Recent Advances in Covalent Organic Frameworks for Heavy Metal Removal Applications
Abstract
1. Introduction
2. Synthetic Methodologies for Covalent Organic Frameworks
2.1. Selection of Functional Side Groups
| Type of Linkage | Benefits | Drawbacks |
|---|---|---|
| Boronate Boroxine | ▪ Thermal stability [28] | ▪ Prone to amorphization or disintegration upon contact with water or protic solvents [59]. |
| Imine | ▪ Enhanced hydrothermal stability [59,60]. ▪ High chemical stability under harsh acidic conditions [60]. | ▪ Lower crystallinity compared to boronate-linked and phenazine-linked COFs [28]. |
| Hydrazone | ▪ Easily controllable, pH-dependent and reversible synthetic route [25]. ▪ Improved stability [29]. | ▪ Exfoliation into thin films under mild conditions, due to weak interlayer interactions [34]. |
| Imide | ▪ High porosity [28] | ▪ Require high temperatures during synthesis [28]. |
| Azine | ▪ Enhanced thermal stability [25,29]. ▪ Resistant to hydrolysis in both acidic and basic media [28,32]. ▪ Production of COFs with narrow pore sizes [59]. | ▪ Less chemical stability than triazine or phenazine linkages [34]. |
| Phenazine | ▪ Stable in various solvents [16,28]. ▪ Extended conjugated networks [34]. | |
| Triazine | ▪ High crystallinity [28]. ▪ High porosity [28]. ▪ Synthesis at room temperature [28] ▪ Extended conjugated networks [34]. | |
| sp2-c | ▪ Ultra-stable frameworks [29,34]. ▪ Maintenance of crystallinity and porosity in water, acidic/alkaline media, and prolonged exposure to air [29,34,40]. | ▪ Complicated synthesis, due to poor reversibility [29,34]. |
2.2. Synthetic Methods of Covalent Organic Framworks
2.2.1. Solvothermal Synthesis
2.2.2. Ionothermal Synthesis
2.2.3. Microwave-Assisted Synthesis
2.2.4. Room-Temperature Synthesis
2.2.5. Other Methods
3. Heavy Metal Removal Applications
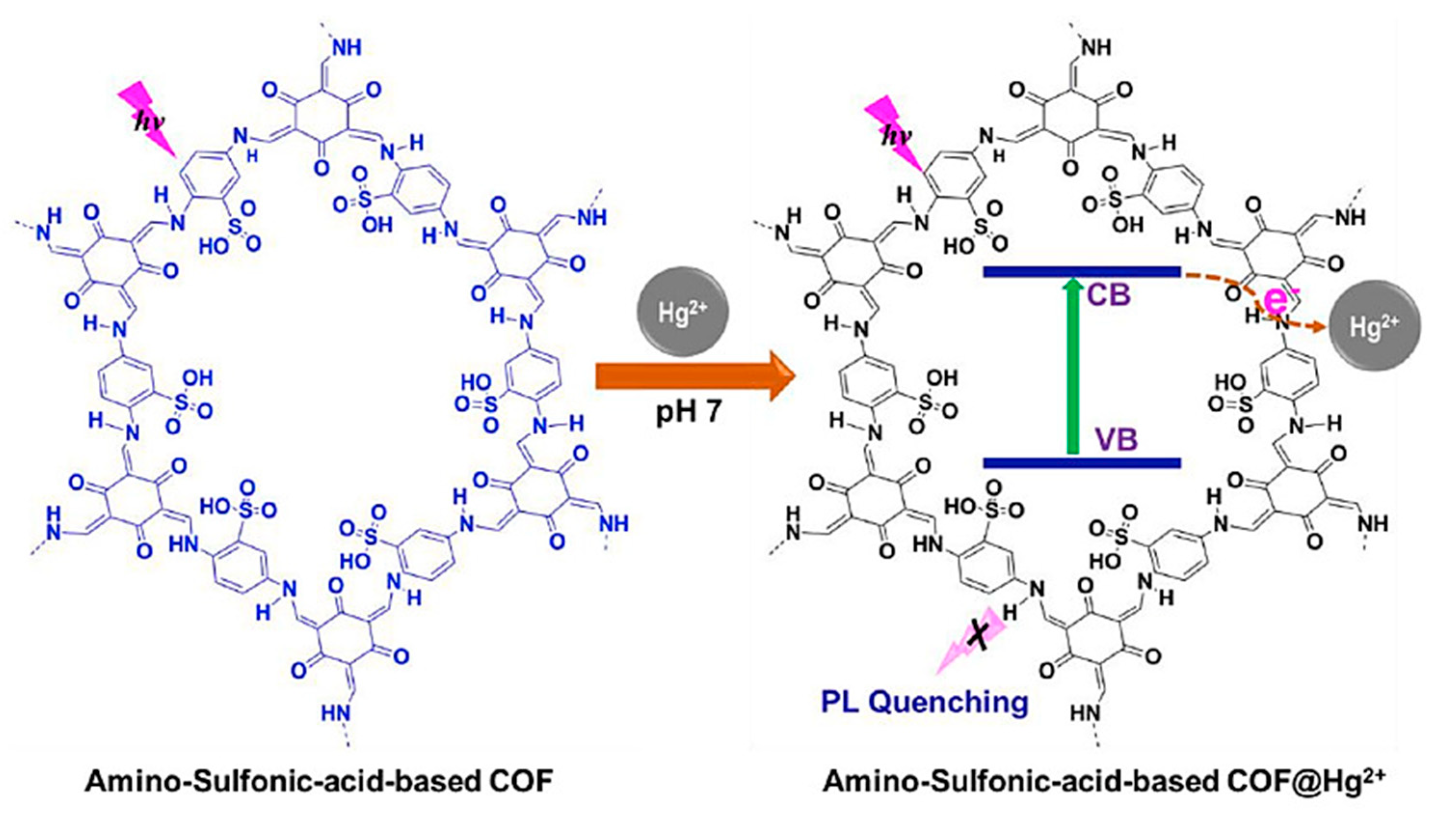
3.1. Removal of Mercury
3.2. Removal of Lead
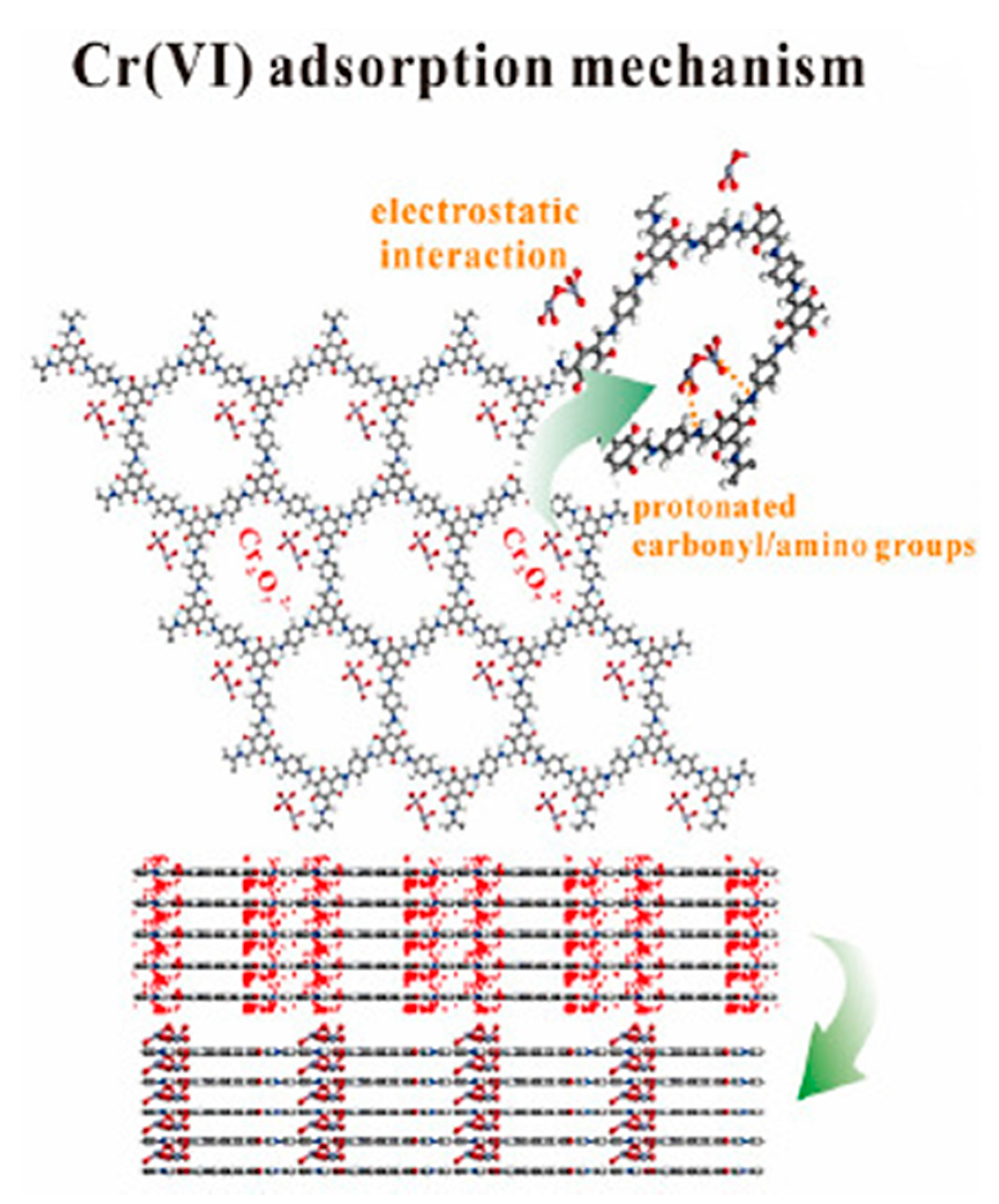
3.3. Removal of Chromium
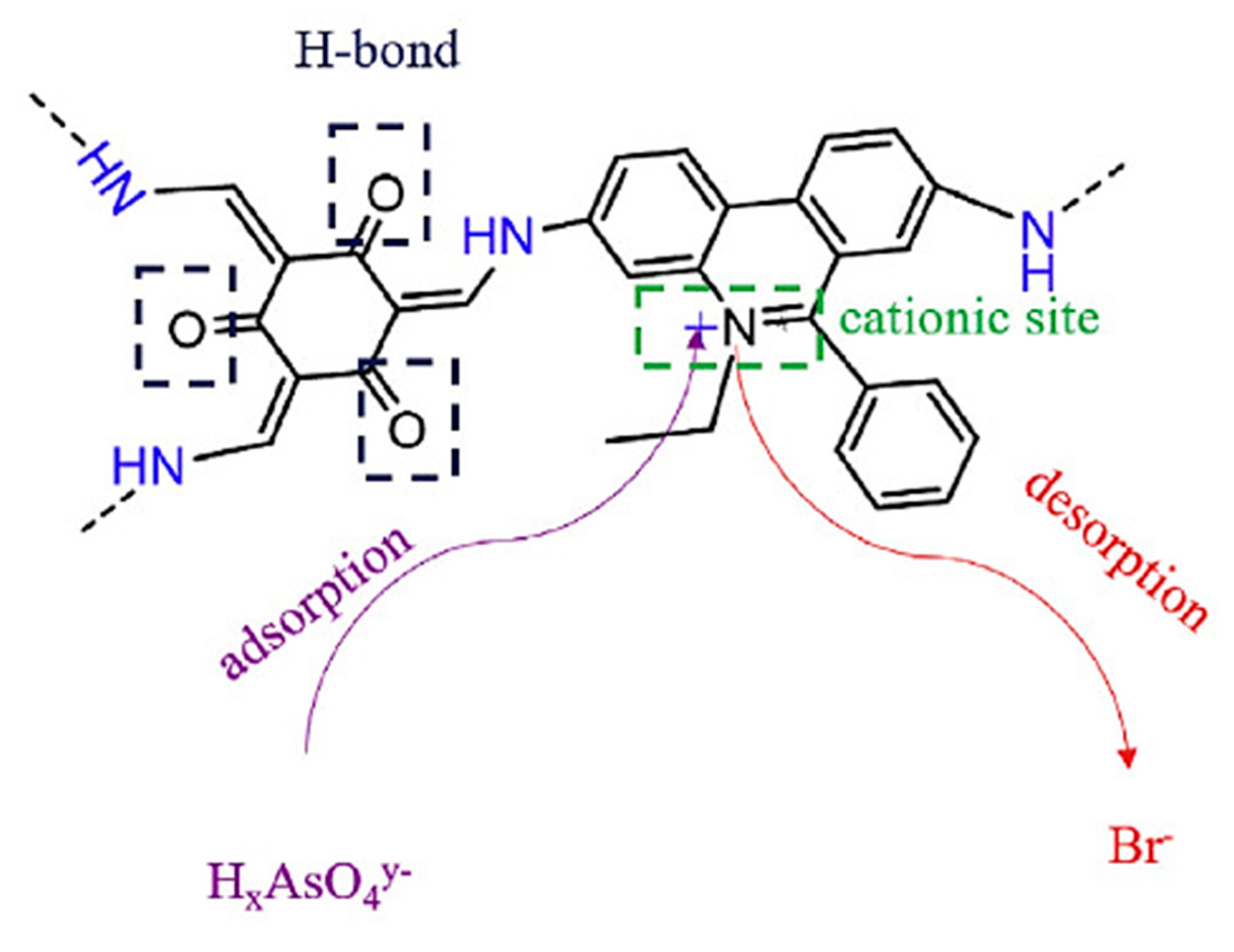
3.4. Removal of Arsenic
3.5. Removal of Cadmium
3.6. Removal of Radioactive Elements
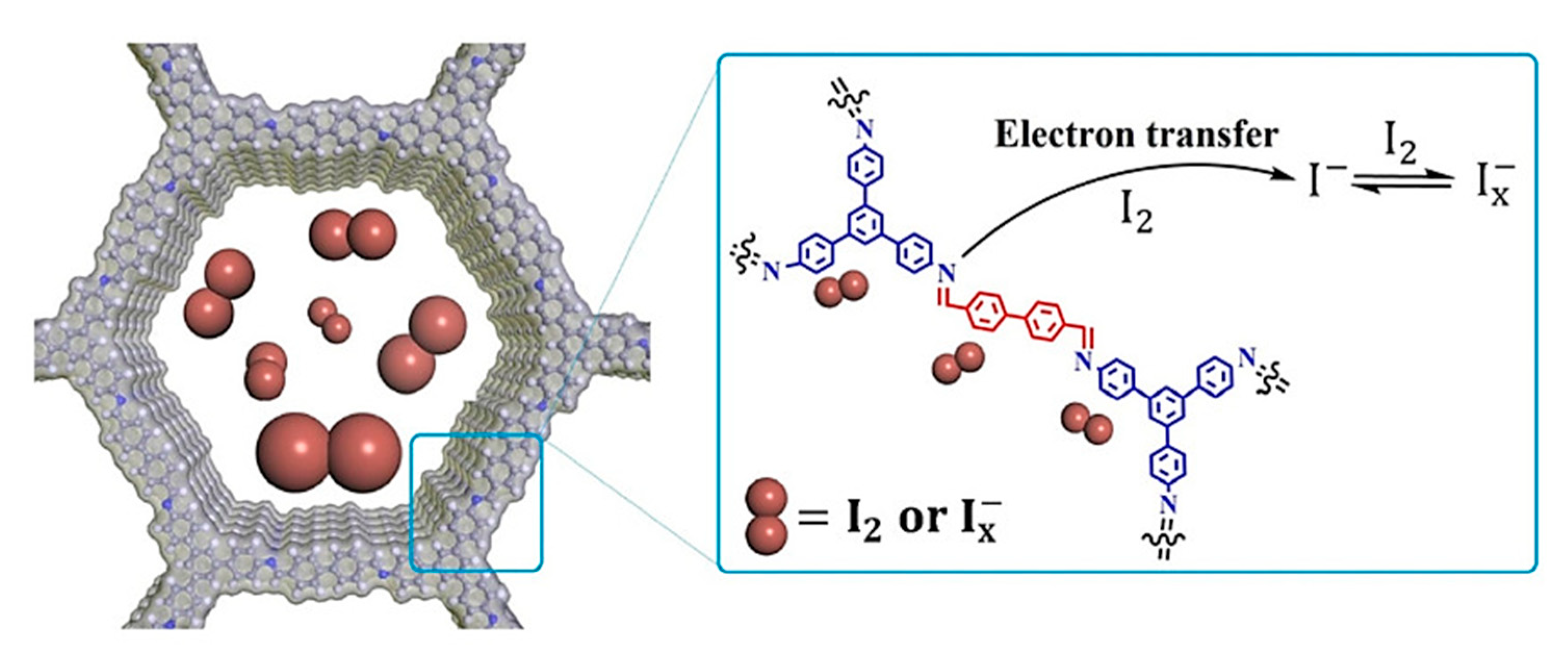
3.6.1. Removal of Iodine
3.6.2. Removal of Uranium
3.7. Other Metals
| Adsorbents | Adsorbates | Adsorption Capacity | References |
|---|---|---|---|
| TPB-DMTP-COF | Hg2+ | 8.5 μg/L | [76] |
| 2D COF | Hg0 Hg2+ | 863 mg/g 1350 mg/g | [77] |
| γ-Fe2O3@CTF-1 | Hg2+ | 165.8 mg/g | [78] |
| SCTN-1 | Hg0 Hg2+ | 813 mg/g 1253 mg/g | [80] |
| AgNPs@COF | Hg2+ | 113 mg/g | [81] |
| COF-SO3− | Hg2+ | 1299 mg/g | [83] |
| Metal-free COF | Hg2+ | 98.42 mg/g | [84] |
| SH-COF | Hg2+ | 1283 mg/g | [85] |
| COF-TP COF-TE | Pb2+ | 140 mg/g 185.7 mg/g | [26] |
| COF-SH | Pb2+ | 239 mg/g | [95] |
| Guanidium-based COF | Cr6+ | 90–200 mg/g | [96] |
| Dual-pore COF | Cr6+ | 384 mg/g | [97] |
| Magnetic COF | Cr6+ | 245.45 mg/g | [98] |
| COF1 COF2 | Cr6+ | 462.96 mg/g 649.35 mg/g | [99] |
| γ-Fe2O3@CTF-1 | As3+ As5+ | 198 mg/g 102.3 mg/g | [78] |
| EB-COF:Br | As5+ | 5.1–53.1 mg/g | [103] |
| Fe0/COF | As3+ | 135.78 mg/g | [104] |
| Heteropore COF | Cd2+ | 116 mg/g | [108] |
| N-riched COF | Cd2+ | 396 mg/g | [105] |
| Heteropore COF | I2 | 4810 mg/g | [110] |
| HCOF | I2 vapor | 2900 mg/g | [111] |
| N-riched COF | I2 | 5.43 g/g | [113] |
| COF@cotton fiber | I2 | 533.9 mg/g | [114] |
| Mesoporous N-COF | I2 | 988.17 mg/g | [115] |
| HBI-COF | U6+ | 81 mg/g | [116] |
| AO-COF | U6+ | 68 mg/g | [117] |
| PAF-1-CH2-AO | U6+ | 300 mg/g | [118] |
| GS-COF | U6+ | 144.2 mg/g | [119] |
| COF-TpPa | U6+ | 152 mg/g | [120] |
| Fe3O4-MOF-COF | Cu2+ | 37.29 mg/g | [124] |
| Adenine-grafted COF | Ag+ | 40 mg/L | [125] |
4. Comparison of Covalent Organic Frameworks with the Competitive Systems
| COF | ZEOLITES | MOF | GO | |||
|---|---|---|---|---|---|---|
| Pb (II) adsorption capacity | 185.7 mg/g [25,26] | 14 mg/g [134,135] | 1348.42 mg/g [132] | 23.46 mg/g [136] | 862.44 mg/g [130] | 204–479 mg/g [133] |
| Hg (II) adsorption capacity | 4395 mg/g [25,82] | 8.0 μmol/g [137] | 718.1 mg/g [41] | 905.5 mg/g [131] | 526.32 mg/g [138] | |
5. Perspectives on the Large-Scale Industrial Applications—Room for Improvement
6. Biocompatibility of COFs: Are There Any Dangers/Drawbacks When Using Them in Aquatic Environments?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, L.; Li, S.; Li, Y.; Liu, Y.; Du, K. Fabrication of superporous cellulose beads via enhanced inner cross-linked linkages for high efficient adsorption of heavy metal ions. J. Clean. Prod. 2020, 253, 120017. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, K.; Zhao, Q.; Huang, M.; Quyang, X. Comparative adsorption of heavy metal ions in wastewater on monolayer molybdenum disulfide. Green Energy Environ. 2020, in press. [Google Scholar] [CrossRef]
- Wang, W.; Fan, X.; Huang, C.; Zheng, H.; Chen, Z.; Fan, B.; Xu, C. Monitoring and comparison analysis of heavy metals in the five great lakes in Jiangsu Province. J. Lake SC 2016, 28, 494–501. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Luan, Y.; Dai, W. Application of algae for heavy metal adsorption: A 20-year meta-analysis. Ecotoxicol. Environ. Saf. 2020, 190, 110089. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indicat. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Ma, R.; Wu, K.; Li, H.; Feng, B.; Li, C.; Shen, Y. Facile synthesis of sulfonated covalent organic framework for the adsorption of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2020, 112, 122–129. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Sher, F.; Bilal, M. Covalent organic frameworks as robust materials for mitigation of environmental pollutants. Chemosphere 2021, 270, 129523. [Google Scholar] [CrossRef]
- d’Halluin, M.; Ru-Barrull, J.; Bretel, G.; Labrugere, C.; Le Grognec, E.; Felpin, F.X. Chemically modified cellulose filter paper for heavy metal remediation in water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chien, S.K.; Jhang, S.R.; Lin, Y.C. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 100, 151–159. [Google Scholar] [CrossRef]
- Shu, J.; Wu, H.; Chen, M.; Peng, H.; Li, B.; Liu, R.; Liu, Z.; Wang, B.; Huang, T.; Hu, Z. Fractional removal of manganese and ammonia nitrogen from electrolytic metal manganese residue leachate using carbonate and struvite precipitation. Water Res. 2019, 153, 229–238. [Google Scholar] [CrossRef]
- Shi, X.; Wang, R.; Xiao, A.; Jia, T.; Sun, S.P.; Wang, Y. Layer-by-layer synthesis of covalent organic frameworks on porous substrates for fast molecular separations. ACS Appl. Nano Mater. 2018, 1, 6320–6326. [Google Scholar] [CrossRef]
- Walekar, L.; Dutta, T.; Kumar, P.; Ok, Y.S.; Pawar, S.; Deep, A.; Kim, K.H. Functionalized fluorescent nanomaterials for sensing pollutants in the environment: A critical review. Trac. Trends Anal. Chem. 2017, 97, 458–467. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, Z.; Li, H.; Pei, W.; Ding, M.; Xie, Z.; Huo, Y.; Li, H. Synergistic removal of organic pollutant and metal ions in photocatalysis-membrane distillation system. Appl. Catal. B 2020, 118463. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the binding performance of amyloid-carbon membranes toward heavy metal ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, J.; Cui, J.; An, W.; Liu, L.; Liang, Y.; Cui, W. Surface oxygen vacancies enriched FeOOH/Bi2MoO6 photocatalysis-Fenton synergy degradation of organic pollutants. J. Hazard. Mater. 2020, 121399. [Google Scholar] [CrossRef]
- Ansari, S. Combination of molecularly imprinted polymers and carbon nanomaterials as a versatile biosensing tool in sample analysis: Recent applications and challenges. Trac. Trends Anal. Chem. 2017, 93, 134–151. [Google Scholar] [CrossRef]
- Acharya, J.; Kumar, U.; Rafi, P.M. Removal of heavy metal ions from wastewater by chemically modified agricultural waste material as potential adsorbent-a review. Int. J. Curr. Eng. Technol. 2018, 8, 526–530. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- He, X.; Qiu, X.; Hu, C.; Liu, Y. Treatment of heavy metal ions in wastewater using layered double hydroxides: A review. J. Dispers. Sci. Technol. 2018, 39, 792–801. [Google Scholar] [CrossRef]
- Meteku, B.E.; Huang, J.; Zeng, J.; Subhan, F.; Feng, F.; Zhang, Y.; Qiu, Z.; Aslam, S.; Li, G.; Yan, Z. Magnetic metal–organic framework composites for environmental monitoring and remediation. Coord. Chem. Rev. 2020, 413, 213261. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. Trac. Trends Anal. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Zhang, N.; Ishag, A.; Li, Y.; Wang, H.; Guo, H.; Mei, P.; Meng, Q.; Sun, Y. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review. J. Clean. Prod. 2020, 277, 123360. [Google Scholar] [CrossRef]
- Li, G.; Ye, J.; Fang, Q.; Liu, F. Amide-based covalent organic frameworks materials for efficient and recyclable removal of heavy metal lead (II). Chem. Eng. J. 2019, 370, 822–830. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19. [Google Scholar] [CrossRef]
- Geng, K.; Arumugam, V.; Xu, H.; Gao, Y.; Jiang, D. Covalent organic frameworks: Polymer chemistry and functional design. Prog. Polym. Sci. 2020, 108, 101288. [Google Scholar] [CrossRef]
- Baldwin, L.A.; Crowe, J.W.; Pyles, D.A.; McGracier, P.L. Metalation of a mesoporous three-dimensional covalent organic framework. J. Am. Chem. Soc. 2016, 138, 15134–15137. [Google Scholar] [CrossRef]
- Cao, S.; Li, B.; Zhu, R.; Pang, H. Design and synthesis of covalent organic frameworks towards energy and environment fields. Chem. Eng. J. 2019, 355, 602–623. [Google Scholar] [CrossRef]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, X.; Zhang, J.; Wu, X.; Liu, Y.; Cui, Y. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 2016, 138, 12332–12335. [Google Scholar] [CrossRef]
- You, J.; Zhao, Y.; Wang, L.; Bao, W. Recent developments in the photocatalytic applications of covalent organic frameworks: A review. J. Clean. Prod. 2021, 291, 125822. [Google Scholar] [CrossRef]
- Guan, X.; Ma, Y.; Li, H.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. [Google Scholar] [CrossRef] [PubMed]
- Bisbey, R.P.; Dichtel, W.R. Covalent organic frameworks as a platform for multidimensional polymerization. ACS Cent. Sci. 2017, 3, 533–543. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 2018, 141, 1807–1822. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Medina, D.D.; Rotter, J.M.; Hu, Y.; Dogru, M.; Werner, V.; Auras, F.; Markiewicz, J.T.; Knochel, P.; Bein, T. Room temperature synthesis of covalent–organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 2015, 137, 1016–1019. [Google Scholar] [CrossRef]
- Jin, E.; Li, J.; Geng, K.; Jiang, Q.; Xu, H.; Xu, Q.; Jiang, D. Designed synthesis of stable light-emitting two-dimensional sp2 carbon-conjugated covalent organic frameworks. Nat. Commun. 2018, 9, 4143. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions. J. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. ACC Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Lyu, H.; Diercks, C.S.; Zhu, C.; Yaghi, O.M. Porous crystalline olefin-linked covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 6848–6852. [Google Scholar] [CrossRef]
- Li, X.; Zhan, C.; Cai, S.; Lei, X.; Altoe, V.; Hong, F.; Urban, J.J.; Ciston, J.; Chan, E.M.; Liu, Y. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Commun. 2018, 9, 2998. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wa, S.; Jin, Y.; Lee, S.H.; Zhang, W. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 2016, 55, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Haase, F.; Troschke, E.; Savasci, G.; Banerjee, T.; Duppel, V.; Dörfler, S.; Grundei, M.M.J.; Burow, A.M.; Ochsenfeld, C.; Kaskel, S.; et al. Topochemical conversion of an imine- into a thiazole-linked covalent organic framework enabling real structure analysis. Nat. Commun. 2018, 9, 2600. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Addicoat, M.; Jin, E.; Zhai, L.; Xu, H.; Huang, N.; Guo, Z.; Lili, L.; Irle, S.; Jiang, D. Locking covalent organic frameworks with hydrogen bonds: General and remarkable effects on crystalline structure, physical properties, and photochemical activity. J. Am. Chem. Soc. 2015, 137, 3241–3247. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Gu, S.; Zheng, J.; Zhuang, Z.; Qiu, S.; Yan, Y. 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis. Angew. Chem. Int. Ed. 2014, 53, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Geng, K.; Lee, K.H.; Jiang, W.; Li, J.; Jiang, Q.; Irle, S.; Jiang, D. Topology-templated synthesis of crystalline porous covalent organic frameworks. Angew. Chem. 2020, 59, 12162–12169. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Yang, C.; Zhang, W.; Xu, J.; Liu, L.; Wu, D.; Wang, X.; Han, Y.; Liang, Q.; Zhang, F. Two-dimensional semiconducting covalent organic frameworks via condensation at arylmethyl carbon atoms. Nat. Commun. 2019, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Acharjya, A.; Pachfule, P.; Roeser, J.; Schmitt, F.J.; Thomas, A. Vinylene-linked covalent organic frameworks by base-catalyzed aldol condensation. Angew. Chem. Int. Ed. 2019, 58, 14865–14870. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Asada, M.; Xu, Q.; Dalapati, S.; Addicoat, M.A.; Brady, M.A.; Xu, H.; Nakamura, T.; Heine, T.; Chen, Q.; et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 2017, 357, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhao, W.; Zhang, F.; Cao, Y.; Liu, F.; Bi, S.; Feng, X. A two-dimensional conjugated polymer framework with fully sp2-bonded carbon skeleton. Polym. Chem. 2016, 7, 4176–4181. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, M.; Mao, H.; Pei, X.; Alshmimri, S.A.; Reimer, J.A.; Yaghi, O.M. Crystalline dioxin-linked covalent organic frameworks from irreversible reactions. J. Am. Chem. Soc. 2018, 140, 12715–12719. [Google Scholar] [CrossRef]
- Waller, P.J.; Lyle, S.J.; Osborn Popp, T.M.; Diercks, C.S.; Reimer, J.A.; Yaghi, O.M. Chemical conversion of linkages in covalent organic frameworks. J. Am. Chem. Soc. 2016, 138, 15519–15522. [Google Scholar] [CrossRef]
- Liu, H.; Chu, J.; Yin, Z.; Cai, X.; Zhuang, L.; Deng, H. Covalent organic frameworks linked by amine bonding for concerted electrochemical reduction of CO2. Chem 2018, 4, 1696–1709. [Google Scholar] [CrossRef]
- Waller, P.J.; AlFaraj, Y.S.; Diercks, C.S.; Jarenwattananon, N.N.; Yaghi, O.M. Conversion of imine to oxazole and thiazole linkages in covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 9099–9103. [Google Scholar] [CrossRef]
- Mahmood, J.; Ahmad, I.; Jung, M.; Seo, J.M.; Yu, S.Y.; Noh, H.J.; Kim, Y.H.; Shin, H.J.; Baek, J.B. Two-dimensional amine and hydroxy functionalized fused aromatic covalent organic framework. Commun. Chem. 2020, 3. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Qian, C.; Qi, Q.Y.; Jiang, G.F.; Cui, F.Z.; Tian, Y.; Zhao, X. Toward covalent organic frameworks bearing three different kinds of pores: The strategy for construction and COF-to-COF transformation via heterogeneous linker exchange. J. Am. Chem. Soc. 2017, 139, 6736–6743. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. Trac. Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Smith, B.J.; Overholts, A.C.; Hwang, N.; Dichtel, W.R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 2016, 52, 3690–3693. [Google Scholar] [CrossRef]
- Koo, B.T.; Heden, R.F.; Clancy, P. Nucleation and growth of 2D covalent organic frameworks: Polymerization and crystallization of COF monomers. Phys. Chem. Chem. Phys. 2017, 19, 9745–9754. [Google Scholar] [CrossRef]
- Singh, V.; Jang, S.; Vishwakarma, N.K.; Kim, D.P. Intensified synthesis and post-synthetic modification of covalent organic frameworks using a continuous flow of microdroplets technique. NPG Asia Mater. 2018, 10, 456. [Google Scholar] [CrossRef]
- Gan, J.S.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review. Int. J. Biol. Macromol. 2021, 167, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gao, Y.; Wang, C.H.H.; Ge, R.; Lu, M.; Zhang, J.; Li, Z.; Shao, P. Synthesis of two-dimensional covalent organic frameworks in ionic liquids. Chem. A Eur. J. 2019, 25, 15488–15492. [Google Scholar] [CrossRef]
- Kuchenbuch, A.; Giernoth, R. Ionic liquids beyond simple solvents: Glimpses at the state of the art in Organic Chemistry. Chem. Open 2015, 4, 677–681. [Google Scholar] [CrossRef]
- Ge, J.; Xiao, J.; Liu, L.; Qiu, L.; Jiang, X. Facile microwave-assisted production of Fe3O4 decorated porous melamine-based covalent organic framework for highly selective removal of Hg2+. J. Porous Mater. 2016, 23, 791800. [Google Scholar] [CrossRef]
- Wei, H.; Chai, S.; Hu, N.; Yang, Z.; Wei, L.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178–12181. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Jadhav, T.; Fang, Y.; Patterson, W.; Liu, C.H.; Hamzehpoo, E.; Perepichka, D.F. 2D Poly(arylene vinylene) covalent organic frameworks via aldol condensation of trimethyltriazine. Angew. Chem. Int. Ed. 2019, 58, 13753–13757. [Google Scholar] [CrossRef] [PubMed]
- Velempini, T.; Pillay, K. Sulphur functionalized materials for Hg (II) adsorption: A review. J. Environ. Chem. Eng. 2019, 7, 103350. [Google Scholar] [CrossRef]
- Wu, S.; Uddin, M.A.; Nagano, S.; Ozaki, M.; Sasaoka, E. Fundamental study on decomposition characteristics of mercury compounds over solid powder by temperature-programmed decomposition desorption mass spectrometry. Energy Fuels 2011, 25, 144–153. [Google Scholar] [CrossRef]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem. Eng. J. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Merí-Bofí, L.; Royuela, S.; Zamora, F.; Ruiz-González, M.L.; Segura, J.L.; Muñoz-Olivas, R.; Mancheño, M.J. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg (II). J. Mater. Chem. A 2017, 5, 17973–17981. [Google Scholar] [CrossRef]
- Sun, B.; Liu, J.; Cao, A.; Song, W.; Wang, D. Interfacial synthesis of ordered and stable covalent organic frameworks on amino-functionalized carbon nanotubes with enhanced electrochemical performance. Chem. Commun. 2017, 53, 6303–6306. [Google Scholar] [CrossRef]
- Leus, K.; Folens, K.; Nicomel, N.R.; Perez, J.P.H.; Filippousi, M.; Meledina, M.; Dirtu, M.M.; Turner, S.; Van Tendeloo, G.; Garcia, Y.; et al. Removal of arsenic and mercury species from water by covalent triazine framework encapsulated γ-Fe2O3 nanoparticles. J. Hazard. Mater. 2018, 353, 312–319. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, W.; Zhang, W.; Huang, Q.; Yan, J.; Pan, C.; Yu, G. Sulfur-rich covalent triazine polymer nanospheRes. for environmental mercury removal and detection. Polym. Chem. 2018, 9, 4125–4131. [Google Scholar] [CrossRef]
- Mondal, S.; Chatterjee, S.; Mondal, S.; Bhaumik, A. Thioether-functionalized covalent triazine nanospheres: A robust adsorbent for mercury removal. ACS Sustain. Chem. Eng. 2019, 7, 7353–7361. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Qiu, Y.; Liu, X.; Huang, W.; Yan, N.; Qu, Z. Utilization of Ag nanoparticles anchored in covalent organic frameworks for mercury removal from acidic waste water. J. Hazard. Mater. 2019, 121824. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Wang, K.; Wang, L. A triarylamine-based fluorescent covalent organic framework for efficient detection and removal of mercury (II) ion. Dye. Pigm. 2020, 173, 107880. [Google Scholar] [CrossRef]
- Tao, Y.; Xiong, X.H.; Xiong, J.B.; Yang, L.X.; Fan, Y.L.; Feng, H.; Luo, F. High-performance removal of mercury ions (II) and mercury vapor by SO3−-anchored covalent organic framework. J. Solid State Chem. 2020, 282, 121126. [Google Scholar] [CrossRef]
- Panda, A.; Yang, Y.; Venkateswarlu, S.; Son, Y.; Bae, T.H.; Yoon, M. Highly durable covalent organic framework for the simultaneous ultrasensitive detection and removal of noxious Hg2+. Microporous Mesoporous Mater. 2020, 306, 110399. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, F.; Liu, N.; Liu, W.; Tong, M. Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg (II) removal from water. J. Hazard. Mater. 2020, 124190. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, G.; Liu, J.; Yuan, D. A recyclable fluorescent covalent organic framework for exclusive detection and removal of mercury (II). Chem. Eng. J. 2020, 401, 126139. [Google Scholar] [CrossRef]
- Awual, M.R. Mesoporous composite material for efficient lead (II) detection and removal from aqueous media. J. Environ. Chem. Eng. 2019, 7, 103124. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Tang, L.; Tang, J.; Zeng, G.; Dong, H.; Deng, Y.; Wang, L.; Liu, Y.; Ren, X.; Zhou, Y. Cu-doped Fe@Fe2O3 core-shell nanoparticle shifted oxygen reduction pathway for high-efficiency arsenic removal in smelting wastewater. Environ. Sci. Nano 2018, 5, 1595–1607. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, J.; Tan, X.; Zhang, W.; Fang, M.; Wang, X. Efficient removal of Pb2+ by Tb-MOFs: Identifying the adsorption mechanism through experimental and theoretical investigations. Environ. Sci. Nano 2019, 6, 261–272. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.; Zou, Y.; Yao, W.; Hou, J.; Xia, L.; Peng, A.; Alsaedi, A.; Hayat, T.; Wang, X. Synthesis of molybdenum disulfide/reduced graphene oxide composites for effective removal of Pb (II) from aqueous solutions. Sci. Bull. 2017, 62, 913–922. [Google Scholar] [CrossRef]
- Gupta, K.M.; Zhang, K.; Jiang, J. Efficient removal of Pb2+ from aqueous solution by an ionic covalent–organic framework: Molecular simulation study. Ind. Eng. Chem. Res. 2018, 57, 6477–6482. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, C.; Huang, W.; Chen, Y.; Wang, Y.; Wang, J. Covalent organic framework as a novel electrochemical platform for highly sensitive and stable detection of lead. Talanta 2018, 188, 578–583. [Google Scholar] [CrossRef]
- Xu, W.; Sun, X.; Huang, M.; Pan, X.; Huang, X.; Zhuang, H. Novel covalent organic framework/PVDF ultrafiltration membranes with antifouling and lead removal performance. J. Environ. Manag. 2020, 269, 110758. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, X.; Zhu, C.; Zhou, S.; Li, R.; Shi, H.; Miao, S.; Vakil, M.; Wang, W.; Qi, D. Sulfhydryl functionalized covalent organic framework as an efficient adsorbent for selective Pb (II) removal. Phys. Eng. Asp. 2020, 600, 125004. [Google Scholar] [CrossRef]
- Jansone-Popova, S.; Moinel, A.; Schott, J.A.; Mahurin, S.M.; Popovs, I.; Veith, G.M.; Moyer, B.A. Guanidinium-based ionic covalent organic framework for rapid and selective removal of toxic Cr (VI) oxoanions from water. Environ. Sci. Technol. 2018, 53, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Liang, R.R.; Qi, Q.Y.; Jiang, G.F.; Zhao, X. Efficient removal of Cr (VI) from aqueous solutions by a dual-pore covalent organic framework. Adv. Sustain. Syst. 2019, 1800150. [Google Scholar] [CrossRef]
- Zhong, X.; Lu, Z.; Liang, W.; Hu, B. The magnetic covalent organic framework as a platform for high-performance extraction of Cr (VI) and bisphenol a from aqueous solution. J. Hazard. Mater. 2020, 393, 122353. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, S.; Zhou, Z.; Li, R.; Ye, J.; Ziyu, X.; Lan, S.; Zhang, Y.; Miao, S.; Wang, W. Highly efficient and selective removal of Cr (VI) by covalent organic frameworks: Structure, performance and mechanism. Colloids Surf. A 2020, 600, 124910. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thakur, A.K.; Goswami, R.; Mazumder, P.; Taki, K.; Vithanage, M.; Kumar, M. Efficacy of agricultural waste derived biochar for arsenic removal: Tackling water quality in the Indo-Gangetic plain. J. Environ. Manag. 2021, 281, 111814. [Google Scholar] [CrossRef] [PubMed]
- Borah, R.; Taki, K.; Gogoi, A.; Da, P.; Kumar, M. Contemporary distribution and impending mobility of arsenic, copper and zinc in a tropical (Brahmaputra) river bed sediment, Assam, India. Ecotoxicol. Environ. Saf. 2018, 161, 769–776. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, R.; Patel, A.K.; Srivastava, M.; Das, N. Scenario, perspectives and mechanism of arsenic and fluoride Co-occurrence in the groundwater: A review. Chemosphere 2020, 249, 126126. [Google Scholar] [CrossRef]
- Yang, C.H.; Chang, J.S.; Lee, D.J. Covalent organic framework EB-COF:Br as adsorbent for phosphorus (V) or arsenic (V) removal from nearly neutral waters. Chemosphere 2020, 253, 126736. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Wang, L.; Qu, Z.; Yan, N. Surface nano-traps of Fe0/COFs for arsenic (III) depth removal from wastewater in non-ferrous smelting industry. Chem. Eng. J. 2020, 381, 122559. [Google Scholar] [CrossRef]
- Dinari, M.; Hatami, M. Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal. J. Environ. Chem. Eng. 2019, 7, 102907. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Pham, T.T.; Vranova, V.; Nguyen, H.T.H.; Nguyen, L.T.N.; Vuong, X.T.; Bui, Q.M. Removal of cadmium from aqueous solution using sonochemically modified clinoptilolite: Optimization and modeling. Environ. Technol. Innov. 2020, 20, 101166. [Google Scholar] [CrossRef]
- Shafiof Sadatal, M.; Nezamzadeh-Ejhieh, A. A comprehensive study on the removal of Cd (II) from aqueous solution on a novel pentetic acid-clinoptilolite nanoparticles adsorbent: Experimental design, kinetic and thermodynamic aspects. Solid State Sci. 2020, 99, 106071. [Google Scholar] [CrossRef]
- Liu, N.; Shi, L.; Han, X.; Qi, Q.Y.; Wu, Z.Q.; Zhao, X. A heteropore covalent organic framework for adsorptive removal of Cd (II) from aqueous solutions with high efficiency. Chin. Chem. Lett. 2020, 31, 386–390. [Google Scholar] [CrossRef]
- El-Shahat, M.; Abdelhamid, A.E.; Abdelhameed, R.M. Capture of iodide from wastewater by effective adsorptive membrane synthesized from MIL-125-NH2 and cross-linked chitosan. Carbohydr. Polym. 2020, 231, 115742. [Google Scholar] [CrossRef]
- Yin, Z.J.; Xu, S.Q.; Zhan, T.G.; Qi, Q.Y.; Wu, Z.Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheRes. formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, X.; Kim, S.T.; Alahakoon, S.B.; Hou, X.; Zhang, Z.; Thomson, C.M.; Smaldone, R.A.; Ke, C. An elastic hydrogen-bonded cross-linked organic framework for effective iodine capture in water. J. Am. Chem. Soc. 2017, 139, 7172–7175. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.; Jiang, W.; Jiang, Q.; Jiang, D. Exceptional iodine capture in 2D covalent organic frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef]
- Guo, X.; Tian, Y.; Zhang, M.; Li, Y.; Wen, R.; Li, X.; Xue, Y.; Ma, L.; Xia, C.; Li, S. Mechanistic insight into hydrogen-bond-controlled crystallinity and adsorption property of covalent organic frameworks from flexible building blocks. Chem. Mater. 2018, 30, 2299–2308. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhao, Q.; Li, L.; Chen, R.; He, C. Cotton fiber functionalized with 2D covalent organic frameworks for iodine capture. Cellulose 2019, 27, 1517–1529. [Google Scholar] [CrossRef]
- Chen, R.; Hu, T.; Li, Y. Stable nitrogen-containing covalent organic framework as porous adsorbent for effective iodine capture from water. React. Funct. Polym. 2021, 159, 104806. [Google Scholar] [CrossRef]
- Li, J.; Suo, L.; Meng, Y.; Li, H. Graph-based fair resource allocation scheme combining interference alignment in femtocell networks. IET Commun. 2015, 9, 211–218. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, M.; Li, B.; Zhao, X.; Zhang, S.; Wang, L.; Li, Y.; Zhang, J.; Ma, L.; Li, S. Modifiable diyne-based covalent organic framework: A versatile platform for in situ multipurpose functionalization. RSC Adv. 2016, 6, 39150–39158. [Google Scholar] [CrossRef]
- Li, B.; Sun, Q.; Zhang, Y.; Abney, C.W.; Aguila, B.; Lin, W.; Ma, S. Functionalized porous aromatic framework for efficient uranium adsorption from aqueous solutions. ACS Appl. Mater. Interfaces 2017, 9, 12511–12517. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Li, Y.; Zhang, M.; Guo, X.; Li, X.; Li, X.; Han, J.; Hu, S.; Tan, W.; Ma, L.; et al. Graphene-synergized 2D covalent organic framework for adsorption: A mutual promotion strategy to achieve stabilization and functionalization simultaneously. J. Hazard. Mater. 2018, 358, 273–285. [Google Scholar] [CrossRef]
- Li, Z.D.; Zhang, H.Q.; Xiong, X.H.; Luo, F. U (VI) adsorption onto covalent organic frameworks-TpPa-1. J. Solid State Chem. 2019, 277, 484–492. [Google Scholar] [CrossRef]
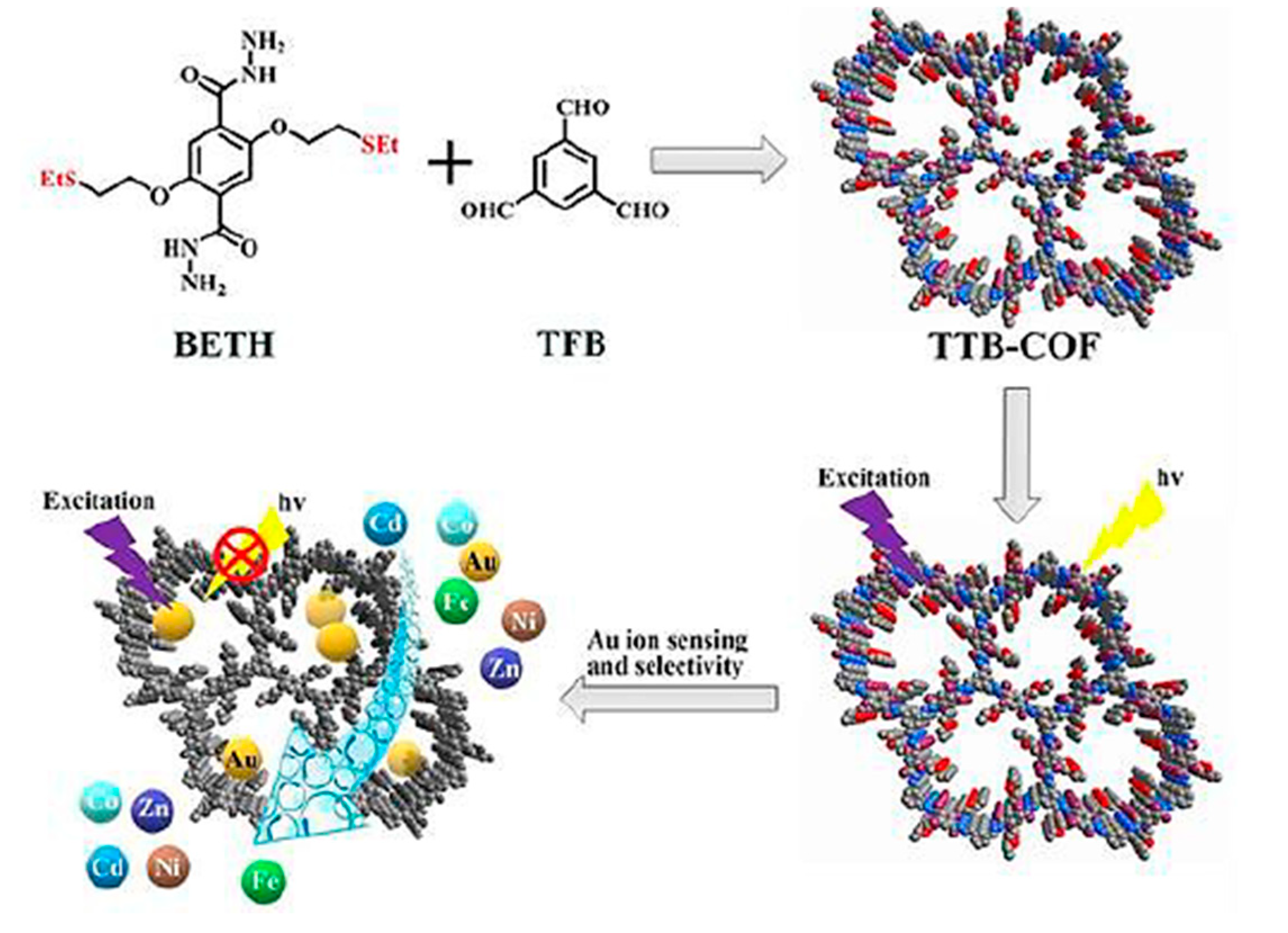
- Zhou, Z.; Zhong, Z.; Cui, K.; Zhuang, Z.; Li, L.; Li, L.; Bi, J.; Yu, Y. A covalent organic framework bearing thioether pendant arms for selective detection and recovery of Au from ultra-low concentration aqueous solution. Chem. Commun. 2018, 54, 9977–9980. [Google Scholar] [CrossRef]
- Lu, Q.; Ma, Y.; Li, H.; Guan, X.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Qiu, S.; Valtchev, V. Postsynthetic functionalization of three-dimensional covalent organic frameworks for selective extraction of lanthanide ions. Angew. Chem. Int. Ed. 2018, 57, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Jiang, Y.; Feng, L.; Hua, Y.; Liu, H.; Fan, C.; Yin, M.; Li, S.; Lv, X.; Wang, H. Q-graphene-scaffolded covalent organic frameworks as fluorescent probes and sorbents for the fluorimetry and removal of copper ions. Anal. Chim. Acta 2019, 1057, 88–97. [Google Scholar] [CrossRef]
- Li, W.T.; Shi, W.; Hu, Z.J.; Yang, T.; Chen, M.L.; Zhao, B.; Wang, J.H. Fabrication of magnetic Fe3O4@metal organic framework@covalent organic framework composite and its selective separation of trace copper. Appl. Surf. Sci. 2020, 530, 147254. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Liang, Y.; Zhao, Y.; Yuan, N.; Sui, Z.; Chen, Q. Adenine-bearing covalent organic frameworks via one-pot tandem reaction for selective adsorption of Ag+. Microporous Mesoporous Mater. 2021, 315, 110923. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M. Green preparation of dual-template chitosan-based magnetic water-compatible molecularly imprinted biopolymer. Carbohydr. Polym. 2020, 236, 116102. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, B.; Mao, Y. Study on force schemes in pseudopotential lattice Boltzmann model for two-phase flows. Math. Probl. Eng. 2018, 1–9. [Google Scholar] [CrossRef]
- Fan, H.; Mundstock, A.; Feldhoff, A.; Knebel, A.; Gu, J.; Meng, H.; Caro, J. Covalent organic framework–covalent organic framework bilayer membranes for highly selective gas separation. J. Am. Chem. Soc. 2018, 140, 10094–10098. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Yang, Q.; Pei, H.; Hu, N.; Suo, Y.; Li, Z.; Zhang, D.; Wang, J. Facile fabrication of robust MOF membranes on cloth via a CMC macromolecule bridge for highly efficient Pb(II) removal. Chem. Eng. J. 2018, 339, 230–239. [Google Scholar] [CrossRef]
- Abdollahi, N.; Razavi, S.A.A.; Morsali, A.; Hu, M.-L. High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl. Mater. Interfaces 2012, 4, 1186. [Google Scholar] [CrossRef] [PubMed]
- Rakhym, A.B.; Seilkhanova, G.A.; Kurmanbayeva, T.S. Adsorption of lead (II) ions from water solutions with natural zeolite and chamotte clay. Mater. Today Proc. 2020, 31, 482–485. [Google Scholar] [CrossRef]
- Mudasir, M.; Karelius, K.; Aprilita, N.H.; Wahyuni, E.T. Adsorption of mercury (II) on dithizone-immobilized natural zeolite. J. Environ. Chem. Eng. 2016, 4, 1839–1849. [Google Scholar] [CrossRef]
- Rasheed, T.; Hassan, A.A.; Bilal, M.; Hussain, T.; Rizwan, K. Metal-organic frameworks based adsorbents: A review from removal perspective of various environmental contaminants from wastewater. Chemosphere 2020, 259, 127369. [Google Scholar] [CrossRef]
- Forghani, M.; Azizi, A.; Livani, M.J.; Kafshgari, L.A. Adsorption of lead (II) and chromium (VI) from aqueous environment onto metal-organic framework MIL-100(Fe): Synthesis, kinetics, equilibrium and thermodynamics. J. Solid State Chem. 2020, 291, 121636. [Google Scholar] [CrossRef]
- Limei, C.; Xiaoyao, G.; Qin, W.; Yaoguang, W.; Liang, G.; Liangguo, Y.; Tao, Y.; Bin, D. Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: Sorption kinetic and uptake mechanism. J. Colloid. Interface Sci. 2015, 439, 112–120. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Dallas, P.; Sanakis, Y.; Stamopoulos, D.; Trapalis, C.; Niarchos, D. Synthesis and characterization of a π-conjugate, covalent layered network derived from condensation polymerization of the 4,4′-bipyridine-cyanuric chloride couple. Eur. Polym. J. 2006, 42, 2940–2948. [Google Scholar] [CrossRef]
- Zhao, W.; Xia, L.; Liu, X. Covalent organic frameworks (COFs): Perspectives of industrialization. CrystEngComm 2018, 20, 1613. [Google Scholar] [CrossRef]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing ultraporous covalent organic frameworks in seconds via an organic terracotta process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiang, Y.; Li, X.; Li, X.; Wang, J.; Wu, Q.; Liu, X. Solvothermal synthesis of microporous, crystalline covalent organic framework nanofibers and their colorimetric nanohybrid structures. ACS Appl. Mater. Interfaces 2013, 5, 8845–8849. [Google Scholar] [CrossRef]
- Das, G.; Shinde, D.B.; Kandambeth, S.; Biswal, B.P.; Banerjee, R. Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 2014, 50, 12615–12618. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Scott, T.F. Approaches and challenges in the synthesis of three-dimensional covalent-organic framework. Commun. Chem. 2018, 98, 1–5. [Google Scholar] [CrossRef]
- Sajid, M.I. Toxicity of Nanoscale Metal-Organic Frameworks in Biological Systems. In Metal-Organic Frameworks for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–395. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Bhunia, S.; Deo, K.A.; Gaharwar, A.K. 2D Covalent Organic Frameworks for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1–27. [Google Scholar] [CrossRef]
- Chen, H.; Lin, T.; Zhang, S.; Chen, W.; Xu, H.; Tao, H. Covalent organic frameworks as an efficient adsorbent for controlling the formation of disinfection by-products (DBPs) in chlorinated drinking water. Sci. Total Environ. 2020, 746, 141138. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, Y.; Darling, S.B. Covalent Organic Frameworks for Water Treatment. Adv. Mater. Interfaces 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; van Puyvelde, P.; van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Salonen, L.M.; Pinela, S.R.; Fernandes, S.P.; Louçano, J.; Carbó-Argibay, E.; Sarriá, M.P.; Rodríguez-Abreu, C.; Peixoto, J.; Espiña, B. Adsorption of marine phycotoxin okadaic acid on a covalent organic framework. J. Chromatogr. A 2017, 1525, 17–22. [Google Scholar] [CrossRef]
- Li, Y.; Dong, G.; Li, J.; Xiang, J.; Yuan, J.; Wang, H.; Wang, X. Ecotoxicology and Environmental Safety A solid-phase microextraction fiber coating based on magnetic covalent organic framework for highly efficient extraction of triclosan and methyltriclosan in environmental water and human urine samples. Ecotoxicol. Environ. Saf. 2021, 219, 112319. [Google Scholar] [CrossRef]
- Fernandes, S.P.; Mellah, A.; Kovář, P.; Sárria, M.P.; Pšenička, M.; Djamila, H.; Salonen, L.M.; Espiña, B. Extraction of ibuprofen from natural waters using a covalent organic framework. Molecules 2020, 25, 3132. [Google Scholar] [CrossRef]
- Kou, X.; Tong, L.; Huang, S.; Chen, G.; Zhu, F.; Ouyang, G. Recent advances of covalent organic frameworks and their application in sample preparation of biological analysis. Trac. Trends Anal. Chem. 2021, 136, 116182. [Google Scholar] [CrossRef]
- Das, G.; Benyettou, F.; Sharama, S.K.; Prakasam, T.; Gandara, F.; de la Pena-O’Shea, V.; Saleh, N.; Pasricha, R.; Jagannathan, R.; Olson, M.A.; et al. Covalent organic nanosheets for bioimaging. Chem. Sci. 2018, 9, 8382–8387. [Google Scholar] [CrossRef]
- Mokhtari, N.; Taymouri, S.; Mirian, M.; Dinari, M. Covalent triazine-based polyimine framework as a biocompatible pH-dependent sustained-release nanocarrier for sorafenib: An in vitro approach. J. Mol. Liq. 2020, 297, 111898. [Google Scholar] [CrossRef]
- Rengaraj, A.; Puthiaraj, P.; Haldorai, Y.; Heo, N.; Hwang, S.K.; Han, Y.K.; Kwon, S.; Ahn, W.S.; Huh, Y.S. Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Appl. Mater. Interfaces 2016, 8, 8947–8955. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Yang, P.; Wang, R.; Unruangsri, J.; Yang, W.; Wang, C.; Guo, J. Stable radical cation-containing covalent organic frameworks exhibiting remarkable structure-enhanced photothermal conversion. J. Am. Chem. Soc. 2019, 141, 14433–14442. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatou, M.-A.; Bika, P.; Stergiopoulos, T.; Dallas, P.; Pavlatou, E.A. Recent Advances in Covalent Organic Frameworks for Heavy Metal Removal Applications. Energies 2021, 14, 3197. https://doi.org/10.3390/en14113197
Gatou M-A, Bika P, Stergiopoulos T, Dallas P, Pavlatou EA. Recent Advances in Covalent Organic Frameworks for Heavy Metal Removal Applications. Energies. 2021; 14(11):3197. https://doi.org/10.3390/en14113197
Chicago/Turabian StyleGatou, Maria-Anna, Panagiota Bika, Thomas Stergiopoulos, Panagiotis Dallas, and Evangelia A. Pavlatou. 2021. "Recent Advances in Covalent Organic Frameworks for Heavy Metal Removal Applications" Energies 14, no. 11: 3197. https://doi.org/10.3390/en14113197
APA StyleGatou, M.-A., Bika, P., Stergiopoulos, T., Dallas, P., & Pavlatou, E. A. (2021). Recent Advances in Covalent Organic Frameworks for Heavy Metal Removal Applications. Energies, 14(11), 3197. https://doi.org/10.3390/en14113197









