Abstract
The interest in employing absorption refrigeration systems is usually related to electricity’s precariousness since these systems generally use thermal rejects for their activation. The application of these systems is closely linked to the concept of energy polygeneration, in which the energy demand to operate them is reduced, which represents their main advantage over the conventional vapor compression system. Currently, the solution pairs used in commercial absorption chillers are lithium bromide/water and ammonia/water. The latter pair has been used in air conditioning and industrial processes due to the ammonia operation’s low temperature. Few review papers on absorption chillers have been published, discussing the use of solar energy as the input source of the systems, the evolution of the absorption refrigeration cycles over the last decades, and promising alternatives to increase the performance of absorption refrigeration systems. There is a lack of consistent studies about designing requirements for absorption chillers, so an updated review covering recent advances and suggested solutions to improve the use and operation of those absorption refrigeration systems using different working fluids is relevant. Hence, this presents a review of the state-of-the-art of ammonia/absorbent based absorption refrigeration systems, considering the most relevant studies, describing the development of this equipment over the years. The most relevant studies in the open literature were collected to describe this equipment’s development over the years, including thermodynamic properties, commercial manufacturers, experimental and numerical studies, and the prototypes designed and tested in this area. The manuscript focuses on reviewing studies in absorption refrigeration systems that use ammonia and absorbents, such as water, lithium nitrate, and lithium nitrate plus water. As a horizon to the future, the uses of absorption systems should be rising due to the increasing values of the electricity, and the environmental impact of the synthetic refrigerant fluids used in mechanical refrigeration equipment. In this context, the idea for a new configuration absorption chiller is to be more efficient, pollutant free to the environment, activated by a heat substantiable source, such as solar, with low cost and compactness structure to attend the thermal needs (comfort thermal) for residences, private and public buildings, and even the industrial and health building sector (thermal processes). To conclude, future recommendations are presented to deal with the improvement of the refrigeration absorption chiller by using solar energy, alternative fluids, multiple-effects, and advanced and hybrid configurations to reach the best absorption chiller to attend to the thermal needs of the residential and industrial sector around the world.
1. Introduction
Absorption refrigeration systems are compression refrigeration systems that use thermal compressors. Such systems have the advantages of reduced electricity consumption, lower maintenance costs, and the elimination of CFC and HCFC use as refrigerants. They also allow cogeneration and solar energy as an input source to drive the system [1,2,3,4].
However, such equipment has the following disadvantages: (i) low COP (coefficient of performance) when compared to vapor compression systems, and (ii) absorption chillers are heavy equipment, and their capital cost is relatively high. Besides the critical points mentioned, the choice of working fluids (refrigerant and absorbent) is crucial for the efficiency and performance of absorption cooling systems, as many authors have demonstrated over the years, by conducting studies in different areas of the cooling thermal comfort or even for refrigeration purposes [5,6].
In commercial manufacturing products, two types of solution pairs are commonly used: lithium bromide/water (LiBr/H2O) [7,8] and ammonia/water (NH3/H2O) [9,10,11]. Usually, the pair LiBr/H2O is used for thermal comfort systems and the pair NH3/H2O for refrigeration systems [12]. As part of the analysis, those equipment have been used for different applications to achieve the energy demands of hospital sector [13], hotel and business buildings [5,6,14,15], refinery industry [16], and also for the configuration of new control strategies on absorption solar systems [17].
In the last decades, new solution pairs have been presented and investigated by different authors [18,19,20] to increase absorption chillers’ heat and mass transfer process. One of the cited pairs, ammonia/lithium nitrate (NH3/LiNO3), is very popular around the world due to its advantages, such as the elimination of the rectifier, high solubility in ammonia, and the absence of corrosion of the metal, among others [19,20,21,22]. Besides the advantages mentioned above, Heard et al. [23] observed a higher tolerance to operation parameters other than the ideal condition, crystallization risk.
Several studies were conducted to evaluate the NH3/LiNO3 solution as an alternative work pair for the absorption systems. Some studies assessed this fluid by employing experimental apparatus [24,25], real prototypes [26,27], heat and mass transfer studies [18], and different components and configurations [28,29]. These studies demonstrated the effective performance of these working fluids in absorption systems and showed their technology limitations.
The thermal energy storage by using absorption systems has been strongly analyzed considering cycles and thermodynamic systems, working fluids, and different system configurations to meet specific thermal demands. Recently, Mehari et al. [30] published a review article on absorption refrigeration systems, aiming to discuss and present the cycles and configurations of thermal absorption energy storage, integrating of storage systems using conventional absorption refrigeration systems, and heat pumps.
To date, a few review papers on absorption chillers have been published [30,31,32,33,34,35]. However, they discuss solar energy as the input source of the systems [36], the absorption refrigeration cycles’ evolution over the last decades [37], or even the progress and new features in solar cooling applications [35]. Regarding the critical description of the literature in the area of absorption refrigeration, Nikbakhti et al. [38] reported in detail, several technologies implemented to improve the COP of absorption refrigeration systems. Promising alternatives were presented and discussed to increase the thermal performance of absorption refrigeration systems, aiming at optimizing the original design of the system. Another critical aspect verified was that the optimization of the absorption chillers’ operational conditions had improved its performance. The discussion on how mathematical or thermodynamic methods had been used to improve their performance is overviewed in Best and Rivera [39]. Even with these studies, updating this subject with new technology and applications on the field is mandatory. It helps the scientific community understand and improve these systems to better technical performance to reach financial feasibility. In the literature, there is a lack of consistent studies about designing requirements for absorption chillers.
It is interesting to see that many authors have been discussing solar powered energy as an input source for absorption chillers [32,33,36]. Others aim towards the type of heat exchanger technologies on those systems [40] and the use of membrane contactor to enhance the absorption process [31]. Figure 1 presents the central paper reviews about absorption chillers presented in the scientific community in the last 10 years. Figure 1 shows no reviews about designing requirements for absorption chillers with the relevance of thermophysical properties in this process and the heat and mass transfer processes in the refrigeration absorption systems using ammonia/nitrate-lithium and water.
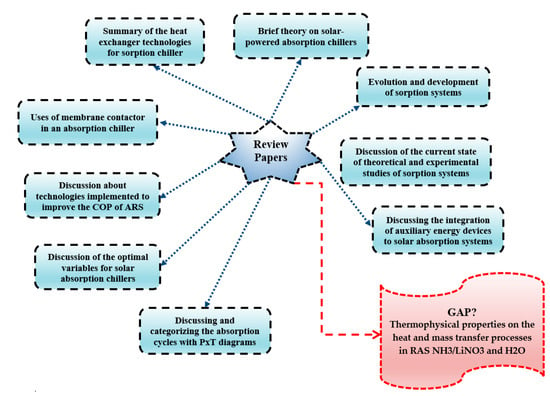
Figure 1.
Review papers on the absorption chillers in the last 10 years and the gap found.
In this context, there is a need to present a more updated review to cover recent advances and suggested solutions to improve the use and operation of those absorption refrigeration systems using different working fluids. Hence, this review tries to fill the gap mentioned above by presenting the state-of-the-art of ammonia/absorbent based absorption refrigeration systems, considering the most relevant studies, describing the development of this equipment over the years.
2. Literature Survey of the State-of-the-Art
The search for related manuscripts was carried out on a scientific repository, Scopus. The keywords were combined with Boolean operators to find the most relevant papers on the chosen topic.
General search keys were firstly used, and then the search continued with more specific terms. In query 1, the investigation was initiated with the search key “chiller and refrigeration”. In the general search, both vapor compression and vapor absorption chillers were found. A second query key, query 2, was used, restricting the results to the absorption chillers. Finally, the keyword “ammonia” was included in the search query to limit the found results to the present work’s specific objective. The results stratification regarding the document types is presented in Table 1. The explicit queries, keywords, and Boolean operators are shown on the notes of the same table.

Table 1.
Overview of the paper founds according to the specified search queries.
The articles found in the present paper were categorized based on the following criteria: (i) thermodynamic properties of mixtures used in systems based on ammonia absorption, (ii) residential and industrial absorption chillers that use NH3/absorbent, (iii) evaluation study of ammonia/absorbent systems, (iv) heat and mass transfer processes in absorption cooling system ammonia/absorbent, (v) chiller activation by absorption through solar energy, and (vi) NH3/absorbent absorption chiller prototypes.
A critical analysis was carried out on the papers found in the literature review. The articles were examined by focusing on the following features: author and year of publication, methodology, goals, and significant findings. Figure 2 group the articles by categories. As can be noticed, 35% of the studies were experimental and 65% numerical and theoretical studies.
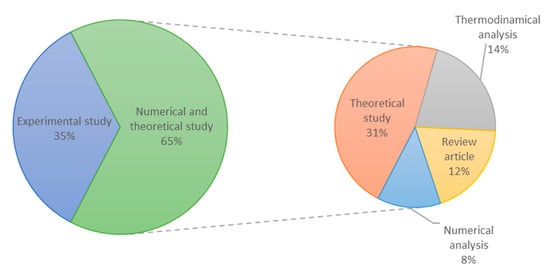
Figure 2.
Overview of the articles found in the literature review.
3. Thermodynamic Properties of Mixtures Used in Systems Based on Ammonia Absorption
Several studies of working fluids’ thermodynamic properties for absorption systems, such as NH3/H2O, have been prepared over the years.
3.1. NH3/H2O Solution
Many researchers have studied correlations and experimental data in the case of the thermodynamic properties of the NH3/H2O solution. Ziegler and Trepp [41] developed an equilibrium correlation for the NH3/H2O solution properties (specific volume, vapor pressure, enthalpy, and equilibrium constant) in the temperature range above 226.85 °C (500 K) and 5000 kPa (50 bar) pressure range based on Schulz’s state equations. These correlations are to be used as a design and testing tool for absorption equipment, especially for heat pumps.
In the study presented by Park and Sonntag [42], a generalized state equation was used to determine the thermodynamic properties of NH3/H2O solution with temperature and pressure ranges above 377 °C and 20,000 kPa (200 bar), respectively, based on the principle of four corresponding state parameters. After that, Ibrahim and Klein [43] proposed a numerical correlation covering liquid pressure and vapor equilibrium temperatures above 11,000 kPa (110 bar) and 327 °C, respectively. Separate state equations were used for liquid phases and vapor for pure ammonia and pure water, assuming the solution’s ideal mixing behavior in the liquid phase. Gibbs’ excess energy was used to leave a change in the solution’s ideal behavior in the other phase (gas). In turn, Pátek and Klomfar [44] proposed different correlations to describe the vapor–liquid equilibrium properties of the NH3/H2O mixture by adjusting experimental data through simple, functional forms.
Simplifying the complex correlations for NH3/H2O mixture properties, Tillner-Roth et al. [45] presented a simple thermodynamic model of a fundamental state equation based on Helmholtz’s free energy, covering a large part of the thermodynamic space between the solid–liquid–vapor boundary and the critical point with a pressure range of 40,000 kPa (400 bar).
El-Shaarawi et al. [46] presented a set of polynomial forms for correlations of properties developed based on experimental data. These correlations are simple, easy to use, and explicitly defined, and can be used as a tool in modeling absorption systems. Napoleao et al. [47] calculated NH3/H2O mixture’s entropy as a function of temperature, pressure, and other important parameters for absorption cooling systems mathematical modeling.
Experimental measurements characterized the prediction of NH3/H2O solution properties to calculate this mixture’s equilibrium properties. Still, for modeling, simulation systems, and to design and optimize the absorption chiller, it is necessary to have numerical correlations to calculate the properties of any operating condition. That is why the proposal made by Tillner-Roth et al. [45] was the easiest, simplest, and most practical way to use in iteration modeling without any problem. Besides, its values are similar to those obtained by Park and Sonntag [42] and Pátek and Klomfar [44].
3.2. NH3/(LiNO3) and NH3/(LiNO3 + H2O) Solution
The working fluids LiBr/H2O and NH3/H2O present some disadvantages such as corrosion problems, crystallization, low operating pressure for LiBr/H2O, toxicity problems, need for an extra component (rectifier) and high operating pressures for the pair NH3/H2O, need for high temperatures for the mixture separation process in the generator, and limitations to operate with solar energy as a driving source [18,19].
The use of the mixture of ammonia and lithium nitrate was proposed as an alternative to prevent and control LiBr/H2 O and H2O/NH3 pairs’ problems. Encouraging results were found, especially for the use of solar energy as the triggering system source. Another advantage is related to the rectifier’s dispensability in the exit of the cooling vapor when using the mixture NH3/LiNO3 [18,19,20].
The study of the NH3/LiNO3 mixture started with the work presented by Davis et al. [48], where several absorbent liquids were examined, and it was found that lithium nitrate had the highest absorbency value and no corrosion effect on carbon steel, which is suitable for this equipment.
Several measurements to determine the vapor pressures of the NH3/LiNO3 mixture were performed for a mass fraction range between 0.24 and 0.42 by Gensh [49]. In the same context, but considering another range of ammonia mass fraction between 0.4 and 0.6, Blytas et al. [50] extended the vapor pressure measurements.
The work presented by Infante-Ferreira [22] proposed different thermodynamic property correlations for the NH3/LiNO3 mixture, but these correlations present divergences with others found in other works at some thermodynamic conditions, such as the one presented by Cuenca et al. [21]. It shows an experimental study of the thermal conductivity of the NH3/LiNO3 mixture at different temperatures with values between 30 (303.15 K) and 80 °C (353.15 K), and different mass fractions from 0.35 to 0.60 at 1500 kPa (15 bar).
Uchibayashi et al. [51] determined the NH3/LiNO mixture’s viscosity for the ammonia mass fraction from 0 to 0.3, with temperature ranging from 223 to 308 K and density for a temperature range from −50.15 to 19.85 °C (223 to 293 K). Extending the study for other properties, Aggarwal and Agarwal [52] determined several thermodynamic properties of the NH3/LiNO3 mixture, such as vapor pressure, solution enthalpy, and latent vaporization heat, considering the 0.3 ammonia mass fraction with a temperature range from −50.15 to 155.85 °C (223 to 429 K). In turn, the work presented by [18,19,20] measured the vapor–liquid balance, density, dynamic viscosity, and thermal capacity of mixtures NH3/LiNO3 and NH3/(LiNO3 + H2O). To improve the mixtures’ available data, the authors also correlated these measurements with analytical equations for each property.
A study of the NH3/LiNO3 mixture’s corrosion on the carbon and stainless-steel materials was carried out by Heard and Ayala [53]. From the results, the authors verified that the three samples are suitable for the construction of an absorption heat pump, and an average corrosion rate of 0.05 mm per year was obtained. Table 2 summarizes of the works found on the binary NH3/LiNO3 working fluid’s thermodynamic properties.

Table 2.
State-of-the-art of thermodynamic properties for the NH3/LiNO3 working fluid.
Even with this binary mixture, the absorption chiller presents a limitation of lithium nitrate’s viscosity, leading to low heat and mass transfer. It was observed that the addition of water in the absorbent (LiNO3) increases heat and mass transfer, besides increasing the affinity of the refrigerant (NH3) with the absorbent, which produces a positive effect on the performance of the absorption chiller [18,19,20].
Some works on the properties of a ternary mixture have been found in the literature. In Ehmke and Renz [54], the effect of water on the solubility and viscosity of the ternary mixture was studied considering the range of water mass fraction between 0.2 and 0.25. It was also determined and correlated with the vapor pressure, and the density data of the same mixture to a water mass fraction equal to 0.2 in the absorbent. In turn, Reiner and Zaltash [55,56] performed an experimental study to measure the viscosity and density of the ternary mixture, considering a mass fraction of ammonia and water of 0.04 and 0.605, respectively. In the same context, Amaris et al. [18], Libotean et al. [19,20] measured the liquid–vapor balance, density, dynamic viscosity, and thermal capacity of the NH3/(LiNO3 + H2O) mixture to increase its evaluated data, besides correlating the measurements with analytical equations for each property, which could be used to design and simulate absorption chillers with such mixture.
To determine the mixture’s thermal conductivity, Cuenca et al. [21] presented an experimental study with temperatures ranging from 303.15 to 353.15 K and mass fractions between 0.35 and 0.60, at 1000 kPa (10 bar), to obtain data that can be correlated to calculate the thermal conductivity of NH3/(LiNO3 + H2O). They found a decreasing thermal conductivity with increasing temperature, and the lowest thermal conductivity was found for the mass fraction of 0.4. Table 3 summarizes works found on the thermodynamic properties of the ternary mixture NH3/(LiNO3 + H2O). Table 4 shows the advantages and disadvantages of ammonia/absorbent working fluids for absorption systems.

Table 3.
State-of-the-art of thermodynamic properties of the NH3/(LiNO3 + H2O) mixture.

Table 4.
Advantages and disadvantages of ammonia/absorbent working fluids for absorption systems.
4. Evaluation Study of Ammonia/Absorbent Systems
The use of mixtures based on ammonia/absorbents in absorption refrigeration processes has been studied, analyzed, and implemented by several authors over the years. Theoretical and experimental studies were carried out to analyze the performance of absorption refrigeration systems, using NH3/H2O [57,58,59,60] or NH3/LiNO3 [61,62] or any other absorbent with NH3 [63,64,65,66]. These techniques were investigated on prototypes or experimental apparatus and by applying the first and second Laws of Thermodynamic, as shown in Figure 3.
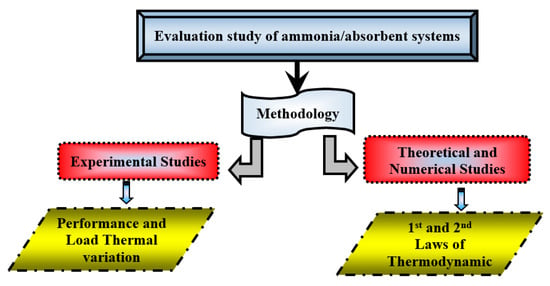
Figure 3.
Flowchart of the methods used for the assessment of ammonia/absorbent systems.
The reuse of energy and renewable energy represent viable alternatives for technological development and expansion of the world energy matrix. In this context, the use of thermodynamic tools that allow the quantification and qualification of energy and its availability have been applied in the literature [67], more specifically, to verify technical feasibility, such as the use of thermal waste to activate absorption chillers [68,69].
The First and Second Laws of Thermodynamics’ application represent essential tools to guarantee the efficient functioning of absorption refrigeration systems (Figure 3). The application of these techniques in refrigeration and heating technologies by vapor absorption systems can be seen in the review presented by Kanabar and Ramani [37]. To study and understand the characteristics of absorption refrigeration cycles, Du et al. [70] proposed a graphical method of relating temperature and thermal load. The method considers different configurations and operating conditions to verify the best operation region in terms of energy of these cycles that use NH3/H2O as the working fluid, and it was demonstrated that the saturation supply condition offered the maximum internal heat recovery point of the cycle.
4.1. Experimental Assessment on Absorption Chiller
Over the years, experimental evaluation has been done to improve the configuration and implement a better control strategy to improve absorption chiller behavior. In this context and studying the possibility of internal heat recovery in these cycles, Du et al. [71] introduced an optimal absorption refrigeration cycle that uses NH3/H2O with internal heat recovery. Thermodynamically, a 20% increase in cycle performance was determined compared to the traditional cycle under typical operating conditions. The improvement was much better for lower evaporation temperatures and higher generation temperatures, despite respecting the refrigerant’s maximum temperature value.
The use of a low-temperature source for the activation of absorption chillers has been a challenge over the years, since it would allow the use of small equipment to produce cold. In this sense, to take advantage of sources of low quality of heat, and to avoid exergetic losses and, therefore, decrease the efficiency of this equipment, Du et al. [72] analyzed how to reuse the maximum of the internal heat over a double-effect absorption refrigeration chiller using NH3/H2O as working fluid., Two operating conditions of the freezing mode (−10 and −30 °C) were considered to determine the chiller’s energetic feasibility, attending these configurations and aiming at the optimal energy value for activation. It was found that the best recovery strategy is through the combination of heat flows at temperature intervals close to the pinch points where the proposed system can be improved by 14% (−10 °C) and 34% (−30 °C) in the operating conditions analyzed when compared to conventional systems.
Du et al. [3] showed the development and experimental survey of an NH3/H2O absorption refrigeration prototype for reusing rejected heat from exhaust gases from diesel engines. The experimental results showed that the operation of the system was more reliable due to the increased variation of the exhaust gases, where a cooling capacity of 33.8 kW and a thermal COP of 0.53 were produced under the conditions of water, refrigerant and exhaust inlet were 26.1, −15.2, and 567 °C, respectively. This led to the approval of a new prototype configuration (Figure 4).
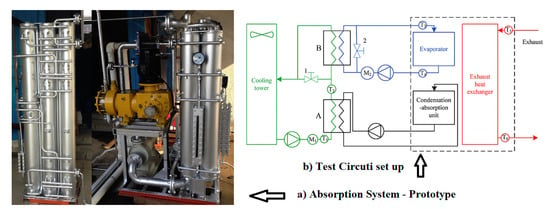
Figure 4.
Absorption refrigeration prototype [3] (Reprinted with permission from Elsevier).
Based on the results obtained in previous works Du and Wang [10], a unified proposal for a single effect NH3/H2O absorption refrigeration system for different freezing, thermal comfort, and heating applications to seek better thermal efficiencies taking into account internal heat recovery depending on the application and demand. The results found from the proposed system verified an increase in the thermal efficiency of 25%, 34%, and 20%, respectively, compared to a conventional absorption system. Table 5 shows the results data from the effects of the feed states on the system’s performance.

Table 5.
Effects of the feed state on the system performance of the system.
Ayala et al. [73,74] developed a theoretical/experimental study of a hybrid cycle (compression and absorption), as presented in Figure 5, using the binary mixture of NH3/LiNO3. The simulation results of the theoretical model determine an increase in the hybrid system’s efficiency compared to an absorption cooling system.
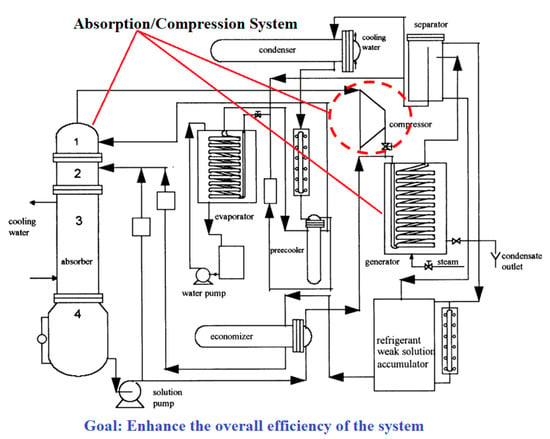
Figure 5.
Absorption/compression refrigeration hybrid cycle. Adapted from Ayala and Heard [73] (Reprinted with permission from Elsevier).
However, a comparison of the numerical and experimental results of the prototype test revealed significant discrepancies between them. This divergence of results was mainly attributed to the veracity of the mixture of thermodynamic properties and problems in bench measurements. It was very common at that time because the numerical correlations they had were not accurate and showed significant discrepancies with the actual thermodynamic process of this solution, as it has been confirmed and discussed by [18,19,20] and Cuenca et al. [21]. However, another problem they might have had would be concerning the instruments and measurements collected in the tests. The importance of using the appropriate numerical correlation in determining the thermodynamic properties of the NH3/LiNO3 mixture can be observed for proper analysis and results consistent with the actual operation of absorption chillers, as it has been confirmed in the study conducted by Vasilescu and Infante-Ferreira [75], where a theoretical and experimental study was done.
Jimínez-García and Rivera [76] carried out an experimental study of new absorption refrigeration equipment that uses stainless steel plate heat exchangers with NH3/LiNO3 mixture working fluids. The objective of the work was to evaluate the equipment experimentally and seek the effect of the input parameters (activation and condensation temperatures, volume and mass flow of the solution, and opening of the expansion valve) on the output variables, such as cooling power, evaporation temperature, and COP. The cooling power of 3 kW was reached with 6 °C of chilled water temperature and COP of 0.62. An increase in the cooling power of 20% was achieved with an increase of 3 kg/min in the solution’s mass flow.
Through the years, many studies have been proposing different configurations associated with absorption systems for cooling or heating, such as multiple absorbers [77], different activation sources [78], and even the combination of sorption and compression systems [79]. Rivera and Rivera [78] performed a theoretical study of an intermittent absorption cooling system operating with the binary mixture of NH3/LiNO3. It was based on the First Law of Thermodynamics, considering a parabolic collector used as a generator. The system’s efficiency throughout the year varied from 0.33 to 0.78 and could produce up to 12 kg of ice at temperatures of the generator and condenser of 120 and 40 °C, respectively.
4.2. Theoretical and Numerical Assessment on Absorption Chiller
The increase of the multiple-effect absorption chiller leads to a better COP, as noted in studies such as [38,80]. That is why the authors have been keeping the study of such absorption systems using different working fluids. Hence, Domínguez-Inzunza et al. [81] performed a comparison of five configurations of absorption refrigeration systems in operation (half, single, double-effect in series, double and triple effect) with NH3/LiNO3. The lowest evaporator temperatures can be achieved with half effect systems at lower generator temperatures, with a COP around 0.3, and can also be used for air conditioning and industrial refrigeration. It was confirmed that the single-effect system is the simplest configuration compared to other systems, and the COP was almost twice as large as that obtained with half-effect systems, requiring, however, higher generator temperatures. With double-effect systems, it was possible to obtain a coefficient of performance as high as 1.12 in condenser temperatures of 30 °C, but they need generator temperatures higher than 140 °C to reach evaporator temperatures as low as 5 °C and presented high pressures. These systems can be used for air conditioning purposes. The highest COP can be achieved with triple-effect systems. However, they require the highest generator temperatures and the pressure is higher than the single- and double-effect systems, which could also increase the system cost. These types of equipment could be used for air conditioning.
Farshi and Asadi [66] performed a comparative analysis of three configurations of double-effect absorption cooling systems (series, parallel, and reverse parallel systems) using the NH3/LiNO3 and NH3/NaSCN working fluids with a single-effect system. The authors observed better performance of the pair NH3/LiNO3 at low generator temperatures than NH3/NaSCN, but this is reversed at higher temperatures. It was also observed that the series system has the worst performance due to its higher probability of crystallization and that the NH3/NaSCN pair has a higher probability of crystallization than the NH3/LiNO3 pair.
Comparing different solutions and introducing a new component to improve the thermal performance of absorption cooling systems, Cai et al. [65] conducted a thermodynamic study on a new air-cooled double-effect absorption cooling system (Figure 6).
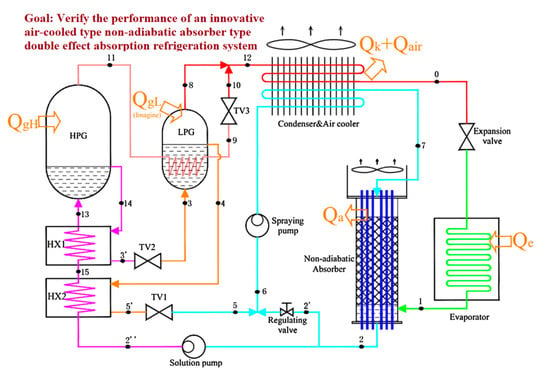
Figure 6.
Absorption/compression refrigeration hybrid cycle. Adapted from Cai et al. [65] (Reprinted with permission from Elsevier).
This cycle performance was enhanced by implementing a nonadiabatic air-cooled absorber. The results allowed determining that the COP of the pair NH3/NaSCN was higher than that obtained by the NH3/LiNO3 based system at evaporation temperature between −10 and −5 °C. However, in conditions of low evaporation temperature, below −10 °C, NH3/LiNO3 was better.
It is noticeable that theoretical and numerical analyses help to understand and design absorption refrigeration systems. However, these models need real and operational data to perform their validations [60], as it was done to validate the thermodynamic modeling of an absorption chiller (Table 6), where the experimental data of water temperatures were used to validate it [82].

Table 6.
Validation of the thermodynamic modeling by experimental data. Adapted from Ochoa et al. [82] (Reprinted with permission from Elsevier).
In work carried out by Ventas et al. [83], a hybrid refrigeration system with a different system configuration was analyzed by Ayala et al. [73,74]. The auxiliary compressor was located between the evaporator and the absorber, which was intended to increase the absorber’s heat and mass transfer process. The model was based on for separate regions of the flat plate exchanger. It is convenient to point that adding a compressor to the absorption chiller introduces a mechanical consumption work that could limit the electric COP, but in this case, it is more efficient than the one supplied to a separate vapor compression chiller with the same refrigerant.
The work presented in Liang et al. [84] proposed a new absorption chiller configuration using two ejectors as an activation source instead of mechanical pumps (Figure 7). A proposed numerical chiller model was developed considering the solutions of NH3/LiNO3 and NH3/NaSCN, as well as the use of an air-cooled absorber, based on the First Law of Thermodynamics to verify thermal performance and compare it with a conventional absorption chiller. The liquid–vapor ejector is located at the absorber inlet, and the steam-powered jet pump is adjusted to the return flow of the solution heat exchanger.
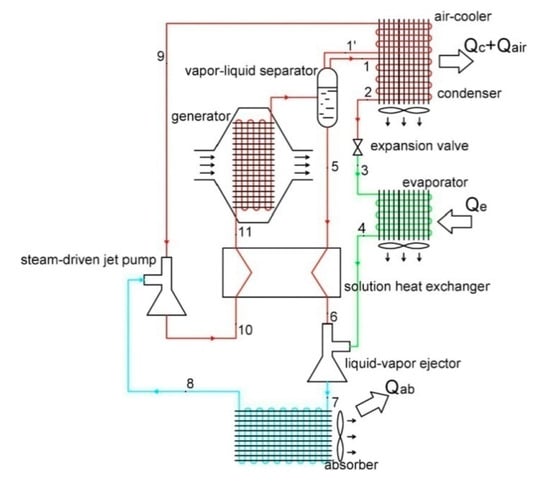
Figure 7.
Proposed combined double ejector-absorption refrigeration cycle. Adapted from Liang et al. [84] (Reprinted with permission from Elsevier).
The thermal COP can reach values of up to 0.64, being sufficient for practical use with exhaust heat sources. As an encouraging result, the use of the two ejectors and the nonadiabatic air-cooled absorber provides benefits for the miniaturization and simplification of the absorption refrigeration system.
To sum up this state-of-the-art research, the theoretical study could be a tool to allow researchers to analyze different scenarios in absorption refrigeration systems that use binary/ternary mixtures without spending too much time and money on research because it is an easy and straightforward way to obtain the results [85,86]. However, this will depend on the sophistication and precision of the model and, increasingly, on the mixture’s properties [63,65,73,74,87]. Several studies have investigated and analyzed compact heat exchangers as a way to reduce the amount of working fluid, as well as reducing the global size of the system, and therefore costs, by the use of compact components in absorption refrigeration systems to use low-temperature sources for the activation [26,85]. Additionally, due to the increasing performance of the refrigeration systems and reduction in energy consumption, new configurations are possible, such as adding multiple absorbers [77], compact heat exchanger components [26,27,76,88] or including ejectors of the systems [84], or combining sorption and mechanical compression systems [80,89], or integrating different systems to use polygeneration plants [67,90].
4.3. Miscellaneous Assessment on Absorption Chiller
Since the 1990s, many studies have been using the Thermodynamic Laws to verify, theoretically or experimentally, the absorption chiller’s behavior using ammonia and other absorbents. Table 7 and Table 8 summarize the studies about absorption chillers using either experimental analysis or numerical and theoretical studies.

Table 7.
Assessment of absorption refrigeration systems using the experimental methodology.

Table 8.
Assessment of absorption refrigeration systems using the theoretical/numerical methodology.
5. Heat and Mass Transfer Processes in Absorption Cooling System Ammonia/Absorbent
The intensification of mass and heat transfer processes in absorption systems represents the main goal of its optimal thermal behavior. That is why many authors have been investigating ways to enhance those processes [85,93,97]. Some possible improvements are the use of different working fluids, water added on the absorbent, configuration of the absorber, and generator, as seen in Figure 8 showing a flow chart on how and why the intensification on the heat and mass transfer has been so important to enhance the performance of the absorption refrigeration systems.
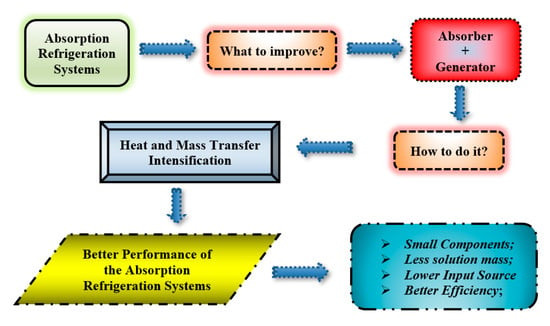
Figure 8.
Flow chart of the way on the intensification on the heat and mass transfer.
Lima et al. [85] evaluated the influence of the ammonia mass fraction and the flows of the absorbent solution and refrigerant vapor to allow the optimization and simulation of absorption cooling systems. The absorption process is one of the limiting factors of the absorption cooling system’s performance. Using the same working fluids, Chandrasekaran et al. [98] presented a characterization of a new microchannel shell-and-tube absorber for absorption refrigeration systems. The idea was to design an absorber that allows significant heat absorption capacities considering a wide range of fluid flow rates and ambient temperatures, using a 10.5 kW NH3/H2O absorption chiller. Numerical modeling was developed and validated with the experimental data collected, and found excellent fit between them (errors lower than 4%). As a main result from the study, it was the development of a sizing and simulation tool that allowed to optimize highly compact and efficient absorbers for absorption systems. Another important advance of this proposal was presented by Kini et al. [99] where through the thermodynamic analysis they verified that the new absorber integrated to the absorption chiller led to excellent behavior proving the scalability of this technology to attend the thermal demand for a residential scale absorption heat pump.
Boiling and absorption processes are the key to sorption refrigeration systems, so their improvement must reach high levels to provide higher refrigeration capacity with less driving energy. In this context, Oronel et al. [93] conducted an experimental study of the boiling and absorption process with the binary mixture (NH3/LiNO3) and ternary mixture (NH3/LiNO3 + H2O) under the operating conditions of absorption refrigeration systems driven by low-temperature heat sources. They were performed using a conjugated flat plate heat exchanger formed by three channels, with the absorption occurring in the central channel. The results showed that the ternary mixture’s mass flow absorption was higher than that obtained with the binary mixture in the same operating conditions. The heat transfer coefficient from the ternary mixture’s solution was higher than that of the binary mixture. The ternary mixture’s low viscosity increased the heat transfer and mass of the absorber compared with the binary mixture.
Studying heat and mass transfer in absorption cooling systems, Aramis et al. [18] performed an experimental analysis using the same working fluids as Oronel et al. [93]. The test’s objective was to characterize the absorption and desorption process using flat plate heat exchangers, looking for a cost and size reduction of the absorption chiller. It was found that the ternary mixture has a higher affinity than the binary mixture, which increases the performance of the absorption and desorption processes.
Using the same working fluids, Taboas et al. [100] presented an experimental study to estimate the heat transfer coefficient by flow boiling and the pressure drop by friction for a plate heat exchanger considering 20% weight of water in the absorbent. The results showed that the solution’s mass flow was the parameter that had the most significant influence on the flow boiling coefficient in the operating conditions applied, representing a characteristic of boiling by convection.
The increase in the flow’s boiling coefficient was significant in low heat flow values when the mass flow was increased for the binary mixture. However, with the ternary mixture, the improvement of the flow’s boiling coefficient, achieved by the increase in the solution’s mass flow, was analogous for all values considered for the heat flow.
Using the NH3/LiNO3 pair, Jiang et al. [92] presented through experimental analysis the influence of parameters such as mass and heat flow, diameter, and titer of vapor on the coefficient of heat transfer by boiling in a horizontal tube. An increase in the heat transfer coefficient with the increase in mass flow and heat flow was observed, where the mass flow was more impactful with high heat flow. A new correlation (Table 9) was also proposed to predict the influence of the analyzed parameters on the boiling heat transfer coefficient, obtaining better results than other works found in the literature.

Table 9.
Proposed correlation to predict the boiling heat transfer coefficient.
Applying the falling film method in the heat and mass transfer process, the authors of [101] presented a parametric analysis to determine the coefficient of performance, cooling capacity, and recirculation of the solution in different operating conditions in an absorber and generator cooling system. Cooling capacities of 4.5 kW, temperatures around 4 °C from the evaporator, COPs between 0.3 and 0.62 were found depending on the operating system.
To improve the thermal performance of heat and mass transfer using an adiabatic absorption process, Zacarías et al. [29] conducted an experimental study of the ammonia vapor absorption process in the NH3/LiNO3 solution using a full cone nozzle and an upstream single pass subcooler to analyze the influence of the absorption rate, subcooling output, mass transfer coefficient, and the equilibrium ratio approach. The results recommended using full cone nozzles in spray absorbers at high mass transfer values due to the high availability, low cost, ease of construction, compacting, and large bore diameter. Equation (1) presented a Sherwood number correlation, expressed as:
It is important to note that the efficiency of absorption systems comes from the effectivity of the heat and mass transfer process through the absorbers, where the intensity of heat and mass transfer is high, and they could represent the element with a greater possibility of bottlenecking the energy process [82,90]. That is why heat exchangers have been identified as an energy barrier element of absorption chillers [40], hence the importance of studying and understanding the operation of heat exchangers. A detailed review of different technologies of heat exchangers in absorption chillers was carried out where detailed works and experimental simulations studies of simple effect absorption systems that use binary solutions with LiBr/H2O, NH3/H2O, and NH3/LiNO3 were presented. The aim, in detail, was on the use of heat exchangers with innovative technologies, conventional and special geometries, mechanical treatments to provide information on the use of heat exchanger technologies and their development in the last 40 years. Another critical aspect discussed was the performance in the heat and mass transfer process and its relevance in choosing the correct heat exchanger configuration, which not only takes the potential for heat and mass transfer performance but factors such as manufacturing costs, type of exchanger and heat source, working fluids, compactness, among others.
By improving the process of the heat and mass transfer in a plate heat exchanger (PHE)-type model NB51 absorber with NH3/LiNO3, Chan et al. [102] conducted an experimental analysis of a bubble mode absorption using a special vapor distributor aiming to increase the mass transfer considering solar cooling operating conditions. A range of 35–50% of solution concentrations and 11.69–35.46 kg s−1 mass flow rates of the dilute solution range were used. As main result from the study, a set of Nusselt number correlations, Equations (2) and (3), governing the ammonia vapor by the NH3/LiNO3 solution in the absorber was determined considering two ranges of Reynolds number.
An experimental analysis to verify the influence of operational conditions on the heat and mass coefficients in a heat and mass transfer process in a horizontal falling film tube absorber using NH3/H2O was carried out in Bohra et al. [103]. The absorber was constructed with transparent housing to visualize flow and heat and mass transfer measurements at the local and component levels, as shown in Figure 9, where a basic schematic drawing is seen [103].
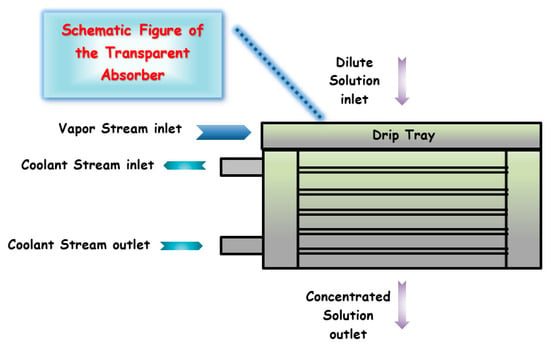
Figure 9.
Absorber assembly and tube array.
The results showed that most of the time, the absorber operates in a falling film mode. It was concluded that while the Nusselt number of the solution increased, Sherwood vapor and liquid numbers remained relatively unchanged by the Reynolds number
Using the falling film technique to analyze the heat and mass transfer process, Nagavarapu and Garimella [104] presented a heat and mass transfer model for NH3/H2O absorption on a bank of microchannel tubes, analyzing three regions in the absorber: solution pool, droplets in evolution, and falling film. It was found that most of the absorption occurred in the region of the film, and 7% of the process occurred in the droplets. The heat transfer coefficients’ values varied between 1788 and 4179 W/m2K, presented through Nusselt numbers. An empirical correlation of the Nusselt number of films was developed as presented in, Equation (4). This correlation can be used to integrate a hydrodynamic model and a heat and mass transfer model. A total of 135 of the185 data points were predicted within ± 25% accuracy.
where , and corresponds to Reynolds and Prandtl numbers of the film , represents the Reynolds numbers of the vapor, respectively.
The applicability of the correlation presented in Equation (4) is limited to the range of parameters shown in Table 10.

Table 10.
Range of applicability of overall Nusselt number correlation, Equation (4) (adapted from Nagavarapu and Garimella [104]).
The unfavorable properties of fluid transport, over the years, have been highlighted as the main factors that prevent the application of ammonia and ionic liquids in absorption refrigeration systems. Despite this information, few studies link this problem to the heat and mass transfer of these ionic fluids. Hence, Wang et al. [61] developed an experimental/numerical study to fill this gap on the process of heat and mass transfer of ionic fluids in absorption chillers using a corrugated plate heat exchanger. The experimental results are used as input for the numerical model developed to study heat and mass transfer performance during the absorption of NH3 vapor in the NH3/ILs pair. The heat transfer coefficient was 1.4 kW/m2-K, and the proposed effective mass diffusivity was exponentially related to the ionic fluid viscosity with an exponent of −1.45 for the analyzed pair.
The use of nanoparticles on the solution absorption fluid pair is also seen in Jiang et al. [105] where a new type of absorber for an absorption refrigeration system that uses NH3/H2O as the working fluids to analyze the processes of heat and mass transfer with different concentrations of TiO2 nanoparticles (0.1%, 0.3%, and 0.5%) was designed and tested. Unlike other studies, the absorber has been integrated into the real absorption system. The absorption performance was mainly determined by the strong solution’s concentration at different evaporation, activation, and inlet cooling water temperatures. It was found that the addition of TiO2 nanoparticles has an essential effect on the absorption process as it allows the temperature of the strong solution to be lower and the concentration of the strong solution to be higher. The phenomena could be explained in Figure 10 where the Prandtl number rises slowly with increasing temperature with the same quantity of nanoparticles. At the same temperature, and a higher concentration of nanoparticles, the Prandtl number is higher, too, since the nanofluid’s viscosity was increased, and the thermal conductivity growths slightly correspondingly. The figure also shows the schematic design used to model the mass and heat transfer [103]. It was also found that the thermal conductivity of nanofluids dominates the absorption process
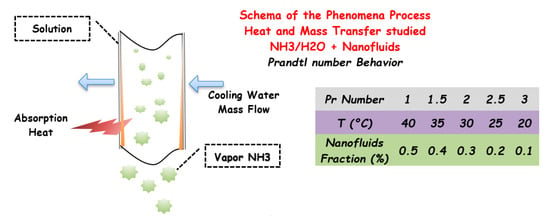
Figure 10.
Absorption process in a falling film tube. Effective coefficient of Prandtl number ratio behavior at different concentrations of nanofluids at different temperatures.
In the second part of this study, Jiang et al. [106] proceeded on the effect of different amounts of TiO2 nanoparticles, but now on the coefficient of performance of the absorption refrigeration system. In this new experiment, the evaporation, activation, and cooling temperature ranges were varied (−18–0 °C), (105–150 °C), and (22–33 °C), respectively. The addition of nanoparticles can lead to an increase of 27% of the heat and mass transfer, as shown in Figure 11, where the increase of the TiO2 nanoparticles increases the COP and the generation and evaporation temperatures. The COP improvement is strongly related to the number of nanoparticles dispersed in the fluid [106]
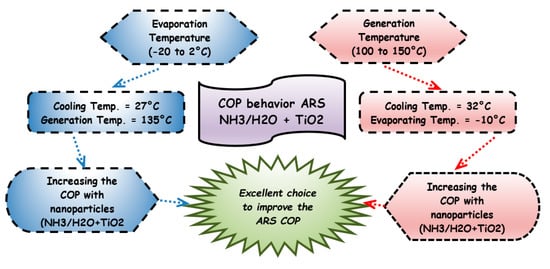
Figure 11.
COP behavior of the absorption systems with different mass fractions of TiO2 nanoparticles under different evaporation and generation temperatures.
It is evident from these studies that the addition of water as an absorbent in the mixture with NH3 allowed a better performance in the absorption and desorption process [18,93,100]. Better results were obtained when this process was performed in plate heat exchangers due to the size, ease of installation, cheaper cost, and ease of testing. Introducing a falling film method and a new component as a full cone nozzle [29,101] could increase heat transfer and mass thermal performance. Therefore, the thermal performance of absorption systems and the appropriate heat exchanger for the absorption chiller could lead to a better COP performance, as seen in Altamirano et al. [40]. Another fact seen around the heat and mass transfer process literature is the positive effect of using ionic nanoparticles on the absorption system, increasing the COP of the chiller [105,106].
6. Chiller Activation by Absorption through Solar Energy
Due to the lower driving temperature, absorption chillers that make use of the absorbent fluid ammonia/absorbents have the possibility of activation by thermal energy obtained through solar collectors [1]. This advantage has led several authors to study this application in generator activation. As shown in Figure 12, it can be seen that the main reason for the use of solar energy as input to drive absorption refrigeration systems is to attend to the residential and industrial sector demand for cooling and refrigeration. The figure also shows how important it is to design small components to compound the absorption chiller, search for different working fluids to operate them, and how the conventional solar and concentrated collector systems impact the technical and financial behavior of those absorption cycles.
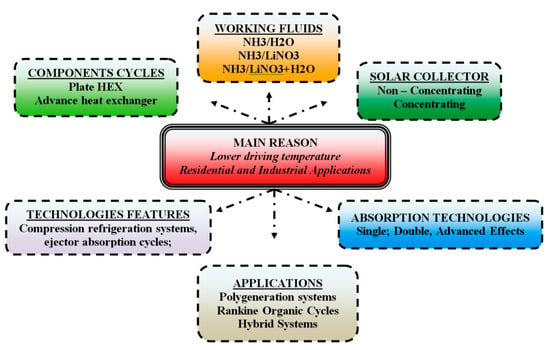
Figure 12.
Schematic graphic of the solar energy source to drive absorption systems with ammonia/absorbents.
Rivera and Rivera [78] performed a theoretical study of an intermittent cycle operating with NH3/LiNO3, using solar energy as the driving source. The authors used data from Texmico, Mexico, and obtained a compound parabolic concentrator efficiency throughout the year with values between 0.33 and 0.78, and the system can produce up to 12 kg of ice when the temperatures of the generator and condenser are 120 and 40 °C, respectively.
Through theoretical analysis, Vasilecu and Infante-Ferreira [75] analyzed a double-effect cooling system with the NH3/LiNO3 work pair driven by solar energy for industrial use. The study was conducted in a dynamic regime considering the Mediterranean summer conditions and horizontal parabolic solar collectors. The authors could conclude that the solar collector’s thermal energy could supply more than 50% of the thermal energy required for the system to operate at maximum cooling load.
Using experimental analysis, the prototype operating with NH3/LiNO3 developed by Rivera et al. [107] has an operating capacity of 8 kg of ice per day using exclusively solar energy. The authors could observe a positive correlation between the COP and the solar radiation. In the same context, Moreno-Quinanar et al. [24] compared the absorption chiller’s performance with the work pairs NH3/LiNO3 and NH3/(LiNO3 + H2O), and driven by solar energy. A better COP was obtained with NH3/(LiNO3 + H2O), 25% higher than the other mixture.
Llamas-Guillén et al. [108] presented the results for a prototype absorption refrigeration system using the NH3/LiNO3 mixture. In an environment with a temperature of 25–35 °C, the system reached a temperature below 10 °C in the evaporator and 110°C in the generator, which was only possible to achieve due to high efficiency evacuated tube collectors. A COP between 0.3 and 0.4 and a thermal load of 4.5 kW was obtained.
The heating and cooling systems powered by solar energy have been proving their efficiency and flexibility, aiming at environmental aspects and saving energy consumption, by using renewable energy that does not harm the environment and saving electricity thanks to a total or partial activation related to solar irradiation. Besides, they have technical and functional feasibility since it represents an alternative to replace conventional energy sources such as fossil fuels and electricity.
A detailed approach on the subject was reported in the review study carried out by Skeikhani et al. [33], where the authors discussed the contributions directed to cooling systems powered by solar energy and integrated with other energy auxiliary devices. The explanation of technologies for capturing solar irradiation, such as flat plate collector, evacuated tube collector, composite parabolic collector, and trough parabolic collector, was conducted. Essential technical and financial parameters (COP, annual energy consumption, payback period, solar implementation systems) were discussed, emphasizing the quality of the activation source used and the configuration effect on the absorption chillers. It is clear that chillers with triple-effect absorption present a better COP but need a higher hot source temperature (around 200–250 °C). This leads to more efficient but, at the same time, more complex and expensive solar collectors, such as evacuated and parabolic collectors. In the case of a single-effect absorption chiller with smaller COP (approximately 0.7) a simpler and cheaper collector can be used (flat plate type).
Sharma et al. [32] presented a review aiming at the importance of these variables (selection of solar collectors and thermal storage) in the performance of absorption chillers powered by solar energy in a critical survey of studies of cooling systems by solar absorption. Based on the information, it was considered that the correct choice of the type of solar collector directly influences the efficiency of the absorption cooling system. Solar energy is intermittent. It is a significant factor in storing this energy and guarantees that the demand for activation source will be attended in periods without solar radiation (night) and moments when the chiller’s operating conditions are excellent. These storage tanks act as buffer absorbers and significantly improve the COP of the chiller.
Regarding the financial viability aspects, other factors might affect the return period of the implementation of the absorption system, such as project, climatic conditions, region, and thermal load, which makes it necessary to subsidize the fixed cost of absorption systems powered by solar energy to encourage the use of this technology and, reduce the return period.
Using a sensitivity analysis as a tool to evaluate the performance of solar absorption chillers at different operational conditions, Luna et al. [109] carried out an experimental analysis of a 5 kW absorption refrigeration system that uses NH3/H2O as the working fluids, where the solar drive system consists of a field of 15 parabolic collectors with a reflective surface made of aluminum, and the absorber tubes made of copper, representing a total area of 38.4 m2. Useful heat of up to 6.5 kW, energy, and exergetic efficiency of up to 20% and 15%, respectively, through the field of the collectors and activation temperatures of up to 105 °C were found and a COP of 0.56 and exergetic efficiency of 0.13 were obtained, considering activation temperatures between 85 and 95 °C, condensation temperatures between 20 and 28 °C, and chilled water up to 6°C.
Through the application of the First and Second Laws of Thermodynamics, absorption chillers of the solar-type can be analyzed considering (i) the type of fluid as a function of the thermal properties for the performance of the system [110], (ii) the type of solar collector and function improving irradiation absorption [111], (iii) the use of nanofluids as a way to increase the efficiency of heat and mass transfer [112], and (iv) the use of integrated polygeneration systems [113].
A double-effect absorption cooling system that uses NH3/H2O as an air-cooled working fluid has been proposed in Du et al. [9] for small applications powered by solar heating. Using a prototype of a rated capacity of 2 kW, it was built to verify the feasibility and performance of this configuration. The system provided a uniform and constant behavior during the tests. The COP stabilized in a range of 0.18–0.25 in thermal comfort conditions in the summer season. According to the promising results, this prototype presents the possibility of developing small systems with low-cost solar energy activation for residential applications.
Pandya et al. [110] presented a thermo-economic comparison of the use of two working fluids (sodium ammonia-thiocyanate and ammonium-lithium nitrate) to evaluate the performance of the 15 kW solar absorption refrigeration systems. Different solar collectors were used, such as flat plate, evacuated tubes, flat plate with parabolic reflectors, and parabolic collectors, integrated with a thermal storage tank. The comparison of COP as a function of working fluids showed that the NH3/LiNO3, coupled to the evacuated tube collectors, was superior to the arrangement with coupled flat plate collectors. Absorption chillers using NH3/LiNO3, together with parabolic collectors, showed higher values of cost and thermal efficiency by 23% and 0.7%, if compared to the system’s values integrated to the evacuated tube collectors. Considering the two working fluids’ performances, it is observed that the NH3/LiNO3 pair was superior to the NH3/NaSCN in all the analyzed solar collector configurations. Therefore, considering the thermodynamic and economic results, using the NH3/LiNO3 mixture in an absorption chiller activated with evacuated tube collectors was recommended.
Likewise, using the same working fluids as Pandya et al. [110], but considering the use of nanofluids in the solar capture system, Mody et al. [112] carried out an energy analysis on solar absorption chiller to evaluate this addition of nanoparticles in the performance of thermal parameters, such as heat transfer coefficient, thermal efficiency, and useful heat gain of the collector. A maximum increase of 122% in the heat transfer coefficient was determined with 2% nanoparticles concentration, the heat transfer coefficient with the use of NH3/NaSCN as the fluid with the best performance 0.12% higher compared to the use of NH3/LiNO3 as fluid. However, in the case of the average COP of the chiller, the use of NH3/LiNO3 was 6% higher than the use of NH3/NaSCN. Thus, the use of NH3/LiNO3 is recommended in absorption refrigeration systems coupled to plate collectors with the addition of nanofluid.
Using the First and Second Laws of Thermodynamics as a technical evaluation tool, Khaliq et al. [113] presented a study of a trigeneration system composed of a heliostat field (Duratherm600 oil), Rankine organic cycle, and a solar-powered absorption chiller (NH3/LiNO3) to produce cold demand at 0 °C. The study analyzed two hydrocarbons (isobutane and propane) as refrigerants in the Rankine organic cycle (ORC). The exergetic flow of the isobutane-operated trigeneration system was increased from 2562 to 4314 kW, while it increased from 1203 to 2028 kW when the system uses propane, considering that the normal direct irradiations were increased from 600 to 1000 W/m2. The results showed that when the ORC uses isobutane as working fluids, 65% of the energy is transformed into useful output energy, and the remaining 35% is lost and exhausted to the environment. On the other hand, in exergetic terms, only 14% is transformed into useful exergy, 85% is destroyed due to irreversibility, and only 1% is transformed into exergetic losses.
Cerezo et al. [114] developed a dynamic model by coupling two computational platforms, Equation Engineering Solver (EES) and TRaNsient System Simulation (TRNSYS), of a single-effect absorption chiller using five working fluids (NH3/H2O, H2O/LiBr, NH3/NaSCN, NH3/LiNO3, and H2O/LiCl) driven by solar energy. The results showed that despite obtaining the best COP among all working fluids, due to the problem of crystallization of the solution, the H2O/LiCl mixture obtained a maximum solar fraction of 0.67 and a minimum heating fraction of 0.33 with a maximum fraction of lost heat of 0.12. The NH3/LiNO3 and NH3/H2O mixtures obtained the most significant energy gain up to 6. Both got a maximum solar fraction of 0.91 and a minimum heating fraction of 0.09, using 89 and 100 m2 of solar collector area.
Some disadvantages of solar absorption refrigeration systems are the complexity of these installations, the required area to capture useful energy for activation, installation control, the type of configuration if it is cold and heating, and, consequently, the high installation and operating costs. In this way, the search for more competitive technologies through hybrid configurations, and combined applications, can represent an effective alternative for the production of heat and air conditioning [36].
The integration of the generator with a vapor separator from an absorption chiller that uses NH3/LiNO3 as the working fluid and considering a field of solar collectors reduces the monetary cost and makes the cooling and solar heating facilities more flexible was proposed by Lecuona-Neumann et al. [111]. The flow established inside the linear receiver tube of the solar collector is driven by gravity and stratified in a counter-flow regime, and modeled in a one-dimensional way adapting convective boiling correlations and including modifications for the effects mixing. A low sensitivity was found to the chosen boiling heat transfer correlation in terms of heat and mass transfer. The theoretical and experimental results showed that the current use of the parabolic collector or Fresnel medium temperature solar collectors in the proposed flow layout was feasible since it allowed to produce vapor with efficiency similar to conventional type vapor generators, significantly if the subcooling length is minimized.
As it has been established and demonstrated, the use of solar energy as input for the cooling absorption systems has been a challenge due to the seasonality and periodicity of it, so the optimization and the uses an efficiency solar collector must be implemented to produce more activation heat, as the experimental studied conducted by Luna et al. [109] showed, where a parabolic collector system was used to produce the heat to drive an ammonia/lithium nitrate absorption chiller with a 5 kW nominal capacity. The goal of them was to assess the operation of the chiller and its solar activation source considering several conditions on the weather of Cuernavaca, Mexico. Particularly comparison and evaluation of the solar absorption chiller, both of the systems (solar collector and absorption chiller) were considered coupled and uncoupled to see their effectiveness, using the first and the second Laws of Thermodynamic. It was determined that the integrated solar collector field system could generate up to 6.5 kW of heat, with up to 20% of thermal efficiency and exergy efficiencies up to 15%, at 105 °C temperature, which is good enough to drive a single effect absorption chiller. Regarding the absorption refrigeration systems, the cooling capacity produced could be up to almost 2 kW with almost 3.5 kW of input heat, with values COP up to 0.56. It was concluded, due of the results obtained, that the parabolic solar absorption cooling configuration system will be allowed to achieve air-conditioning demands with rational performance.
From the studies presented, it is evident that the use solar energy as driving input to activate the absorption refrigeration systems has been one of the main goals of investigations on absorption refrigeration systems, as confirmed by Sheikhani et al. [33] and Sharma et al. [32]. The motivation is that it allows reducing electrical consumption to activate cooling and air conditioning systems, using renewable energy as an input, and a decrease in the use of fossil resources [109,115]. However, this kind of resource is directly associated with specific regions and climatic conditions since solar energy can only be used directly during the day and depends on solar irradiation. It is, then, necessary to use storage systems, collection systems with better efficiency [75,78], and the use of considerable areas, depending on the cooling capacity and activation of the absorption systems [114].
Due to these implications, several authors have directed efforts in the use of alternative mixtures to reduce the activation capacity, lower temperatures [108], combining technologies as mechanical, ejector absorption cycles [36], advanced technologies of heat exchanger [111], the addition of substances [112], among others, in order to activate absorption chillers exclusively/partially with solar energy [113].
7. Ammonia/Absorbent Absorption Chiller Prototypes
In recent years, studies on the absorption cooling system using the binary mixture have taken a direction in designing and constructing prototypes [27,34,116].
As mentioned, NH3/H2O is one of those usual working fluids of absorption chillers [3,9,10,117,118]. That is why many authors are still researching to achieve better performances on different operation conditions. Hence, searching for better ways to minimize exergy destruction, Du et al. [71] proposed a novel cycle considering the maximum internal heat recovery applying the pinch method technology. This application was able to verify that this proposed cycle’s performance was considerably improved, at least by 20%, compared with the traditional cycles, and even better at low evaporating temperature and when the highest generation temperature was considered. Du et al. [70] also presented an analysis of the same system applying a graphical method to identify the characteristics of different cycles with different internal heat recovery strategies and find out the key points that significantly influence internal heat recovery.
After dealing with a single-effect, Du et al. [72] continued to work using the pinch technology on a double-effect NH3/H2O ARS, aimed at the internal heat recovery of a mass-coupled considering freezing temperatures conditions to verify the reduction on the losses by irreversibility and to quantify the gain of uses of this kind of implementation on absorption chillers. The COP values from the derived refrigeration double-effect absorption chiller showed a significant increase, between 14% and 34%, under the tested conditions.
Currently, cooling demands are significant to the total energy consumption in buildings. Therefore, the focus on the design of more efficient and sustainable refrigeration systems is especially important, Neyer et al. [116] using NH3/H2O as a working fluid, energetically and financially analyzed the influence of different heat rejection sources in a single-effect and half-effect ACH powered by solar or cogeneration energy. A functional chiller was developed and built based on flat plate heat exchangers. Models developed were used to simulate other operating conditions through the TRNSYS computational platform and evaluate the annual impact on the new single and half-effect absorption refrigeration prototypes. It was verified that the chiller presented a good and stable performance in different operating conditions, and the system was able to operate with a heat rejection temperature of up to 45 °C, which provides its use in hot and arid climates. Savings of primary nonrenewable energy of up to 70% were verified on this prototype powered by solar energy and cogeneration when compared to the conventional ones.
Considering the NH3/LiNO3 working fluids, Rivera et al. [107] presented a performance analysis of the intermittent absorption cooling system. The developed prototype has a nominal capacity of 8 kg of ice per day and was based on the theoretical study developed by Rivera and Rivera [78]. The measurements performed on the reported prototype evaporator presented temperatures as low as 11 °C obtained for several hours with solar coefficients of performance up to 0.08. It was verified that the coefficient of performance increased with the increase of solar radiation and the solution’s concentration, and there was no dependence on the coefficient of performance with the temperature of the cooling water. However, there were significant discrepancies between the numerical and experimental results of the CPC efficiency. This may be associated with inappropriate numerical correlations for the thermodynamic properties of NH3/LiNO3. Even with these errors, the proposed system could work exclusively with solar energy as the driving source and produces 8 kg of ice/day.
In Moreno-Quintanar et al. [24], an experimental comparison of the solar-driven intermittent absorption cooling system developed by Rivera et al. [107] considering the two mixtures NH3/LiNO3 and NH3/(LiNO3 + H2O) was performed. The idea was to verify which mixtures present better performance. It was found that the evaporator temperature reaches 8 °C for 8 h driven exclusively by solar energy. The ternary mixtures’ system performance was better than the binary mixtures, obtaining a COP 25% higher. This increase in the COP may be related to the fact that the generator temperature was 5 °C lower than the binary mixture and the pressure reduces with the increase of water in the ternary mixture, reducing the pump’s consumption in comparison to the binary mixture.
Zacarías et al. [119] performed an experimental analysis to evaluate ammonia and lithium nitrate solution’s adiabatic absorption. The authors used a flat plate absorber with a flat fan nozzle, and in the upstream, a single pass subcooler, obtaining a heat transfer coefficient twice as high as that obtained for the tubular vertical absorber in bubbles.
In Zacarías et al. [120], a study of an absorber using NH3/LiNO3 solution was performed. The injection of the absorbent solution was performed through a mist injector. The adiabatic equilibrium factor was 3.7% higher, and the mass transfer coefficient was half of the value obtained in Zacarías et al. [119], respectively. In both studies, correlations for the equilibrium factor and Sherwood’s number were presented.
Testing a prototype ARS, Hernández-Magallanes et al. [25] analyzed a single-effect system with 3 kW nominal cooling capacity operating with NH3/LiNO3 as a working fluid designed and built for food conservation and air conditioning purposes. The developed prototype is presented in Figure 13.
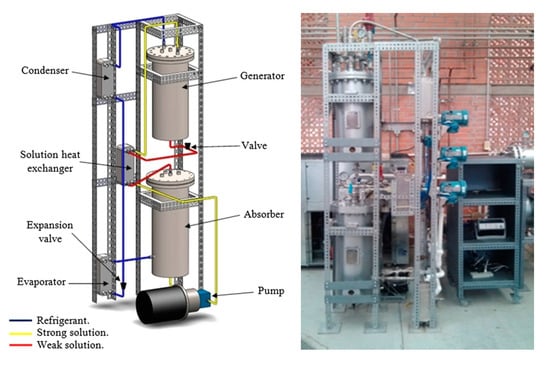
Figure 13.
Prototype scheme and real absorption system built [25] (Reprinted with permission from Elsevier).
The generator and absorber are heat exchangers with internal coils and the condenser, evaporator, and solution HEX are compact plate heat exchangers. It has been reported that the system produces up to 3 kW of cooling capacity with a hot water temperature of 95 °C and can reach evaporator temperatures around 1 °C. Additionally, the COP can range from 0.45 to 0.70. It was found that the system can work with a hot water temperature of 80 °C, which is adequate for the use of solar energy as a driving source.
There were significant discrepancies between the numerical and experimental results that the authors attributed the components’ inefficiencies and the heat losses to the environment but did not mention the uses of the correlations, found from the literature, to determinate thermodynamic properties. The previous studies found in the literature [19,20,21,73,74], confirmed the imprecision on thermodynamic properties’ use could bring inefficiencies of the ARS.
As mentioned before, there are no manufacturers that produce absorption chillers with these working fluids (binary or ternary solution), but there is specific research studying this type of absorption chiller. Zamora et al. [26] developed two preindustrial absorption chillers, a water-cooled and an air-cooled one, both using welded plate heat exchangers. Figure 14 shows the absorption chiller prototype’s schematic and the test bench installed at the Rovira and Virgili University in Spain, where the water circuits are presented and the part-load circuit configuration, adapted from Zamora et al. [26,27].
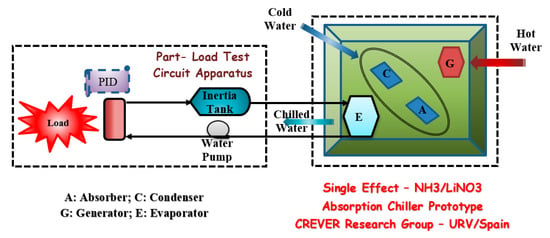
Figure 14.
Schematic partial-load test circuit apparatus used to assess the absorption chiller prototypes.
A new rotary pump replaced the circulation pump with lower energy consumption. The water-cooled prototype produced almost 13 kW of cooling capacity and an electric COPelec of 19 when operating at 15, 90, and 35 °C of chilled, hot, and cooling water, respectively. In the air-cooled prototype, a cooling capacity of 9 kW and an electric COPelec of 6.5 to 15, 90, and 35 °C of chilled, hot, and air temperatures, respectively. Table 11 shows the coefficients of performance, thermally and electrically, achieved by this absorption chiller prototype considering the driving temperature and two evaporation temperatures and its operating condition.

Table 11.
Coefficients of performance of the absorption chiller prototype.
The COP values, from a thermal point of view, are those expected for single-effect ARS reaching values of 0.5–0.6. However, what is essential to highlight were the COPs electrically achieved which represent significant values (varying from 19 to 27), making it an excellent alternative to ARS prototype. This system meets energetic demands for thermal comfort processes and even lower temperature systems, such as data centers.
Figure 15 shows the two absorption chiller prototypes’ global electricity consumption distribution presented in Zamora et al. [26,27].
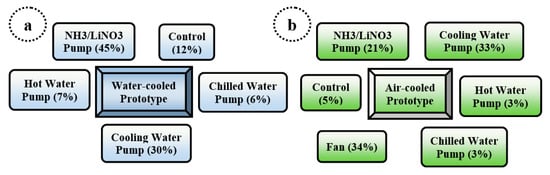
Figure 15.
Distribution of the global electricity consumption of the two absorption chiller prototypes: (a) Water-Cooled Prototype (b) Air-Cooled Prototype.
It is possible to see in Figure 15 that there is still a potential space for improving the performance of the prototype chillers due to the high electricity consumption of the cooling water pump. Hence, characterizing in partial load mode, Zamora et al. [27] conducted an experimental test. The electrical performance coefficient’s partial load curve was obtained by adjusting the experimental data to the curve shape proposed in the standard prEN-14825:2011 for air–water chillers. These prototypes were described by the characteristic equation of the experimental data collected. The uses of welded plate heat exchanger have transitory response times similar to those of vapor compression machines, and the results in partial load operation achieve higher electric COP, where it was better to use an ON-OFF control than to modify the hot water temperature. These prototypes were intended to produce a commercial absorption cooling chiller for residential use, driven exclusively by solar sources. This was a project in cooperation with the Engineering Department of Rovira and Virgili University and CIATESA Corporation.
There is still no mass marketing of absorption refrigeration systems that use ammonia with different water absorbers. However, several studies have shown the technical and financial viability of some prototypes that allow the use of LiNO3, LiNO3 + H2O, and others, as seen in Zamora et al. [26], through the use of new configurations of absorbers [29,77,119,120], as the main component in the process of heat and mass transfer in absorption refrigeration systems [93,103,104]. An important fact found in this critical survey of the state-of-the-art of refrigeration systems by absorption of ammonia/absorbents is the use of hot sources at low temperatures, aiming at the use of solar energy to activate the systems [107] for different applications, such as food preservation [25], ice production [24], and air-conditioning [25].
Therefore, the present work shows the importance of these applied materials in the pursuit of bringing to the market other sorbents that can help to reduce the size of the absorption refrigeration equipment [27], as well as in energy flexibility with the complete introduction of solar energy as an activating source of absorption chillers [25].
8. Final Considerations on the Absorption Refrigeration Systems (NH3/Absorbents)
The survey of the state-of-the-art of absorption refrigeration systems that use ammonia as a refrigerant, and water, lithium nitrate, and lithium-water nitrate as absorbent substances, have been studied exhaustively. The following aspects were considered: thermodynamic properties of the mixtures and their numerical correlations, heat and mass transfer processes in absorbers and generators, theoretical and experimental studies of the First and Second Laws of Thermodynamics, and active and passive applications of the solar-type models. It also presents the detailing of prototypes and alternative equipment in the search for systems that allow operating with adequate activation temperatures and lower than those used in commercial chillers, offered by several manufacturers (using LiBr/H2O as a working fluid).
An important aspect that was verified in the dimensioning and construction of absorption refrigeration equipment was the choice of the working fluids, specifically, the thermophysical properties and their uncertainties, since the incorrect selection can lead to unrealistic results and, therefore, to nonalternative and efficient systems for the production of chilled water with low activation temperatures, as was perceived in the works presented in the open literature [19,21,22,121].
8.1. Critical Discussion of the Results Raised in the State-of-the-Art on the NH3/Absorbents Absorption Chillers
NH3/H2O mixture is commonly used in commercial type absorption chillers for refrigeration and air conditioning applications. Due to the mixture’s behavior, it is necessary to add a rectifier, to assure the ammonia’s purity throughout the refrigeration circuit. This component’s addition leads to a greater degree of complexity of the system and an increase in the initial cost. Additionally, the system activation temperatures are relatively high for single-effect systems, which often do not allow full use of solar energy as an activation source, limiting its use in the residential sector and places where solar radiation is limited, a fact highlighted in the literature [18,19,60]. Among these limitations, the search for alternative working fluids led to the development of the NH3/LiNO3 and NH3/(LiNO3 + H2O) working fluids to minimize the complexity of the absorption refrigeration system that uses NH3/H2O [85,122], as well as problems related to vacuum pressures, corrosion, and crystallization of the solution, as verified in use and LiBr/H2O in several works [90,123,124].
An essential aspect in the dimensioning and manufacturing of the NH3/LiNO3 working fluids for absorption refrigeration systems is the no need for rectification components and with the possibility of using solar energy as the exclusive activation source as shown [19,20,121,125]. Many factors have been considered in the selection and use of these chemical components in the working fluids, as well as the use of compact heat exchangers that use loads of relatively small mixtures, leading to the construction of smaller absorption chillers [26,27,62,126], but with capacities similar to the commercial ones [127,128].
Another fact was the improvement of the heat and mass transfer process in the integrating components of the thermal compressor of the ARS [82], aiming at the generator and the absorber. In this context, the use of plate exchangers as a basic component in heat and mass transfer was highlighted, where a flat plate heat exchanger [126,129] was studied to reduce the cost and size of the absorption chiller. In these studies it was noticed that even with the use of the alternative NH3/LiNO3 mixture [20,40,121,125,130] and improvements in the overall performance of the absorption chiller [62,81], compared to ARS that use NH3/H2O [10,11,59,106], the problem of the viscosity of the mixture still represented an energy limitation, as established in the literature [95,125,130,131]. This led to the addition of water in the LiNO3 absorber as a way to minimize these limitations. Thus, several studies [100,130] were carried out to determine the concentration needed to reduce the pressure drop, that is, to reduce the solution’s viscosity. Taboas et al. [100,122] found that a 20% concentration in water would improve the heat transfer coefficient by boiling the flow and reducing the pressure drop in plate heat exchangers and the best energy gains (COP) in most of the ARS was using NH3/(LiNO3 + H2O) working fluids. The use of ionic fluids in the solution to increase the efficiency of the heat and mass transfer process in absorbers and generators of absorption refrigeration systems was discussed by several authors [61,92,106].
As mentioned throughout the discussion, solar energy as an activation source was one of the main targets using of absorption refrigeration systems that use these alternative working fluids (NH3/LiNO3 and NH3/LiNO3 + H2O). Solar use is aimed at the total activation of the equipment [114], exclusively with the energy generated from the solar source to be used in the residential sector in thermal comfort applications [36,75] to meet thermal demand in a Mediterranean city, the production of ice was another application applied by the Mexican University group [78,115]. It was emphasized that the activation of such ARS is limited to the climatic conditions of the installation site, workload, as well as the solar system of capture, storage, and distribution [132], and as main types used are the solar plate, either of flat plates [33], evacuated tubes [108] and parabolic [75,109], as well as the area available for the installation of the solar energy system [110].
Among the advantages brought by the use of solar energy that were pointed out in this survey was the use NH3/absorbents, where an electrical consumption reduction, and, therefore, a decrease in fossil resources [115], as well as the expansion of the energy matrix with renewable energy was mentioned [32,33].
As a drawback, the renewable source, in this case solar, is linked to regions with specific climatic conditions. Solar energy can only be used directly during the day and also, depending on the amount of solar radiation, which makes necessary the use of storage systems, collection systems with better efficiency [133,134], in addition to the use of considerable areas, depending on the cooling capacity and activation of the absorption systems [25,114,135]. Some alternatives to overcome these disadvantages were the proposal and study of the integration and coupling of different technologies, that is, hybrid systems (integration of mechanical systems, absorption, and ejectors), as well as improvements in the transfer of heat and mass, the addition of ionic and nanofluid substances [108,111,112,113].
The search for systems with better thermal performance, not only of the fluid used, but with the modification of the cycle through the introduction of a component has been done, as shown by Zacarías et al. [77] with the addition of several absorbers, or with the use of more compact exchangers [26,27,76,88] or by the integration of ejectors into the system [84], or simply through joining refrigeration systems by vapor compression and absorption [80,89], integration of cycles in the production of electricity and cold [136], and obviously in the use of these ARS in energy polygeneration plants, analyzed and verified technically and financially [67,137].
Finally, this study reviewed thermodynamic studies and the search for alternative working fluids. It showed the most recent advances of refrigerant equipment to meet residential and commercial type demands. Details of design, construction, and testing of absorption refrigeration prototypes capable of meeting, partially or entirely, these limitations found in commercial type absorption chillers, such as those proposed by manufacturers were also presented [127,128]. Thus, the prototypes’ construction elaborated by different groups around the world, such as the ARS prototypes presented in this review survey [24,25,26,27,115,116,136,138,139], indicates a way to make it possible to apply solar energy as a driven source for them.
8.2. Profiles of the COP Values of the NH3/Absorbents Absorption Chillers
As final considerations, Figure 16, Figure 17 and Figure 18 present the values of the performance coefficients and driving temperatures of the different works analyzed in this state-of-the-art article, considering three main mixtures: NH3/H2O, NH3/LiNO3, and NH3/(LiNO3 + H2O), through single/double/triple-effect systems and advanced cycles considering changes in the fluid circuit, such as the integration of mechanical compressor, ejectors, pumps, and other components.
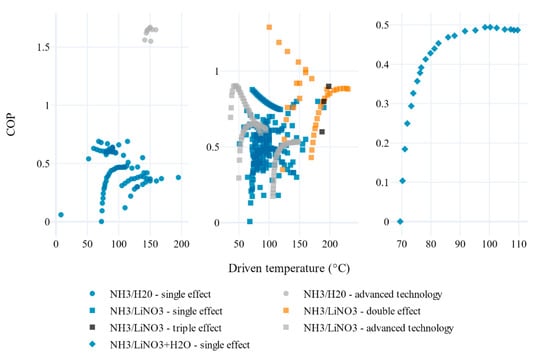
Figure 16.
COP against driving temperature for each of the three working fluids (NH3/H2O, NH3/LiNO3, and NH3/LiNO3 + H2O) and each of the four technologies (single, double, triple, and advanced technology).
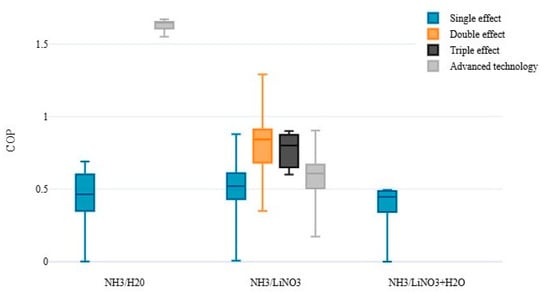
Figure 17.
Summary of the COP for each of the three working fluids (NH3/H2O, NH3/LiNO3, and NH3/LiNO3 + H2O) for each of the four technologies (single, double, triple, and advanced technology).
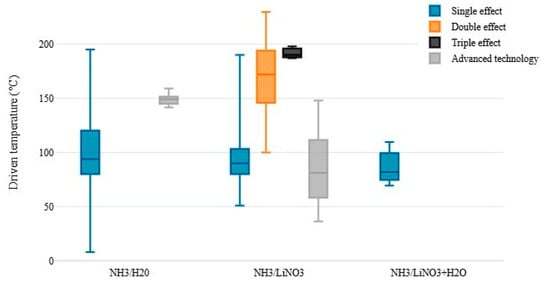
Figure 18.
Summary of the driving temperature for each of the three working fluids (NH3/H2O, NH3/LiNO3, and NH3/LiNO3 + H2O) for each of the four technologies (single, double, triple, and advanced technology).
By using several references of state-of-the-art evaluated here, the behavior of the COP of the ARS was mapped, taking into account the generator temperature for the next mixtures: NH3/H2O, NH3/LiNO3, NH3/(LiNO3 + H2O). Figure 16 shows the COP as a function of the driving temperature for the three different NH3 pairs, H2O, LiNO3, LiNO3 + H2O, and the four technologies single/double/triple, and advanced technology.
For the NH3/H2O single-effect absorption chiller [11,57,58,59,63,100,105,114,122,140], Figure 16, shows that the COP values of the single-effect absorption chiller vary up to 0.7, which represents the common values, considering driving temperature among 50–200 °C. For some references, the values between 50 and 100 °C showed a good fit for using solar energy as an input source of the ARS. However, for values beyond 100 °C, it could be a limitation since, for high-temperature values, the COPs are low, around 0.35.
According to advanced technology, higher values for the COP could be found for NH3/H2O chillers, as presented in Lu et al. [141]. This prototype was built with an intermediary process to increase the system’s performance to attend the residential district heating’s thermal demands because conventional absorption heat pumps undergo significant performance deterioration at low ambient temperatures. This prototype with an intermediate process could offer up to 30 kW heating capacity to heat the water, leading to an increased coefficient of performance around 1.66 (Figure 16).
For the NH3/LiNO3 mixture, Figure 16 shows the COP values for a single-, double-, triple-effect chiller, and the COP values of the advanced modified absorption chiller, respectively [11,16,17,41,42,45,46,47,49,50,51,52,55,56,58,59,64,69,80,88,90,92,93,101,125,130,131]. The results are coherent with the conventional COP values of the single effect absorption chiller, which are 0.6–0.7, but the most important fact is that those values were obtained with driving temperatures (60–90 °C), which suits the use of solar energy as an input source.
The COP values for the double-effect absorption chillers proposed in the literature are similar to the conventional ARS (LiBr/H2O and NH3/H2O) but have lower driving temperatures. This information is an excellent indicator to take thsese mixtures and ACH technology as an alternative to attend the residential sector’s thermal demand by using just solar energy.
The COP values of the triple-effect ARS are notably lower, around 0.9, which is not suitable for this configuration. It has one of the highest values of COP for the three configuration NH3/LiNO3 chillers. The triple-effect absorption chiller requires the highest generator temperatures, and that equipment is very complex, restricting their application to air conditioning processes [81].
Aiming at improving chiller COP, some advanced technologies were incorporated in NH3/LiNO3 chillers. In Ventas et al. [83], the introduction of a hybrid system was proposed by integrating a booster compressor between the evaporator and the absorber. In Ventas et al. [87], aiming for the double-effect absorption chiller’s behavior, the integration of a power cycle was proposed and simulated to attend to the electricity and cooling demands, generating innovative schemes. In Wang et al. [96] to reduce the generator’s input heat and improve the absorption chiller’s performance, an absorption-compression hybrid chiller system was proposed by recovering condensation heat for energy generation.
As shown in Figure 16, the modification or incorporation of a component to improve ARS performance using the NH3/LiNO3 has been achieved since the COP values were better than conventional ARS. Wang et al. [96] presented COP values up to 0.9, and 0.65, as presented in Ventas et al. [79]. The study’s case made by Ventas et al. [87] shows that the values are lower than those produced by conventional single-effect absorption chiller. This proposed cycle allows to generate power and cooling simultaneously, which is an important finding regarding the achievement of two products for any sector.
For the NH3/LiNO3 + H2O mixture, Figure 16 shows the COP values for a single-effect chiller [100,122]. The values shown in these studies are coherent with the values for a conventional machine of absorption chiller around 0.5, but its solution pump electric consumption was less than by using the regular NH3/LiNO3 or NH3/H2O.
Figure 17 and Figure 18 present box-whiskers plots of the presented scenario for the absorption chillers, using different working fluids and different technologies. Those figures help to summarize the results from the present research. As presented in Figure 17, the best COP is found for the NH3/H20 chiller with advanced technology at the cost of a higher driving temperature (Figure 18), but it is still suitable for practical applications.
For the NH3/LiNO3 pair, the best COP was found for double- and triple-effect, but also at the cost of a higher driving temperature, which, in this case, limits the application of the technology. The NH3/LiNO3 + H2O pair obtained lower COP compared to the previous cases.
9. Conclusions
A critical survey of the state-of-the-art of absorption refrigeration systems that use ammonia and absorbents (H2O, LiNO3, and LiNO3 + H2O) was studied exhaustively. The main aspects considered in this work are the thermodynamic properties of the mixtures, numerical correlations, improvement heat and mass transfer processes in the thermal compressor, energetic and exergetic studies, active and passive applications using solar energy, and available prototypes and alternative equipment.
Considered in this work were the following: active and passive applications using solar energy as input, detailing of prototypes and alternative equipment made around the world, among us. The following conclusions can be drawn from this review study:
- The thermophysical properties accuracy and their uncertainties could lead to unrealistic results and, therefore, to nonalternative and efficient systems for the production of chilled water with low activation temperatures, as was perceived in open literature;
- Due to disadvantages of the commercial mixtures LiBr/H2O and NH3/H2O such as corrosion, crystallization problems, low-pressure operation, toxicity problem, the working fluids discussed, NH3/LiNO3 and NH3/(LiNO3 + H2O), showed better results, especially when considering the use of solar energy as a driving source for the system;
- The addition of water as an absorbent in the mixture with NH3 allowed a better performance in the absorption and desorption process. Even better when these processes are performed in plate heat exchangers, due to the size, lower cost, ease of installation;
- An important fact found in this critical survey of the state-of-the-art of ARS of ammonia/absorbents was the use of hot sources low temperatures, aiming at the use of solar energy to drive them for different applications, like food preservation, ice production, air conditioning;
- The intensification in the heat and mass transfer process led to an increase in the absorption process, hence, increasing the thermal performance of the absorption chillers, so the uses of plate exchangers as a basic component in heat and mass transfer was highlighted since those led to reduce the cost and size of the absorption chiller;
- The alternative addition of water to the absorbent (LiN3) in the NH3/LiNO3 mixture improved the absorption chiller’s overall performance compared to ARS using NH3/H2O, since the high viscosity of the mixture still represented an energy limitation. Another important aspect on the heat and mass transfer is the use of ionic fluids in the solution since it helps to increase the efficiency of the heat and mass transfer process in absorbers and generators of absorption refrigeration systems;
- The solar energy as an activation source represents one of the main goals in absorption refrigeration systems that use these alternative working fluids (NH3/LiNO3 and NH3/LiNO3 + H2O). Among the advantages, it has been established that the use of solar energy led to a reduction in the electrical consumption and, therefore, a decrease in fossil resources and the expansion of the energy matrix with renewable energy; the disadvantages of this energy were linked to technical specificities in the regions, and climatic conditions since the source could be only used directly during the day and also depending on the intensity of solar radiation, which makes mandatory the use of storage systems and collection systems with better efficiency;
- The integration of advanced technology and multiple-effect absorption chillers, such as double- and triple-effect, can overcome the negative point of the solar source. Alternatives configuration systems, such as the integration of mechanical and absorption refrigeration systems (double- and triple-effect with higher COP), and also and ejector components, have been demonstrated by many authors improve the thermal and power efficiency;
- The thermodynamic studies’ importance has been leading to the search for alternative working fluids, and mostly, for more efficient, compact and flexible absorption refrigeration, to achieve the thermal demands for different sectors, such as residential and industrial. This has brought better design, construction, and testing of absorption refrigeration prototypes capable of attending, partially or completely, the limitations that have been found in commercial type absorption chillers, over the years;
- The COP values of the advanced modified absorption chiller have been coherent with the conventional COP values of the single effect absorption chiller, (around 0.6–0.7), but the most important fact is that those values were obtained with driving temperatures (60–90 °C), which suits the use of solar energy as an input source on the uses these alternatives working fluids. The COP values for the double-effect absorption chillers proposed in the literature are similar to the conventional ARS (LiBr/H2O and NH3/H2O) but lower driving temperatures. This information is an excellent indicator to take these mixtures and ACH technology as an alternative to attend the residential sector’s thermal demand by using just solar energy. For some triple-effect ARS systems, COP values were notably lower, around 0.9, which is not suitable for this configuration. It has one of the highest values of COP for the three configurations of NH3/LiNO3 chillers. The triple-effect absorption chiller requires the highest generator temperatures, and that equipment is very complex, restricting their application to air conditioning processes.
Based on the current state-of-the-art of the absorption refrigeration systems, it can be seen that there is still more research to be done to understand how to build more efficient absorbers and generators to increase the intensification of the heat and mass transfer and to allow a better absorption process, and reduction in energy consumption and, hence, better COP. In this context, the recommendations for the absorption refrigeration systems future are as follows:
- ✓
- Reducing the cost and size of the absorption refrigeration chillers, by developing a better configuration of heat exchangers, especially, absorber and generators for the thermal compressor;
- ✓
- Development of alternative ionic fluids to increase the heat and mass transfer and reduce the amount of the solution carried for the ARS, aiming to reduce the size and space, hence, the cost. Another direction is to build more efficient heat exchanger components to use lower heat source drive;
- ✓
- Investigation and development of new alternative working fluids operating with lower temperatures using sustainable sources, such as solar. These systems should produce cooling capacities to attend the needs of thermal comfort for the residential sector and small and medium capacities buildings.
Author Contributions
A.A.S.L., conceptualization; methodology; formal analysis; writing—original draft; funding acquisition; G.d.N.P.L., conceptualization; methodology; formal analysis; writing—original draft, A.A.V.O., conceptualization; formal analysis; writing—review and editing; supervision; funding acquisition., C.A.C.d.S., conceptualization; methodology; software; project administration; writing—original draft; funding acquisition., J.A.P.d.C., formal analysis; visualization; investigation; writing—review and editing., P.S.A.M., writing—review and editing; supervision., A.M.A.C., formal analysis; writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to acknowledge the support of the National Council for Scientific and Technological Development (CAPES, Brazil), through a PhD. The CNPq/Brazil for the scholarship in the program science without border of the post-doctoral study (PDE:203489/2014-4), also, for the financial support of the Universal project nº 402323/2016-5, and Research Productivity grants nº 309154/2019-7. Thanks are extended to IFPE for its financial support within the framework of Call 10/2019/Propesq, and FACEPE/CNPq for the financial support regarding research project APQ-0151-3.05/14.
Acknowledgments
The authors thank the CAPEs for the scholarship of the master’s and Doctorate’s degree. Additionally, Professor Alberto Coronas for his support during the post-doctoral study at the Rovira and Virgili University and the Staff from the same university, especially the CREVER group.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Bo | Boiler Number | [-] |
| Cp | Specific Heat | [kJ kg−1 K−1] |
| Coefficient of Performance | [-] | |
| d | diameter | [m] |
| h | Convective heat transfer coefficient | [ kW m−2 K−1] |
| H2O | Water | [-] |
| k | Thermal Conductivity | [kW m−1 K−1] |
| L | Length | [m] |
| LiNO3 | Lithium Nitrate | [-] |
| NH3 | Ammonia | [-] |
| P | Pressure | [kPa] |
| q | Heat Flow | [kW m−2] |
| T | Temperature | [°C or K] |
| UA | Overall heat transfer coefficients per unit of area | [kW K−1] |
| X1, X3 | Concentration of component LiNO3 and H2O | [%] |
| Dimensionless Numbers | ||
| X | Lockhart-Martinelli parameter | [-] |
| S1, S2 | S1 expressed the function of X, Bo and We, and S2 the function of Re and d | [-] |
| Prandtl | [-] | |
| Re | Reynolds | |
| Sh | Sherwood | |
| Sc | solution Schmidt | |
| Nu | Nusselt | |
| We | Weber Numbers | |
| Greek Letters | ||
| ρ | Density | [kg m−3] |
| µ | Dynamic Viscosity | [kg m−1 s−1] |
| Subscripts | ||
| cs | Concentrated solution | |
| Driven | Driven | |
| Electric | Electric | |
| evaporate | evaporate | |
| eff | Effective | |
| Film | film | |
| i | internal | |
| m | mean | |
| nb, cv | Nucleate, convective | |
| s | solution | |
| tp | Two-phase | |
| Thermal | Thermal | |
| l, vap, sat | Liquid, vapor, saturation phase | |
| w | Water | |
References
- Ochoa, A.A.V.; Dutra, J.C.C.; Henríquez, J.R.G.; Rohatgi, J. Energetic and exergetic study of a 10RT absorption chiller integrated into a microgeneration system. Energy Convers. Manag. 2014, 88. [Google Scholar] [CrossRef]
- Cézar, K.L.; Caldas, A.G.A.; Caldas, A.M.A.; Cordeiro, M.C.L.; Dos Santos, C.A.C.; Ochoa, A.A.V.; Michima, P.S.A. Development of a novel flow control system with arduino microcontroller embedded in double effect absorption chillers using the LiBr/H2O pair. Int. J. Refrig. 2020, 111, 124–135. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.Z.; Chen, X. Development and experimental study of an ammonia water absorption refrigeration prototype driven by diesel engine exhaust heat. Energy 2017, 130, 420–432. [Google Scholar] [CrossRef]
- Matjanov, E. Gas turbine efficiency enhancement using absorption chiller. Case study for Tashkent CHP. Energy 2020, 192, 116625. [Google Scholar] [CrossRef]
- Jaradat, M.; Al-Addous, M.; Albatayneh, A.; Dalala, Z.; Barbana, N. Potential study of solar thermal cooling in sub-mediterranean climate. Appl. Sci. 2020, 10, 2418. [Google Scholar] [CrossRef]
- Da Marques, A.S.; Carvalho, M.; Ochoa, Á.A.V.; Souza, R.J.; dos Santos, C.A.C. Exergoeconomic assessment of a compact electricity-cooling cogeneration unit. Energies 2020, 13, 5417. [Google Scholar] [CrossRef]
- Cezar, K.; MacArio, A.; Cabral Dos Santos, C.A.; Ochoa, A.; Charamba Dutra, J.C. Flow Control for Absorption Chillers Using the Par H2O/LiBr Driven in Recirculation Pumps of Low Power. IEEE Lat. Am. Trans. 2016, 14, 1624–1629. [Google Scholar] [CrossRef]
- Butt, I.B.; Tan, J.; Waqas, A.; Ali, M.; Javed, A.; Ali, A.Y. Effect of modified flow schemes of heat transfer fluid on the performance of a solar absorption-cooling system for an educational building in Pakistan. Appl. Sci. 2020, 10, 3327. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.Z.; Lin, P.; Xu, Z.Z.; Pan, Q.W.; Xu, S.C. Experimental studies on an air-cooled two-stage NH3-H2O solar absorption air-conditioning prototype. Energy 2012, 45, 581–587. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.Z. A unified single stage ammonia-water absorption system configuration with producing best thermal efficiencies for freezing, air-conditioning and space heating applications. Energy 2019, 174, 1039–1048. [Google Scholar] [CrossRef]
- de Araújo, J.J.; dos Santos, C.A.C.; de Holanda, C.A.; Duarte, J.B.F.; Ochoa, A.A.V.; Dutra, J.C.C. Energetic analysis of a commercial absorption refrigeration unit using an ammonia-water mixture. Acta Sci. Technol. 2017, 39, 439. [Google Scholar] [CrossRef]
- Caldas, A.M.A.; Caldas, A.G.A.; Dos Santos, C.A.C.; Ochoa, A.A.V.; Cézar, K.L.; Michima, P.S.A. Design, development and construction of Hall effect-based turbine meter type to measure flow in low-cost lithium bromide salt: Proposed flowmeter and first results. Int. J. Refrig. 2020, 112, 240–250. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; D’Accadia, M.D.; Libertini, L.; Vicidomini, M. Dynamic simulation and thermoeconomic analysis of a trigeneration system in a hospital application. Energies 2020, 13, 3558. [Google Scholar] [CrossRef]
- Martínez, P.J.; Martínez, P.; Soto, V.M.; Bujedo, L.A.; Rodriguez, J. Design of a 35 kW solar cooling demonstration facility for a hotel in spain. Appl. Sci. 2020, 10, 496. [Google Scholar] [CrossRef]
- Ren, J.; Qian, Z.; Yao, Z.; Gan, N.; Zhang, Y. Thermodynamic Evaluation of LiCl-H2O and LiBr-H2O Absorption Refrigeration Systems Based on a Novel Model and Algorithm. Energies 2019, 12, 3037. [Google Scholar] [CrossRef]
- Tahmasebzadehbaie, M.; Najafi Nobar, S.; Derahaki, M. Thermodynamic analysis of the NGL plant in a sample gas refinery and problem solving by designing an absorption chiller. Appl. Therm. Eng. 2019, 159, 113963. [Google Scholar] [CrossRef]
- Albers, J. Improved solar operation control for a solar cooling system of an IT center. Appl. Sci. 2020, 10, 3354. [Google Scholar] [CrossRef]
- Amaris, C.; Bourouis, M.; Vallès, M.; Salavera, D.; Coronas, A. Thermophysical Properties and Heat and Mass Transfer of New Working Fluids in Plate Heat Exchangers for absorption refrigeration systems. Heat Transfer Eng. 2014, 36, 37–41. [Google Scholar] [CrossRef]
- Libotean, S.; Salavera, D.; Valles, M.; Esteve, X.; Coronas, A. Vapor-liquid equilibrium of ammonia + lithium nitrate + water and ammonia + lithium nitrate solutions from (293.15 to 353.15) K. J. Chem. Eng. Data 2007, 52, 1050–1055. [Google Scholar] [CrossRef]
- Libotean, S.; Martı, A.; Salavera, D.; Valles, M.; Esteve, X.; Coronas, A. Densities, Viscosities, and Heat Capacities of Ammonia + Lithium Nitrate and Ammonia + Lithium Nitrate + Water Solutions between (293.15 and 353.15) K. J. Chem. Eng. 2008, 2383–2388. [Google Scholar] [CrossRef]
- Cuenca, Y.; Salavera, D.; Vernet, A.; Teja, A.S.; Vallès, M. Thermal conductivity of ammonia + lithium nitrate and ammonia + lithium nitrate + water solutions over a wide range of concentrations and temperatures. Int. J. Refrig. 2014, 38, 333–340. [Google Scholar] [CrossRef]
- Infante Ferreira, C.A. Thermodynamic and physical property data equations for ammonia-lithium nitrate and ammonia-sodium thiocyanate solutions. Sol. Energy 1984, 32, 231–236. [Google Scholar] [CrossRef]
- Heard, C.L.; Rivera, W.; Best, R. Characteristics of an ammonia/lithium nitrate double effect heat pump-transformer. Appl. Therm. Eng. 2016, 99, 518–527. [Google Scholar] [CrossRef]
- Moreno-Quintanar, G.; Rivera, W.; Best, R. Comparison of the experimental evaluation of a solar intermittent refrigeration system for ice production operating with the mixtures NH3/LiNO3 and NH3/LiNO3/H2O. Renew. Energy 2012, 38, 62–68. [Google Scholar] [CrossRef]
- Hernández-Magallanes, J.A.; Domínguez-Inzunza, L.A.; Gutiérrez-Urueta, G.; Soto, P.; Jiménez, C.; Rivera, W. Experimental assessment of an absorption cooling system operating with the ammonia/lithium nitrate mixture. Energy 2014, 78, 685–692. [Google Scholar] [CrossRef]
- Zamora, M.; Bourouis, M.; Coronas, A.; Vallès, M. Pre-industrial development and experimental characterization of new air-cooled and water-cooled ammonia/lithium nitrate absorption chillers. Int. J. Refrig. 2014, 45, 189–197. [Google Scholar] [CrossRef]
- Zamora, M.; Bourouis, M.; Coronas, A.; Vallès, M. Part-load characteristics of a new ammonia/lithium nitrate absorption chiller. Int. J. Refrig. 2015, 56, 43–51. [Google Scholar] [CrossRef]
- Asfand, F.; Stiriba, Y.; Bourouis, M. CFD simulation to investigate heat and mass transfer processes in a membrane-based absorber for water-LiBr absorption cooling systems. Energy 2015, 91, 517–530. [Google Scholar] [CrossRef]
- Zacarías, A.; Venegas, M.; Lecuona, A.; Ventas, R.; Carvajal, I. Experimental assessment of vapour adiabatic absorption into solution droplets using a full cone nozzle. Exp. Therm. Fluid Sci. 2015, 68, 228–238. [Google Scholar] [CrossRef]
- Mehari, A.; Xu, Z.Y.; Wang, R.Z. Thermal energy storage using absorption cycle and system: A comprehensive review. Energy Convers. Manag. 2020, 206. [Google Scholar] [CrossRef]
- Asfand, F.; Bourouis, M. A review of membrane contactors applied in absorption refrigeration systems. Renew. Sustain. Energy Rev. 2015, 45, 173–191. [Google Scholar] [CrossRef]
- Sharma, D.K.; Sharma, D.; Ali, A.H.H. A state of the art on solar-powered vapor absorption cooling systems integrated with thermal energy storage. Environ. Sci. Pollut. Res. 2020, 27, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Sheikhani, H.; Barzegarian, R.; Heydari, A.; Kianifar, A.; Kasaeian, A.; Gróf, G.; Mahian, O. A review of solar absorption cooling systems combined with various auxiliary energy devices. J. Therm. Anal. Calorim. 2018, 134, 2197–2212. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wang, R.Z. Absorption refrigeration cycles: Categorized based on the cycle construction. Int. J. Refrig. 2016, 62, 114–136. [Google Scholar] [CrossRef]
- Ayou, D.S.; Coronas, A. New Developments and Progress in Absorption Chillers for Solar Cooling Applications. Appl. Sci. 2010, 10, 4073. [Google Scholar] [CrossRef]
- Shirazi, A.; Taylor, R.A.; Morrison, G.L.; White, S.D. Solar-powered absorption chillers: A comprehensive and critical review. Energy Convers. Manag. 2018, 171, 59–81. [Google Scholar] [CrossRef]
- Kanabar, B.K.; Ramani, B.M. Energy and Exergy Analysis of Vapour Absorption Refrigeration Cycle—A Review. J. Inst. Eng. Ser. C 2016, 97, 479–491. [Google Scholar] [CrossRef]
- Nikbakhti, R.; Wang, X.; Hussein, A.K.; Iranmanesh, A. Absorption cooling systems—Review of various techniques for energy performance enhancement. Alex. Eng. J. 2020, 59, 707–738. [Google Scholar] [CrossRef]
- Best, R.; Rivera, W. A review of thermal cooling systems. Appl. Therm. Eng. 2015, 75, 1162–1175. [Google Scholar] [CrossRef]
- Altamirano, A.; Stutz, B.; Le Pierrès, N. Review of small-capacity single-stage continuous absorption systems operating on binary working fluids for cooling: Compact exchanger technologies. Int. J. Refrig. 2020, 114, 118–147. [Google Scholar] [CrossRef]
- Ziegler, B.; Trepp, C. Equation of state for ammonia-water mixtures. Int. J. Refrig. 1984, 7, 101–106. [Google Scholar] [CrossRef]
- Park, Y.M.; Sonntag, R.E. Thermodynamic properties of ammonia-water mixtures: A generalized equation-of-state approach. ASHRAE Trans. 1990, 96, 150–159. [Google Scholar]
- Ibrahim, O.M.; Klein, S.A. Thermodynamic Properties of Air Water Mixtures. ASHRAE Trans. 1993, 99, 1495. [Google Scholar]
- Pátek, J.; Klomfar, J. Simple functions for fast calculations of selected thermodynamic properties of the ammonia-water system. Int. J. Refrig. 1995, 18, 228–234. [Google Scholar] [CrossRef]
- Tillner-Roth, R.; Harms-Watzenberg, F.; Baehr, H. A new fundamental equation for ammonia Eine neue Fundamentalgleichung fuer Ammoniak. In Proceedings of the German Refrigeration and Air Conditioning Conference and Annual Meeting of Deutscher Kaelte- und Klimatechnischer Verein e.V. (DKV), Nuernberg, Germany, 17–19 November 1993. [Google Scholar]
- El-Shaarawi, M.A.I.; Said, S.A.M.; Siddiqui, M.U. New correlation equations for ammonia-water vapor-liquid equilibrium (VLE) thermodynamic properties. ASHRAE Trans. 2013, 119, 293–298. [Google Scholar]
- dos Napoleao, D.A.S.; Silveira, J.L.; Giacaglia, G.E.O.; de Lamas, W.Q.; Araujo, F.H.M. Diagrammes entropiques pour des mélanges ammoniac-eau: Applications aux systèmes frigorifiques à absorption. Int. J. Refrig. 2017, 82, 335–347. [Google Scholar] [CrossRef]
- Davis, R.; Olmstead, L.; Lundstrum, F. Vapor pressure of lithium nitrte: Ammonia System. J. Am. Chem. Soc. 1921, 43, 1575–1580. [Google Scholar] [CrossRef]
- Gensch, K. Lithium nitrat ammonia katals Absortions flus—Sigkeit fur Kaltemaschinen. Z. GesamteKalte-Ind. 1937, 44, 24–29. [Google Scholar]
- Blytas, G.C.; Kertesz, D.J.; Daniels, F. Concentrated Solutions in Liquid Ammonia: Solubility of NaNO3 and KBr and Other Salts; Vapor Pressures of LiNO3-NH3 Solutions. J. Am. Chem. Soc. 1962, 84, 1083–1085. [Google Scholar] [CrossRef]
- Uchibayashi, K.; Nilbe, M.; Nakamura, Y. Viscosity and Lithium-7 Nuclear Magnetic Resonance Relaxation Time of Concentrated Lithium Nitrate—Ammonia Solutions. J. Chem. Eng. Data 1985, 30, 214–216. [Google Scholar] [CrossRef]
- Aggarwal, M.K.; Agarwal, R.S. Thermodynamic properties of lithium Nitrate-Ammonia Mixtures. Energy Res. 1986, 10, 59–68. [Google Scholar] [CrossRef]
- Heard, C.L.; Ayala, R. Carbon and stainless steel in solutions of lithium nitrate in ammonia at moderate temperatures. Mater. Corros. 2003, 54, 609–611. [Google Scholar] [CrossRef]
- Ehmke, H.J.; Renz, M. Ternary working fluids for absorption systems with salt-liquid mixtures as absorber. In Proceedings of the IIF, IIR Congress Commission B1, Paris, France, 31 August–7 September 1983. [Google Scholar]
- Reiner, R.H.; Zaltash, A. Evaluation of Ternary Ammonia-Water Fluids for GAX and Regenerative Absorption Cycles; Report ORNL/CF-91/263; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1991.
- Reiner, R.; Zaltash, A. Densities and viscosities of ternary ammonia/water fluids. In Proceedings of the ASME Winter Annual Meeting, New Orleans, LA, USA, 28 November–3 December 1993. [Google Scholar]
- Said, S.A.M.; Spindler, K.; El-Shaarawi, M.A.; Siddiqui, M.U.; Schmid, F.; Bierling, B.; Khan, M.M.A. Design, construction and operation of a solar powered ammonia-water absorption refrigeration system in Saudi Arabia. Int. J. Refrig. 2016, 62, 222–231. [Google Scholar] [CrossRef]
- Zotter, G.; Rieberer, R. Experimental analysis of a novel concept of a “thermally driven” solution pump operating a small-capacity ammonia/water absorption heat pumping system. Int. J. Refrig. 2015, 60, 190–205. [Google Scholar] [CrossRef]
- Le Lostec, B.; Galanis, N.; Millette, J. Simulation of an ammonia-water absorption chiller. Renew. Energy 2013, 60, 269–283. [Google Scholar] [CrossRef]
- de Araújo, J.J.P.; dos Santos, C.A.C.; de Holanda, C.A.M.; Duarte, J.B.F.; Villa, A.A.O.; Dutra, J.C.C. Análise energética de um chiller de refrigeração por absorção comercial utilizando a mistura amônia-água. Acta Sci. Technol. 2017, 39, 439–448. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wang, B.; Wu, W.; Song, P.; Shi, W.; Wang, M.; He, L.; Infante Ferreira, C.A. Ammonia absorption in ionic liquids-based mixtures in plate heat exchangers studied by a semi-empirical heat and mass transfer framework. Procedia Eng. 2017, 134, 1302–1317. [Google Scholar] [CrossRef]
- Lima, A.A.; Ochoa, A.A.; Da Costa, J.Â.; Dos Santos, C.A.; Lima, M.; Menezes, F. Energetic analysis of an absorption chiller using NH3/LiNO3 as an alternative working fluid. Braz. J. Chem. Eng. 2019, 36, 1061–1073. [Google Scholar] [CrossRef]
- Acuña, A.; Velázquez, N.; Cerezo, J. Energy analysis of a diffusion absorption cooling system using lithium nitrate, sodium thiocyanate and water as absorbent substances and ammonia as the refrigerant. Appl. Therm. Eng. 2013, 51, 1273–1281. [Google Scholar] [CrossRef]
- Farshi, L.G.; Infante Ferreira, C.A.; Mahmoudi, S.M.S.; Rosen, M.A. First and second law analysis of ammonia/salt absorption refrigeration systems. Int. J. Refrig. 2014, 40, 111–121. [Google Scholar] [CrossRef]
- Cai, D.; He, G.; Tian, Q.; Bian, Y.; Xiao, R.; Zhang, A. First law analysis of a novel double effect air-cooled non-adiabatic ammonia/salt absorption refrigeration cycle. Energy Convers. Manag. 2015, 98, 1–14. [Google Scholar] [CrossRef]
- Farshi, L.; Asadi, S. Ammonia lithium nitrate and ammonia sodium thiocyanate double effect absorption refrigeration systems: Thermodynamic analysis. Appl. Therm. Eng. 2018, 138, 374–385. [Google Scholar] [CrossRef]
- Silva, H.C.N.; Dutra, J.C.C.; Costa, J.A.P.; Ochoa, A.A.V.; dos Santos, C.A.C.; Araújo, M.M.D. Modeling and simulation of cogeneration systems for buildings on a university campus in Northeast Brazil—A case study. Energy Convers. Manag. 2019, 186, 334–348. [Google Scholar] [CrossRef]
- Mirzaee, M.; Zare, R.; Sadeghzadeh, M.; Maddah, H.; Ahmadi, M.H.; Acıkkalp, E.; Chen, L. Thermodynamic analyses of different scenarios in a CCHP system with micro turbine—Absorption chiller, and heat exchanger. Energy Convers. Manag. 2019, 198. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, N.; Yang, S. Thermodynamic and parametric analysis of a coupled LiBr/H2O absorption chiller/Kalina cycle for cascade utilization of low-grade waste heat. Energy Convers. Manag. 2020, 205. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.Z.; Xia, Z.Z. Graphical analysis on internal heat recovery of a single stage ammonia-water absorption refrigeration system. Energy 2015, 80, 687–694. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.Z.; Xia, Z.Z. Optimal ammonia water absorption refrigeration cycle with maximum internal heat recovery derived from pinch technology. Energy 2014, 68, 862–869. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.Z.; Chen, X. Analysis on maximum internal heat recovery of a mass-coupled two stage ammonia water absorption refrigeration system. Energy 2017, 133, 822–831. [Google Scholar] [CrossRef]
- Ayala, R.; Heard, C.L.; Holland, F.A. Ammonia/lithium nitrate absorption/compression refrigeration cycle. Part II. Experimental. Appl. Therm. Eng. 1998, 18, 661–670. [Google Scholar] [CrossRef]
- Ayala, R.; Heard, C.L.; Holland, F.A. Ammonia/lithium nitrate absorption/compression refrigeration cycle. Part I. Simulation. Appl. Therm. Eng. 1997, 17, 223–233. [Google Scholar] [CrossRef]
- Vasilescu, C.; Infante Ferreira, C. Solar driven double-effect absorption cycles for sub-zero temperatures. Int. J. Refrig. 2014, 39, 86–94. [Google Scholar] [CrossRef]
- Jiménez-García, J.C.; Rivera, W. Parametric analysis on the performance of an experimental ammonia/lithium nitrate absorption cooling system. Int. J. Energy Res. 2018, 42, 4402–4416. [Google Scholar] [CrossRef]
- Zacarías, A.; Quiroz, J.A.; Gutiérrez-Urueta, G.L.; Venegas, M.; Carvajal, I.; Rubio, J. Comparison between adiabatic and nonadiabatic absorption chillers using ammonia–lithium nitrate and water–lithium bromide solutions. Heat Transf. Res. 2020, 51, 609–621. [Google Scholar] [CrossRef]
- Rivera, C.O.; Rivera, W. Modeling of an intermittent solar absorption refrigeration system operating with ammonia-lithium nitrate mixture. Sol. Energy Mater. Sol. Cells 2003, 76, 417–427. [Google Scholar] [CrossRef]
- Ventas, R.; Lecuona, A.; Zacarías, A.; Venegas, M. Ammonia-lithium nitrate absorption chiller with an integrated low-pressure compression booster cycle for low driving temperatures. Appl. Therm. Eng. 2010, 30, 1351–1359. [Google Scholar] [CrossRef]
- Ventas, R.; Lecuona, A.; Vereda, C.; Legrand, M. Two-stage double-effect ammonia/lithium nitrate absorption cycle. Appl. Therm. Eng. 2016, 94, 228–237. [Google Scholar] [CrossRef]
- Domínguez-Inzunza, L.A.; Hernández-Magallanes, J.A.; Sandoval-Reyes, M.; Rivera, W. Comparison of the performance of single-effect, half-effect, double-effect in series and inverse and triple-effect absorption cooling systems operating with the NH3–LiNO3 mixture. Appl. Therm. Eng. 2014, 66, 612–620. [Google Scholar] [CrossRef]
- Ochoa, A.A.V.; Dutra, J.C.C.; Henríquez, J.R.G.; dos Santos, C.A.C.; Rohatgi, J. The influence of the overall heat transfer coefficients in the dynamic behavior of a single effect absorption chiller using the pair LiBr/H2O. Energy Convers. Manag. 2017, 136. [Google Scholar] [CrossRef]
- Ventas, R.; Lecuona, A.; Legrand, M.; Rodríguez-Hidalgo, M.C. On the recirculation of ammonia-lithium nitrate in adiabatic absorbers for chillers. Appl. Therm. Eng. 2010, 30, 2770–2777. [Google Scholar] [CrossRef][Green Version]
- Liang, X.; Zhou, S.; Deng, J.; He, G.; Cai, D. Thermodynamic analysis of a novel combined double ejector-absorption refrigeration system using ammonia/salt working pairs without mechanical pumps. Energy 2019, 185, 895–909. [Google Scholar] [CrossRef]
- Lima, A.A.; Ochoa, A.A.; Da Costa, J.A.; Henríquez, J. CFD simulation of heat and mass transfer in an absorber that uses the pair ammonia/water as a working fluid. Int. J. Refrig. 2019, 98, 514–525. [Google Scholar] [CrossRef]
- Cai, D.; Jiang, J.; He, G.; Li, K.; Niu, L.; Xiao, R. Experimental evaluation on thermal performance of an air-cooled absorption refrigeration cycle with NH3-LiNO3 and NH3-NaSCN refrigerant solutions. Energy Convers. Manag. 2016, 120, 32–43. [Google Scholar] [CrossRef]
- Ventas, R.; Lecuona, A.; Vereda, C.; Rodriguez-Hidalgo, M.C. Performance analysis of an absorption double-effect cycle for power and cold generation using ammonia/lithium nitrate. Appl. Therm. Eng. 2017, 115, 256–266. [Google Scholar] [CrossRef]
- de Vega, M.; Venegas, M.; García-Hernando, N. Modeling and performance analysis of an absorption chiller with a microchannel membrane-based absorber using LiBr-H2O, LiCl-H2O, and LiNO3-NH3. Int. J. Energy Res. 2018, 42, 3544–3558. [Google Scholar] [CrossRef]
- Dixit, M.; Arora, A.; Kaushik, S.C. Energy, exergy, environment and economic analyses and optimization of two-stage absorption–compression combined refrigeration system. Clean Technol. Environ. Policy 2017, 19, 2215–2229. [Google Scholar] [CrossRef]
- Ochoa, A.A.V.; Dutra, J.C.C.; Henríquez, J.R.G.; Dos Santos, C.A.C. Dynamic study of a single effect absorption chiller using the pair LiBr/H2O. Energy Convers. Manag. 2016, 108. [Google Scholar] [CrossRef]
- Antonopoulos, K.A.; Rogdakis, E.D. Performance of solar-driven ammonia-lithium nitrate and ammonia-sodium thiocyanate absorption systems operating as coolers or heat pumps in athens. Appl. Therm. Eng. 1996, 16, 127–147. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Y.; He, G.; Liu, Y.; Cai, D.; Liang, X. Experimental investigations and an updated correlation of flow boiling heat transfer coefficients for ammonia/lithium nitrate mixture in horizontal tubes. Int. J. Heat Mass Transf. 2017, 112, 224–235. [Google Scholar] [CrossRef]
- Oronel, C.; Amaris, C.; Vallès, M.; Bourouis, M.; Coronas, A. Performance Comparison of a Bubble Absorber with Ammonia/Lithium Nitrate and Ammonia/(Lithium Nitrate + Water) for Absorption Chillers. In Proceedings of the International Sorption Heat Pump Conference ISHPC-2011, Padua, Italy, 6–8 April 2011. [Google Scholar]
- Yang, L.; Jiang, W.; Chen, X.; Du, K. Caractéristiques dynamiques d’un frigorigène respectueux de l’environnement: Nanofluides TiO2 à base d’ammoniac-eau. Int. J. Refrig. 2017, 82, 366–380. [Google Scholar] [CrossRef]
- Ochoa, A.; Coronas, A. Aplicação do método da equação característica num chiller por absorção com NH3/LiNO3. In Proceedings of the Cytef—VIII Iberrian/VI Ibero-American Congress—Refrigeration Sciences and Technologies, Coimbra, Portugal, 22–24 May 2016. [Google Scholar]
- Wang, J.; Li, X.; Wang, B.; Wu, W.; Song, P.; Shi, W. Performance Comparison between an Absorption-compression Hybrid Refrigeration System and a Double-effect Absorption Refrigeration Sys-tem. Procedia Eng. 2017, 205, 241–247. [Google Scholar] [CrossRef]
- Oronel, C.; Amaris, C.; Vallès, M.; Bourouis, M. Experiments on the characteristics of saturated boiling heat transfer in a plate heat exchanger for ammonia/lithium nitrate and ammonia/(lithium nitratewater). In Proceedings of the 2010 3rd International Conference on Thermal Issues in Emerging Technologies Theory and Applications, Cairo, Egypt, 19–22 December 2010; pp. 217–225. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Hughes, M.; Kini, G.; Garimella, S. A microchannel shell-and-tube absorber for ammonia-water absorption. Appl. Therm. Eng. 2020, 116321. [Google Scholar] [CrossRef]
- Kini, G.; Chandrasekaran, S.; Tambasco, M.; Garimella, S. A residential absorption chiller for high ambient temperatures. Int. J. Refrig. 2020, 120, 31–38. [Google Scholar] [CrossRef]
- Taboas, F.; Bourouis, M.; Valles, M. Boiling heat transfer and pressure drop of NH3/LiNO3 and NH3/(LiNO3 + H2O) in a plate heat exchanger. Int. J. Therm. Sci. 2016, 105, 182–194. [Google Scholar] [CrossRef]
- Domínguez-Inzunza, L.A.; Hernández-Magallanes, J.A.; Soto, P.; Jiménez, C.; Gutiérrez-Urueta, G.; Rivera, W. Experimental assessment of an absorption cooling system utilizing a falling film absorber and generator. Appl. Therm. Eng. 2016, 103, 1105–1111. [Google Scholar] [CrossRef]
- Chan, J.J.; Best, R.; Cerezo, J.; Barrera, M.A.; Lezama, F.R. Experimental study of a bubble mode absorption with an inner vapor distributor in a plate heat exchanger-type absorber with NH3-LiNO3. Energies 2018, 11, 2137. [Google Scholar] [CrossRef]
- Bohra, L.K.; Lee, S.; Garimella, S. Heat and mass transfer models for horizontal-tube falling-film ammonia-water absorption. Int. J. Refrig. 2019, 107, 84–97. [Google Scholar] [CrossRef]
- Nagavarapu, A.K.; Garimella, S. Experimentally validated models for falling-film absorption around microchannel tube banks: Heat and mass transfer. Int. J. Heat Mass Transf. 2019, 139, 303–316. [Google Scholar] [CrossRef]
- Jiang, W.; Li, S.; Yang, L.; Du, K. Experimental investigation on enhancement of ammonia absorption process with TiO2 nanoparticles in newly designed absorber. Int. J. Refrig. 2019, 100, 93–103. [Google Scholar] [CrossRef]
- Jiang, W.; Li, S.; Yang, L.; Du, K. Experimental investigation on performance of ammonia absorption refrigeration system with TiO2 nanofluid. Int. J. Refrig. 2019, 98, 80–88. [Google Scholar] [CrossRef]
- Rivera, W.; Moreno-Quintanar, G.; Rivera, C.O.; Best, R.; Martínez, F. Evaluation of a solar intermittent refrigeration system for ice production operating with ammonia/lithium nitrate. Sol. Energy 2011, 85, 38–45. [Google Scholar] [CrossRef]
- Llamas-Guillén, S.U.; Cuevas, R.; Best, R.; Gómez, V.H. Experimental results of a direct air-cooled ammonia-lithium nitrate absorption refrigeration system. Appl. Therm. Eng. 2014, 67, 362–369. [Google Scholar] [CrossRef]
- Luna, Y.R.G.; Franco, W.R.G.; Carrasco, U.D.; Domínguez, R.J.R.; García, J.C.J. Integration of the experimental results of a parabolic trough collector (PTC) solar plant to an absorption air-conditioning system. Appl. Sci. 2018, 8, 2163. [Google Scholar] [CrossRef]
- Pandya, B.; Modi, N.; Kumar, V.; Upadhyai, R.; Patel, J. Performance comparison and optimal parameters evaluation of solar-assisted NH3–NaSCN and NH3–LiNO3 type absorption cooling system. J. Therm. Anal. Calorim. 2019, 135, 3437–3452. [Google Scholar] [CrossRef]
- Lecuona-Neumann, A.; Famiglietti, A.; Legrand, M. Theoretical study of direct vapor generation for energy integrated solar absorption machines. Renew. Energy 2019, 135, 1335–1353. [Google Scholar] [CrossRef]
- Modi, N.; Pandya, B.; Patel, J. Comparative analysis of a solar-driven novel salt-based absorption chiller with the implementation of nanoparticles. Int. J. Energy Res. 2019, 43, 1563–1577. [Google Scholar] [CrossRef]
- Khaliq, A.; Mokheimer, E.M.A.; Yaqub, M. Thermodynamic investigations on a novel solar powered trigeneration energy system. Energy Convers. Manag. 2019, 188, 398–413. [Google Scholar] [CrossRef]
- Cerezo, J.; Romero, R.J.; Ibarra, J.; Rodríguez, A.; Montero, G.; Acuña, A. Dynamic simulation of an absorption cooling system with differentworking mixtures. Energies 2018, 11, 259. [Google Scholar] [CrossRef]
- Moreno-Quintanar, G.; Rivera, W.; Best, R. Development of a Aolar Intermittent Refrigeration System for Ice Production. In Proceedings of the World Renewable Energy Congress-Sweden, Linköping, Sweden, 8–13 May 2011; Linköping University Electronic Press: Linköping, Sweden, 2011; Volume 57, pp. 4033–4040. [Google Scholar] [CrossRef]
- Neyer, D.; Ostheimer, M.; Hauer, N.; Halmdienst, C.; Pink, W. Application of an adapted single-/half-effect NH3/H2O absorption chiller in tri-generation and solar cooling systems. Sol. Energy 2018, 173, 715–727. [Google Scholar] [CrossRef]
- Mirl, N.; Doil, M.; Spindler, K.; Stergiaropoulos, K. Comparison of ammonia/water equations of state under operating conditions of absorption systems. Fluid Phase Equilib. 2020, 526, 112748. [Google Scholar] [CrossRef]
- Garimella, S.; Ponkala, M.J.; Goyal, A.; Staedter, M.A. Waste-heat driven ammonia-water absorption chiller for severe ambient operation. Appl. Therm. Eng. 2019, 154, 442–449. [Google Scholar] [CrossRef]
- Zacarías, A.; Venegas, M.; Ventas, R.; Lecuona, A. Experimental assessment of ammonia adiabatic absorption into ammonia-lithium nitrate solution using a flat fan nozzle. Appl. Therm. Eng. 2011, 31, 3569–3579. [Google Scholar] [CrossRef]
- Zacarías, A.; Venegas, M.; Lecuona, A.; Ventas, R. Experimental evaluation of ammonia adiabatic absorption into ammonia-lithium nitrate solution using a fog jet nozzle. Appl. Therm. Eng. 2013, 50, 781–790. [Google Scholar] [CrossRef]
- Salavera, D.; Libotean, S.; Patil, K.R.; Esteve, X.; Coronas, A. Densities and heat capacities of the ammonia plus water plus NaOH and ammonia plus water plus KOH solutions. J. Chem. Eng. Data 2006, 51, 1020–1025. [Google Scholar] [CrossRef]
- Táboas, F.; Bourouis, M.; Vallès, M. Analysis of ammonia/water and ammonia/salt mixture absorption cycles for refrigeration purposes in fishing ships. Appl. Therm. Eng. 2014, 66, 603–611. [Google Scholar] [CrossRef]
- Villa, A.A.O.; Dutra, J.C.C.; Guerrero, J.R.H.; Dos Santos, C.A.C.; da Costa, J.Â.P. Dynamic experimental analysis of a LiBr/H2O single effect absorption chiller with nominal capacity of 35 kW of cooling. Acta Sci. Technol. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Kohlenbach, P.; Ziegler, F. A dynamic simulation model for transient absorption chiller performance. Part II: Numerical results and experimental verification. Int. J. Refrig. 2008, 31, 226–233. [Google Scholar] [CrossRef]
- Al-Falahi, A.; Alobaid, F.; Epple, B. Design and Thermo-Economic Comparisons of an Absorption Air Conditioning System Based on Parabolic Trough and Evacuated Tube Solar Collectors. Energies 2020, 13, 3198. [Google Scholar] [CrossRef]
- Oronel, C.; Amaris, C.; Bourouis, M.; Vallès, M. Heat and mass transfer in a bubble plate absorber with NH3/LiNO3 and NH3/(LiNO3 + H2O) mixtures. Int. J. Therm. Sci. 2013, 63, 105–114. [Google Scholar] [CrossRef]
- Pink Chiller. PC19. Available online: https://www.pink.co.at/inc.download.php?dlf=77 (accessed on 30 November 2020).
- RoburCorp. Available online: http://www.robur.com/ (accessed on 30 November 2020).
- Oronel, C.; Vallès, M.; Bourouis, M.; Coronas, A. Absorption process with ammonia/lithium nitrate in plate heat exchangers for absorption refrigeration systems. Int. Sorption Heat Pump Conf. 2008, 2008, 23–26. [Google Scholar]
- Oronel, C.; Amaris, C.; Bourouis, M.; Vallès, M. Boiling heat transfer in a plate heat exchanger of ammonia based working fluids for absorption refrigeration systems. In Proceedings of the 6th European Thermal Sciences Conference (Eurotherm 2012), Poitiers, France, 4–7 September 2012. [Google Scholar]
- Cuenca, Y.; Salavera, D.; Vernet, A.; Vallès, M. Thermal conductivity of ammonia + water mixtures over a wide range of concentrations. Int. J. Refrig. 2013, 36, 998–1003. [Google Scholar] [CrossRef]
- Braimakis, K. Solar ejector cooling systems: A review. Renewable 2020, 164, 566–602. [Google Scholar] [CrossRef]
- de Novaes Pires Leite, G.; Weschenfelder, F.; Araújo, A.M.; Villa Ochoa, Á.A.; da Franca Prestrelo Neto, N.; Kraj, A. An economic analysis of the integration between air-conditioning and solar photovoltaic systems. Energy Convers. Manag. 2019, 185, 836–849. [Google Scholar] [CrossRef]
- Lima, T.P.; Dutra, J.C.C.; Primo, A.R.M.; Rohatgi, J.; Ochoa, A.A.V. Solar water heating for a hospital laundry: A case study. Sol. Energy 2015, 122. [Google Scholar] [CrossRef]
- Weschenfelder, F.; de Novaes Pires Leite, G.; Araújo da Costa, A.C.; de Castro Vilela, O.; Ribeiro, C.M.; Villa Ochoa, A.A.; Araújo, A.M. A review on the complementarity between grid-connected solar and wind power systems. J. Clean. Prod. 2020, 257, 120617. [Google Scholar] [CrossRef]
- Rivera, W.; Sánchez-Sánchez, K.; Hernández-Magallanes, J.A.; Jiménez-García, J.C.; Pacheco, A. Modeling of novel thermodynamic cycles to produce power and cooling simultaneously. Processes 2020, 8, 320. [Google Scholar] [CrossRef]
- Ochoa, A.A.; Dutra, J.C.C.; Henríquez, J.R.; dos Santos, C.A.C. Techno-economic and Exergoeconomic Analysis of a micro cogeneration system for a residential use. Acta Sci. Technol. 2016, 38, 327. [Google Scholar] [CrossRef][Green Version]
- Lu, Z.S.; Wang, R.Z. Experimental performance investigation of small solar air-conditioning systems with different kinds of collectors and chillers. Sol. Energy 2014, 110, 7–14. [Google Scholar] [CrossRef]
- Hernández-Magallanes, J.A.; Rivera, W.; Coronas, A. Comparison of single and double stage absorption and resorption heat transformers operating with the ammonia-lithium nitrate mixture. Appl. Therm. Eng. 2017, 125, 53–68. [Google Scholar] [CrossRef]
- Boman, D.B.; Hoysall, D.C.; Staedter, M.A.; Goyal, A.; Ponkala, M.J.; Garimella, S. Méthode de comparaison des couples de fluides actifs dans les pompes à chaleur à absorption. Int. J. Refrig. 2017, 77, 149–175. [Google Scholar] [CrossRef]
- Lu, D.; Bai, Y.; Zhao, Y.; Dong, X.; Gong, M.; Luo, E.; Chen, G.; Xu, Q.; Shen, J. Experimental investigations of an absorption heat pump prototype with intermediate process for residential district heating. Energy Convers. Manag. 2020, 204, 112323. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).