1. Introduction
Thermal drying plays a crucial role in several industries, such as chemical, pharmaceutical, agricultural, and food production. It involves heating a wet product to evaporate its liquid fraction and generating a thermally induced mass flux [
1]. According to the dominant energy transfer mechanism, thermal drying can be categorized into convective [
2], conductive [
3], and radiative drying [
4]. The present study focuses on convective drying operated as a continuous process on a horizontal fluidized bed (
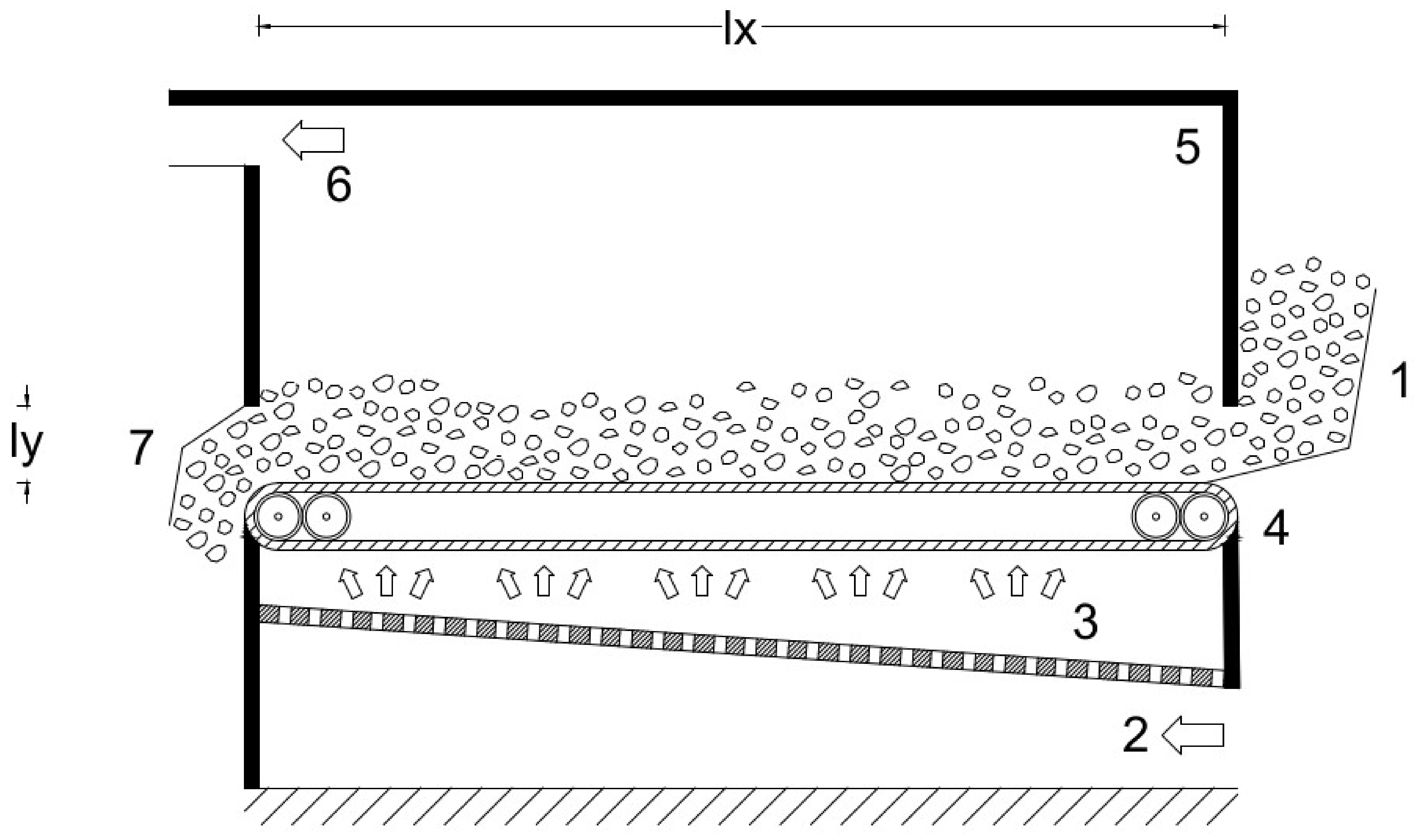
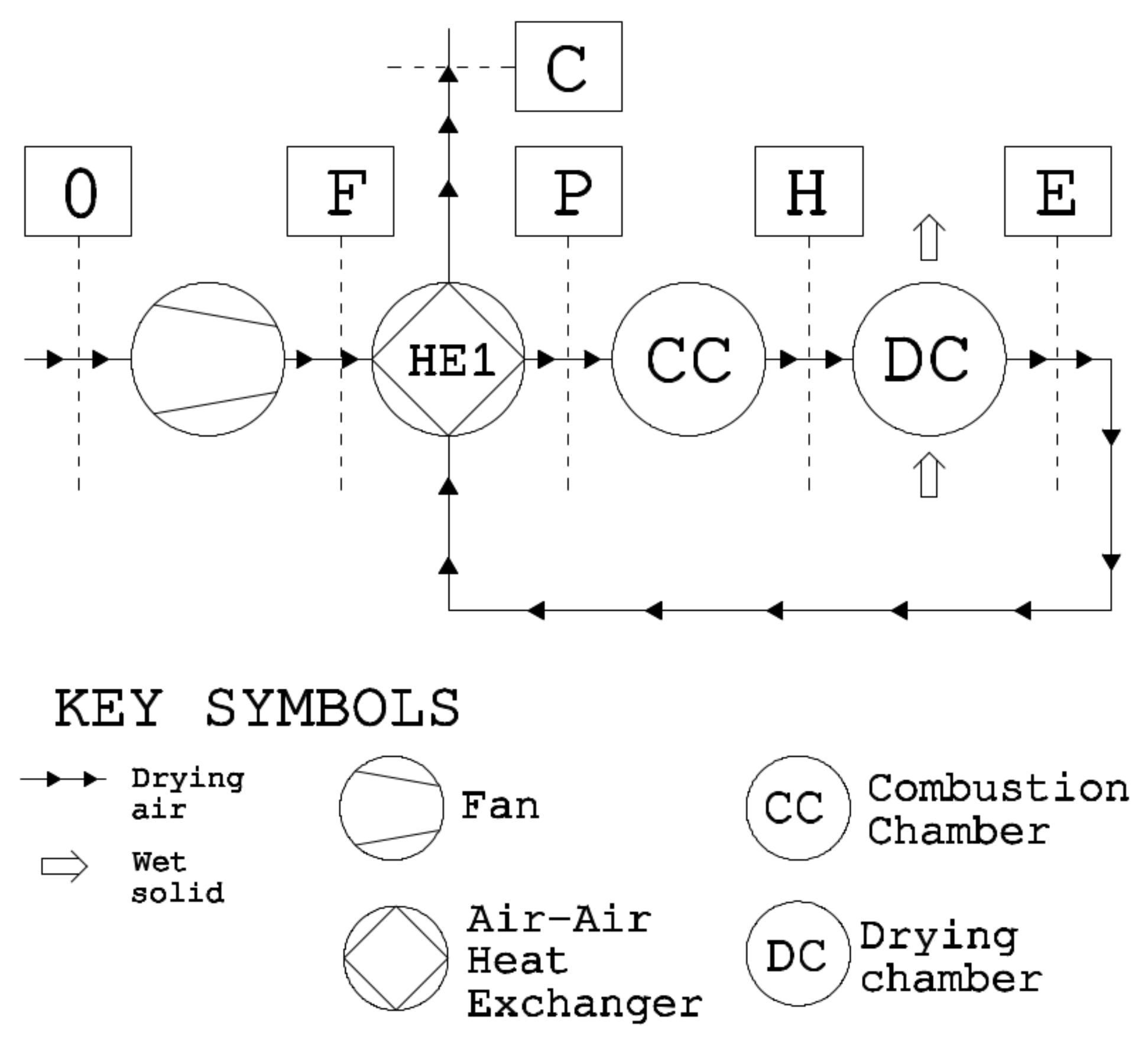
Figure 1).
The fluidized bed systems present several advantages as a good mixing quality (homogeneous temperature distribution along the bed) and high heat and mass transfer rate caused by the extended contact surface between the solid particles, and the gaseous phase [
5,
6]. However, reaching the optimal thermodynamic performance of such systems needs careful tuning of numerous operating parameters that influence the final energy consumption, product quality, and environmental effects.
For example, the finest particles (powder) tend to agglomerate, affecting the evaporation rate and quality of the final product [
7]. This effect increases the bed pressure drop; thus, a faster airflow becomes necessary to preserve product quality, but it increases the final energy consumption, and costs [
8]. Further limitations of the global performance derive from the unavoidable inefficiencies of the process: the sensible heating of the dry fraction, the heat losses of the drying chamber, and the low thermal conductivity of the heat transfer media (air), which demands high operative temperatures to drive adequate heat flux [
9]. Another issue regards the effect of the environmental conditions on the final energy use: the analysis of Reference [
10] reveal the drying chamber as particularly susceptible to the external temperature; its performance deteriorate with temperature fluctuations of approx. 5
C. Finally, some specific applications such as the convective drying of biomass and hazardous materials, release several pollutants into the atmosphere; this deteriorates local air quality and contributes to greenhouses gas emissions [
11,
12,
13]. For such reasons, stringent environmental regulations limit their functioning.
In the design and optimization practice, predicting all the effects mentioned above can be challenging because of the relations among the drying parameters and the mutual dependencies among the system components [
14]. The standard approach studies the relation between the drying conditions and final performance by the energy analysis of the drying chamber. Dincer et al. [
15] calculated the efficiency of the cycle (i.e., drying efficiency) as the ratio between the energy invested in the evaporation process over the total energy entering the drying chamber by hot airflow. Further studies [
16] included the specific energy consumption, calculated as the amount of consumed energy per unit mass of the evaporated moisture.
Energy analysis by itself cannot calculate the operative costs and any form of environmental effect because it does not distinguish the primary energy source; such an approach does not provide any information regarding the effects of the climate on system productivity. To overcome these limitations, several authors included the exergy analysis in the evaluation of system performances.
The first exergy analysis of convective drying is presented in Reference [
17], wherein the authors calculate the exergy efficiency of the drying chamber as the ratio between the exergy invested in the evaporation process to the total exergy of the entering airflow. Assuming the entering product is at a dead-state, the exergy investment is the exergy of the total evaporated moisture leaving the drying chamber. Several works follow the approaches presented above. Akpinar et al. [
18] related the energy utilization of the drying chamber with an evaporation rate and initial product moisture; they measured an increase in exergy losses using the drying air temperature and velocity. Yogendrasasidhar et al. [
19] analyzed the effect of the several drying parameters on the energy utilization, exergy losses, and exergy efficiency of the system: by increasing the wall temperature and air velocity, the energy usage augments but the exergy losses decreases with benefits on the final exergy efficiency; the prolonging of drying time produces a two-fold benefit by reducing energy usage and exergy losses and by increasing exergy efficiency. Aviara et al. [
20] showed a linear dependence between energy efficiency and the drying air temperature.
More recent research on convective drying oriented the exergy analysis toward optimization by comparing different system configurations and operative conditions. Icier et al. [
21] compared the exergy performance of two open drying cycles and a closed cycle, where drying air was recovered from the drying chamber and heated by a heat pump. Xiang et al. [
22] analyzed a drying system coupled to a heat pump; in particular, they investigated how the system performance changes under different operative conditions, varying the amount of recirculating air (from an open to a fully-closed cycle). Cay et al. [
23,
24] analyzed and compared two open cycles, in which internal and external combustion chambers heat the drying air, respectively. Erbay [
10], and Gungor [
25] investigated how dead-state temperature affects the exergy performance of a drying system fed by a ground source and a gas heat pump.
All studies presented above refer to the given system configurations; their results are valid only under the working conditions observed for the analyzed system and can be used for designing the drying cycle of a specific material. Furthermore, limiting the performance analysis to the drying chamber misses the most recent and accurate optimization techniques of energy systems that extend the exergy analysis to a multicomponent level (they calculate the exergy destruction in a single component by considering interdependencies with all other components) [
26,
27]. Thus, the research gap in the current literature is based on the design approach specifically formulated for convective drying, which considers the effects of changing the system configuration and operative conditions (e.g., dry another product, adding another component, and varying the climate) for the optimization of thermodynamics and costs [
28].
The design of a drying system needs a theoretical model to calculate the state of working flows in the different components (i.e., thermodynamic equilibrium model). The Euler–Euler description is a common approach to simulate the thermodynamic equilibrium of a drying process due to its low computational costs and the capability to simulate fluids with a high concentration of the dispersed phase [
29,
30,
31]. Assari et al. [
32,
33] studied the drying of wheat grain: first, they formulated a two-fluid model to investigate the effects of varying the operative conditions on the main operative parameters of the drying bed (e.g., void percentage and air humidity); in the following study, they analyzed the exergy performance. Li et al. [
34] modeled two fluids, the bubbly and emulsion phases, a mixture of an interstitial gas, and a solid phase; using this formulation, they performed a sensitivity analysis of the drying performance with respect to the state of inlet air, particle diameter, and wall temperature of the drying chamber. Ranjbaran et al. [
35] developed a two-fluid model for paddy drying; they investigated the temporal variation of the energy and exergy efficiency during the drying process with effects of air temperature and flow rate; a source term in transport equations is used to model the evaporation of moisture. Rosli et al. [
36] simulate the drying of sago waste by a two-fluid model of the drying column; they investigate by CFD the effects of different drying conditions (e.g., the air velocity, temperature, and particle size) on the fluidization of the bed. Jang et al. [
37] simulate a fluidized bed dryer by an Euler-Euler model coupled to empirical correlations representing the inter-phase exchanges; the authors investigate the advantages of such a model in the design and scaling-up of pharmaceutical applications.
The above studies simulated the drying process by two homogeneous phases—the drying air and the wet solid—without focusing on their chemical composition. A multispecies approach enhances the versatility and accuracy of the two-fluids theory—it comprehends the effects of each species on the mass and energy fluxes [
38]. Furthermore, this approach can simulate applications of reactive flows according to the stoichiometry of the ongoing chemical reactions (e.g., combustion of a hydrocarbon for air heating). The multicomponent theory generally finds applications in petroleum distillation [
39,
40], while the available literature lacks references for applications to convective drying.
In addition to the thermodynamic equilibrium model, the design of a drying system needs characteristic equations describing the heat and mass transfer phenomena that occur in each component; as an example, the characteristic equation of the drying chamber is the evaporation model. Defraeye et al. [
41] derive the characteristic equation of drying by a theoretical approach—the authors analyze the convective drying of a porous flat plate by solving the transport phenomena at the interface between the porous media and the airflow, explicitly. Such an approach, known as conjugate modeling, describes in detail the physics of the heat and mass transfer; however, despite its accuracy, just a few academic applications use this technique because of the high complexity, and computational costs [
30]. Quite the opposite, the empirical or non-conjugated models derive from experimental observations and describe the heat and mass transfer by constant coefficients, with a limited understanding of the involved physics. Some well-known examples of empirical models are Newton’s law of cooling, and the evaporation model of Page [
42].
Our study investigates the thermodynamic performance of convective drying under different operating conditions and system configurations; the final aim is distinguishing the parameters with the most significant effects on the energy use and product quality to define the setup with the best performance. We follow a theoretical approach, named off-design analysis applied successfully for the optimization and control strategies of various energy systems [
43,
44,
45]. The novelty of our work is the nature of the studied application: in the literature, there is not any off-design analysis of convective drying; in particular, the current design practice lacks a theoretical formulation for modeling the state of working flows in the drying chamber and all system components, including the devices for heat and mass recovery. The level of detail of our theoretical approach is a further innovation in the field of drying modeling: we formulate the two-fluids theory describing the thermodynamic equilibrium of the single chemical species to simulate all components of the drying system. Finally, due to the adaptability of the solving algorithm, we present an innovative tool for both design new drying cycles and verify the states of working flows in existing systems.
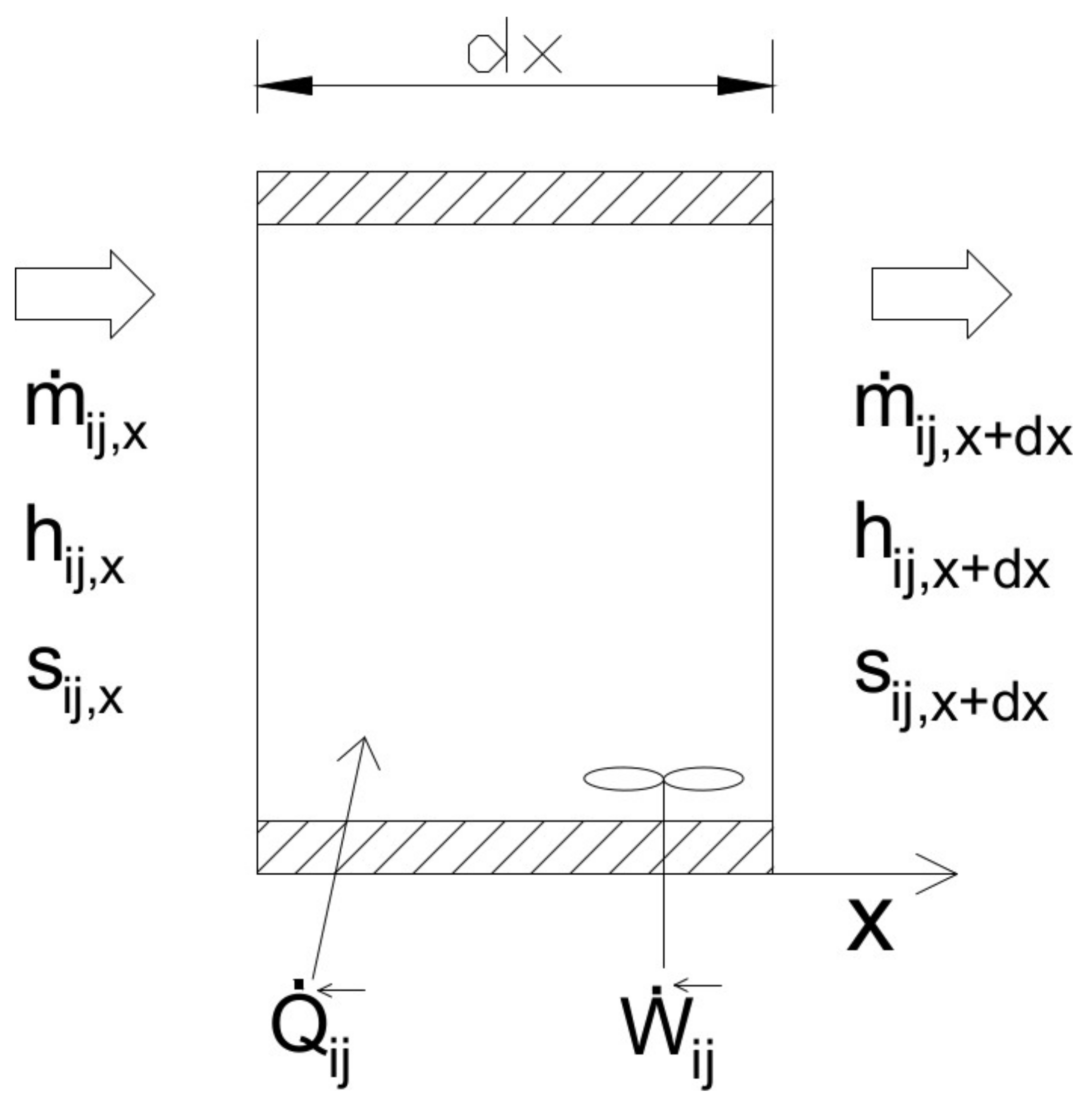
The presented equilibrium model includes the multispecies approach in the two-fluid theory to simulate all the components of a drying system; in particular, we follow a two-fluid multispecies Euler–Euler (TFMM) approach to simulate the following processes.
Mass and energy exchange between nonreactive phases: Convective drying of a wet solid and air-water counter-current mixing;
Mass and energy exchange between reactive phases: combustion of airflow by a hydrocarbon jet;
Energy exchange between nonreactive phases: Air-to-air and air-to-water heat exchange within a tube bank.
Our analysis aims to support the design practice of a drying system rather than investigate drying physics in detail. Thus, we simulate heat and mass exchanges by empirical equations because of their appropriate accuracy for the scale of simulated processes (macro-scale between 0.01–10 m [
30]), as well as the advantages of a simple mathematical formulation and low computational costs.
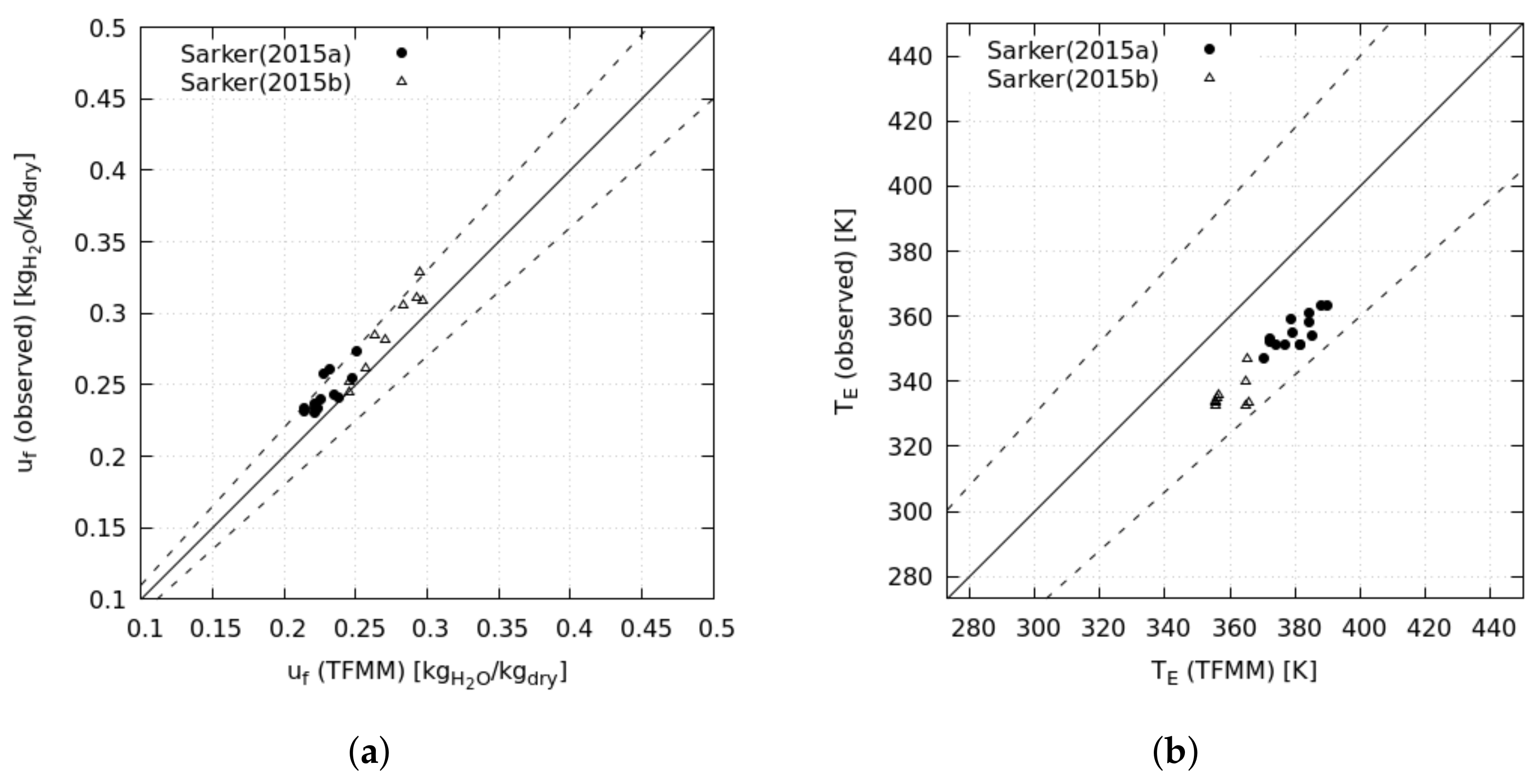
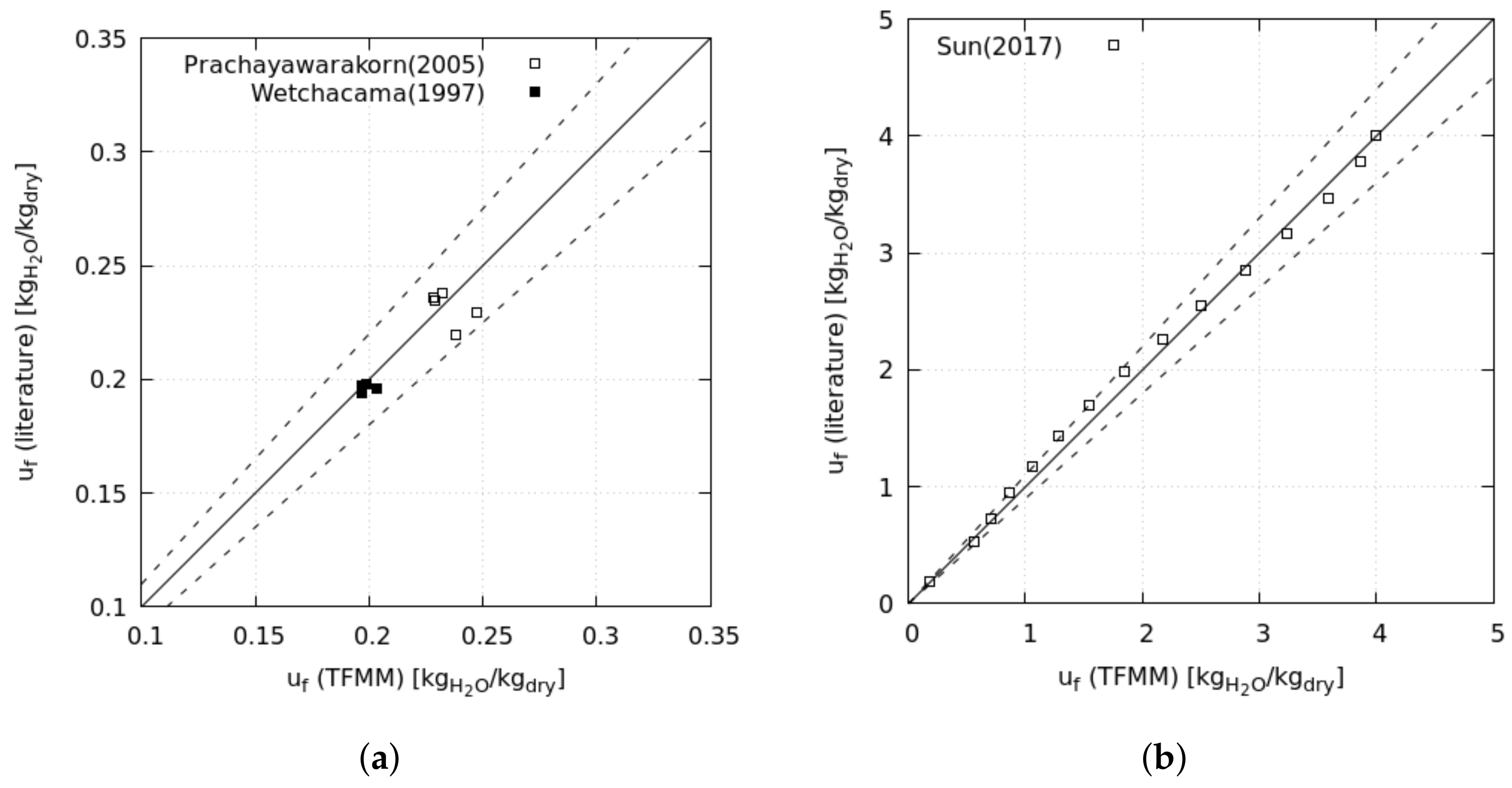
The analysis starts with the exergy analysis of a reference drying cycle, named the baseline scenario, based on an existing industrial system; this scenario mounts the essential components of convective drying: fan, combustion chamber, and drying chamber (
Figure 1). We calculate the baseline performance at the operative conditions of the existing system and validate the results against experimental data. Later, we change the drying conditions, dead-state conditions, and dried material to investigate the effects on energy consumption and exergy efficiency. By the last set of operative conditions, we assess the drying of municipal sewage sludge, thereby aiming to contribute to this crucial but scarcely investigated sector. Finally, we modified the reference system to investigate the effects on the global performance of heat and mass recovery by three different layouts.
7. Conclusions
The performance of convective drying systems depends on a wide range of interrelated operating parameters that affect the energy use, exergy efficiency, dimensions (i.e., costs) of system components, and product quality. We proposed a methodology that calculates the system performance considering exogenous variables (climatic conditions, initial product moisture, and physical properties of the dried product), energy\mass intake, and cycle configuration.
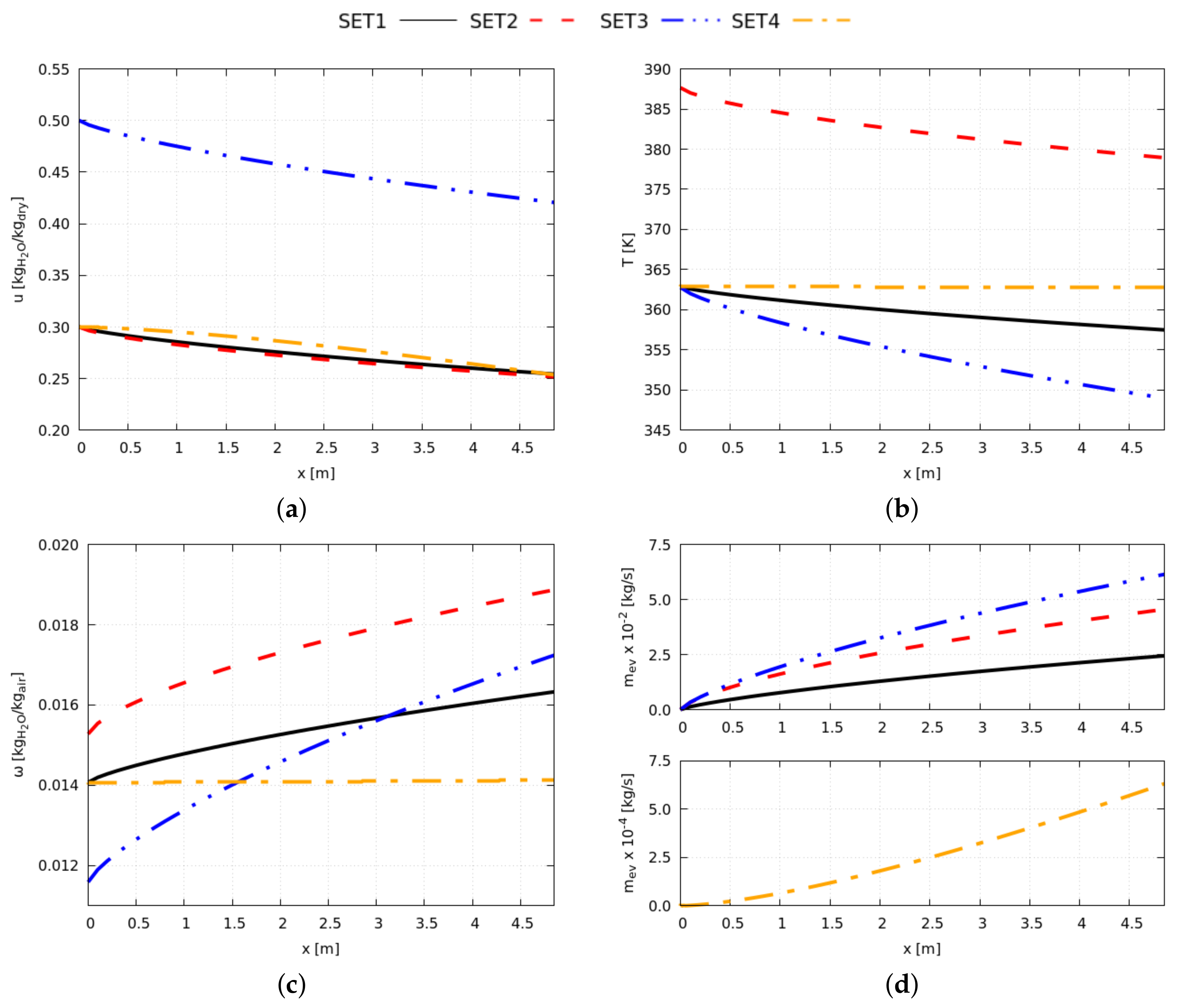
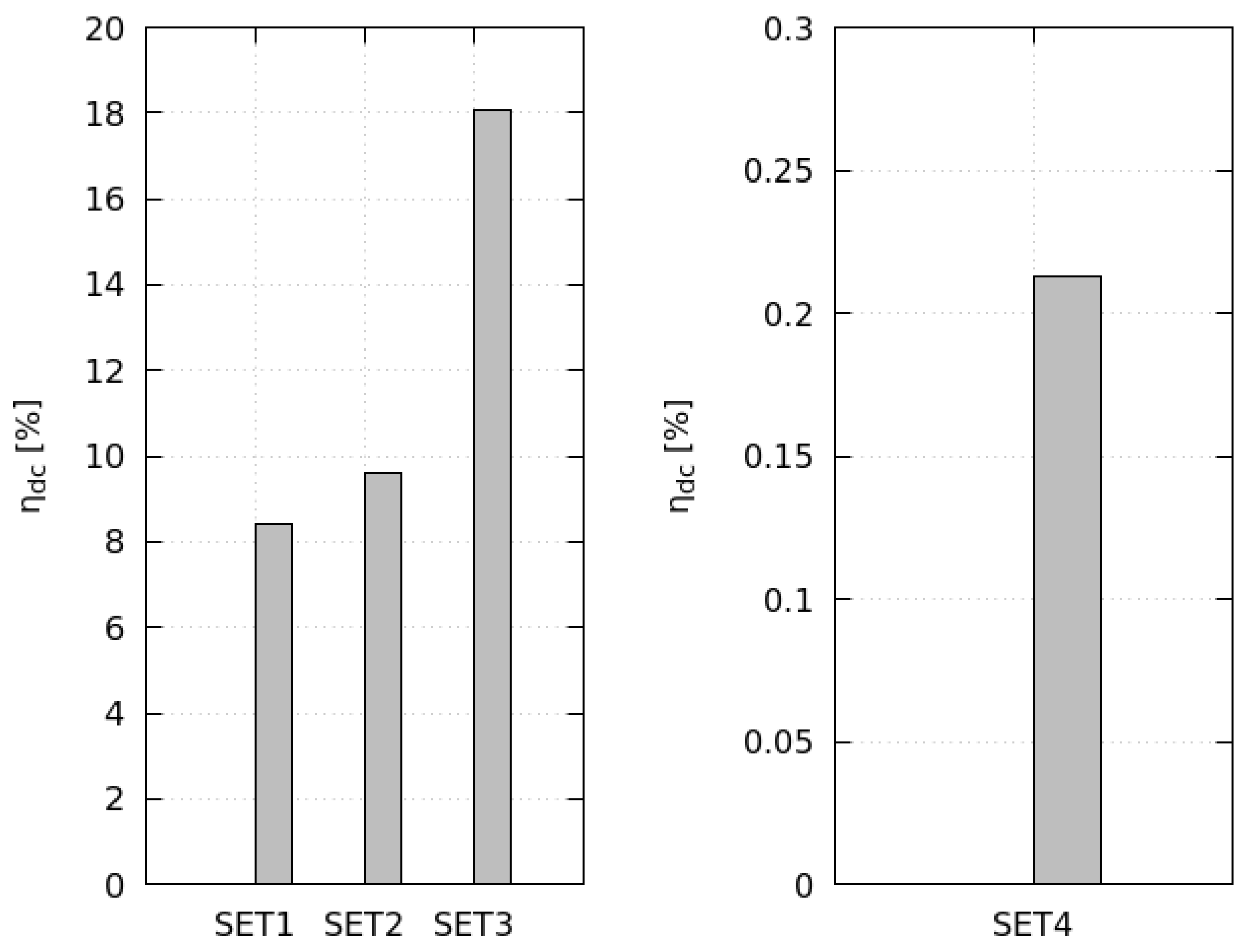
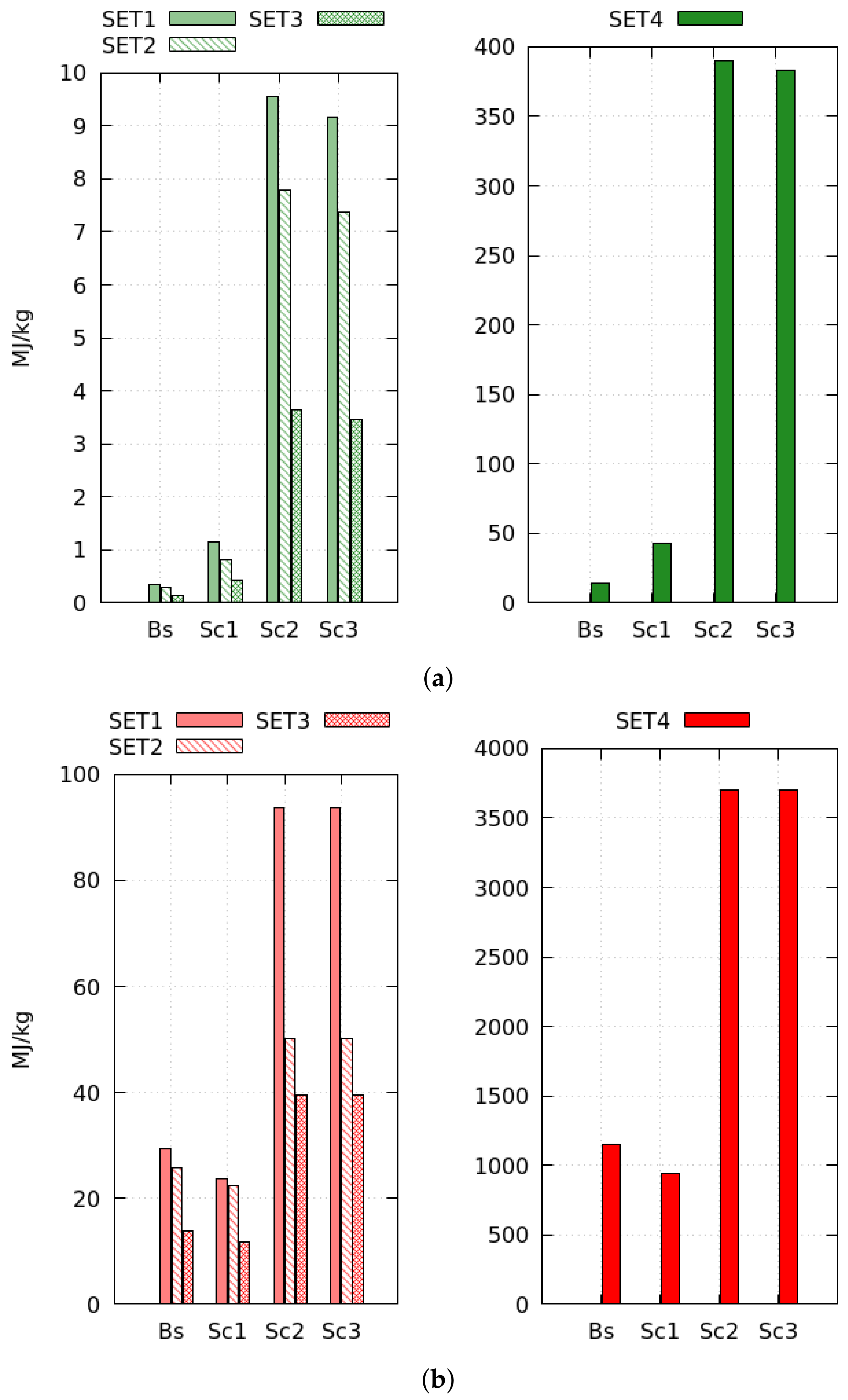
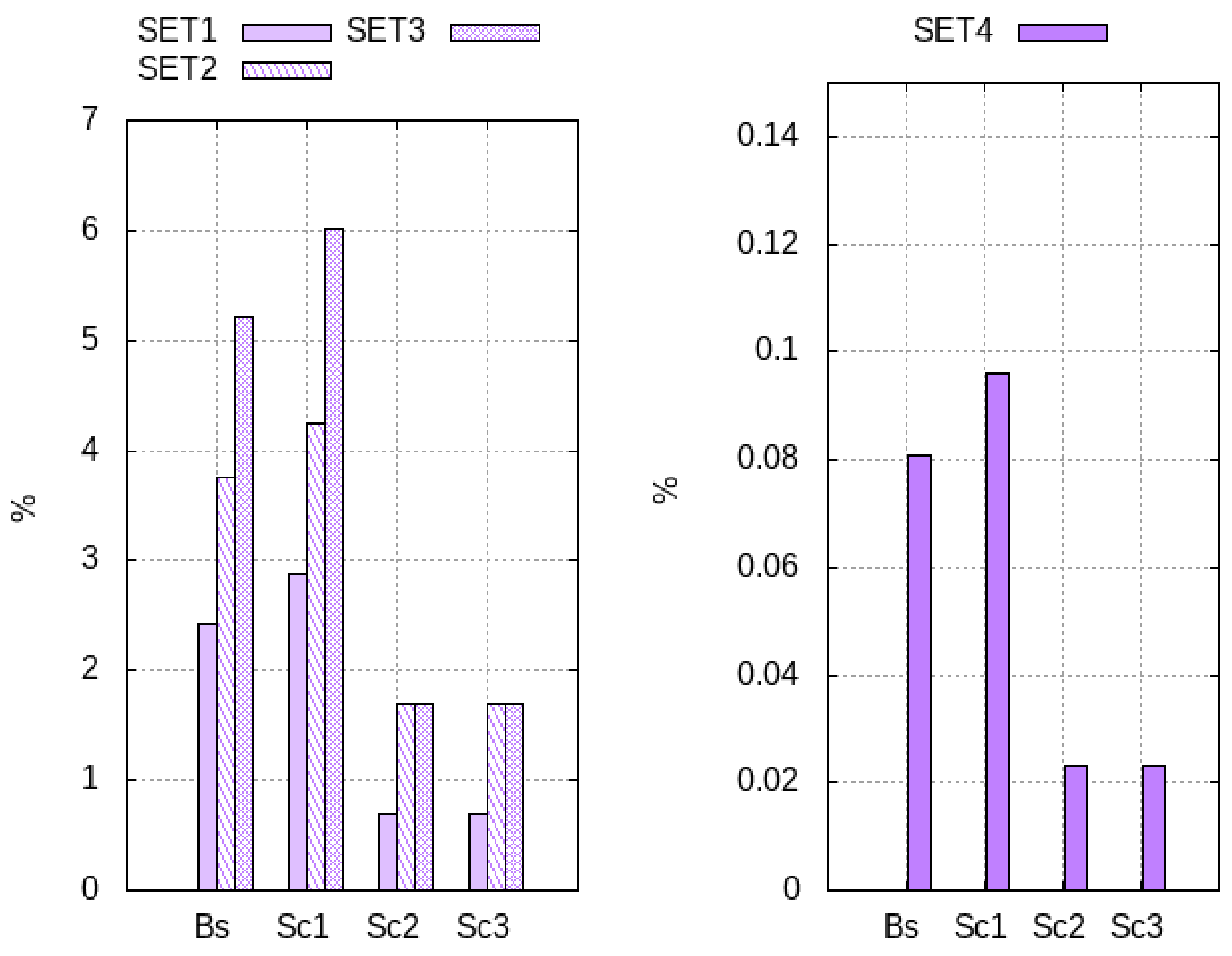
On comparing the results of different to the reference , the evaporation rate showed the most significant effect on the global performance because it determines the energy and exergy productively invested in the drying process. When increasing heating and flowing loads (), the system evaporates a deeper moisture layer with benefits on the exergy efficiency (+97%). Higher initial product moisture () augments the evaporation rate, and the drying cycles presents the best performance in terms of drying efficiency (+114%) and exergy efficiency (+127%). When drying the MSS (), the evaporation rate reduces by approximately times, worsening the exergy performance (−97%); such results reveal the dimensions of the drying bed inadequate to dry that particular product.
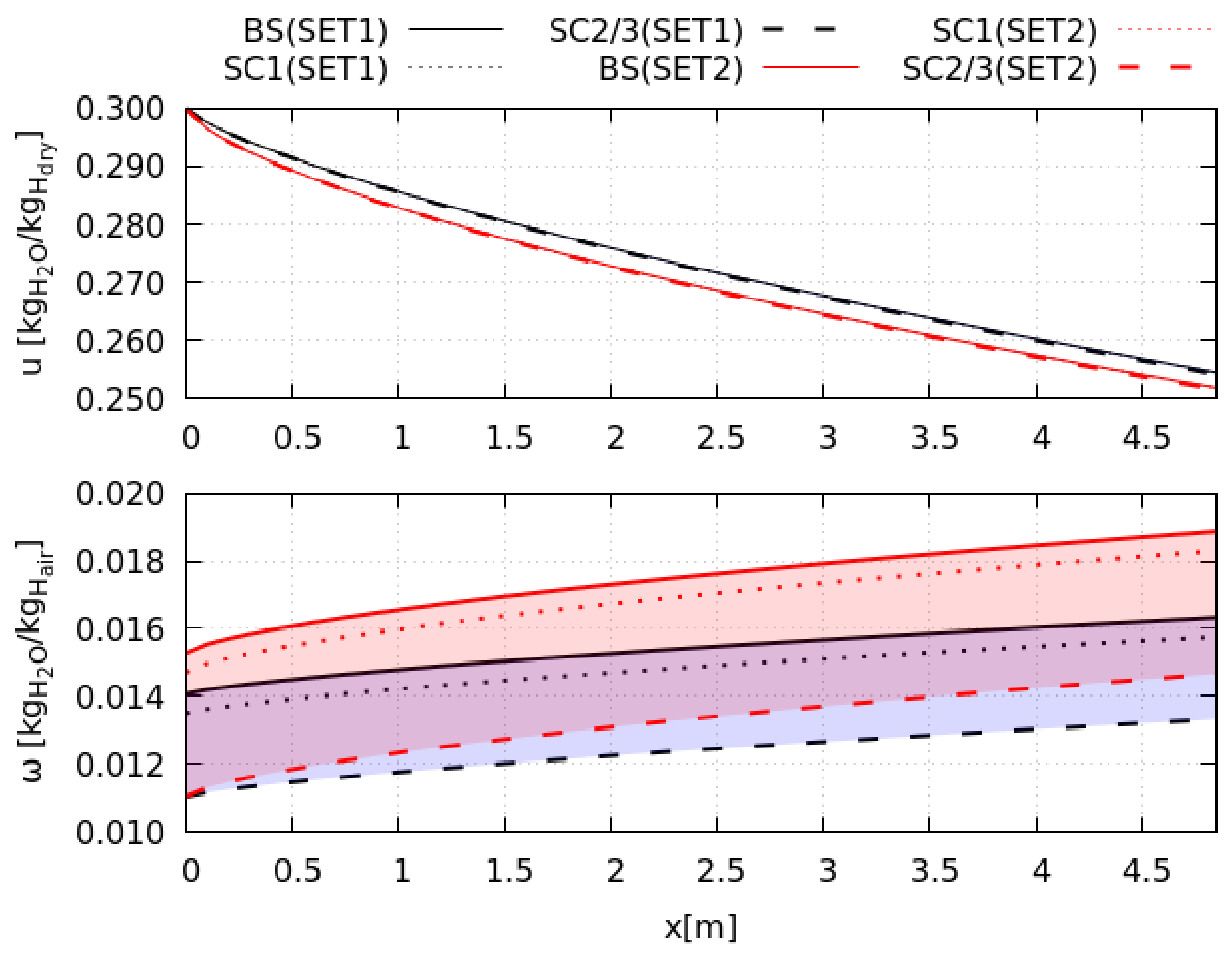
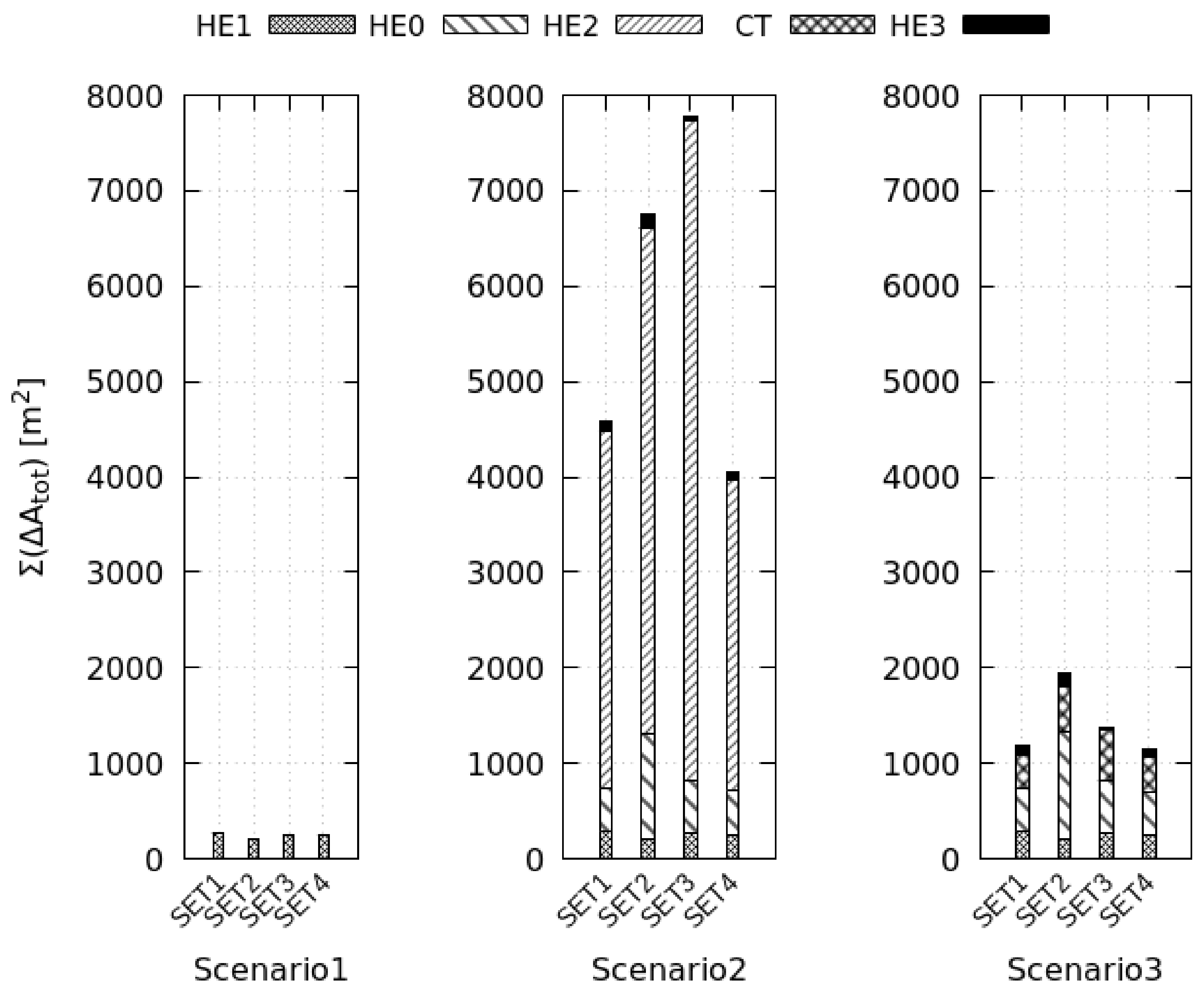
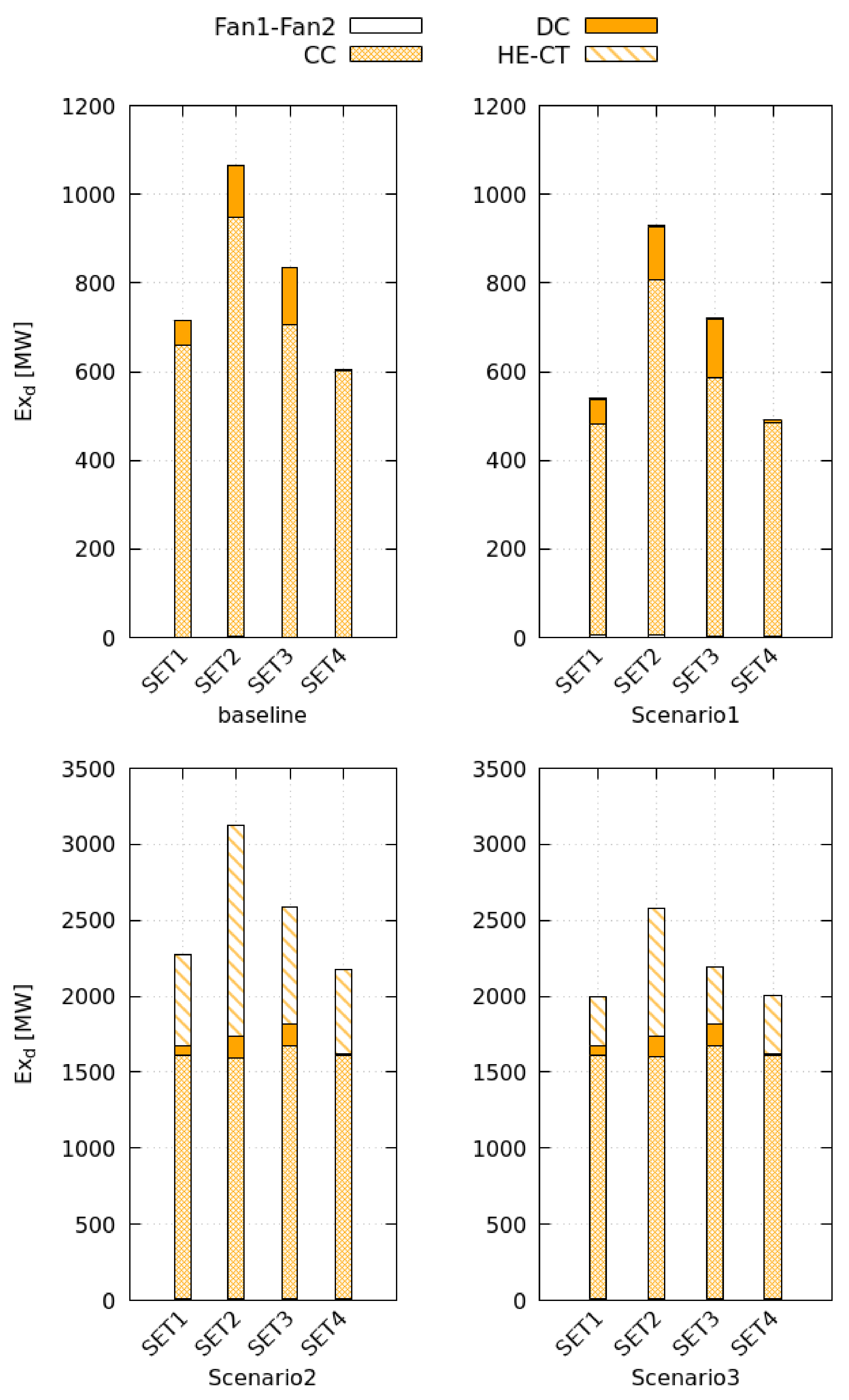
Scenario 1 is the optimal system configuration. The heat-recovery increases the baseline electrical consumption and reduces the thermal consumption by maximum +211% and −17%, respectively, with benefits on the exergy efficiency (+17%). The efficiency of heat recovery depends on the wasted energy; at low drying efficiency, or when the fan dissipates more shaft work, the HE1 fulfills the production target reducing the exchange surface by 25%. Thus, the heat-recovery is more convenient in low efficiency processes when the material is difficult to dry.
Closed cycles cut the exergy efficiency by maximum −67%. These systems use four additional heat exchangers increasing the electric consumption by 20 times. Moreover, external combustion and air regeneration processes augment the thermal energy needs (+180%). The performances of Scenario 3 are slightly better than that of Scenario 2 because the tower presents a faster cooling-rate than HE2, especially in the saturation step. This process occurs almost instantaneously in the tower that reaches the target cooling conditions with a 10 times smaller surface than HE2. Furthermore, when the saturation step becomes shorter, the total exergy destruction rate diminishes (−48%), and its spatial distribution tends to become more uniform (−39%). Thus, the tower reduces system irreversibility.
Based on the above results, we derive some technical recommendations that can help the designer optimize the energy and exergy use of a convective drying system.
The performance of the system varies along the climatic year because of the variations of the initial temperature and humidity of the working flows (air intake and processed product). However, the designer can ensure the production targets are unaltered by adjusting the energy input with benefits on the exergy efficiency. Best performances are observed in the cold season.
Operating conditions shall be oriented to maximize the evaporation rate; the increasing thermal loads is valid to this purpose; however, it is limited by some adverse effects as the depletion of the product quality and the humidification of drying air by combustion. As an alternative, the designer can augment the dimensions of the drying bed and extend the residence time of the processed product in the drying chamber.
When the bed cannot be enlarged, the drying efficiency is low, and a heat recovery unit can reuse a fraction of the heat wasted by the drying chamber to preheat the external air intake; this practice is particularly advantageous in high-temperature processes where small units significantly increase energy and exergy efficiency.
Air recirculation dramatically reduces the performance of the system because of air regeneration processes. More than an optimization practice, the cycle closure can be a safety procedure for drying hazardous materials and limit the emissions of harmful substances in the environment. The cooling tower is particularly suitable for this purpose; compared to an indirect cooling system, it presents the highest energy and exergy efficiency and is configurable as a wet scrubber to wash away toxic species from the airflow and restore its initial conditions.
Future developments will overcome the limitations of the current work. Using a real gas model (e.g., Van der Walls), the TFMM can simulate the compression and throttle of a refrigerant fluid and predict the performances of a drying cycle driven by the heat pump, thereby promising remarkable enhancement of the exergy efficiency caused by the low-temperature of the heat generation process. Finally, the coupling of the TFMM to an analytical model (e.g., upscaled porosity model) will increase the accuracy of TFMM to describe the drying phenomenology and simulate the process at a higher level of detail.