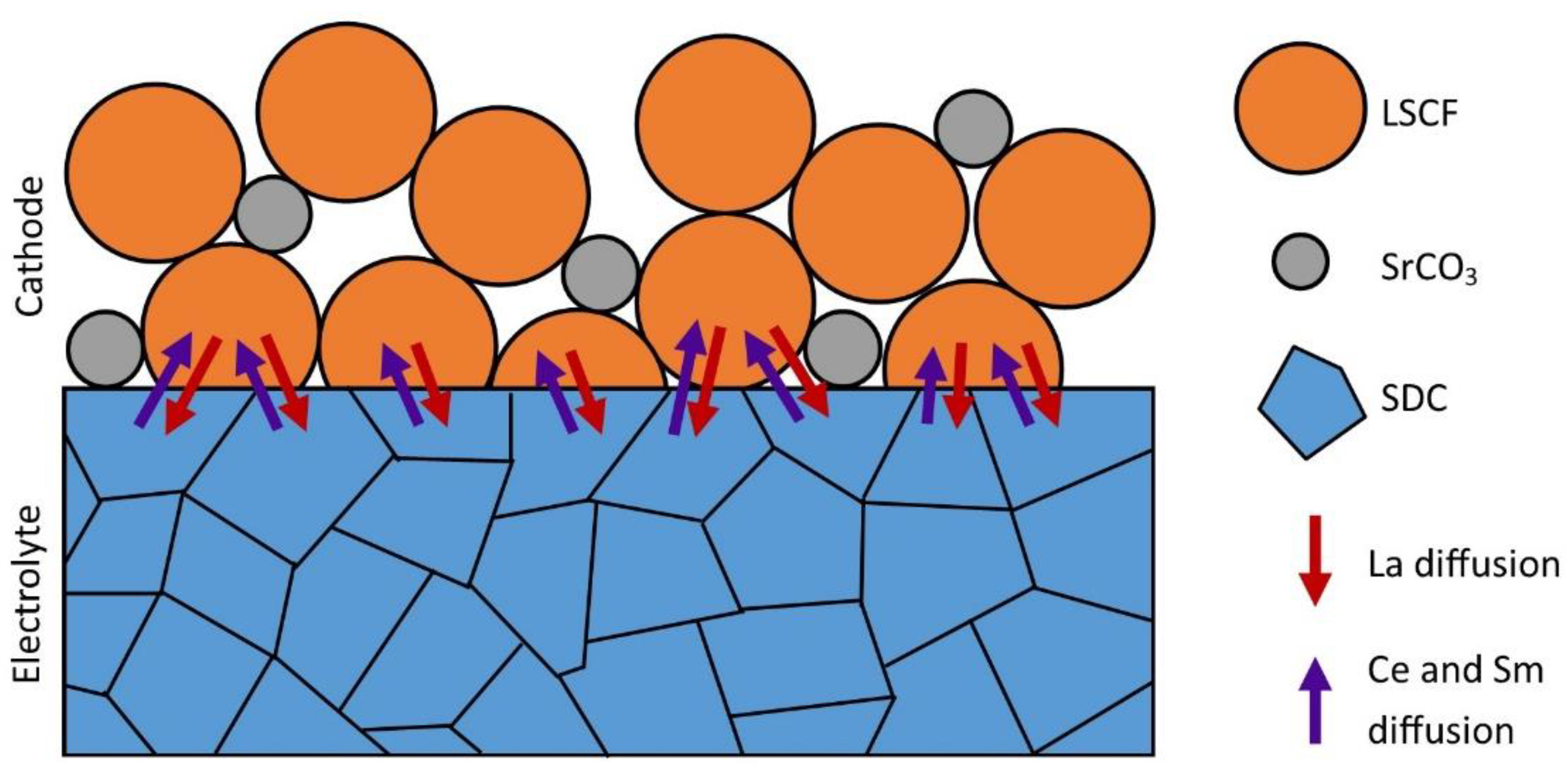
- That lanthanum diffused into the SDC electrolyte layer while cerium and samarium diffused into the cathode layer, the preferred diffusion paths appearing along the grain boundaries. This occurred both during co-sintering at 1380 °C and cell operation, leading to the formation of La- and Sm-doped ceria and resulting in a slight increase in the ohmic resistance.
- The incorporation of Sm and Ce into the LSCF structure involved an increase in the resistance related to the charge transfer at the cathode–electrolyte interface. On the other hand, strontium segregation into SrCO3 clusters at the interface and at the surface of LSCF grains was also highlighted.
- This mechanism occurred during sintering and possibly persisted during cell operation, leading to a significant increase in the cathode polarization resistance. This chemical degradation was caused by the destabilization of the perovskite structure during thermal treatment due to the segregation of the strontium ions out of the LSCF phase.
- Finally, strontium segregation was very detrimental for the cell performance, while lanthanum diffusion into Sm-doped ceria only resulted in a small increase in the reduction of cerium.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wachsman, E.D.; Lee, K.T. Lowering the Temperature of Solid Oxide Fuel Cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.-L.; Hagen, A.; Hendriksen, P.V.; Poulsen, F.W. LSM–YSZ Reactions in Different Atmospheres. Fuel Cells 2009, 9, 833–840. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Zhao, Z.; Tu, B.; Ou, D.; Cui, D.; Cheng, M. Insight into the Oxygen Reduction Reaction on the LSM|GDC Interface of Solid Oxide Fuel Cells through Impedance Spectroscopy Analysis. Catal. Sci. Technol. 2016, 6, 4945–4952. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. Development of Interconnect Materials for Solid Oxide Fuel Cells. Mater. Sci. Eng. A 2003, 348, 227–243. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A Review on Oxygen Storage Capacity of CeO2-Based Materials: Influence Factors, Measurement Techniques, and Applications in Reactions Related to Catalytic Automotive Emissions Control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Harris, D.; Stephenson, H.; Boghosian, S.; Efstathiou, A.M. A Novel Analysis of Transient Isothermal 18O Isotopic Exchange on Commercial CexZr1−xO2-Based OSC Materials. Top. Catal. 2019, 62, 219–226. [Google Scholar] [CrossRef]
- Anirban, S.; Dutta, A. Structural and Ionic Transport Mechanism of Rare Earth Doped Cerium Oxide Nanomaterials: Effect of Ionic Radius of Dopant Cations. Solid State Ion. 2017, 309, 137–145. [Google Scholar] [CrossRef]
- Eguchi, K.; Setoguchi, T.; Inoue, T.; Arai, H. Electrical Properties of Ceria-Based Oxides and Their Application to Solid Oxide Fuel Cells. Solid State Ion. 1992, 52, 165–172. [Google Scholar] [CrossRef]
- Anjaneya, K.C.; Nayaka, G.P.; Manjanna, J.; Govindaraj, G.; Ganesha, K.N. Preparation and Characterization of Ce1−xSmxO2−δ (x = 0.1–0.3) as Electrolyte Material for Intermediate Temperature SOFC. Solid State Sci. 2013, 26, 89–96. [Google Scholar] [CrossRef]
- Coduri, M.; Checchia, S.; Longhi, M.; Ceresoli, D.; Scavini, M. Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties. Front. Chem. 2018, 6, 526. [Google Scholar] [CrossRef]
- Balazs, G. Ac Impedance Studies of Rare Earth Oxide Doped Ceria. Solid State Ion. 1995, 76, 155–162. [Google Scholar] [CrossRef]
- Giuliano, A.; Carpanese, M.P.; Clematis, D.; Boaro, M.; Pappacena, A.; Deganello, F.; Liotta, L.F.; Barbucci, A. Infiltration, Overpotential and Ageing Effects on Cathodes for Solid Oxide Fuel Cells: La0.6Sr0.4Co0.2Fe0.8O3-δ versus Ba0.5Sr0.5Co0.8Fe0.2O3-δ. J. Electrochem. Soc. 2017, 164, F3114–F3122. [Google Scholar] [CrossRef]
- Anjaneya, K.C.; Nayaka, G.P.; Manjanna, J.; Govindaraj, G.; Ganesha, K.N. Studies on Structural, Morphological and Electrical Properties of Ce0.8Ln0.2O2−δ (Ln=Y3+, Gd3+, Sm3+, Nd3+ and La3+) Solid Solutions Prepared by Citrate Complexation Method. J. Alloy. Compd. 2014, 585, 594–601. [Google Scholar] [CrossRef]
- Koettgen, J.; Grieshammer, S.; Hein, P.; Grope, B.O.H.; Nakayama, M.; Martin, M. Understanding the Ionic Conductivity Maximum in Doped Ceria: Trapping and Blocking. Phys. Chem. Chem. Phys. 2018, 20, 14291–14321. [Google Scholar] [CrossRef]
- Priya, N.S.C.; Sandhya, K.; Rajendran, D.N. Study on Electrical Conductivity and Activation Energy of Doped Ceria Nanostructures. Electrochem. Energy Technol. 2018, 3, 49–53. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid Oxide Fuel Cells (SOFCs): A Review of an Environmentally Clean and Efficient Source of Energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Da Silva, F.S.; de Souza, T.M. Novel Materials for Solid Oxide Fuel Cell Technologies: A Literature Review. Int. J. Hydrog. Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shao, Z.; Mori, T.; Jiang, S.P. Development of Nickel Based Cermet Anode Materials in Solid Oxide Fuel Cells—Now and Future. Mater. Rep. Energy 2021, 1, 100003–100029. [Google Scholar]
- Fu, C.; Sun, K.; Zhang, N.; Chen, X.; Zhou, D. Electrochemical Characteristics of LSCF–SDC Composite Cathode for Intermediate Temperature SOFC. Electrochim. Acta 2007, 52, 4589–4594. [Google Scholar] [CrossRef]
- Parbey, J.; Wang, Q.; Lei, J.; Espinoza-Andaluz, M.; Hao, F.; Xiang, Y.; Li, T.; Andersson, M. High-Performance Solid Oxide Fuel Cells with Fiber-Based Cathodes for Low-Temperature Operation. Int. J. Hydrog. Energy 2020, 45, 6949–6957. [Google Scholar] [CrossRef]
- Zhou, W.; Ran, R.; Shao, Z. Progress in Understanding and Development of Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Basedcathodes for Intermediate-Temperature Solid-Oxide Fuel Cells: A Review. J. Power Sour. 2009, 192, 231–246. [Google Scholar] [CrossRef]
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cell Applications. Ceram. Int. 2020, 46, 23314–23325. [Google Scholar] [CrossRef]
- Wang, F.; Chen, D.; Shao, Z. Sm0.5Sr0.5CoO3−δ-Infiltrated Cathodes for Solid Oxide Fuel Cells with Improved Oxygen Reduction Activity and Stability. J. Power Sour. 2012, 216, 208–215. [Google Scholar] [CrossRef]
- Tai, L.-W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and Electrical Properties of La1−xSrxCo1−yFeyO3. Part 2. The System La1−xSrxCo0.2Fe0.8O3. Solid State Ion. 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Develos-Bagarinao, K.; De Vero, J.; Kishimoto, H.; Ishiyama, T.; Yamaji, K.; Horita, T.; Yokokawa, H. Oxygen Surface Exchange Properties and Surface Segregation Behavior of Nanostructured La0.6Sr0.4Co0.2Fe0.8O3-δ Thin Film Cathodes. Phys. Chem. Chem. Phys. 2019, 21, 7183–7195. [Google Scholar] [CrossRef]
- Wang, F.; Brito, M.E.; Yamaji, K.; Cho, D.-H.; Nishi, M.; Kishimoto, H.; Horita, T.; Yokokawa, H. Effect of Polarization on Sr and Zr Diffusion Behavior in LSCF/GDC/YSZ System. Solid State Ion. 2014, 262, 454–459. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stevenson, J.W. Degradation Mechanisms of La–Sr–Co–Fe–O3 SOFC Cathodes. Electrochem. Solid-State Lett. 2006, 9, A478–A481. [Google Scholar] [CrossRef]
- Yu, Y.; Ludwig, K.F.; Woicik, J.C.; Gobalan, S.; Pal, U.B.; Kaspar, T.C.; Basu, S.N. Effect of Sr Content and Strain on Sr Surface Segregation of La1−xSrxCo0.2Fe0.8O3−δ as Cathode Material for Solid Oxide Fuel Cells. Appl. Mater. Interfaces 2016, 8, 26704–26711. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A Short Review of Cathode Poisoning and Corrosion in Solid Oxide Fuel Cell. Int. J. Hydrog. Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Mercadelli, E.; Montaleone, D.; Gondolini, A.; Pinasco, P.; Sanson, A. Tape-Cast Asymmetric Membranes for Hydrogen Separation. Ceram. Int. 2017, 43, 8010–8017. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, J.; Cao, J.; Wang, S.; Wen, T. A Study of Multilayer Tape Casting Method for Anode-Supported Planar Type Solid Oxide Fuel Cells (SOFCs). J. Alloy. Compd. 2007, 437, 264–268. [Google Scholar] [CrossRef]
- Hildenbrand, N.; Boukamp, B.A.; Nammensma, P.; Blank, D.H.A. Improved Cathode/Electrolyte Interface of SOFC. Solid State Ion. 2011, 192, 12–15. [Google Scholar] [CrossRef]
- Giannici, F.; Canu, G.; Chiara, A.; Gambino, M.; Aliotta, C.; Longo, A.; Buscaglia, V.; Martorana, A. Cation Diffusion and Segregation at the Interface between Samarium-Doped Ceria and LSCF or LSFCu Cathodes Investigated with X-Ray Microspectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 44466–44477. [Google Scholar] [CrossRef] [PubMed]
- Sayan, Y.; Venkatesan, V.; Guk, E.; Wu, H.; Kim, J.-S. Single-Step Fabrication of an Anode Supported Planar Single-Chamber Solid Oxide Fuel Cell. Int. J. Appl. Ceram. Technol. 2018, 15, 1375–1387. [Google Scholar] [CrossRef] [Green Version]
- Carpanese, M.P.; Cerisola, G.; Viviani, M.; Piccardo, P.; Vladikova, D.; Stoynov, Z.; Barbucci, A. Study of the Rate Limiting Step of the Cathodic Process in Anode Supported Solid Oxide Fuel Cell. J. Fuel Cell Sci. Technol. 2008, 5, 011010. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of Effective Capacitance and Film Thickness from Constant-Phase-Element Parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Generalised Equivalent Circuits for Mass and Charge Transport: Chemical Capacitance and Its Implications. Phys. Chem. Chem. Phys. 2001, 3, 1668–1678. [Google Scholar] [CrossRef]
- Chen, D.; Bishop, S.R.; Tuller, H.L. Nonstoichiometry in Oxide Thin Films Operating under Anodic Conditions: A Chemical Capacitance Study of the Praseodymium−Cerium Oxide System. Chem. Mater. 2014, 26, 6622–6627. [Google Scholar] [CrossRef]
- Huang, Y.L.; Pellegrinelli, C.; Wachsman, E.D. Fundamental Impact of Humidity on SOFC Cathode ORR. J. Electrochem. Soc. 2016, 163, F171–F182. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Ding, D.; Bai, Y.; He, T.; Liu, M. An Efficient SOFC Based on Samaria-Doped Ceria (SDC) Electrolyte. J. Electrochem. Soc. 2012, 159, B661–B665. [Google Scholar] [CrossRef]
- Tuttle, R. Alloy Steels. Metals 2018, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 754–767. [Google Scholar]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shi, Y.; Eyring, E.M.; Ernst, R.D. Concentration of Ce3+ and Oxygen Vacancies in Cerium Oxide Nanoparticles. Chem. Mater. 2006, 18, 5144–5146. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, L.; Lu, C.; Ni, Y.; Xu, Z. Effects of Oxygen Defects on Structure and Properties of Sm0.5Sr0.5CoO3-δ Annealed in Different Atmospheres. J. Rare Earths 2013, 31, 1183–1190. [Google Scholar] [CrossRef]
- Chang, H.-C.; Tsai, D.-S.; Chung, W.-H.; Huang, Y.-S.; Le, M.-V. A Ceria Layer as Diffusion Barrier between LAMOX and Lanthanum Strontium Cobalt Ferrite along with the Impedance Analysis. Solid State Ion. 2009, 180, 412–417. [Google Scholar] [CrossRef]
- Volkova, N.E.; Khvostova, L.V.; Gavrilova, L.Y.; Cherepanov, V.A. Role of Sm Content to the Crystal Structure and Properties of Sr1-Sm FeO3-δ. J. Solid State Chem. 2018, 267, 113–118. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Zhao, X.; Tang, W.; Yang, S.; Zhong, S.; Wei, M. Effects of Cerium Doping on the Performance of LSCF Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2018, 43, 18946–18954. [Google Scholar] [CrossRef]
- Mandal, B.P.; Grover, V.; Tyagi, A.K. Phase Relations, Lattice Thermal Expansion in Ce1−xEuxO2−x/2 and Ce1−xSmxO2−x/2 Systems and Stabilization of Cubic RE2O3 (RE: Eu, Sm). Mater. Sci. Eng. A 2006, 430, 120–124. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3−δ/CeO2Heterostructured Composite Nanofibersas a Highly Active and Robust Cathode Catalyst for Solid Oxide FuelCells. Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Guan, K.; Wei, Z.; Meng, J.; Meng, J.; Liu, X. Enhancing Activity and Durability of A-Site-Deficient (La0.6 Sr0.4)0.95 Co0.2 Fe0.8 O3−δ Cathode by Surface Modification with PrO2−δ Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 3367–3380. [Google Scholar] [CrossRef]
- Hardy, J.S.; Templeton, J.W.; Edwards, D.J.; Lu, Z.; Stevenson, J.W. Lattice Expansion of LSCF-6428 Cathodes Measured by in Situ XRD during SOFC Operation. J. Power Sources 2012, 198, 76–82. [Google Scholar] [CrossRef]
- Kuntaiah, K.; Sudarsanam, P.; Reddy, B.M.; Vinu, A. Nanocrystalline Ce12xSmxO2-d (x = 0.4) Solid Solutions: Structural Characterization versus CO Oxidation3. RSC Adv. 2013, 11, 7953–7962. [Google Scholar] [CrossRef] [Green Version]
- Acharya, S.A.; Gaikwad, V.M.; D’Souza, S.W.; Barman, S.R. Gd/Sm Dopant-Modified Oxidation State and Defect Generation in Nano-Ceria. Solid State Ion. 2014, 260, 21–29. [Google Scholar] [CrossRef]
- Kundu, S.; Sutradhar, N.; Thangamuthu, R.; Subramanian, B.; Panda, A.B.; Jayachandran, M. Fabrication of Catalytically Active Nanocrystalline Samarium (Sm)-Doped Cerium Oxide (CeO2) Thin FIlms Using Electron Beam Evaporation. J. Nanopart Res. 2012, 16. [Google Scholar] [CrossRef]
- Liang, S.; Broitman, E.; Wang, Y.; Cao, A.; Veser, G. Highly Stable, Mesoporous Mixed Lanthanum–Cerium Oxides with Tailored Structure and Reducibility. J. Mater. Sci. 2011, 46, 2928–2937. [Google Scholar] [CrossRef] [Green Version]
- Loche, D. Determining the Maximum Lanthanum Incorporation in the Fluorite Structure of La-Doped Ceria Nanocubes for Enhanced Redox Ability. RSC Adv. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-D.; Mrabet, D.; Do, T.-O. Controlled Self-Assembly of Sm2 O3 Nanoparticles into Nanorods: Simple and Large Scale Synthesis Using Bulk Sm2 O3 Powders. J. Phys. Chem. C 2008, 112, 15226–15235. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Han, F.; Sun, J.; Bi, H.; Wang, H.; Yu, S.; Pei, L. Ni1—XCux–SDC Anodes for Intermediate Temperature Solid Oxide Fuel Cell. Solid State Ion. 2016, 288, 115–119. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS Studies of Metal Oxides, Hydroxides and Peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Feltham, R.D.; Brant, P. XPS Studies of Core Binding Energies in Transition Metal Complexes. 2. Ligand Group Shifts. Am. Chem. Soc. 1982, 104, 641–645. [Google Scholar] [CrossRef]
- Drouet, C.; Laberty, C.; Fierro, J.L.G.; Alphonse, P.; Rousset, A. X-Ray Photoelectron Spectroscopic Study of Non-Stoichiometric Nickel and Nickel–Copper Spinel Manganites. Int. J. Inorg. Mater. 2000, 2, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Allen, G.C.; Harris, S.J.; Jutson, L. A Study of a Number of Mixed Transition Metal Oxide Spinels Using X-Ray Photoelecton Spectroscopy. Appl. Surf. Sci. 1989, 37, 111–134. [Google Scholar] [CrossRef]
- Natile, M.M.; Galenda, A.; Glisenti, A. From La2O3 to LaCoO3: XPS Analysis. Surf. Sci. Spectra 2008, 15, 14. [Google Scholar] [CrossRef]
- Li, J.P.H.; Zhou, X.; Pang, Y.; Zhu, L.; Vovk, E.I.; Cong, L.; van Bavel, A.P.; Li, S.; Yang, Y. Understanding of Binding Energy Calibration in XPS of Lanthanum Oxide by in Situ Treatment. Phys. Chem. Chem. Phys. 2019, 21, 22351–22358. [Google Scholar] [CrossRef]
- Zhang, L. Integrating the Cationic Engineering and Hollow Structure Engineering into Perovskites Oxides for Efficient and Stable Electrocatalytic Oxygen Evolution. Electrochim. Acta 2019, 11. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Geng, C.; Cao, H.; Xu, C.; Cheng, J.; Hong, T. A Novel Core-Shell LSCF Perovskite Structured Electrocatalyst with Local Hetero-Interface for Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 11824–11833. [Google Scholar] [CrossRef]
- Sunding, M.F. XPS Characterisation of in Situ Treated Lanthanum Oxide and Hydroxide Using Tailored Charge Referencing and Peak Fitting Procedures. J. Electron. Spectrosc. Relat. Phenom. 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Opitz, A.K.; Rameshan, C.; Kubicek, M.; Rupp, G.M.; Nenning, A.; Götsch, T.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Rupprechter, G.; et al. The Chemical Evolution of the La0.6Sr0.4CoO3−δ Surface under SOFC Operating Conditions and Its Implications for Electrochemical Oxygen Exchange Activity. Top. Catal 2018, 61, 2129–2141. [Google Scholar] [CrossRef] [Green Version]
- Newby, D.; Kuyyalil, J.; Laverock, J.; Ludwig, K.F.; Yu, Y.; Davis, J.; Gopalan, S.; Pal, U.B.; Basu, S.; Smith, K.E. Surface Evolution of Lanthanum Strontium Cobalt Ferrite Thin Films at Low Temperatures. Thin Solid Films 2015, 589, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Oishi, J.; Otomo, J.; Oshima, Y.; Koyama, M. The Effects of Minor Components in LSCF Cathode on Oxygen Reduction Reaction. ECS Trans. 2013, 57, 1909–1916. [Google Scholar] [CrossRef]
- Young, V.; Otagawa, T. XPS Studies on Strontium Compounds. Appl. Surf. Sci. 1985, 20, 228–248. [Google Scholar] [CrossRef]
- Vasquez, R.P. SrCO3 by XPS. Surf. Sci. Spectra 1992, 1, 112–116. [Google Scholar] [CrossRef]
- Wei, W.-C.; Huang, D.-R.; Wang, D. (Bi,Sr) (Fe1−x,Mx)O3−δ (M = Co, Ni and Mn) Cathode Materials with Mixed Electro-Ionic Conductivity. Materials 2016, 9, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowjanya, C.; Mandal, R.; Abhinay, S.; Mohanta, A.; Das, S.; Pratihar, S.K. Effect of B-Site Substitution on the Crystal Structure, Electrical Conductivity and Oxygen Transport Properties of La0.5Sr0.5M0.2Fe0.8O3-δ (M = Co, Al, and Zn) Perovskite. J. Solid State Chem. 2020, 285, 121237. [Google Scholar] [CrossRef]
- Wang, H.; Yakal-Kremski, K.J.; Yeh, T.; Rupp, G.M.; Limbeck, A.; Fleig, J.; Barnett, S.A. Mechanisms of Performance Degradation of (La,Sr)(Co,Fe)O 3-δ Solid Oxide Fuel Cell Cathodes. J. Electrochem. Soc. 2016, 163, F581–F585. [Google Scholar] [CrossRef] [Green Version]
- Kubicek, M.; Limbeck, A.; Fromling, T.; Hutter, H. Relationship between Cation Segregation and the Electrochemical Oxygen Reduction Kinetics of La0.6Sr0.4CoO32d Thin Film Electrodes. J. Electrochem. Soc. 2011, 8. [Google Scholar] [CrossRef]
- Dai, H.; Kou, H.; Tao, Z.; Liu, K.; Xue, M.; Zhang, Q.; Bi, L. Optimization of Sintering Temperature for SOFCs by a Co-Firing Method. Ceram. Int. 2020, 46, 6987–6990. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Yang, W.; Hong, T.; Zhu, Z.; Zhang, Y.; Wu, X.; Xia, C. Mechanism for the Enhanced Oxygen Reduction Reaction of La0.6 Sr0.4 Co0.2 Fe0.8 O3−δ by Strontium Carbonate. Phys. Chem. Chem. Phys. 2017, 19, 503–509. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Zhang, L.; Zhang, X.; Chan, S.H. Effect of Sr Surface Segregation of La0.6Sr0.4Co0.2Fe0.8O3−δElectrode on Its Electrochemical Performance in SOC. J. Electrochem. Soc. 2015, 162, F1316–F1323. [Google Scholar] [CrossRef]
- Feng, B.; Sugiyama, I.; Hojo, H.; Ohta, H.; Shibata, N.; Ikuhara, Y. Atomic Structures and Oxygen Dynamics of CeO2 Grain Boundaries. Sci. Rep. 2016, 6, 20288–20294. [Google Scholar] [CrossRef] [Green Version]
- Izuki, M.; Brito, M.E.; Yamaji, K.; Kishimoto, H.; Cho, D.-H.; Shimonosono, T.; Horita, T.; Yokokawa, H. Interfacial Stability and Cation Diffusion across the LSCF/GDC Interface. J. Power Sour. 2011, 196, 7232–7236. [Google Scholar] [CrossRef]
- Kwak, N.W.; Lim, D.; Jeong, S.J.; Byeon, P.; Chung, S.; Jung, W. Diffusion of Cation Impurities through Ceria Grain Boundaries. Adv. Mater. Interfaces 2020, 7, 2000688. [Google Scholar] [CrossRef]
- Jung, G.-B.; Huang, T.-J.; Chang, C.-L. Effect of Temperature and Dopant Concentration on the Conductivity of Samaria-Doped Ceria Electrolyte. J. Solid State Electrochem. 2002, 6, 225–230. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Singh, P. Fuel Cells. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 511–547. ISBN 978-0-08-099424-6. [Google Scholar]
- Yun, J.W.; Yoon, S.P.; Park, S.; Han, J.; Nam, S.W.; Lim, T.-H.; Kim, J.-S. Modifying the Cathodes of Intermediate-Temperature Solid Oxide Fuel Cells with a Ce0.8Sm0.2O2 Sol–Gel Coating. Int. J. Hydrog. Energy 2009, 34, 9213–9219. [Google Scholar] [CrossRef]
- Morales, M.; Miguel-Pérez, V.; Tarancón, A.; Slodczyk, A.; Torrell, M.; Ballesteros, B.; Ouweltjes, J.P.; Bassat, J.M.; Montinaro, D.; Morata, A. Multi-Scale Analysis of the Diffusion Barrier Layer of Gadolinia-Doped Ceria in a Solid Oxide Fuel Cell Operated in a Stack for 3000 h. J. Power Sour. 2017, 344, 141–151. [Google Scholar] [CrossRef]

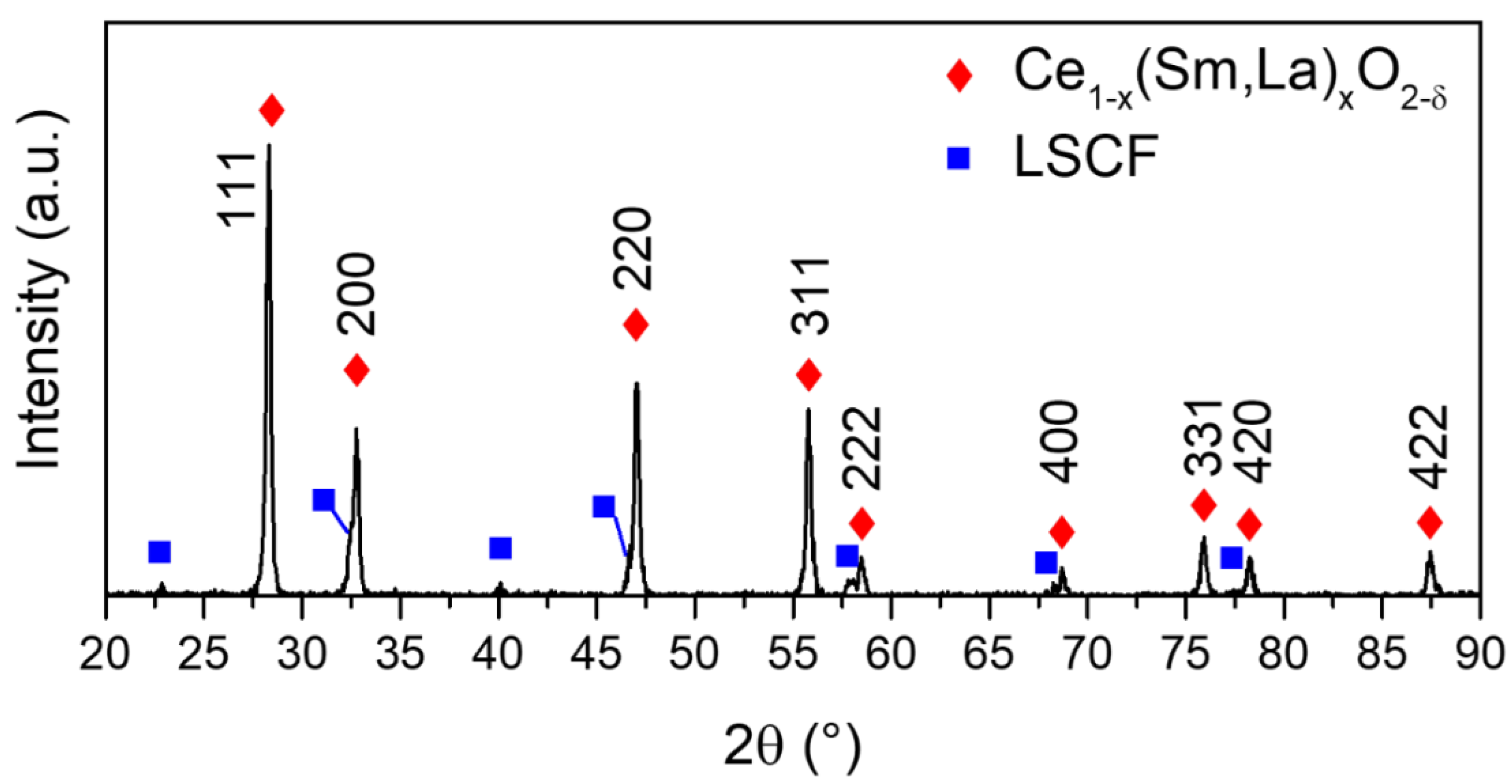
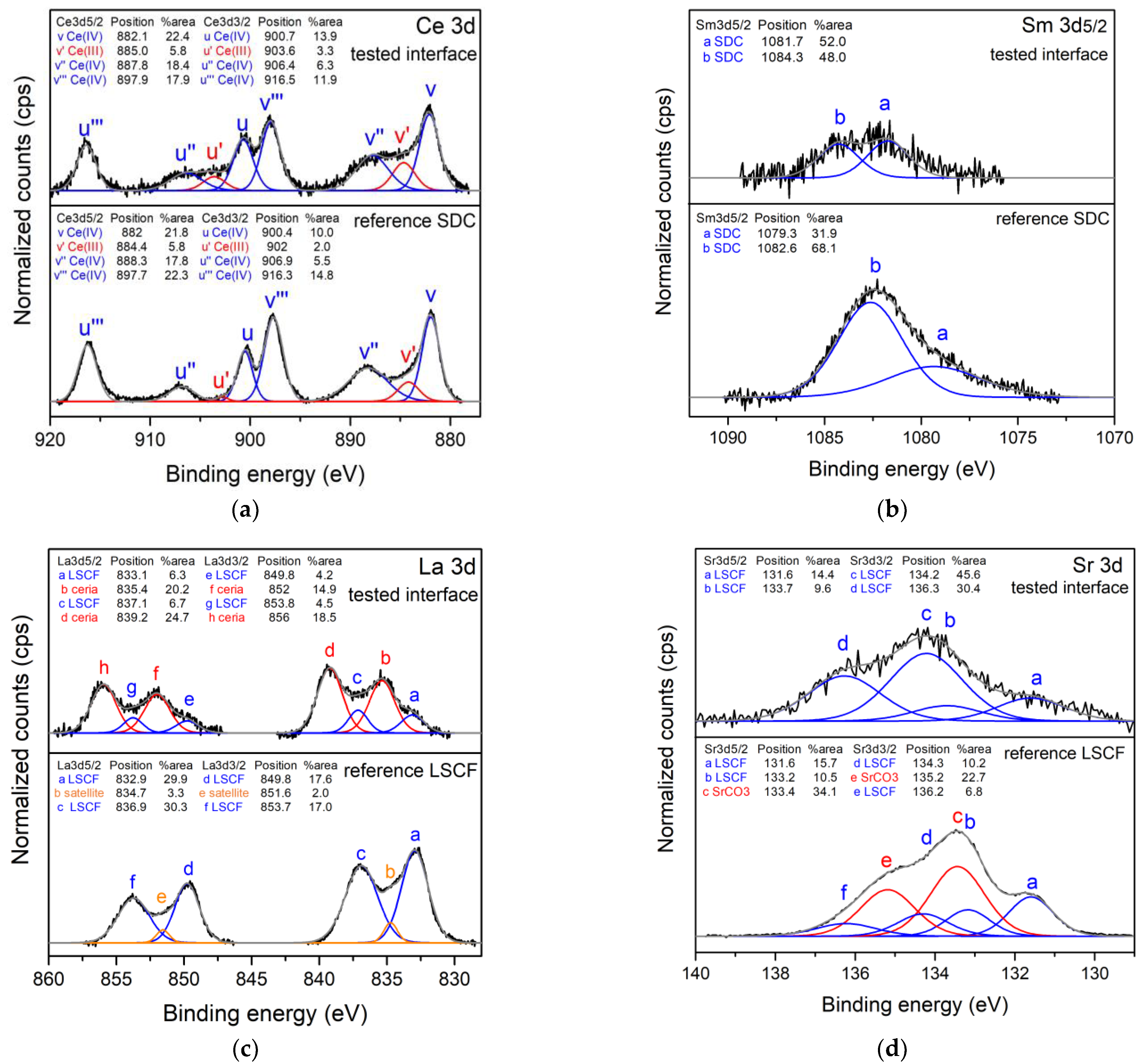
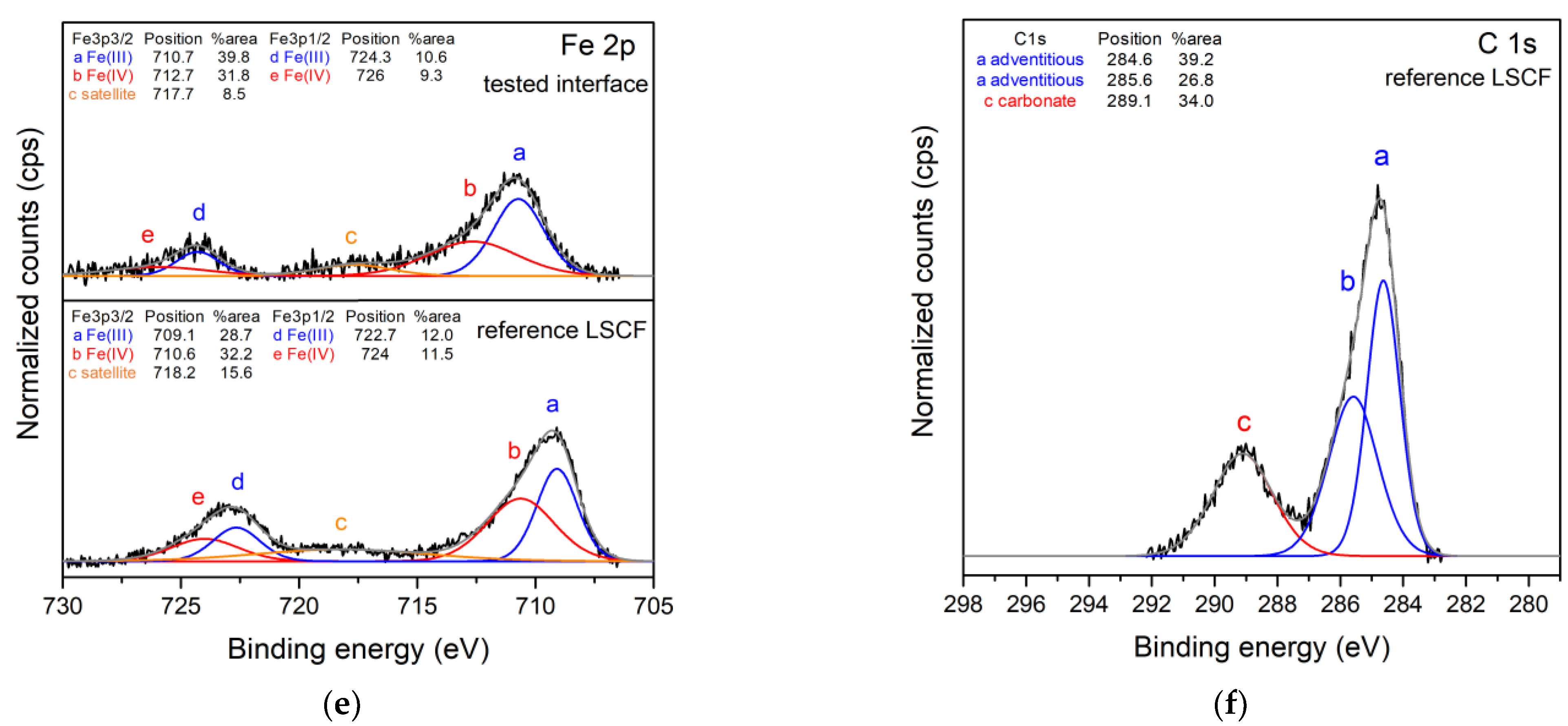
| Slurries | Powder (g) | Graphite (g) | Ethanol (g) | MEK (g) | TEA (g) | PVB (g) | PEG (g) | BBP (g) |
|---|---|---|---|---|---|---|---|---|
| Cathode (LSCF) | 25 | 1.9 | 5.9 | 5.6 | 1.2 | 2 | 0.8 | 0.8 |
| Cathode Functional layer (LSCF-SDC) | LSCF-SDC 12-12 | 1.9 | 5.9 | 5.6 | 0.8 | 2 | 0.8 | 0.8 |
| Electrolyte (SDC) | 22 | 0 | 6.8 | 6.8 | 0.8 | 2 | 0.8 | 0.8 |
| AFL (NiO-SDC) | 22 | 0 | 4.5 | 4.5 | 1 | 1.75 | 0.7 | 0.7 |
| Anode (NiO-SDC) | 63 | 5 | 13 | 13 | 2 | 5 | 2 | 2 |
| Time | L (H) | RΩ (Ω·cm2) | Rhf (Ω·cm2) | CPEhf (F) (nhf) | τhf (s) | Rmf (Ω·cm2) | CPEmf (F) (nmf) | τmf (s) | Rlf (Ω·cm2) | CPElf (F) (nlf) | τlf (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 1.3 10−6 | 22.4 | 0.6 | 2.4 10−3 (0.60) | 3.4 10−4 | 3.0 | 2.3 10−2 (0.60) | 2.3 10−2 | 24.4 | 7.6 10−2 (0.87) | 5.5 |
| 17 h | 1.2 10−6 | 25.2 | 0.5 | 3.5 10−4 (0.75) | 2.1 10−4 | 5.5 | 3.1 10−2 (0.45) | 1.8 10−2 | 31.6 | 5.2 10−2 (0.88) | 4.9 |
| 40 h | 6.6 10−7 | 33.8 | 3.3 | 1.9 10−3 (0.57) | 1.7 10−4 | 4.7 | 7.3 10−3 (0.74) | 1.8 10−2 | 37.6 | 3.3 10−2 (0.86) | 4.4 |
| Equivalent Capacitance, Ceq [F cm−2] | |||
|---|---|---|---|
| Aging Time | hf | Mf | Lf |
| 0 h | 3.06 × 10−5 | 3.87 × 10−3 | 8.33 × 10−2 |
| 17 h | 1.94 × 10−5 | 3.57 × 10−3 | 5.56 × 10−2 |
| 40 h | 4.14 × 10−5 | 2.23 × 10−3 | 3.41 × 10−2 |
| Area | C | Ce | Sm | La | Sr | Fe | O |
|---|---|---|---|---|---|---|---|
| Tested interface | ND | 10.2 | 2.2 | 4.6 | 4.1 | 11.3 | 67.7 |
| Reference SDC | ND | 19.4 | 4.4 | ND | ND | ND | 76.2 |
| Reference LSCF | 8.5 | ND | ND | 4.7 | 15.4 | 5.7 | 65.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
François, M.; Carpanese, M.P.; Heintz, O.; Lescure, V.; Clematis, D.; Combemale, L.; Demoisson, F.; Caboche, G. Chemical Degradation of the La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Sm0.2O2−δ Interface during Sintering and Cell Operation. Energies 2021, 14, 3674. https://doi.org/10.3390/en14123674
François M, Carpanese MP, Heintz O, Lescure V, Clematis D, Combemale L, Demoisson F, Caboche G. Chemical Degradation of the La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Sm0.2O2−δ Interface during Sintering and Cell Operation. Energies. 2021; 14(12):3674. https://doi.org/10.3390/en14123674
Chicago/Turabian StyleFrançois, Mélanie, Maria Paola Carpanese, Olivier Heintz, Victoire Lescure, Davide Clematis, Lionel Combemale, Frédéric Demoisson, and Gilles Caboche. 2021. "Chemical Degradation of the La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Sm0.2O2−δ Interface during Sintering and Cell Operation" Energies 14, no. 12: 3674. https://doi.org/10.3390/en14123674
APA StyleFrançois, M., Carpanese, M. P., Heintz, O., Lescure, V., Clematis, D., Combemale, L., Demoisson, F., & Caboche, G. (2021). Chemical Degradation of the La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Sm0.2O2−δ Interface during Sintering and Cell Operation. Energies, 14(12), 3674. https://doi.org/10.3390/en14123674