Photoelectrochemical Hydrogen Production by Screen-Printed Copper Oxide Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodes Development
2.2. Inks and Electrodes Characterization
2.3. Hydrogen Production
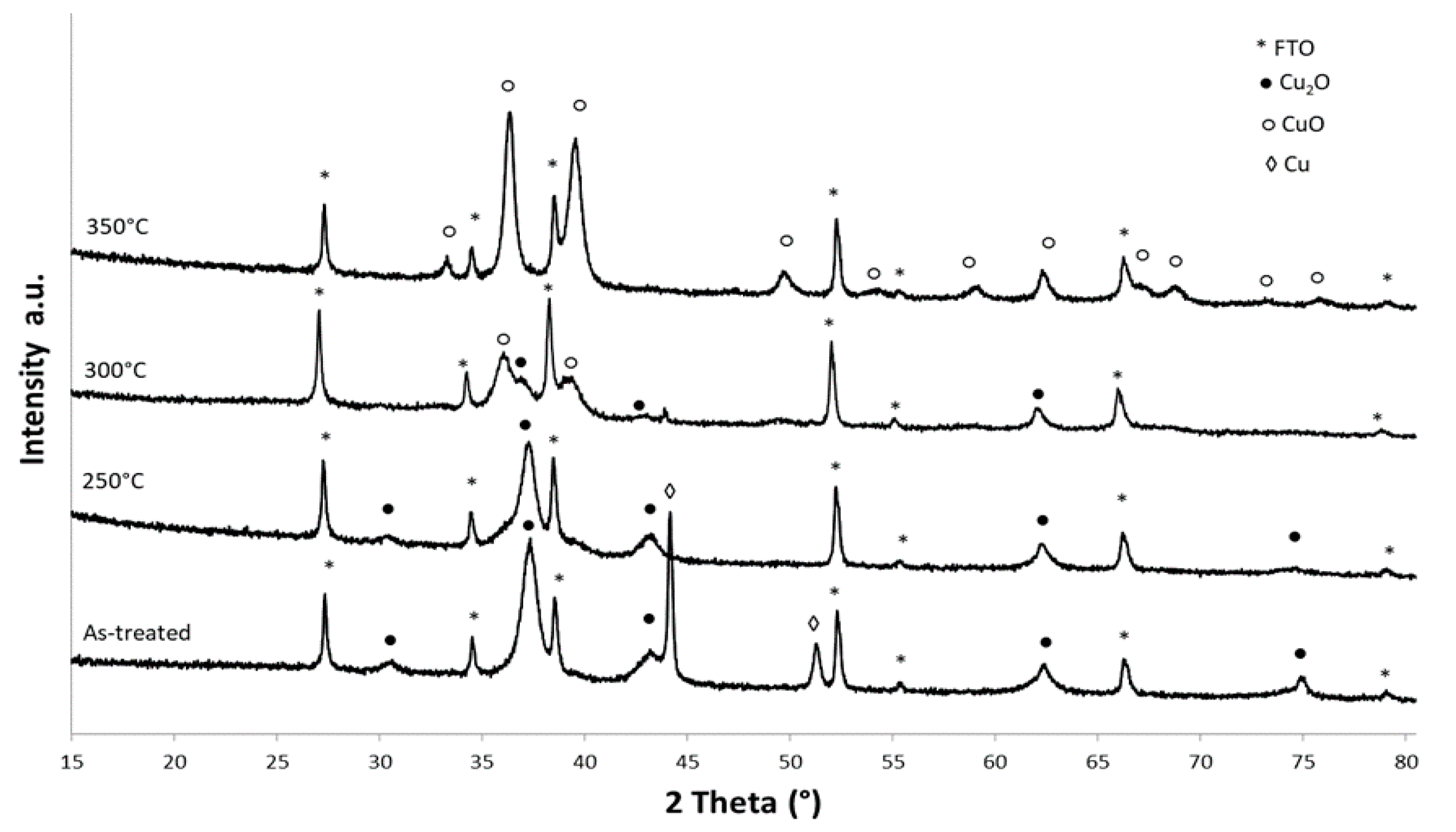
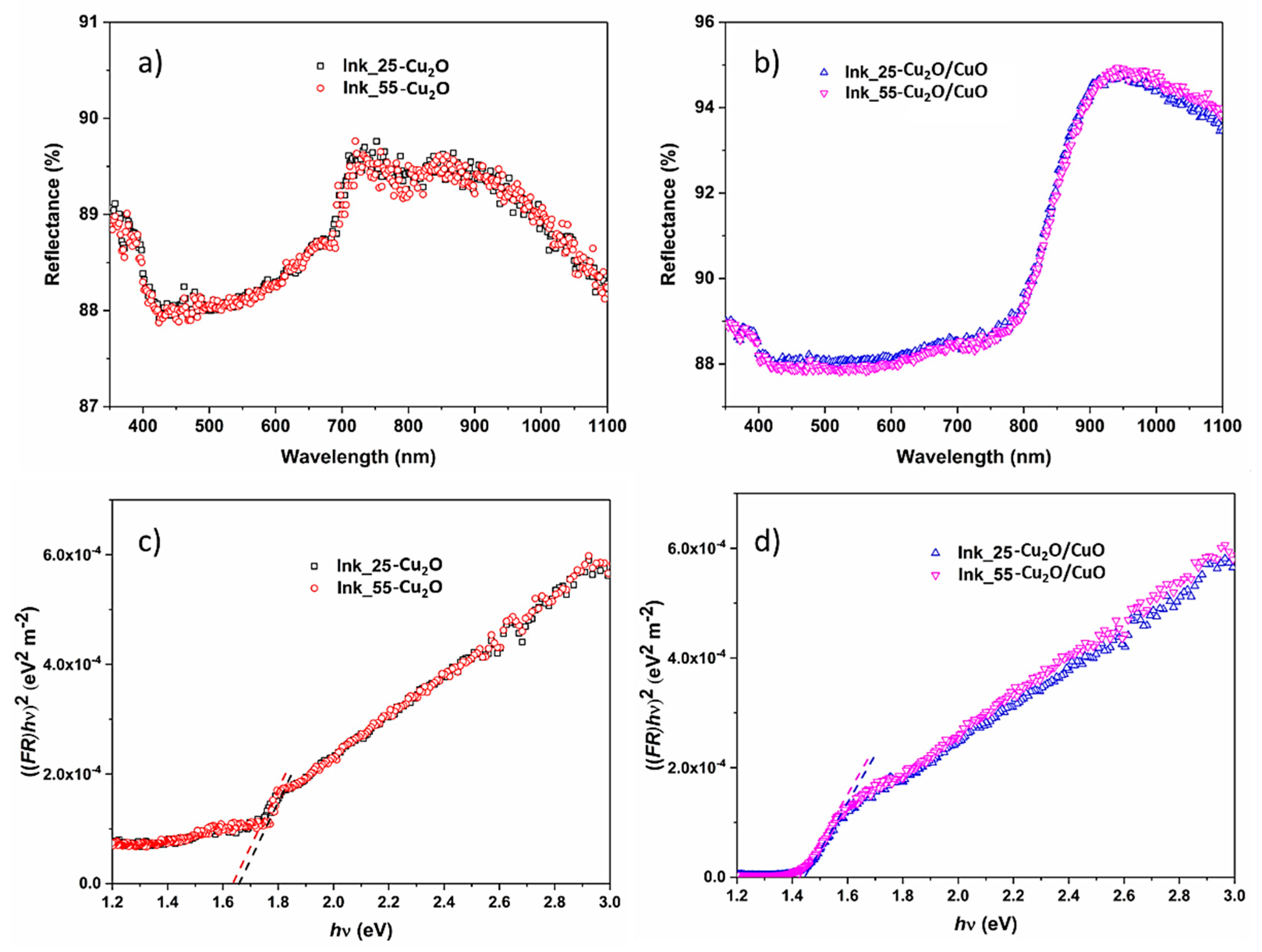
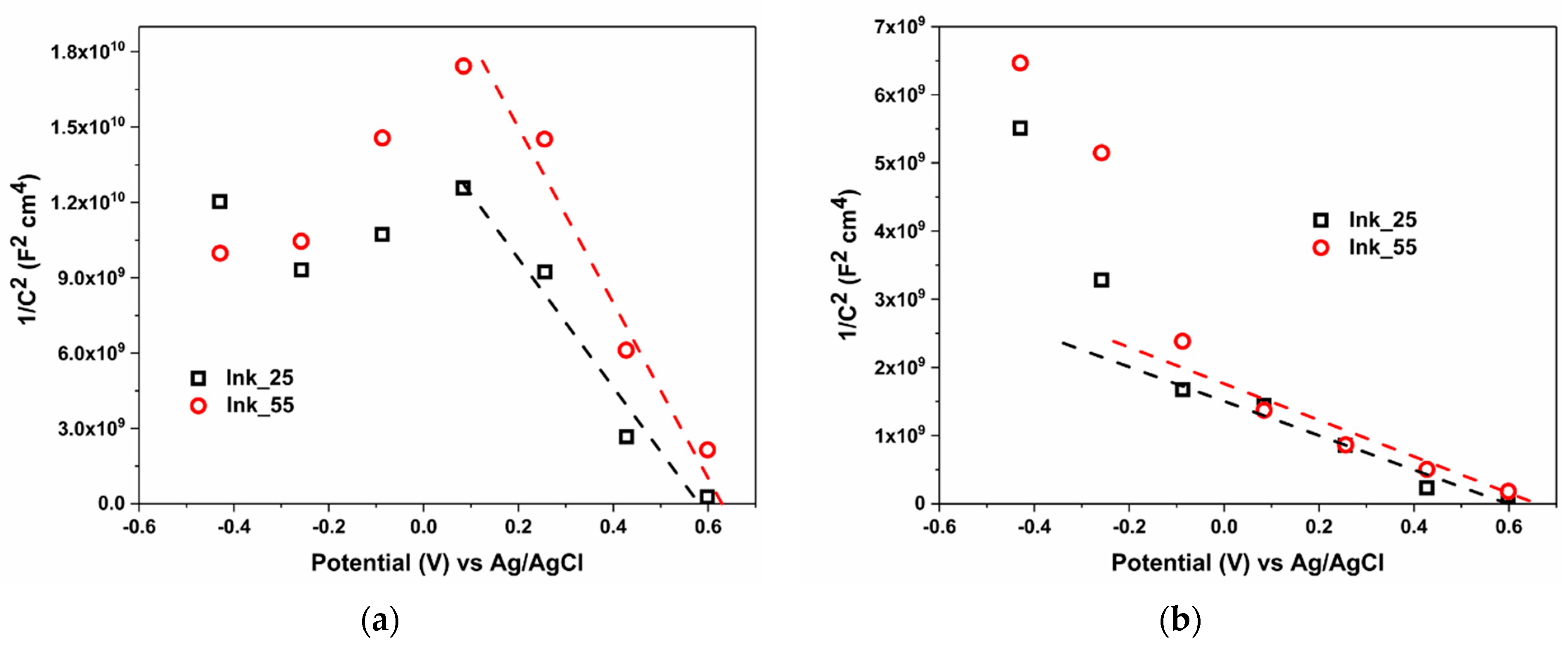
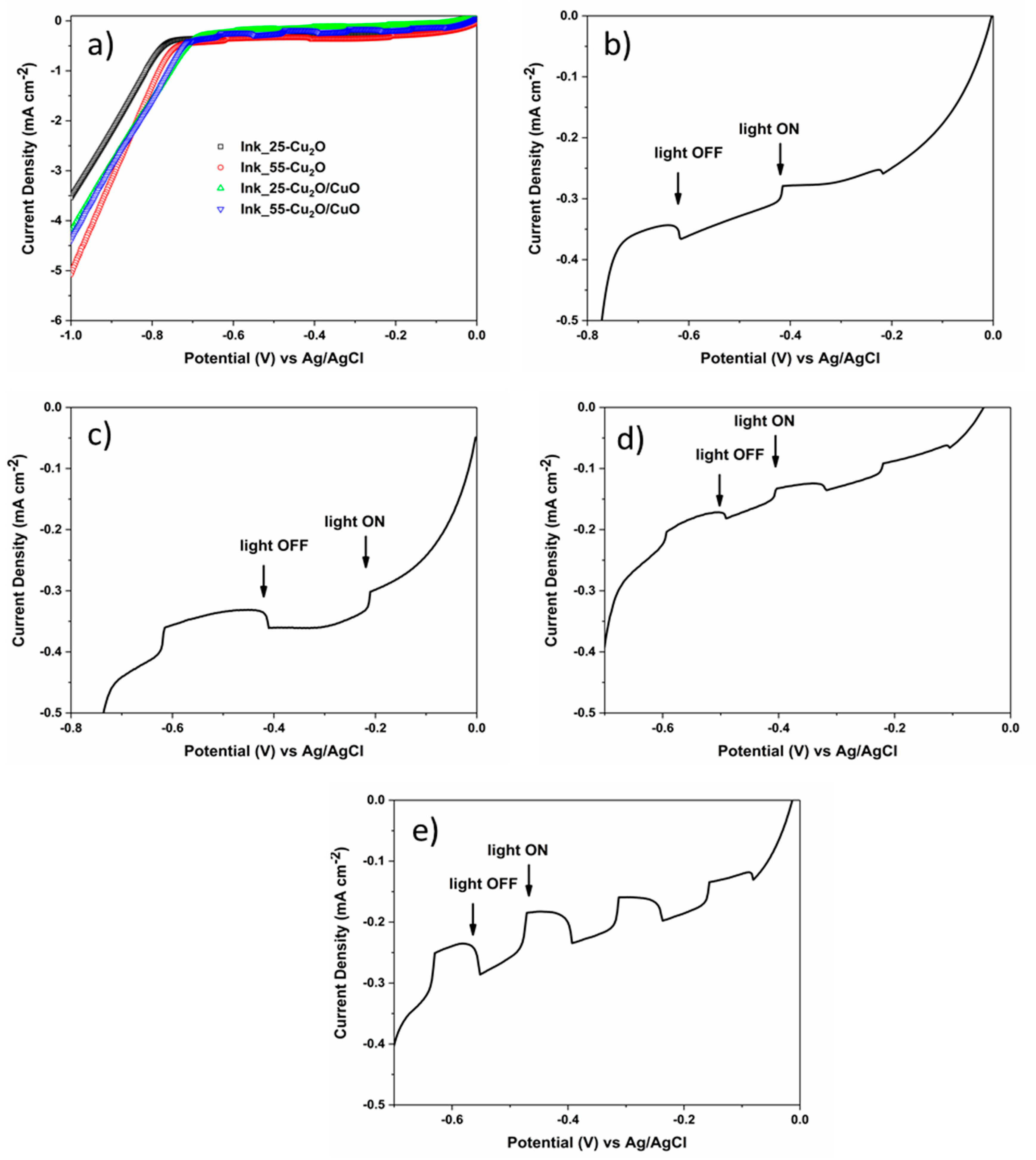
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samsudin, M.F.R.; Sufian, S.; Mohamed, N.M.; Bashiri, R.; Wolfe, F.; Ramli, R.M. Enhancement of Hydrogen Production over Screen-Printed TiO2/BiVO4 Thin Film in the Photoelectrochemical Cells. Mater. Lett. 2018, 211, 13–16. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Rodene, D.D.; Gupta, R.B. Recent Advancements in Semiconductor Materials for Photoelectrochemical Water Splitting for Hydrogen Production Using Visible Light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Tuci, G.; Sanson, A.; Peruzzini, M.; Giambastiani, G. Metal-Free Carbon-Based Materials for Electrocatalytic and Photo-Electrocatalytic CO2 Reduction. Rend. Fis. Acc. Lincei 2019, 30, 497–513. [Google Scholar] [CrossRef]
- Günnemann, C.; Curti, M.; Eckert, J.G.; Schneider, J.; Bahnemann, D.W. Tailoring the Photoelectrochemical Activity of TiO2 Electrodes by Multilayer Screen-Printing. ChemCatChem 2019, 11, 6439–6450. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Yusoff, N.; Pandikumar, A.; Huang, N.M.; Akbarzadeh, O.; Sagadevan, S.; Wahab, Y.A.; Johan, M.R. Tailoring Morphological Characteristics of Zinc Oxide Using a One-Step Hydrothermal Method for Photoelectrochemical Water Splitting Application. Int. J. Hydrogen Energy 2019, 44, 17535–17543. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Kong Eugene, Y.-J.; Dasineh Khiavi, N.; Katal, R.; Gong, X. Aluminum-Incorporated p-CuO/n-ZnO Photocathode Coated with Nanocrystal-Engineered TiO2 Protective Layer for Photoelectrochemical Water Splitting and Hydrogen Generation. J. Mater. Chem. A 2018, 6, 11951–11965. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly Active Oxide Photocathode for Photoelectrochemical Water Reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol-Gel Deposited Cu2O and CuO Thin Films for Photocatalytic Water Splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Reyes, M.; Camposeco-Solís, R.; Zanella, R.; Rodríguez González, V. Hydrogen Production by Tailoring the Brookite and Cu2O Ratio of Sol-Gel Cu-TiO2 Photocatalysts. Chemosphere 2017, 184, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Dubale, A.A.; Pan, C.-J.; Tamirat, A.G.; Chen, H.-M.; Su, W.-N.; Chen, C.-H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.-F.; et al. Heterostructured Cu2O/CuO Decorated with Nickel as a Highly Efficient Photocathode for Photoelectrochemical Water Reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Waldner, G.; Krýsa, J. Photocurrents and Degradation Rates on Particulate TiO2 Layers: Effect of Layer Thickness, Concentration of Oxidizable Substance and Illumination Direction. Electrochim. Acta 2005, 50, 4498–4504. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, J.S. Photoelectrochemical Water Splitting with P-Type Metal Oxide Semiconductor Photocathodes. ChemSusChem 2019, 12, 1835–1845. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Aroh, K.; Franson, N.; Satsangi, V.R.; Dass, S.; Ehrman, S. Copper Oxide Nanoparticle Made by Flame Spray Pyrolysis for Photoelectrochemical Water Splitting—Part II. Photoelectrochemical Study. Int. J. Hydrogen Energy 2011, 36, 15519–15526. [Google Scholar] [CrossRef]
- Chang, Y.; Braun, A.; Deangelis, A.; Kaneshiro, J.; Gaillard, N. Effect of Thermal Treatment on the Crystallographic, Surface Energetics, and Photoelectrochemical Properties of Reactively Cosputtered Copper Tungstate for Water Splitting. J. Phys. Chem. C 2011, 115, 25490–25495. [Google Scholar] [CrossRef]
- Thongthep, P.; Moonmangmee, S.; Ponchio, C. Solar/Photoelectrocatalytic Cell Development for H2 Production and Simultaneous Organic Dye Degradation. Mater. Sci. Semicond. Process. 2021, 124, 105597. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Haigh, J. Strengths and Advantages of Electrodeposition as a Semiconductor Growth Technique for Applications in Macroelectronic Devices. J. Electrochem. Soc. 2005, 153, 47. [Google Scholar] [CrossRef]
- Gondolini, A.; Mercadelli, E.; Sangiorgi, A.; Sanson, A. Integration of Ni-GDC Layer on a NiCrAl Metal Foam for SOFC Application. J. Eur. Ceram. Soc. 2017, 37, 1023–1030. [Google Scholar] [CrossRef]
- Gondolini, A.; Mercadelli, E.; Constantin, G.; Dessemond, L.; Yurkiv, V.; Costa, R.; Sanson, A. On the Manufacturing of Low Temperature Activated Sr0.9La0.1TiO3-δ-Ce1-XGdxO2-δ Anodes for Solid Oxide Fuel Cell. J. Eur. Ceram. Soc. 2018, 38, 153–161. [Google Scholar] [CrossRef]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical Characterization of Squaraine-Sensitized Nickel Oxide Cathodes Deposited via Screen-Printing for p-Type Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Obata, K.; van de Krol, R.; Schwarze, M.; Schomäcker, R.; Abdi, F.F. In Situ Observation of PH Change during Water Splitting in Neutral PH Conditions: Impact of Natural Convection Driven by Buoyancy Effects. Energy Environ. Sci. 2020, 13, 5104–5116. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Aversa, L.; Tatti, R.; Verucchi, R.; Sanson, A. Spectrophotometric Method for Optical Band Gap and Electronic Transitions Determination of Semiconductor Materials. Opt. Mater. 2017, 64, 18–25. [Google Scholar] [CrossRef]
- Murali, D.S.; Kumar, S.; Choudhary, R.J.; Wadikar, A.D.; Jain, M.K.; Subrahmanyam, A. Synthesis of Cu2O from CuO Thin Films: Optical and Electrical Properties. AIP Adv. 2015, 5, 047143. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [PubMed]
- Gondolini, A.; Mercadelli, E.; Zin, V.; Barison, S.; Sanson, A. Easy Preparation Method of Stable Copper-Based Nanoparticle Suspensions in Lubricant Engine Oil. Lubr. Sci. 2020, 32, 205–217. [Google Scholar] [CrossRef]
- Moreno, R. Better Ceramics through Colloid Chemistry. J. Eur. Ceram. Soc. 2020, 40, 559–587. [Google Scholar] [CrossRef]
- Gondolini, A.; Fasolini, A.; Mercadelli, E.; Basile, F.; Sanson, A. Freeze Cast Porous Membrane Catalyst for Hydrogen Production via Oxy-Reforming. Fuel Process. Technol. 2021, 213, 106658. [Google Scholar] [CrossRef]
- Sanson, A.; Mercadelli, E.; Roncari, E.; Licheri, R.; Orrù, R.; Cao, G.; Merlone-Borla, E.; Marzorati, D.; Bonavita, A.; Micali, G.; et al. Influence of Processing Parameters on the Electrical Response of Screen Printed SrFe0.6Ti0.4O3−δ Thick Films. Ceram. Int. 2010, 36, 521–527. [Google Scholar] [CrossRef]
- Sanson, A.; Gardini, D.; Montanari, G.; Galassi, C.; Roncari, E. Key Role of Milling in the Optimization of TiO2 Nanoinks. J. Mater. Res. 2006, 21, 1561–1569. [Google Scholar] [CrossRef]
- Lanzini, A.; Guerra, C.; Leone, P.; Santarelli, M.; Smeacetto, F.; Fiorilli, S.; Gondolini, A.; Mercadelli, E.; Sanson, A.; Brandon, N.P. Influence of the Microstructure on the Catalytic Properties of SOFC Anodes under Dry Reforming of Methane. Mater. Lett. 2016, 164, 312–315. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H Embedding and Kinetic Effects in the Formation of Suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal Engineering of Sputter-Grown CuO Photocathode for Visible-Light-Driven Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef]
- Aktar, A.; Ahmmed, S.; Hossain, J.; Md. Ismail, A.B. Solution-Processed Synthesis of Copper Oxide (CuxO) Thin Films for Efficient Photocatalytic Solar Water Splitting. ACS Omega 2020, 5, 25125–25134. [Google Scholar] [CrossRef] [PubMed]
- Nian, J.-N.; Hu, C.-C.; Teng, H. Electrodeposited P-Type Cu2O for H2 Evolution from Photoelectrolysis of Water under Visible Light Illumination. Int. J. Hydrogen Energy 2008, 33, 2897–2903. [Google Scholar] [CrossRef]
- Bagal, I.V.; Chodankar, N.R.; Hassan, M.A.; Waseem, A.; Johar, M.A.; Kim, D.-H.; Ryu, S.-W. Cu2O as an Emerging Photocathode for Solar Water Splitting—A Status Review. Int. J. Hydrogen Energy 2019, 44, 21351–21378. [Google Scholar] [CrossRef]
- Toe, C.Y.; Scott, J.; Amal, R.; Ng, Y.H. Recent Advances in Suppressing the Photocorrosion of Cuprous Oxide for Photocatalytic and Photoelectrochemical Energy Conversion. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 191–211. [Google Scholar] [CrossRef]
- Toe, C.Y.; Zheng, Z.; Wu, H.; Scott, J.; Amal, R.; Ng, Y.H. Photocorrosion of Cuprous Oxide in Hydrogen Production: Rationalising Self-Oxidation or Self-Reduction. Angew. Chem. Int. Ed. 2018, 57, 13613–13617. [Google Scholar] [CrossRef]
- Enzweiler, H.; Yassue-Cordeiro, P.H.; Schwaab, M.; Barbosa-Coutinho, E.; Olsen Scaliante, M.H.N.; Fernandes, N.R.C. Catalyst Concentration, Ethanol Content and Initial PH Effects on Hydrogen Production by Photocatalytic Water Splitting. J. Photochem. Photobiol. A Chem. 2020, 388, 112051. [Google Scholar] [CrossRef]
- Peng, R.; Baltrusaitis, J.; Wu, C.M.; Koodali, R.T. Pd–Ti-MCM-48 Cubic Mesoporous Materials for Solar Simulated Hydrogen Evolution. Int. J. Hydrogen Energy 2015, 40, 905–918. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Xiao, Y.; Li, Y.; Delaunay, J.-J. Earth-Abundant Cu-Based Metal Oxide Photocathodes for Photoelectrochemical Water Splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
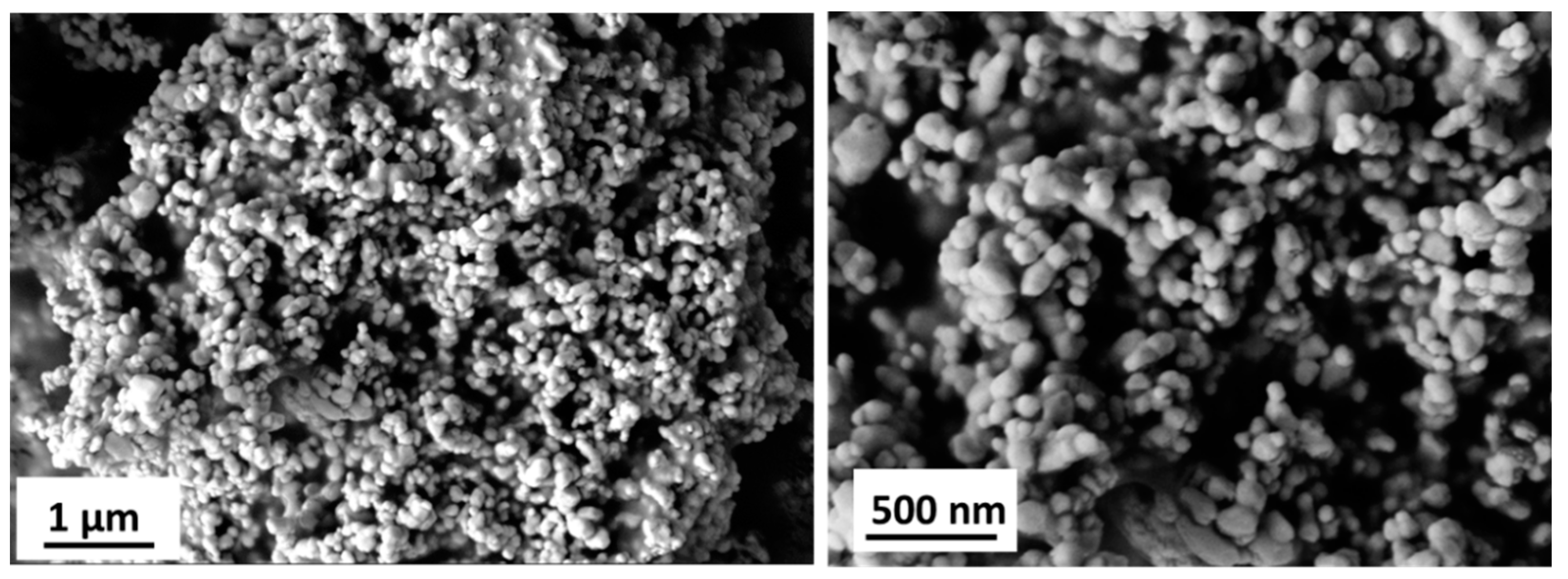

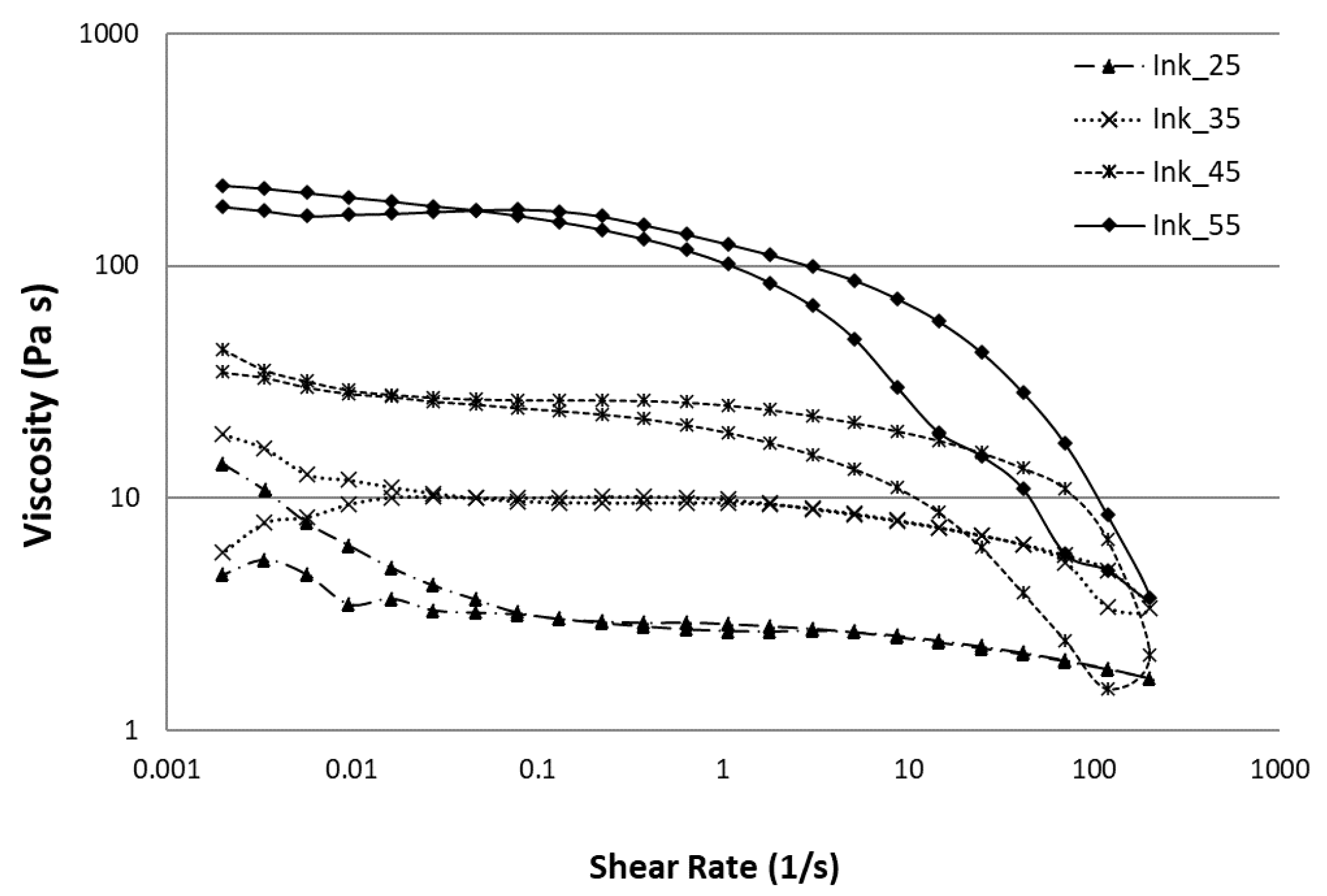
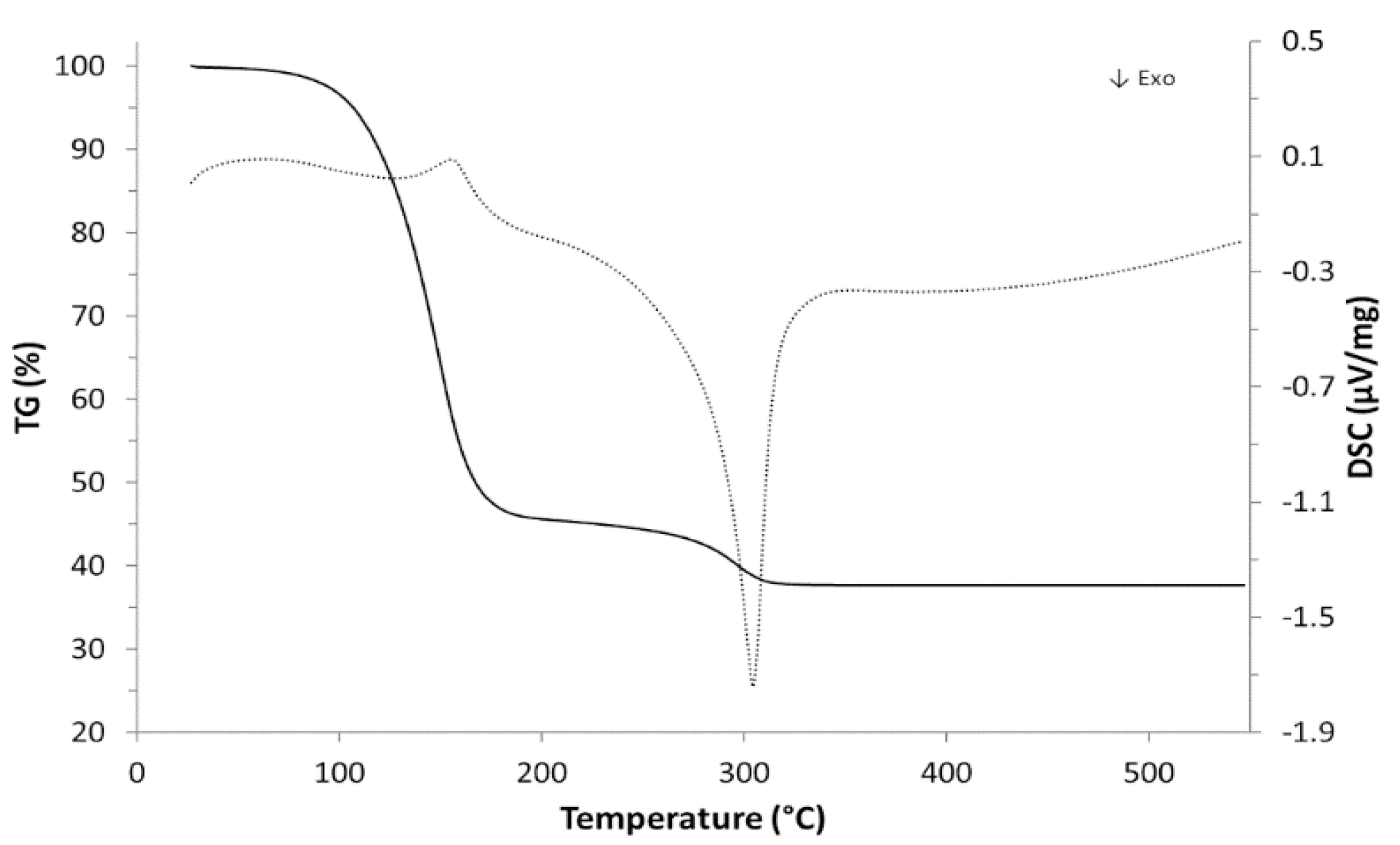
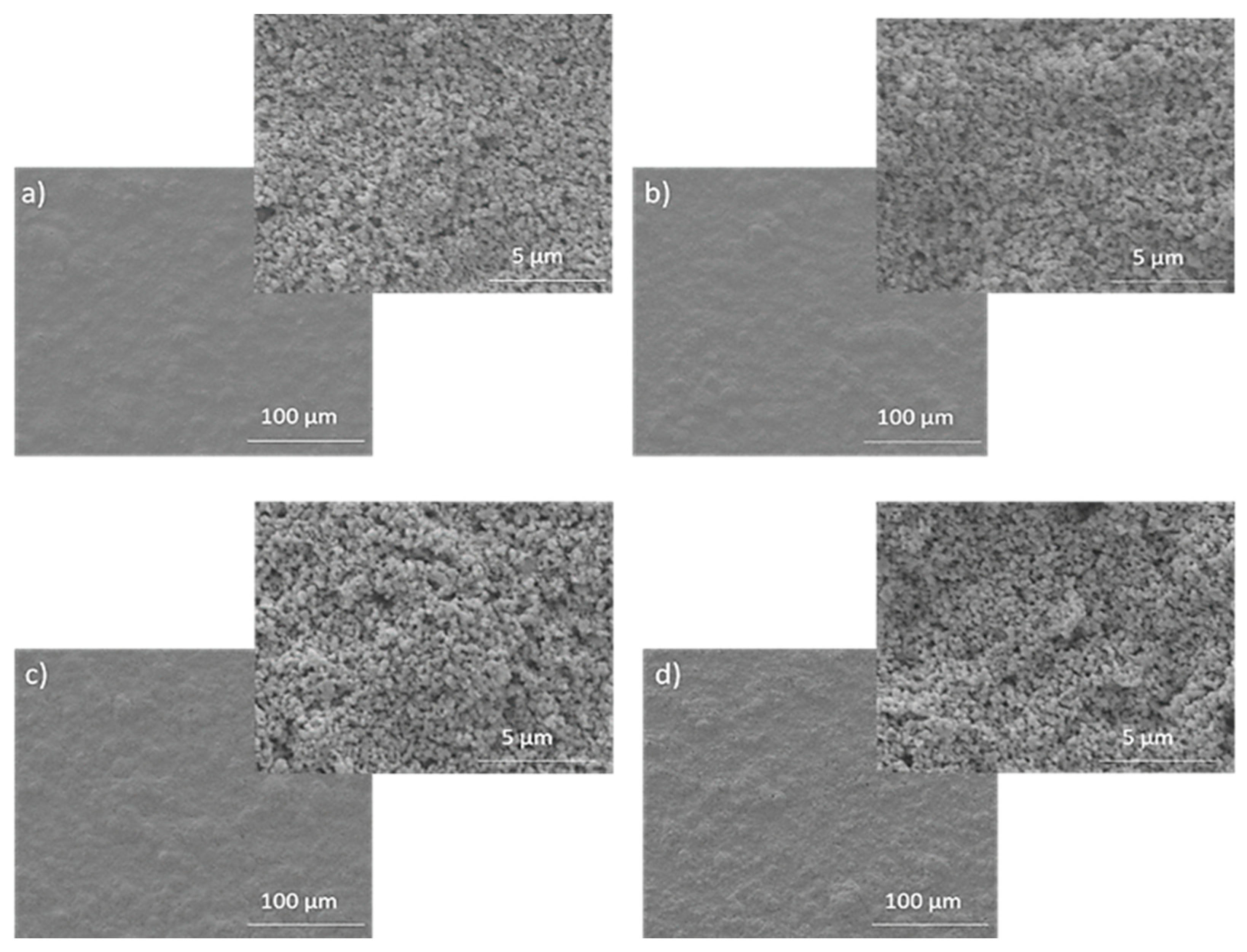

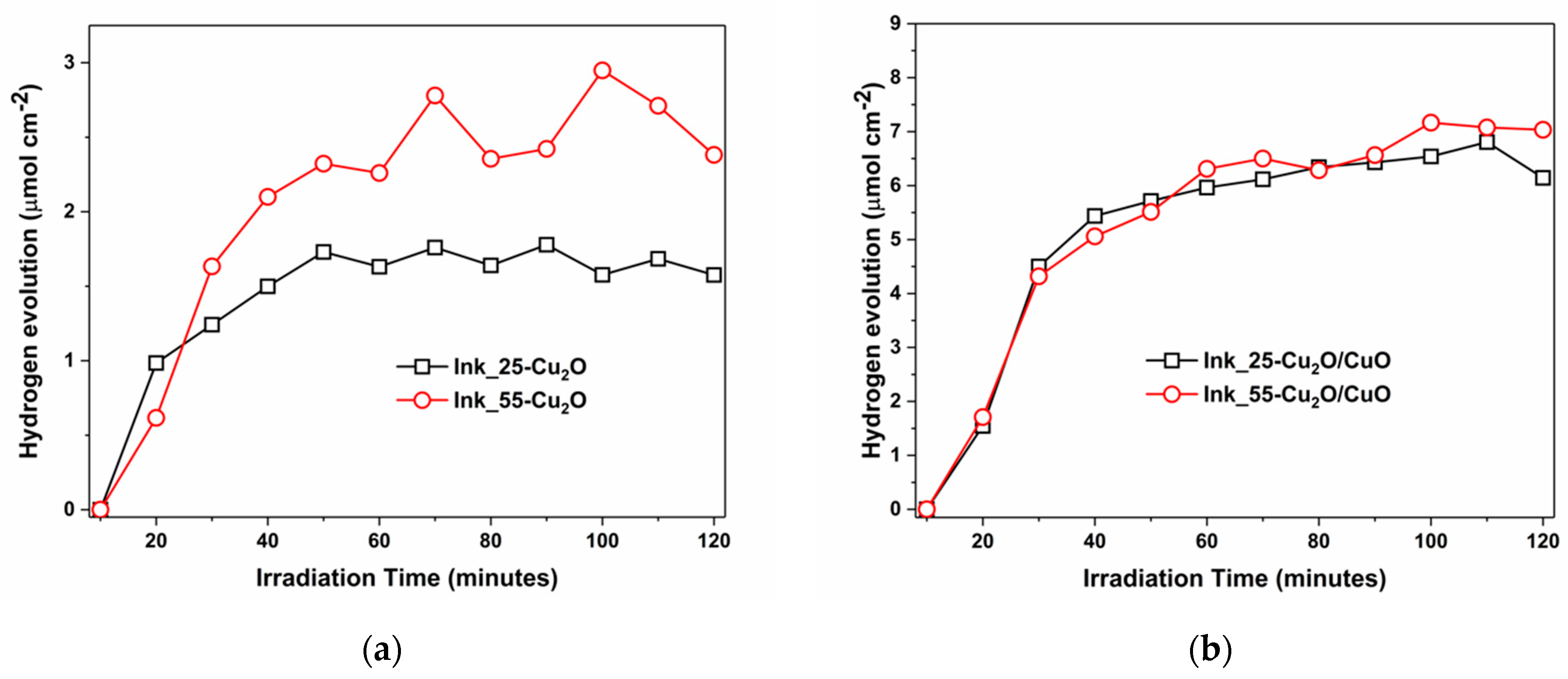
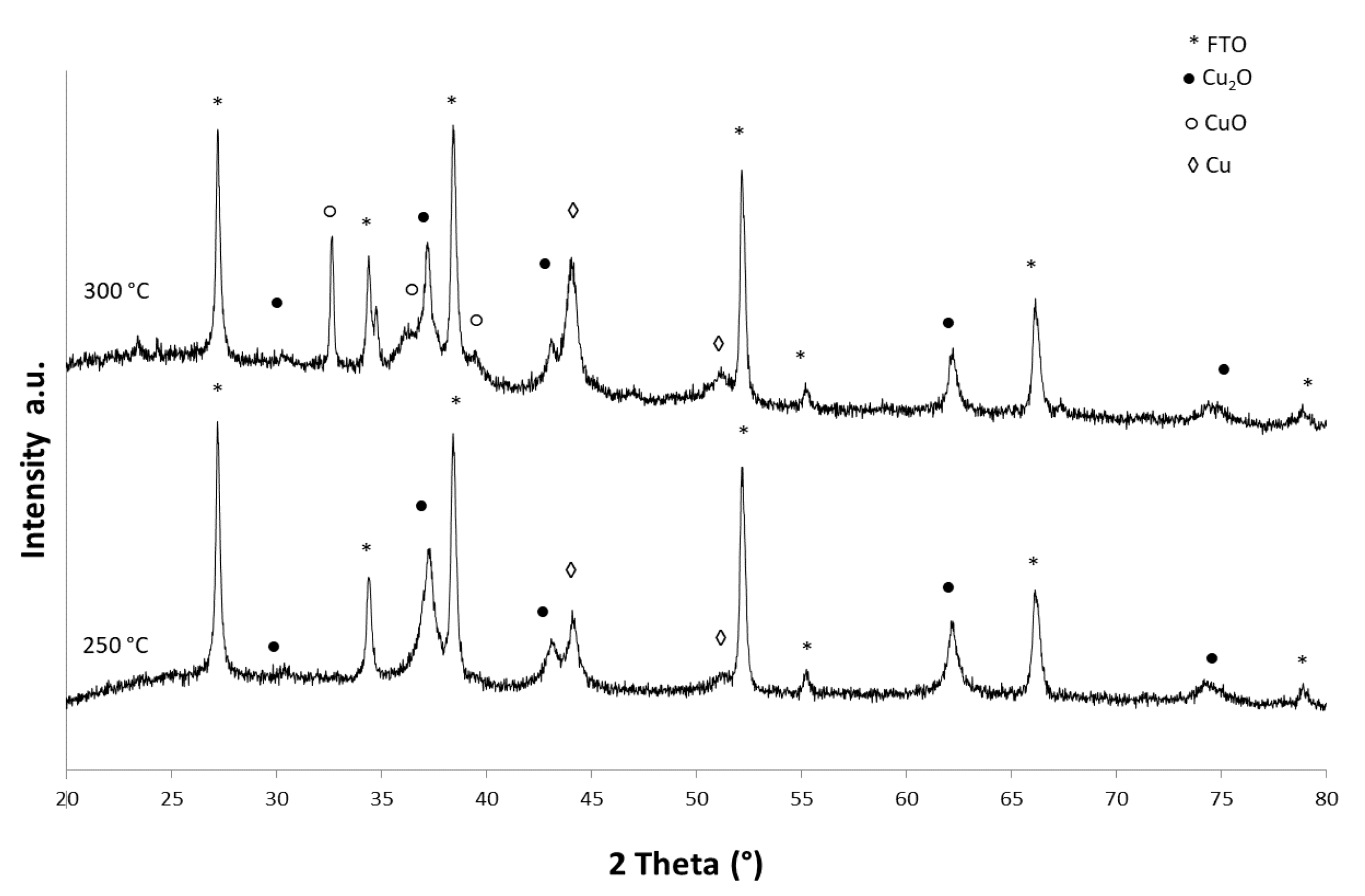
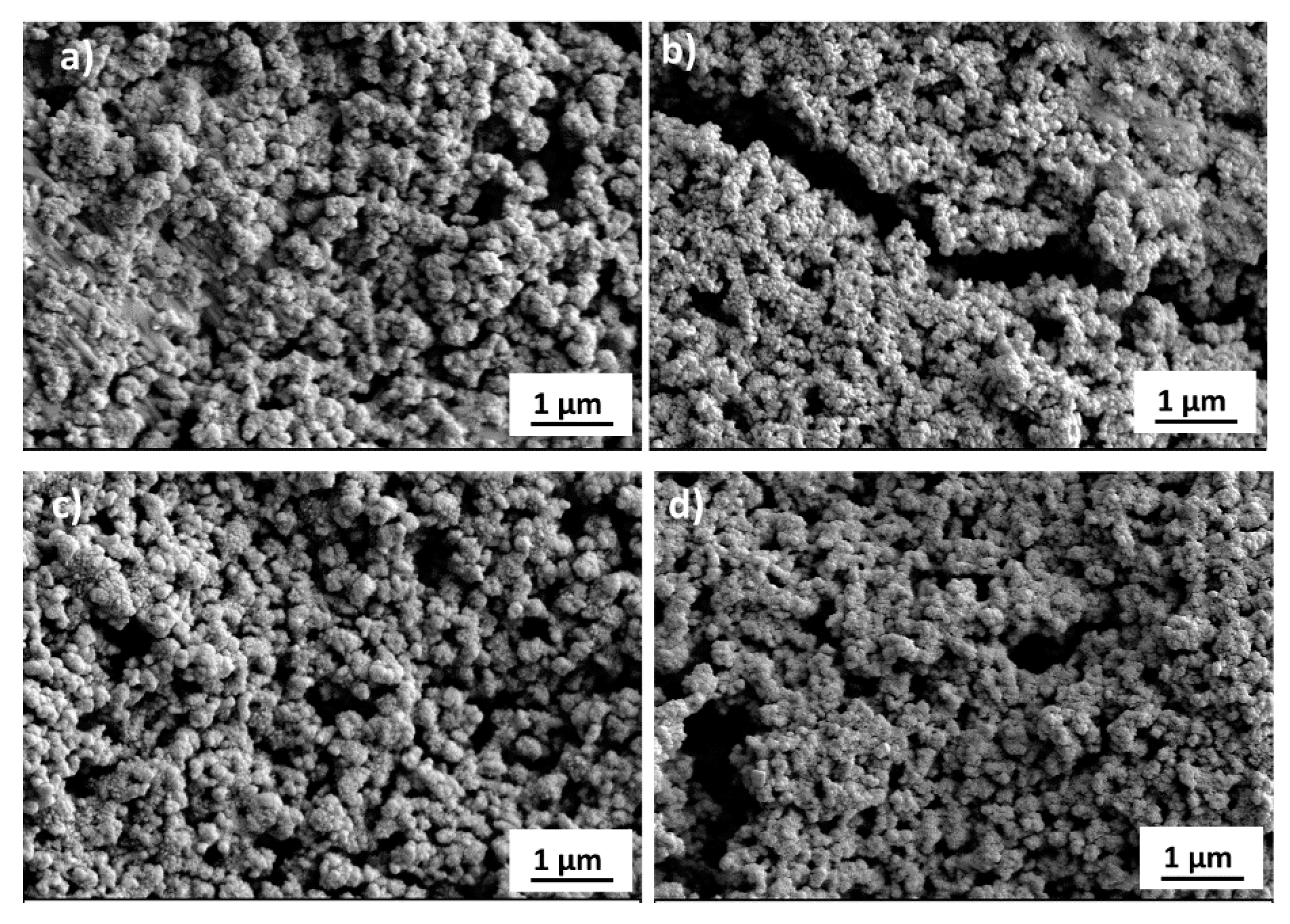
| Material | Ink_25 | Ink_35 | Ink_45 | Ink_55 |
|---|---|---|---|---|
| CuO | 8.54 | 8.39 | 8.20 | 7.94 |
| Terpineol | 85.40 | 83.92 | 81.99 | 79.37 |
| GTO | 3.21 | 3.16 | 3.08 | 2.98 |
| EC | 2.85 | 4.53 | 6.72 | 9.71 |
| Parameter | Ink_25 | Ink_35 | Ink_45 | Ink_55 |
|---|---|---|---|---|
| Ra (µm) | 0.515 ± 0.063 | 0.412 ± 0.059 | 0.415 ± 0.085 | 0.372 ± 0.076 |
| Phase | Inks | Eg (eV) | Efb (V) vs. Ag/AgCl | Efb (V) a vs. NHE | NA (cm−3) |
|---|---|---|---|---|---|
| Cu2O-based | Ink_25 | 1.65 | 0.58 | 1.12 | 2.6 × 1027 |
| Ink_55 | 1.63 | 0.62 | 1.16 | 2.3 × 1027 | |
| Cu2O/CuO-based | Ink_25 | 1.46 | 0.61 | 1.15 | 2.5 × 1028 |
| Ink_55 | 1.44 | 0.66 | 1.20 | 2.4 × 1028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondolini, A.; Sangiorgi, N.; Sangiorgi, A.; Sanson, A. Photoelectrochemical Hydrogen Production by Screen-Printed Copper Oxide Electrodes. Energies 2021, 14, 2942. https://doi.org/10.3390/en14102942
Gondolini A, Sangiorgi N, Sangiorgi A, Sanson A. Photoelectrochemical Hydrogen Production by Screen-Printed Copper Oxide Electrodes. Energies. 2021; 14(10):2942. https://doi.org/10.3390/en14102942
Chicago/Turabian StyleGondolini, Angela, Nicola Sangiorgi, Alex Sangiorgi, and Alessandra Sanson. 2021. "Photoelectrochemical Hydrogen Production by Screen-Printed Copper Oxide Electrodes" Energies 14, no. 10: 2942. https://doi.org/10.3390/en14102942
APA StyleGondolini, A., Sangiorgi, N., Sangiorgi, A., & Sanson, A. (2021). Photoelectrochemical Hydrogen Production by Screen-Printed Copper Oxide Electrodes. Energies, 14(10), 2942. https://doi.org/10.3390/en14102942







