Design Study on Customised Piezoelectric Elements for Energy Harvesting in Total Hip Replacements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Finite Element Model
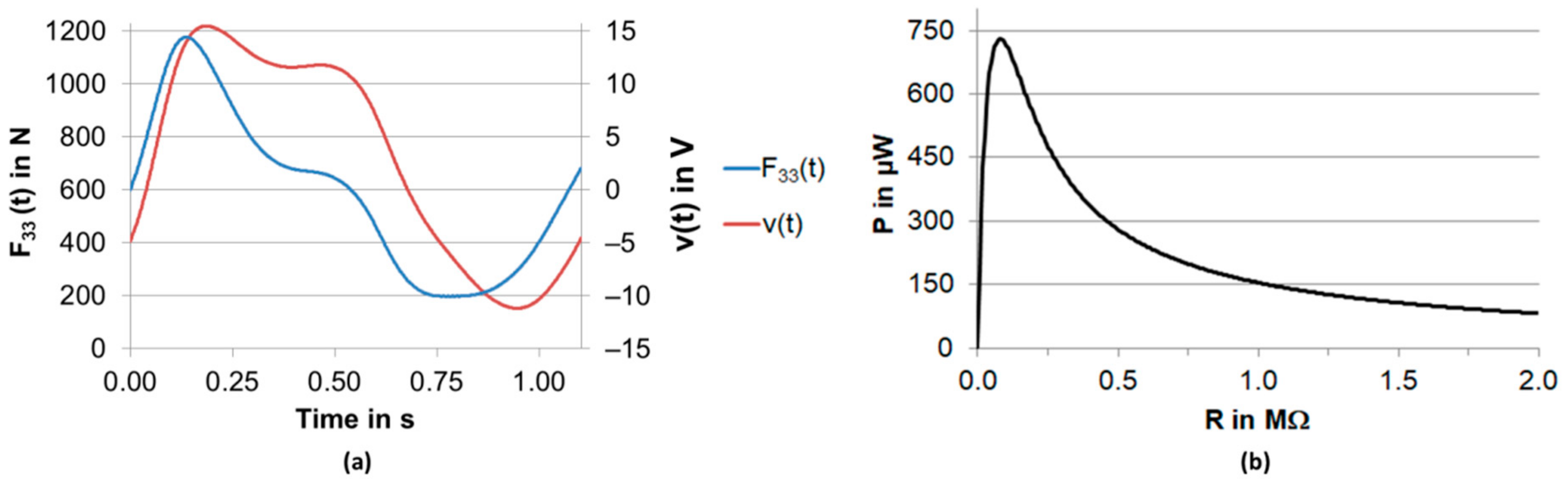
2.2. Postprocessing
2.3. Geometry Variants
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Ledet, E.H.; Liddle, B.; Kradinova, K.; Harper, S. Smart implants in orthopedic surgery, improving patient outcomes: A review. Innov. Entrep. Health 2018, 5, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.; Zimmermann, U.; Van Rienen, U. Modeling of an Optimized Electrostimulative Hip Revision System under Consideration of Uncertainty in the Conductivity of Bone Tissue. IEEE J. Biomed. Health Inform. 2015, 19, 1321–1330. [Google Scholar] [CrossRef]
- Raben, H.; Kämmerer, P.W.; Bader, R.; Van Rienen, U. Establishment of a Numerical Model to Design an Electro-Stimulating System for a Porcine Mandibular Critical Size Defect. Appl. Sci. 2019, 9, 2160. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, M.P.S.; Marote, A.; Santos, T.; Torrão, J.; Ramos, A.; Simões, J.A.O.; da Cruz e Silva, O.A.B.; Furlani, E.P.; Vieira, S.I.; Ferreira, J.A.F. New cosurface capacitive stimulators for the development of active osseointegrative implantable devices. Sci. Rep. 2016, 6, 30231. [Google Scholar] [CrossRef]
- Platt, S.R.; Farritor, S.; Garvin, K.; Haider, H. The Use of Piezoelectric Ceramics for Electric Power Generation within Orthopedic Implants. IEEE/ASME Trans. Mechatron. 2005, 10, 455–461. [Google Scholar] [CrossRef]
- Platt, S.R.; Farritor, S.; Haider, H. On Low-Frequency Electric Power Generation with PZT Ceramics. IEEE/ASME Trans. Mechatron. 2005, 10, 240–252. [Google Scholar] [CrossRef]
- Almouahed, S.; Gouriou, M.; Hamitouche, C.; Stindel, E.; Roux, C. Self-powered instrumented knee implant for early detection of postoperative complications. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5121–5124. [Google Scholar] [CrossRef]
- Almouahed, S.; Hamitouche, C.; Stindel, E. Optimized Prototype of Instrumented Knee Implant: Experimental Validation. IRBM 2017, 38, 250–255. [Google Scholar] [CrossRef]
- Wilson, B.E. Modeling and Experimentation for Evaluation of Piezoelectric Sensors for In-Vivo Monitoring. Master’s Thesis, Tennessee Technological University, Cookeville, TN, USA, 2015. [Google Scholar]
- Safaei, M. A Piezoelectric Instrumented Total Knee Replacement for Sensing and Energy Harvesting. Ph.D. Thesis, Tennessee Technological University, Cookeville, TN, USA, 2019. [Google Scholar]
- Luciano, V.; Sardini, E.; Serpelloni, M.; Baronio, G. An energy harvesting converter to power sensorized total human knee prosthesis. Meas. Sci. Technol. 2014, 25, 25702. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Jain, M.; Salman, E.; Willing, R.; Towfighian, S. A smart knee implant using triboelectric energy harvesters. Smart Mater. Struct. 2019, 28, 025040. [Google Scholar] [CrossRef]
- Morais, R.; Silva, N.; Santos, P.; Frias, C.; Ferreira, J.; Ramos, A.; Simõesd, J.; Baptista, J.; Reis, M. Permanent magnet vibration power generator as an embedded mechanism for smart hip prosthesis. Procedia Eng. 2010, 5, 766–769. [Google Scholar] [CrossRef]
- Santos, M.; Ferreira, J.; Ramos, A.; Pascoal, R.; Morais, R.; Silva, N.; Simoes, J.; Reis, M.J.C.S.; Boeri, C.N.; Festas, A.; et al. Multi-Source Energy Harvesting Power Generators for Instrumented Implants—Towards the Development of a Smart Hip Prosthesis. In Biodevices 2012, Proceedings of the International Conference on Biomedical Electronics and Devices, Vilamoura, Algarve, Portugal, 1–4 February 2012; [integrated in BIOSTEC (International Joint Conference on Biomedical Engineering Systems and Technologies)]. International Conference on Biomedical Electronics and Devices; Gabriel, J., Ed.; SciTePress: Setubal, Portugal, 2012; pp. 71–81. ISBN 978-989-8425-91-1. [Google Scholar]
- Lange, H.-E.; Hohlfeld, D.; Bader, R.; Kluess, D. A piezoelectric energy harvesting concept for an energy-autonomous instrumented total hip replacement. Smart Mater. Struct. 2020, 29, 115051. [Google Scholar] [CrossRef]
- Soodmand, E.; Kluess, D.; Varady, P.A.; Cichon, R.; Schwarze, M.; Gehweiler, D.; Niemeyer, F.; Pahr, D.; Woiczinski, M. Interlaboratory comparison of femur surface reconstruction from CT data compared to reference optical 3D scan. Biomed. Eng. Online 2018, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Kluess, D.; Soodmand, E.; Lorenz, A.; Pahr, D.; Schwarze, M.; Cichon, R.; Varady, P.A.; Herrmann, S.; Buchmeier, B.; Schröder, C.; et al. A round-robin finite element analysis of human femur mechanics between seven participating laboratories with experimental validation. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Heller, M.; Bergmann, G.; Deuretzbacher, G.; Dürselen, L.; Pohl, M.; Claes, L.; Haas, N.; Duda, G. Musculo-skeletal loading conditions at the hip during walking and stair climbing. J. Biomech. 2001, 34, 883–893. [Google Scholar] [CrossRef]
- Heller, M.; Bergmann, G.; Kassi, J.-P.; Claes, L.; Haas, N.; Duda, G. Determination of muscle loading at the hip joint for use in pre-clinical testing. J. Biomech. 2005, 38, 1155–1163. [Google Scholar] [CrossRef]
- Speirs, A.; Heller, M.; Duda, G.N.; Taylor, W.R. Physiologically based boundary conditions in finite element modelling. J. Biomech. 2007, 40, 2318–2323. [Google Scholar] [CrossRef]
- Nuño, N.; Groppetti, R.; Senin, N. Static coefficient of friction between stainless steel and PMMA used in cemented hip and knee implants. Clin. Biomech. 2006, 21, 956–962. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Li, H.; Gao, G.; Zhang, X. Friction and wear characteristics of ultrahigh molecular weight polyethylene (UHMWPE) composites containing glass fibers and carbon fibers under dry and water-lubricated conditions. Wear 2017, 380–381, 42–51. [Google Scholar] [CrossRef]
- Taddei, F.; Schileo, E.; Helgason, B.; Cristofolini, L.; Viceconti, M. The material mapping strategy influences the accuracy of CT-based finite element models of bones: An evaluation against experimental measurements. Med. Eng. Phys. 2007, 29, 973–979. [Google Scholar] [CrossRef]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus–density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Kempf, I.; Leung, K.S.; Grosse, A.; Haarman, H.J.T.M.; Seidel, H.; Taglang, G. Practice of Intramedullary Locked Nails: Scientific Basis and Standard Techniques Recommended by AIOD; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 3642563309. [Google Scholar]
- Saha, S.; Pal, S. Mechanical properties of bone cement: A review. J. Biomed. Mater. Res. 1984, 18, 435–462. [Google Scholar] [CrossRef]
- Kurtz, S.M. UHMWPE biomaterials handbook. In Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, 2nd ed.; Academic: London, UK, 2009; ISBN 9780123747211. [Google Scholar]
- PI Ceramic GmbH. Material Coefficients PIC255: V4.3; PI Ceramic GmbH: Lederhose, Germany, 2017. [Google Scholar]
- Zysset, P.K.; Guo, X.E.; Hoffler, C.E.; Moore, K.E.; Goldstein, S.A. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef]
- Ashman, R.; Cowin, S.; Van Buskirk, W.; Rice, J. A continuous wave technique for the measurement of the elastic properties of cortical bone. J. Biomech. 1984, 17, 349–361. [Google Scholar] [CrossRef]
- PI Ceramic GmbH. Data Sheet Round PICMA® Chip Actuators: Miniature Multilayer Piezo Actuators with and without Inner Hole. Available online: https://static.piceramic.com/fileadmin/user_upload/physik_instrumente/files/datasheets/PD0xx-Datasheet.pdf (accessed on 15 January 2019).
- Safaei, M.; Meneghini, R.M.; Anton, S.R. Force detection, center of pressure tracking, and energy harvesting from a piezoelectric knee implant. Smart Mater. Struct. 2018, 27, 114007. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Meneghini, R.M.; Anton, S.R. Parametric analysis of electromechanical and fatigue performance of total knee replacement bearing with embedded piezoelectric transducers. Smart Mater. Struct. 2017, 26, 094002. [Google Scholar] [CrossRef]
- Safaei, M.; Meneghini, R.M.; Anton, S.R. Energy Harvesting and Sensing With Embedded Piezoelectric Ceramics in Knee Implants. IEEE/ASME Trans. Mechatron. 2018, 23, 864–874. [Google Scholar] [CrossRef]
- Chen, H.; Liu, M.; Jia, C.; Wang, Z. Power harvesting using PZT ceramics embedded in orthopedic implants. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 2010–2014. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Deng, Z.D. Energy harvesting from low frequency applications using piezoelectric materials. Appl. Phys. Rev. 2014, 1, 041301. [Google Scholar] [CrossRef] [Green Version]
- Safaei, M.; Sodano, H.A.; Anton, S.R. A review of energy harvesting using piezoelectric materials: State-of-the-art a decade later (2008–2018). Smart Mater. Struct. 2019, 28, 113001. [Google Scholar] [CrossRef]
- Almouahed, S.; Hamitouche, C.; Poignet, P.; Stindel, E. Battery-free force sensor for instrumented knee implant. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; ISBN 978-1-5386-1392-4. [Google Scholar]
- Anton, S.R.; Sodano, H.A. A review of power harvesting using piezoelectric materials (2003–2006). Smart Mater. Struct. 2007, 16, R1–R21. [Google Scholar] [CrossRef]
- Okazaki, Y. Comparison of Fatigue Properties and Fatigue Crack Growth Rates of Various Implantable Metals. Materials 2012, 5, 2981–3005. [Google Scholar] [CrossRef] [Green Version]
- Lange, H.-E.; Bader, R.; Kluess, D. Endurance testing and finite element simulation of a modified hip stem for integration of an energy harvesting system. Proc. Inst. Mech. Eng. Part H 2021. [Google Scholar] [CrossRef]
- Wang, H.; Cooper, T.A.; Lin, H.-T.; Wereszczak, A.A. Fatigue responses of lead zirconate titanate stacks under semibipolar electric cycling with mechanical preload. J. Appl. Phys. 2010, 108, 084107. [Google Scholar] [CrossRef]
- CTS Company. Actuators for Dynamic Applications: Tutorial. Version 1501. 2021. Available online: http://www.noliac.com/fileadmin/user_upload/documents/Tutorials/Tutorials_Actuator_2.pdf (accessed on 31 March 2021).
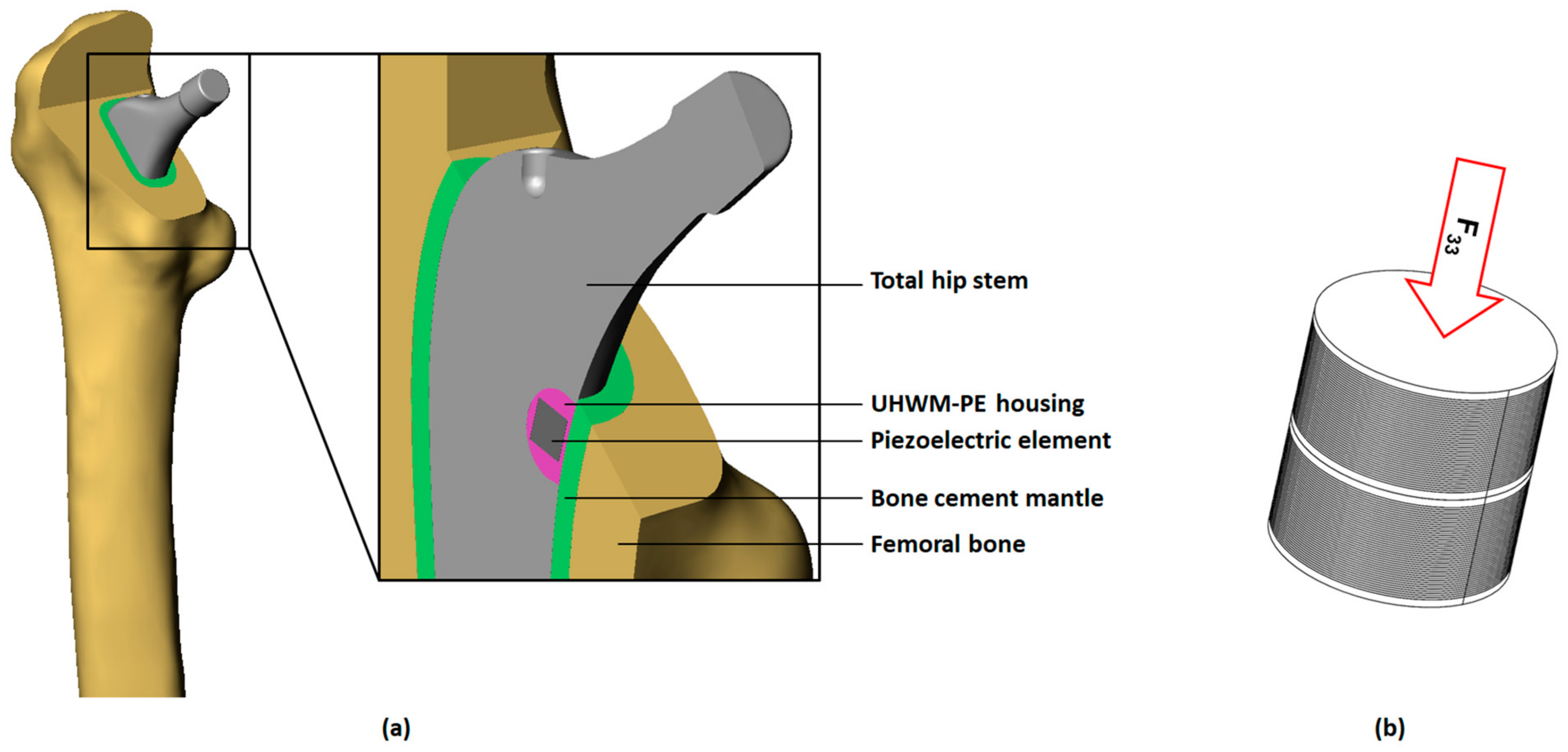
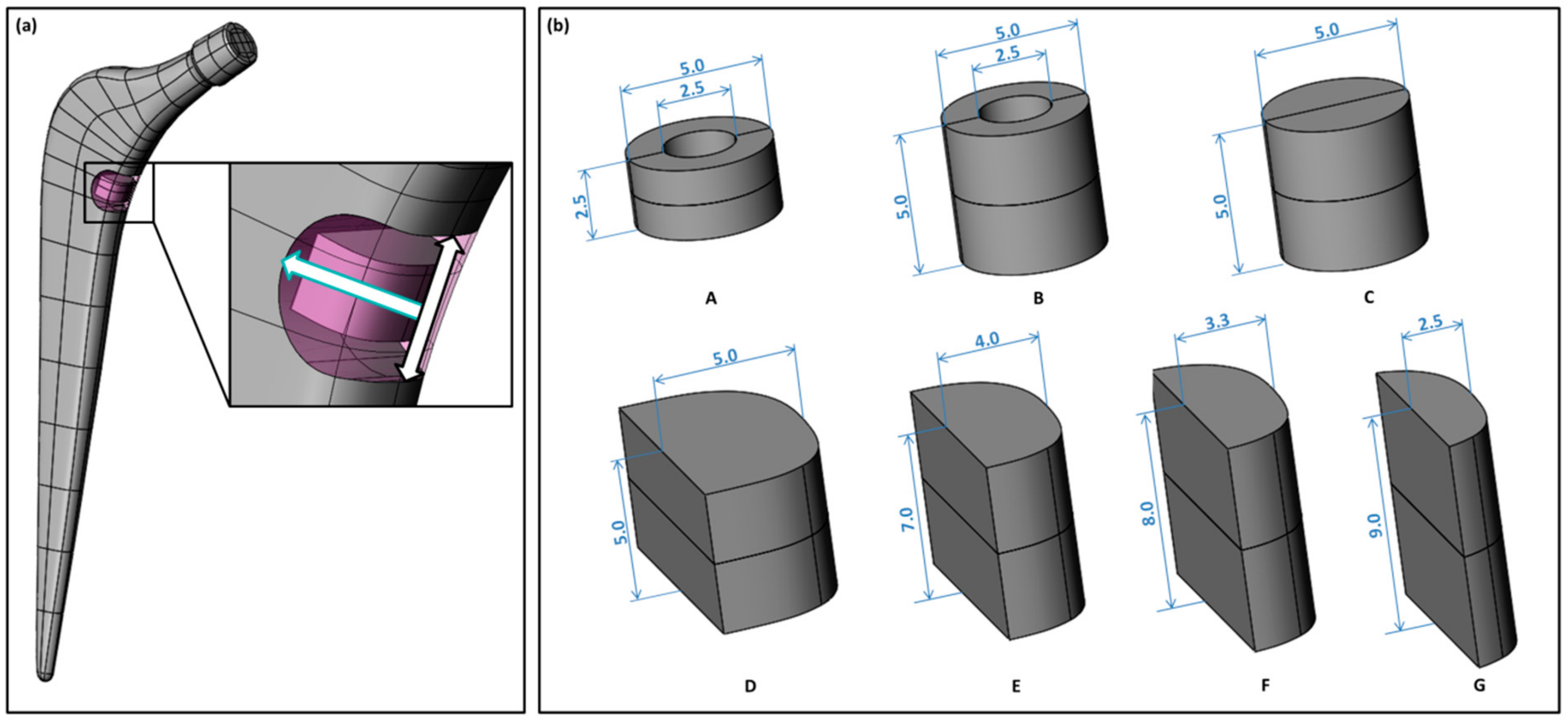
| Component | Young’s Modulus (GPa) | Poisson’s Ratio 1 (-) |
|---|---|---|
| Metallic implant | 195 [26] | 0.3 |
| Bone cement mantle | 2.3 [27] | 0.3 |
| UHMW-PE housing | 0.83 [28] | 0.46 [28] |
| PZT piezoelectric element | 52.4 [29] | 0.35 [29] |
| Femoral bone | 6850 × ρapp1.49 [25], max. value 20 GPa [30,31] | 0.3 |
| Parameter | Value |
|---|---|
| A | Variable |
| h | Variable, minus passive layers |
| hlayer | 0.05 mm |
| nlayer | Variable, defined by h and hlayer |
| 1751 [29] | |
| d33 | 3.996E − 10 m/V [29] |
| Symbol | Output Parameter | Limit |
|---|---|---|
| σImp | Stress maximum at cavity base (metallic implant component) | 290.9 MPa |
| σPiez | Stress maximum in the piezoelectric element’s mid plane | 30.0 MPa |
| F33 | Contact force acting on the piezoelectric element’s end face | n/a |
| P | Approximated power output | To be maximised |
| Variant | σImp (MPa) | σPiez (MPa) | F33 (N) | P (µW) | |
|---|---|---|---|---|---|
| Single | Layers | 269.4 | 14.0 | 141.6 | 8.1 |
| No layers | 269.6 | 14.1 | 140.6 | 8.0 | |
| Difference (%) | 0.08% | 0.64% | −0.67% | −1.33% | |
| Stack | Layers | 267.2 | 16.9 | 196.3 | 31.1 |
| No layers | 267.6 | 15.1 | 192.0 | 29.8 | |
| Difference (%) | 0.14% | −10.71% | −2.20% | −4.36% |
| Variant |  |  |  | 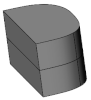 |  |
|---|---|---|---|---|---|
| Single | Stacked | Stacked | Initial  Final Final | ||
| Off-The-Shelf | Full Cylinder | Customised Geometry | |||
| σImp (MPa) | 269.6 | 267.6 | 266.6 | 256.6 | 273.4 |
| σPiez (MPa) | 14.1 | 15.1 | 11.9 | 16.9 | 29.7 |
| F33 (N) | 140.6 | 192.0 | 207.7 | 536.0 | 1218.7 |
| P (µW) | 8.0 | 29.8 | 26.1 | 77.8 | 729.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, H.-E.; Bader, R.; Kluess, D. Design Study on Customised Piezoelectric Elements for Energy Harvesting in Total Hip Replacements. Energies 2021, 14, 3480. https://doi.org/10.3390/en14123480
Lange H-E, Bader R, Kluess D. Design Study on Customised Piezoelectric Elements for Energy Harvesting in Total Hip Replacements. Energies. 2021; 14(12):3480. https://doi.org/10.3390/en14123480
Chicago/Turabian StyleLange, Hans-E., Rainer Bader, and Daniel Kluess. 2021. "Design Study on Customised Piezoelectric Elements for Energy Harvesting in Total Hip Replacements" Energies 14, no. 12: 3480. https://doi.org/10.3390/en14123480






