The Potential Use of Fly Ash from the Pulp and Paper Industry as Thermochemical Energy and CO2 Storage Material
Abstract
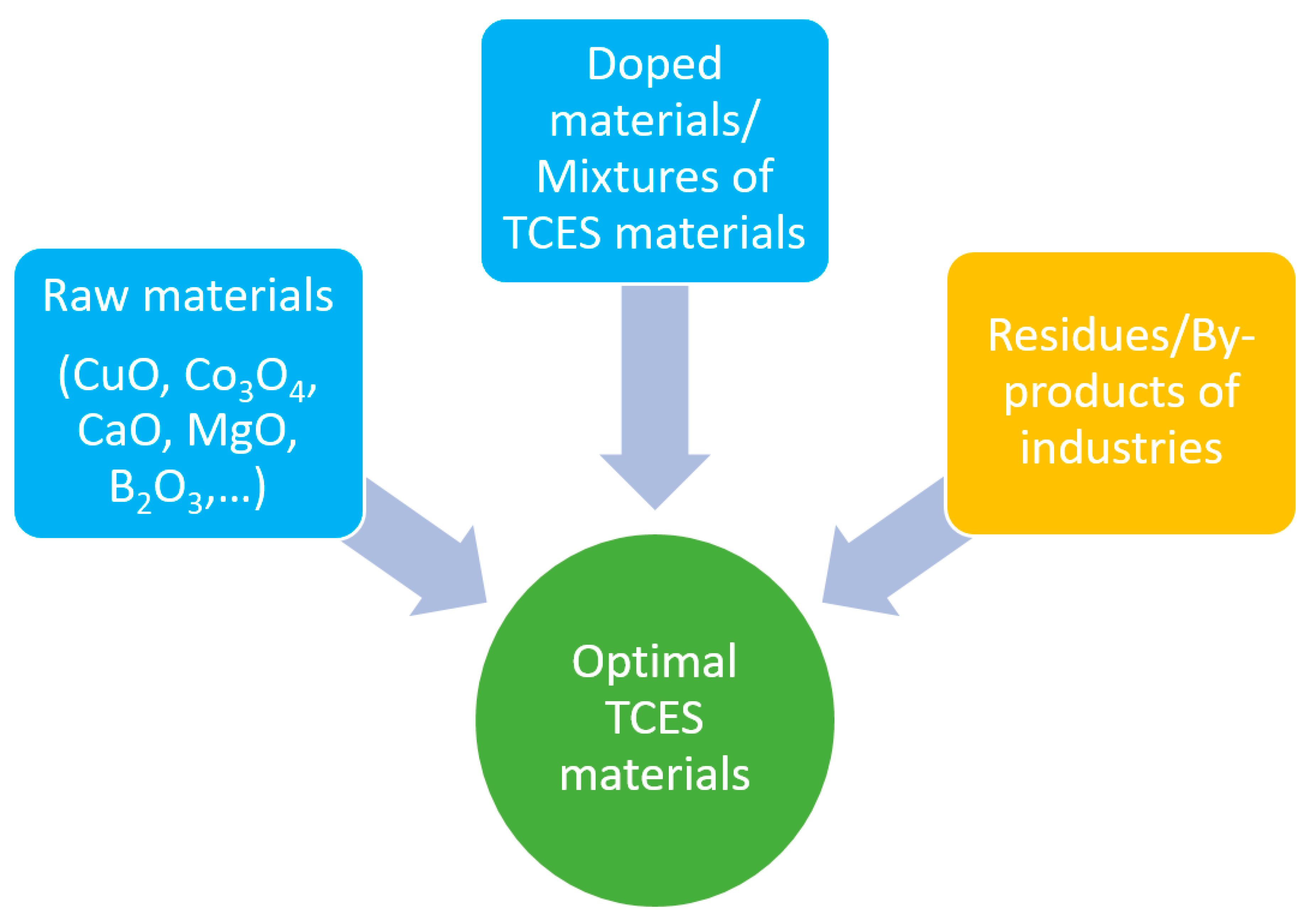
1. Introduction
2. Materials and Methods
2.1. General Physical Properties of Fly Ash Samples
2.2. Particle Size Distribution (PSD)
2.3. X-ray Fluorescence Spectroscopy (XRF)
2.4. Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES)
2.5. X-ray Diffractometry (XRD)
2.6. Simultaneous Thermal Analysis (STA)
2.7. Leaching Test
2.8. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. X-ray Fluorescence Spectroscopy (XRF)
3.2. Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES)
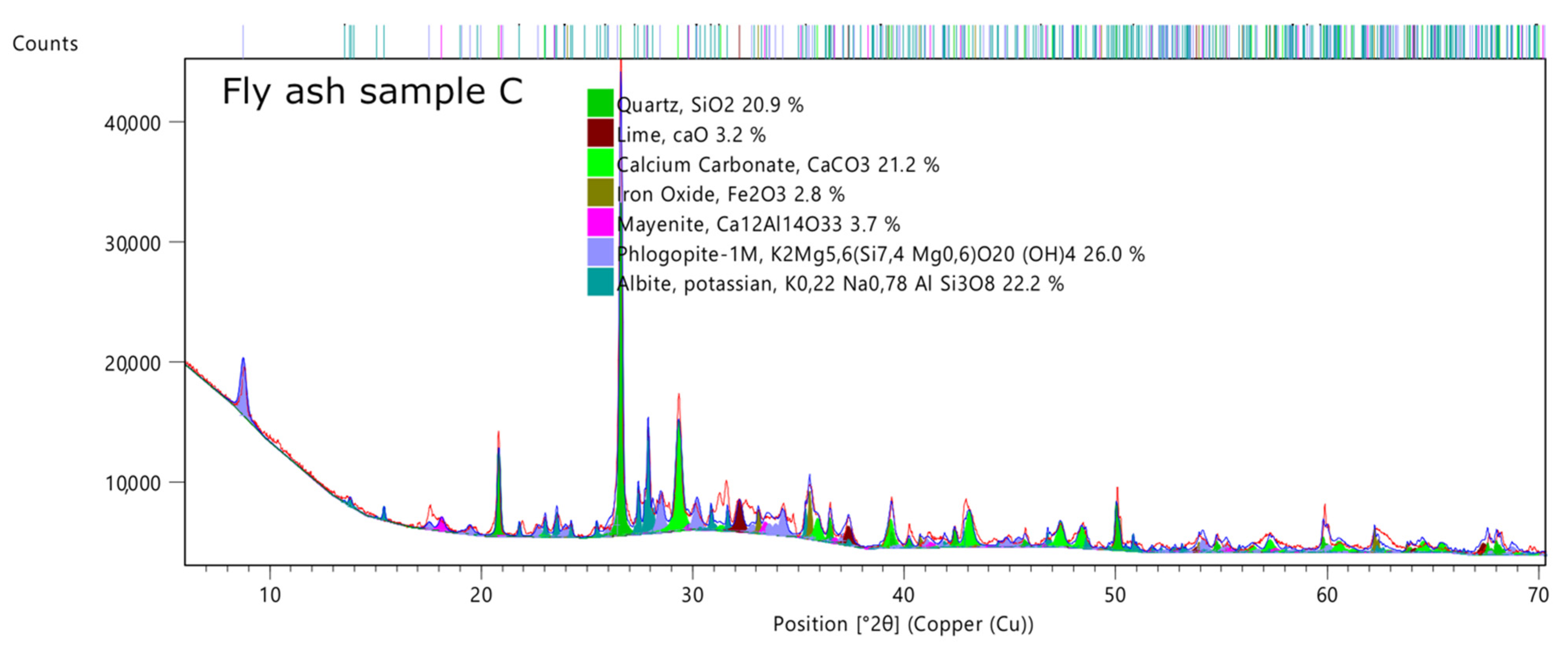
3.3. X-ray Diffractometry (XRD)
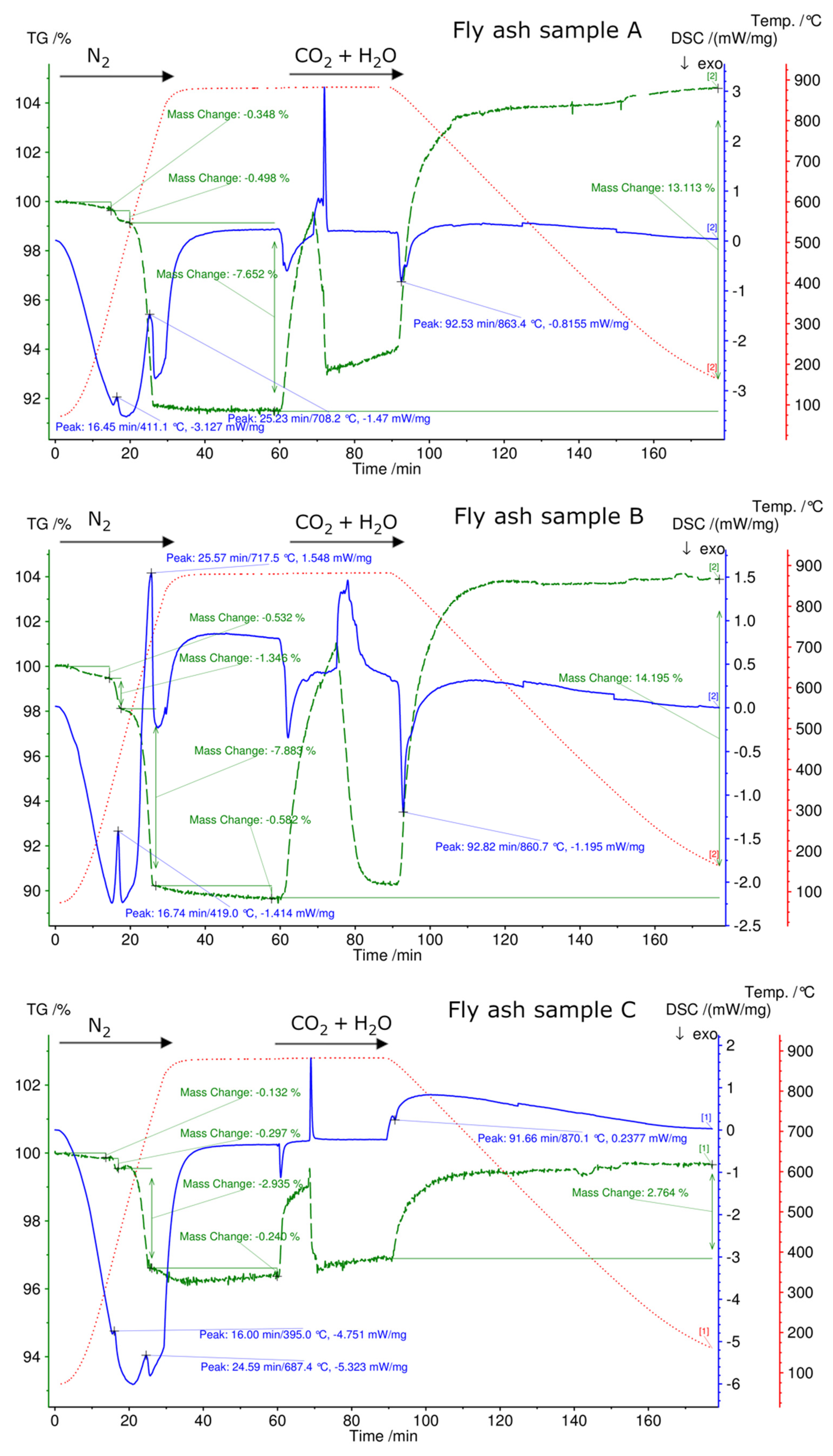
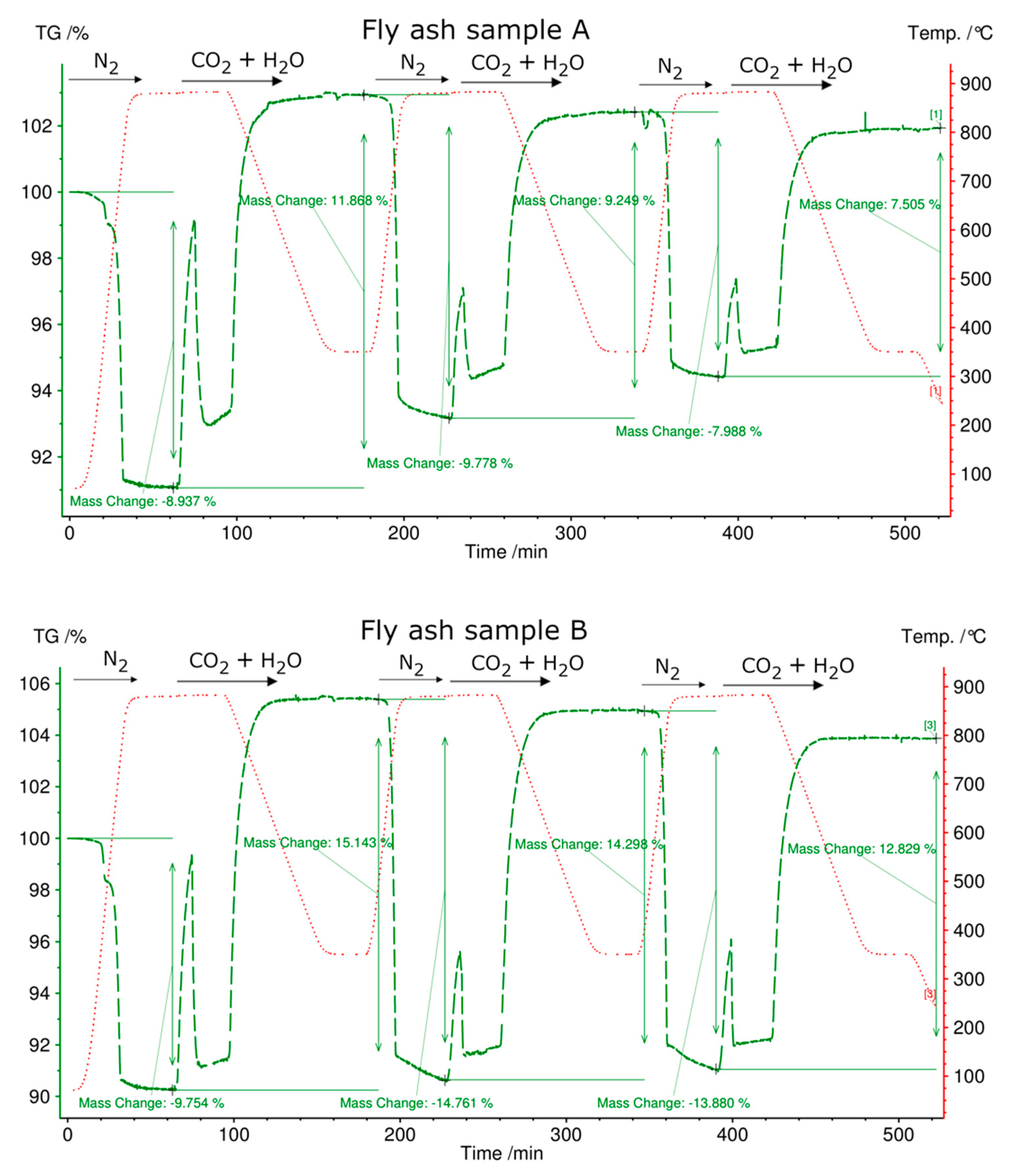
3.4. Simultaneous Thermal Analysis (STA)
3.5. Leaching Test
3.6. Particle Size Distribution (PSD)
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahromy, S.S. From High-Value to Byproduct and Waste Materials for Thermochemical Energy and CO2 Storage. Ph.D. Thesis, TU Wien, Vienna, Austria, 2019. [Google Scholar]
- International Energy Agency (IEA). World Energy Outlook; International Energy Agency (IEA): Paris, France, 2017. [Google Scholar]
- Jahromy, S.S.; Birkelbach, F.; Jordan, C.; Huber, C.; Harasek, M.; Werner, A.; Winter, F. Impact of Partial Pressure, Conversion, and Temperature on the Oxidation Reaction Kinetics of Cu2O to CuO in Thermochemical Energy Storage. Energies 2019, 12, 508. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Abedin, A.H.R.; Marc, A. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Huber, C.; Jahromy, S.S.; Jordan, C.; Schreiner, M.; Harasek, M.; Werner, A.; Winter, F. Boric Acid: A High Potential Candidate for Thermochemical Energy Storage. Energies 2019, 12, 1086. [Google Scholar] [CrossRef]
- Ortiz, C.; Chacartegui, R.; Valverde, J.; Carro, A.; Tejada, C. Increasing the solar share in combined cycles through thermochemical energy storage. Energy Convers. Manag. 2021, 229, 113730. [Google Scholar] [CrossRef]
- Obermeier, J. Thermodynamische Analyse Thermochemischer Energiespeicher auf Basis Reversibler Gas-Feststoff-Reaktionen. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Bayern, Germany, 2017. [Google Scholar]
- Deutsch, M.; Müller, D.; Aumeyr, C.; Jordan, C.; Gierl-Mayer, C.; Weinberger, P.; Winter, F.; Werner, A. Systematic search algorithm for potential thermochemical energy storage systems. Appl. Energy 2016, 183, 113–120. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the performance of MgO for thermochemical energy storage by dehydration—From fundamentals to phase impurities. Appl. Energy 2019, 253, 253. [Google Scholar] [CrossRef]
- Deutsch, M.; Horvath, F.; Knoll, C.; Lager, D.; Gierl-Mayer, C.; Weinberger, P.; Winter, F. High-Temperature Energy Storage: Kinetic Investigations of the CuO/Cu2O Reaction Cycle. Energy Fuels 2017, 31, 2324–2334. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Ruh, T.; Artner, W.; Welch, J.M.; Peterlik, H.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Blaha, P.; et al. Calcium Doping Facilitates Water Dissociation in Magnesium Oxide. Adv. Sustain. Syst. 2018, 2, 1700096. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; O’Shea, V.A.D.L.P.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the Thermochemical Energy Storage Performance of the Mn2O3/Mn3O4 Redox Couple by the Incorporation of Iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Jahromy, S.S.; Jordan, C.; Azam, M.; Werner, A.; Harasek, M.; Winter, F. Fly Ash from Municipal Solid Waste Incineration as a Potential Thermochemical Energy Storage Material. Energy Fuels 2019, 33, 5810–5819. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Yanase, I.; Maeda, T.; Kobayashi, H. The effect of addition of a large amount of CeO2 on the CO2 adsorption properties of CaO powder. Chem. Eng. J. 2017, 327, 548–554. [Google Scholar] [CrossRef]
- Jing, J.-Y.; Li, T.-Y.; Zhang, X.-W.; Wang, S.-D.; Feng, J.; Turmel, W.A.; Li, W.-Y. Enhanced CO2 sorption performance of CaO/Ca3Al2O6 sorbents and its sintering-resistance mechanism. Appl. Energy 2017, 199, 225–233. [Google Scholar] [CrossRef]
- Khosa, A.A.; Yan, J.; Zhao, C. Investigating the effects of ZnO dopant on the thermodynamic and kinetic properties of CaCO3/CaO TCES system. Energy 2021, 215, 119132. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Wu, S. A new nano CaO-based CO2 adsorbent prepared using an adsorption phase technique. Chem. Eng. J. 2013, 218, 39–45. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Duan, Y.; Zhao, C. Reactivity enhancement of calcium based sorbents by doped metal oxides through the sol-gel process. Appl. Energy 2016, 162, 390–400. [Google Scholar] [CrossRef]
- Lu, S.; Wu, S. Calcination-carbonation durability of nano CaCO3 doped with Li2SO4. Chem. Eng. J. 2016, 294, 22–29. [Google Scholar] [CrossRef]
- Jahromy, S.S.; Azam, M.; Huber, F.; Jordan, C.; Wesenauer, F.; Huber, C.; Naghdi, S.; Schwendtner, K.; Neuwirth, E.; Laminger, T.; et al. Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2. Materials 2019, 12, 3358. [Google Scholar] [CrossRef]
- Del Valle-Zermeño, R.; Barreneche, C.; Cabeza, L.F.; Formosa, J.; Fernandez, A.I.; Chimenos, J.M. MSWI bottom ash for thermal energy storage: An innovative and sustainable approach for its reutilization. Renew. Energy 2016, 99, 431–436. [Google Scholar] [CrossRef]
- Gutierrez, A.; Miró, L.; Gil, A.; Rodríguez-Aseguinolaza, J.; Barreneche, C.; Calvet, N.; Py, X.; Fernández, A.I.; Grágeda, M.; Ushak, S.; et al. Advances in the valorization of waste and by-product materials as thermal energy storage (TES) materials. Renew. Sustain. Energy Rev. 2016, 59, 763–783. [Google Scholar] [CrossRef]
- Lin, W.Y.; Li, T.; Akasyah, L.; Lim, J.W.M.; Xu, H.; Py, X.; Rawat, R.S.; Romagnoli, A. Comparison of sintering condition and radio frequency plasma discharge on the conversion of coal/biomass fly ash into high-temperature thermal energy storage material. Energy Convers. Manag. 2019, 192, 180–187. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Bian, Z.; Yan, X.; Wang, Z.; Liu, W. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles. Energy Convers. Manag. 2019, 197. [Google Scholar] [CrossRef]
- Maaten, B.; Konist, A.; Siirde, A. Potential of solid residues from power plants as thermochemical energy storage materials. J. Therm. Anal. Calorim. 2020, 142, 1799–1805. [Google Scholar] [CrossRef]
- Azam, M.; Jahromy, S.S.; Raza, W.; Wesenauer, F.; Schwendtner, K.; Winter, F. Comparison of the Characteristics of Fly Ash Generated from Bio and Municipal Waste: Fluidized Bed Incinerators. Materials 2019, 12, 2664. [Google Scholar] [CrossRef]
- Scheepers, G.P.; du Toit, B. Potential use of wood ash in South African forestry: A review. South. For. J. For. Sci. 2016, 78, 255–266. [Google Scholar] [CrossRef]
- Haiying, Z.; Youcai, Z.; Jingyu, Q. Thermal characterization of fly ash from one municipal solid waste incinerator (MSWI) in Shanghai. Process. Saf. Environ. Prot. 2010, 88, 269–275. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y.; Wilson, N.; Voncken, J. Thermal behaviour of ESP ash from municipal solid waste incinerators. J. Hazard. Mater. 2009, 166, 567–575. [Google Scholar] [CrossRef]
- Viet, D.B.; Chan, W.-P.; Phua, Z.-H.; Ebrahimi, A.; Abbas, A.; Lisak, G. The use of flyashes from waste-to-energy processes as mineral CO2 sequesters and supplementary cementitious materials. J. Hazard. Mater. 2020, 398, 122906. [Google Scholar] [CrossRef] [PubMed]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Li, Z.-S.; Fang, F.; Tang, X.-Y.; Cai, N.-S. Effect of Temperature on the Carbonation Reaction of CaO with CO2. Energy Fuels 2012, 26, 2473–2482. [Google Scholar] [CrossRef]
- Khosa, A.A.; Zhao, C. Heat storage and release performance analysis of CaCO3/CaO thermal energy storage system after doping nano silica. Sol. Energy 2019, 188, 619–630. [Google Scholar] [CrossRef]
- Ordinance by the Federal Minister for the Environment on Waste Disposal Sites. Wien, Austria. 1996, Volume 164, p. 49. Available online: https://www.ecolex.org/details/legislation/landfill-ordinance-lex-faoc033823/ (accessed on 11 October 2019).
- Cherian, C.; Siddiqua, S. Pulp and Paper Mill Fly Ash: A Review. Sustainability 2019, 11, 4394. [Google Scholar] [CrossRef]
- Azam, M.; Jahromy, S.S.; Raza, W.; Raza, N.; Lee, S.S.; Kim, K.-H.; Winter, F. Status, characterization, and potential utilization of municipal solid waste as renewable energy source: Lahore case study in Pakistan. Environ. Int. 2020, 134, 105291. [Google Scholar] [CrossRef]








| Parameter | Plant A | Plant B | Plant C |
|---|---|---|---|
| Fuel type | Bark, sludge, biogas, natural gas | Bark, wood, wood residue, process sludge | Biomass, Biological sludge < 2% |
| Incinerator type | BFBC | CFBC | CFBC |
| Heat Capacity (MW) | 15 | 48 | 91 |
| Ash type | ESP/Filter bags | Filter bags | ESP |
| Temperature free zone (°C) | 800–900 °C | 800–900 °C | 810–900 °C |
| Amount of ash per year (t/y) | 25,000 | 45,000 | 5000 |
| Utilization | Cement industries | Cement industries | Cement industries |
| Elements | Fly Ash Sample A | Fly Ash Sample B | Fly Ash Sample C |
|---|---|---|---|
| SnO | 0.01 | 0.02 | n.d. |
| ZrO2 | 0.04 | 0.05 | 0.06 |
| SrO | 0.2 | 0.23 | 0.18 |
| PbO | 0.01 | 0.03 | 0.02 |
| As2O3 | n.d. | 0.01 | n.d. |
| ZnO | 0.03 | 0.16 | 0.31 |
| CuO | 0.08 | 0.12 | 0.04 |
| NiO | 0.01 | 0.02 | 0.04 |
| Fe2O3 | 0.66 | 1.17 | 5.87 |
| MnO | 0.1 | 0.05 | 1.39 |
| Cr2O3 | 0.02 | 0.03 | 0.09 |
| TiO2 | 0.28 | 0.77 | 0.87 |
| CaO | 61.6 | 62.69 | 25.3 |
| K2O | 0.47 | 0.43 | 4.38 |
| Cl | 0.18 | 0.36 | 0.2 |
| SO3 | 1.11 | 1.6 | 3.14 |
| P2O5 | 0.47 | 0.87 | 4.21 |
| SiO2 | 18.14 | 16.1 | 34.63 |
| Al2O3 | 14.51 | 12.14 | 12.67 |
| MgO | 1.87 | 2.11 | 5.29 |
| Na2O | 0.61 | 1.05 | 1.32 |
| Elements | Limit Value (mg/kg) | Fly Ash Sample A (mg/kg) | Fly Ash Sample B (mg/kg) | Fly Ash Sample C (mg/kg) |
|---|---|---|---|---|
| Sb | 144.77 | 69.83 | ||
| As | 5000 | 8.5 | 6.4 | 19.11 |
| Ba | 706.13 | 661.67 | ||
| Pb | 628.43 | 464.12 | ||
| Cd | 5000 | 4.76 | 3.19 | 11.63 |
| Cr | 46.69 | 39.99 | ||
| Co | 11.05 | 9.34 | ||
| Cu | 591.99 | 567.32 | ||
| Mo | 5.46 | 4.58 | ||
| Ni | 24.62 | 21.63 | ||
| Hg | 20 | 0.64 | 0.44 | 2.1 |
| Zn | 3368.4 | 2562.8 | ||
| Sn | 111.26 | 52.61 | ||
| Al | 45,396 | 45,396.9 | ||
| Fe | 6520.96 | 5913.12 |
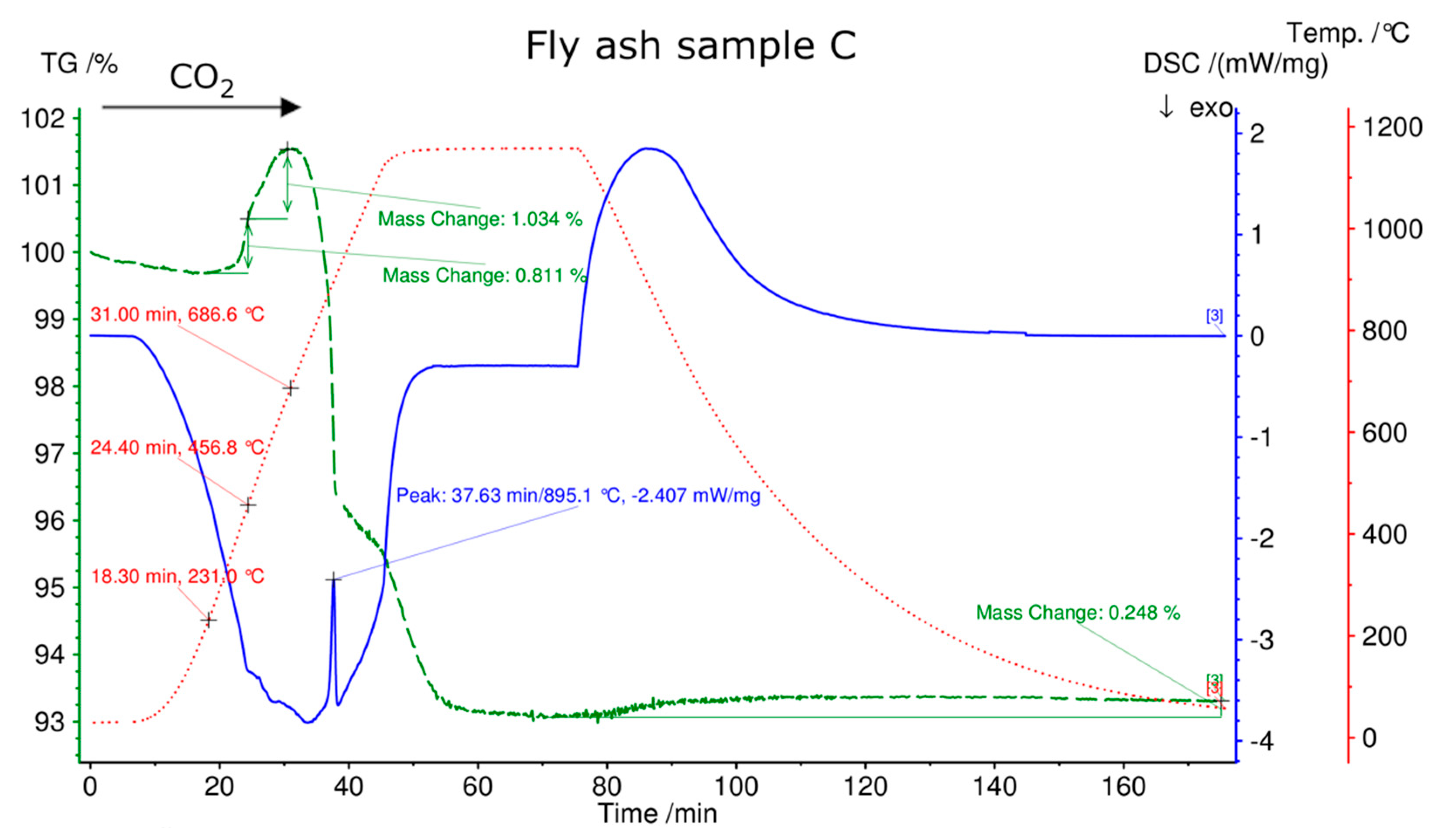
| TR °C | ML (%) FA A | DSC Endo/Exo | TR °C | ML (%) FA B | DSC Endo/Exo | TR °C | ML (%) FA C | DSC Endo/Exo |
|---|---|---|---|---|---|---|---|---|
| 70–356 | 0.3 | -- | 70–335 | 0.5 | -- | 70–313 | 0.1 | |
| 356–534 | 0.4 | Endo | 336–445 | 1.3 | Endo | 313–434 | 0.2 | |
| 534–880 | 7.6 | Endo | 445–734 | 7.8 | Endo | 434–716 | 2.9 | Endo |
| -- | -- | 734–880 | 0.5 | -- | 716–880 | 0.2 | Endo | |
| Total | 8.3 | 10.1 | 3.4 |
| TR °C | MG (%) FA A | DSC Endo/Exo | TR °C | MG (%) FA B | DSC Endo/Exo | TR °C | MG (%) FA C | DSC Endo/Exo |
|---|---|---|---|---|---|---|---|---|
| 873–70 | 13.1 | Exo | 874–70 | 14.2 | Exo | 874–70 | 2.7 | Exo |
| Fly Ash Sample | Charging (kJ/kg) | Discharging (kJ/kg) |
|---|---|---|
| A | 225 | 938 |
| B | 504 | 1011 |
| C | 75 | 195 |
| Energy Content in kJ/kg | 1. Cycle | 2. Cycle | 3. Cycle |
|---|---|---|---|
| Charging fly ash sample, A (heating to 880 °C, N2 atmosphere) | 335 | 700 | 570 |
| Discharging fly ash sample, A (cooling from 880 °C to 350 °C, CO2 and H2O atmosphere) | 852 | 664 | 541 |
| Charging fly ash sample B (heating to 880 °C, N2 atmosphere) | 504 | 1061 | 996 |
| Discharging fly ash sample B (cooling from 880 °C to 350 °C, CO2 and H2O atmosphere) | 1090 | 1025 | 924 |
| Charging fly ash sample C (heating to 880 °C, N2 atmosphere) | 162 | 151 | 115 |
| Discharging fly ash sample C (cooling from 880 °C to 350 °C, CO2 and H2O atmosphere) | 158 | 115 | 86 |
| Element Components | Limits Value (mg/kg) | Fly Ash Sample A (mg/kg) | Fly Ash Sample B (mg/kg) | Fly Ash Sample C (mg/kg) |
|---|---|---|---|---|
| Al | 143 | 174.1 | 232.1 | |
| Sb | 0.7 (2.1) | <0.02 | <0.02 | 0.11 |
| As | 2 | 0.06 | 0.08 | 0.21 |
| Ba | 100 (300) | 59.7 | 68.45 | 5.12 |
| Pb | 10 (30) | 5.69 | 5.97 | <0.42 |
| Cd | 1 | <0.01 | <0.01 | <0.01 |
| Cr | 10 (20) | 0.09 | 0.32 | 0.88 |
| Co | 5 | 0.09 | <0.09 | <0.09 |
| Fe | 0.06 | 0.70 | 0.69 | |
| Cu | 50 | 0.04 | 0.03 | <0.03 |
| Mo | <0.20 | 0.17 | 1.96 | |
| Ni | 10 | 0.1 | 0.13 | 0.03 |
| Hg | 0.1 | 0.01 | 0.01 | 0.02 |
| Ag | 1 | 0.43 | <0.03 | <0.03 |
| Zn | 50 (100) | 0.02 | 1.34 | 0.33 |
| Sn | 20 | 12.62 | 0.05 | 0.02 |
| pH | 10.21 | 12.58 | 12.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setoodeh Jahromy, S.; Azam, M.; Jordan, C.; Harasek, M.; Winter, F. The Potential Use of Fly Ash from the Pulp and Paper Industry as Thermochemical Energy and CO2 Storage Material. Energies 2021, 14, 3348. https://doi.org/10.3390/en14113348
Setoodeh Jahromy S, Azam M, Jordan C, Harasek M, Winter F. The Potential Use of Fly Ash from the Pulp and Paper Industry as Thermochemical Energy and CO2 Storage Material. Energies. 2021; 14(11):3348. https://doi.org/10.3390/en14113348
Chicago/Turabian StyleSetoodeh Jahromy, Saman, Mudassar Azam, Christian Jordan, Michael Harasek, and Franz Winter. 2021. "The Potential Use of Fly Ash from the Pulp and Paper Industry as Thermochemical Energy and CO2 Storage Material" Energies 14, no. 11: 3348. https://doi.org/10.3390/en14113348
APA StyleSetoodeh Jahromy, S., Azam, M., Jordan, C., Harasek, M., & Winter, F. (2021). The Potential Use of Fly Ash from the Pulp and Paper Industry as Thermochemical Energy and CO2 Storage Material. Energies, 14(11), 3348. https://doi.org/10.3390/en14113348








