Determining the Heat of Fusion and Specific Heat of Microencapsulated Phase Change Material Slurry by Thermal Delay Method
Abstract
1. Introduction
1.1. Microencapsulated PCM Slurry as a Latent Functional Thermal Fluid
1.2. Specific Heat and Heat of Fusion of mPCM Slurry—A Previous Study
2. Materials and Methods
2.1. Slurry Properties
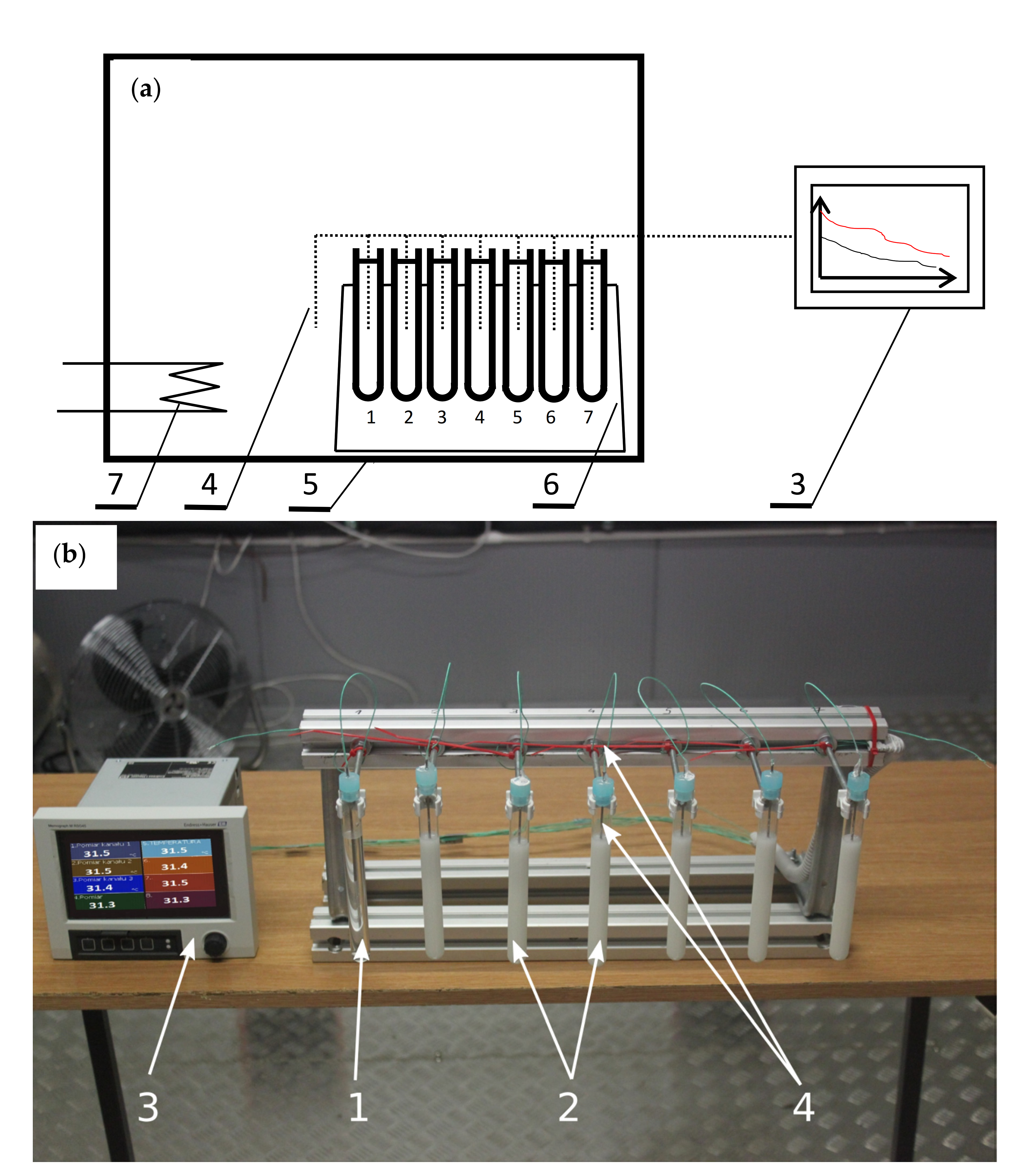
2.2. Experimental Set-Up
2.3. Test Conditions and Procedure
3. Results and Discussion
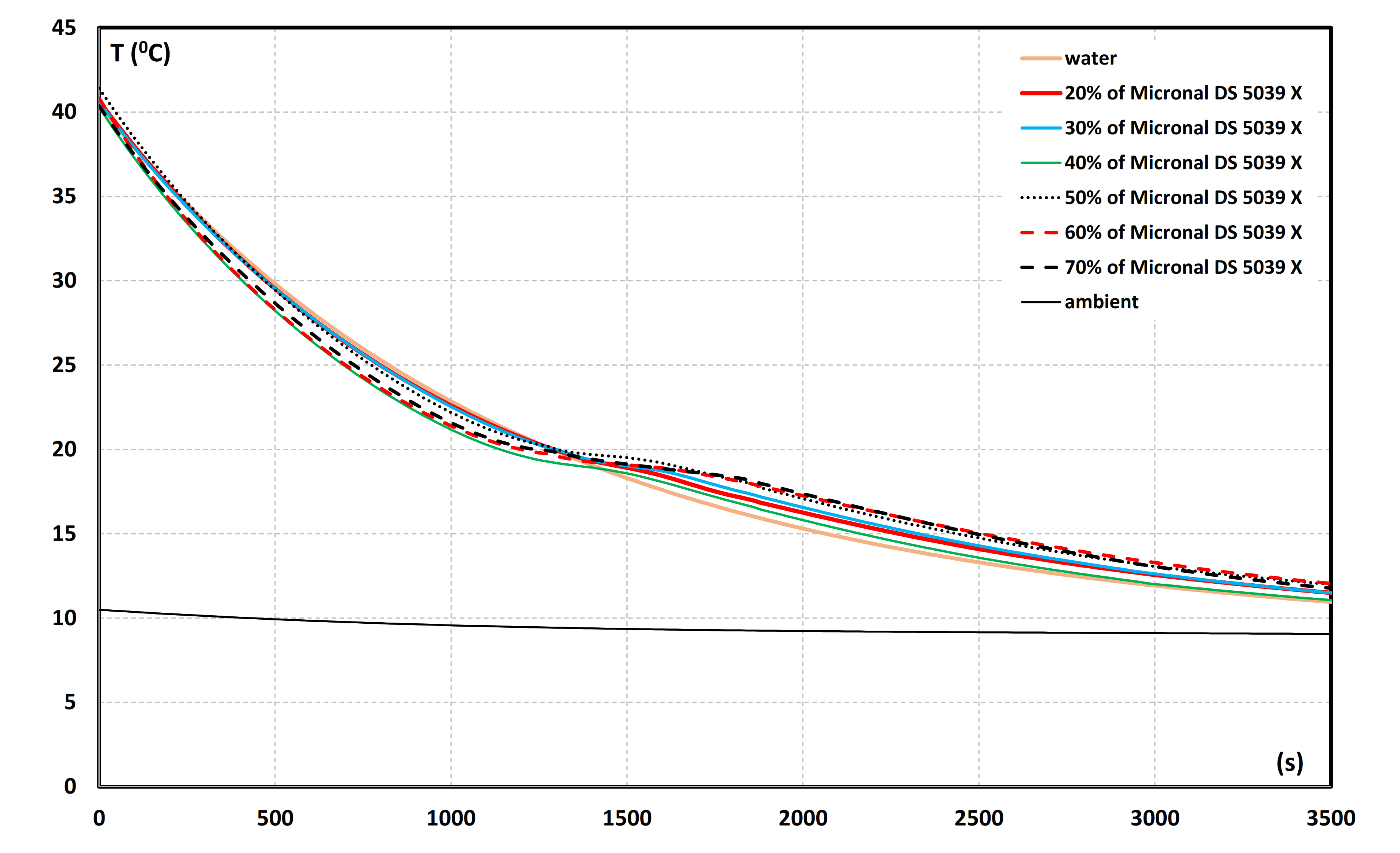
3.1. T-History Cooling Curves for the mPCM Slurries
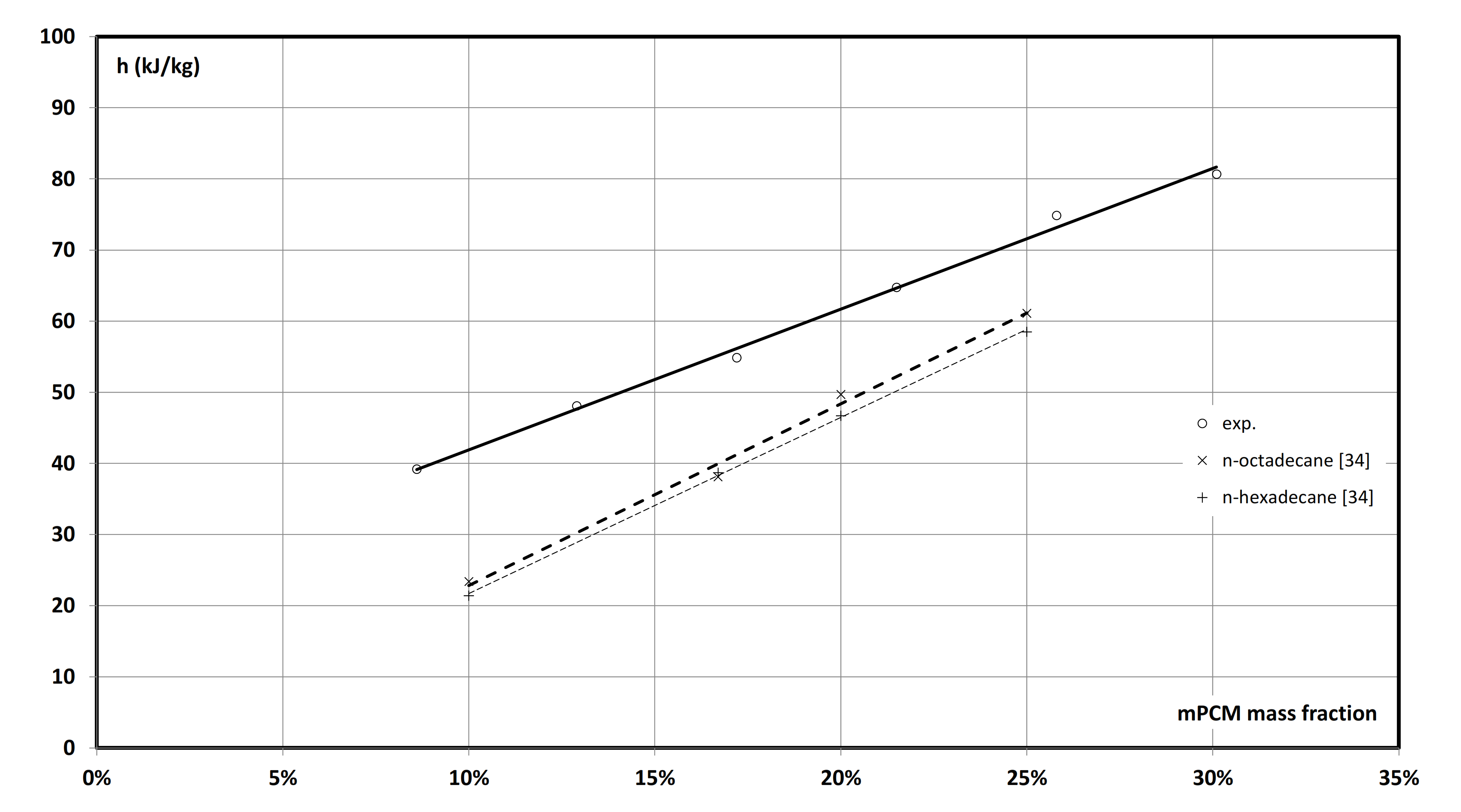
3.2. Specific Heat and Latent Heat of mPCM Slurries
4. Summary and Conclusions
- Phase transition of PCM microencapsulated (analogous to PCM emulsions) may occur at a lower temperature, depending on the rate of cooling of the sample. The value of the main peak crystallization temperature of mPCM slurry was about 18.5 °C.
- An increase in the concentration of microcapsules caused a proportional increase in specific heat in the main peak melting temperature of mPCM slurry. The value of the maximum specific heat changed from 9.2 kJ/(kgK) to 33.7 kJ/(kgK) respectively for 8.6 wt%, and 30.1 wt% of mPCM.
- The specific heat of the slurry when PCM in microcapsules is in liquid form is lower than the specific heat of the slurry when PCM in microcapsules is in liquid form.
- Specific heat of the slurry (when PCM in microcapsules is in liquid form) decreased from 4.04 kJ/(kgK) to 3.77 kJ/(kgK) respectively for 8.6 wt% and 30.1 wt% of mPCM.
- The specific heat of the slurry (when the PCM in the microcapsules is in the form of a solid) increased from 4.45 kJ/(kgK) to 5.23 kJ/(kgK) for 8.6 wt%, and 30.1 wt% of mPCM respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Z.; Ma, X.; Li, P.; Zhao, X.; Wright, A. Micro-encapsulated phase change material (MPCM) slurries: Characterization and building applications. Renew. Sustain. Energy Rev. 2016, 77, 246–262. [Google Scholar] [CrossRef]
- Rasta, I.M.; Suamir, I.N. The role of vegetable oil in water based phase change materials for medium temperature refrigeration. J. Energy Storage 2018, 15, 368–378. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Advancement in phase change materials for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2017, 172, 82–92. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Pandey, A.K.; Hossain, M.S.; Tyagi, V.V.; Rahim, N.A.; Selvaraj, J.A.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2017, 82, 281–323. [Google Scholar] [CrossRef]
- Bose, P.; Amirtham, V.A. A review on thermal conductivity enhancement of paraffin wax as latent heat energy storage material. Renew. Sustain. Energy Rev. 2016, 65, 81–100. [Google Scholar] [CrossRef]
- Allouche, Y.; Varga, S.; Bouden, C.; Oliveira, C. Experimental determination of the heat transfer and cold storage characteristics of a microencapsulated phase change material in a horizontal tank. Energy Convers. Manag. 2015, 94, 275–285. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Z.; Wang, J.; Li, Y.; Rao, Z. Experimental research on flow and heat transfer characteristics of latent functional thermal fluid with microencapsulated phase change materials. Int. J. Heat Mass Transf. 2017, 115, 737–742. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Hawlader, M.; Uddin, M.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Fu, W.; Liang, X.; Xie, H.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Thermophysical properties of n -tetradecane@polystyrene-silica composite nanoencapsulated phase change material slurry for cold energy storage. Energy Build. 2017, 136, 26–32. [Google Scholar] [CrossRef]
- Wu, W.; Bostanci, H.; Chow, L.C.; Ding, S.J.; Hong, Y.; Su, M.; Kizito, J.P.; Gschwender, L.; Snyder, C.E. Jet impingement and spray cooling using slurry of nanoencapsulated phase change materials. Int. J. Heat Mass Transf. 2011, 54, 2715–2723. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Bai, Z.Y.; Ye, J. Rheological and energy transport characteristics of a phase change material slurry. Energy 2016, 106, 63–72. [Google Scholar] [CrossRef]
- Kruzel, M.; Bohdal, T.; Sikora, M. Heat transfer and pressure drop during refrigerants condensation in compact heat exchangers. Int. J. Heat Mass Transf. 2020, 161, 120283. [Google Scholar] [CrossRef]
- Bohdal, T.; Charun, H.; Kruzel, M.; Sikora, M. An Investigation of Heat Transfer Coefficient during Refrigerants Condensation in Vertical Pipe Minichannels. In Proceedings of the 17th International Conference Heat Transfer and Renewable Sources of Energy (HTRSE-2018), Międzyzdroje, Poland, 2–5 September 2018. [Google Scholar]
- Kruzel, M.; Bohdal, T. Refrigerant condensation in vertical pipe minichannels under various heat flux density level. Int. J. Heat Mass Transf. 2020, 146. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Sopian, K.; Chaichan, M.T.; Kazem, H.A.; Ibrahim, A.; Mat, S.; Ruslan, M.H. Evaluation of the nanofluid and nano-PCM based photovoltaic thermal (PVT) system: An experimental study. Energy Convers. Manag. 2017, 151, 693–708. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Zhao, X.; Li, P.; Ali, S. Experimental investigation of the energy performance of a novel Micro-encapsulated Phase Change Material (MPCM) slurry based PV/T system. Appl. Energy 2016, 165, 260–271. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.; Lin, Y.; Alva, G.; Fang, G. Performance evaluation of a novel solar photovoltaic–thermal collector with dual channel using microencapsulated phase change slurry as cooling fluid. Energy Convers. Manag. 2017, 145, 30–40. [Google Scholar] [CrossRef]
- Smuga-Kogut, M.; Walendzik, B.; Szymanowska-Powalowska, D.; Kobus-Cisowska, J.; Wojdalski, J.; Wieczorek, M.; Cielecka-Piontek, J. Comparison of Bioethanol Preparation from Triticale Straw Using the Ionic Liquid and Sulfate Methods. Energies 2019, 12, 1155. [Google Scholar] [CrossRef]
- Smuga-Kogut, M.; Wnuk, A.D.; Zgórska, K.; Kubiak, M.S.; Wojdalski, J.; Kupczyk, A.; Szlachta, J.; Luberański, A. Production of ethanol from wheat straw. Pol. J. Chem. Technol. 2015, 17, 89–94. [Google Scholar] [CrossRef]
- Siddiqui, O.K.; Yilbas, B.S.; Shuja, S.Z.; Wang, E. Volumetric solar absorption in a channel with presence of phase change material in a carrier fluid. Appl. Therm. Eng. 2016, 102, 1059–1068. [Google Scholar] [CrossRef]
- Serale, G.; Fabrizio, E.; Perino, M. Design of a low-temperature solar heating system based on a slurry Phase Change Material (PCS). Energy Build. 2015, 106, 44–58. [Google Scholar] [CrossRef]
- Huang, M.J.; Eames, P.C.; McCormack, S.; Griffiths, P.; Hewitt, N.J. Microencapsulated phase change slurries for thermal energy storage in a residential solar energy system. Renew. Energy 2011, 36, 2932–2939. [Google Scholar] [CrossRef]
- Li, L.Y.; Zou, D.; Ma, X.F.; Liu, X.S.; Hu, Z.G.; Guo, J.R.; Zhu, Y.Y. Preparation and flow resistance characteristics of novel microcapsule slurries for engine cooling system. Energy Convers. Manag. 2017, 135, 170–177. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Wang, R. An overview of phase change material slurries: MPCS and CHS. Renew. Sustain. Energy Rev. 2010, 14, 598–614. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, M.; Zhang, D. Testing method of phase change temperature and heat of inorganic high temperature phase change materials. Exp. Therm. Fluid Sci. 2013, 44, 697–707. [Google Scholar] [CrossRef]
- Browne, M.C.; Norton, B.; McCormack, S.J. Heat retention of a photovoltaic/thermal collector with PCM. Sol. Energy 2016, 133, 533–548. [Google Scholar] [CrossRef]
- Yinping, Z.; Yi, J. A simple method, the T-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar] [CrossRef]
- Kravvaritis, E.D.; Antonopoulos, K.A.; Tzivanidis, C. Experimental determination of the effective thermal capacity function and other thermal properties for various phase change materials using the thermal delay method. Appl. Energy 2011, 88, 4459–4469. [Google Scholar] [CrossRef]
- Yang, R.; Xu, H.; Zhang, Y. Preparation, physical property and thermal physical property of phase change microcapsule slurry and phase change emulsion. Sol. Energy Mater. Sol. Cells 2003, 80, 405–416. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107. [Google Scholar] [CrossRef]
- Fischer, L.J.; Von Arx, S.; Wechsler, U.; Züst, S.; Worlitschek, J. Phase change dispersion properties, modeling apparent heat capacity. Int. J. Refrig. 2017, 74, 240–253. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, Y.; Lin, X.; Xie, N.; Chen, H. Experimental study on natural convective heat transfer of tube immersed in microencapsulated phase change material suspensions. Appl. Therm. Eng. 2016, 99, 583–590. [Google Scholar] [CrossRef]
- Morimoto, T.; Togashi, K.; Kumano, H.; Hong, H. Thermophysical properties of phase change emulsions prepared by D-phase emulsi_cation. Energy Convers. Manag. 2016, 122, 215–222. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsi_er for thermal energy storage. Solar Energy Mater. Solar Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Ho, C.J.; Chang, P.C.; Yan, W.M.; Amani, M. Microencapsulated n-eicosane PCM suspensions: Thermophysical properties measurement and modeling. Int. J. Heat Mass Transf. 2018, 125, 792–800. [Google Scholar] [CrossRef]
- Sivapalan, B.; Chandran, M.N.; Manikandan, S.; Saranprabhu, M.K.; Pavithra, S.; Rajan, K.S. Paraffin wax–water nanoemulsion: A superior thermal energy storage medium providing higher rate of thermal energy storage per unit heat exchanger volume than water and paraffin wax. Energy Convers. Manag. 2018, 162, 109–117. [Google Scholar] [CrossRef]
- Buttitta, G.; Serale, G.; Cascone, Y. Enthalpy-temperature Evaluation of Slurry Phase Change Materials with T-history Method. Energy Procedia 2015, 78, 1877–1882. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, C.Y. Thermal and rheological properties of microencapsulated phase change materials. Renew. Energy 2011, 36, 2959–2966. [Google Scholar] [CrossRef]
- Manikandan, S.; Rajan, K.S. New hybrid nanouid containing encapsulated paraffin wax and sand nanoparticles in propylene glycol-water mixture: Potential heat transfer fluid for energy management. Energy Convers. Manag. 2017, 137, 74–85. [Google Scholar] [CrossRef]
- Xie, S.; Sun, J.; Wang, Z.; Liu, S.; Han, L.; Ma, G.; Jing, Y.; Jia, Y. A thermally stable phase change material with high latent heat based on an oxalic acid dihydrate/boric acid binary eutectic system. Solar Energy Mater. Solar Cells 2017, 168, 38–44. [Google Scholar] [CrossRef]
- Gimenez-Gavarrell, P.; Fereres, S. Glass encapsulated phase change materials for high temperature thermal energy storage. Renew. Energy 2017, 107, 497–507. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Stalin, J.; Marçal, M.; Delubac, R.; Karabanova, A.; Chiu, J.N.W.; Martin, V. Erythritol, glycerol, their blends, and olive oil, as sustainable phase change materials. Energy Procedia 2017, 135, 249–262. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Chiu, J.N.; Martin, V.; Hedström, P. The experimental phase diagram study of the binary polyols system erythritol-xylitol. Sol. Energy Mater. Sol. Cells 2018, 174, 248–262. [Google Scholar] [CrossRef]
- Rathgeber, C.; Schmit, H.; Miró, L.; Cabeza, L.F.; Gutierrez, A.; Ushak, S.; Hiebler, S. Enthalpy-temperature plots to compare calorimetric measurements of phase change materials at different sample scales. J. Energy Storage 2018, 15, 32–38. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R. Preparation and enhanced thermal performance of novel (solid to gel) form-stable eutectic PCM modified by nano-graphene platelets. J. Energy Storage 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Luo, Z.; Gao, X.; Fang, X.; Fang, Y.; Zhang, Z. Characterization of medium-temperature phase change materials for solar thermal energy storage using temperature history method. Solar Energy Mater. Solar Cells 2018, 179, 152–160. [Google Scholar] [CrossRef]
- Sefidan, A.M.; Sojoudi, A.; Saha, S.C.; Cholette, M. Multi-layer PCM solidification in a finned triplex tube considering natural convection. Appl. Therm. Eng. 2017, 123, 901–916. [Google Scholar] [CrossRef]
- Mazo, J.; Delgado, M.; Peñalosa, C.; Dolado, P.; Miranda, P.; Lazaro, A.; Marín, J.M.; Zalba, B. Evaluation of the suitability of different calorimetric methods to determine the enthalpy-temperature curve of granular PCM composites. Appl. Therm. Eng. 2017, 125, 306–316. [Google Scholar] [CrossRef]
- Shao, J.; Darkwa, J.; Kokogiannakis, G. Development of a novel phase change material emulsion for cooling systems. Renew. Energy 2016, 87, 509–516. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental investigation of the effects of mass fraction and temperature on the viscosity of microencapsulated PCM slurry. Int. J. Heat Mass Transf. 2018, 126, 390–399. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental research of viscosity of microencapsulated PCM slurry at the phase change temperature. Int. J. Heat Mass Transf. 2019, 134, 1209–1217. [Google Scholar] [CrossRef]
- Microtek Labs, MICRONAL® DS 5039 X Technical Data. Available online: https://cdn2.hubspot.net/hubfs/4153344/Microtek%20Laboratories%20December2017/PDF/MPDS3300-0045%20Rev%201.pdf?t=1516975227818 (accessed on 10 October 2020).
- Kousksou, T.; El Rhafiki, T.; Mahdaoui, M.; Bruel, P.; Zeraouli, Y. Crystallization of supercooled PCMs inside emulsions: DSC applications. Sol. Energy Mater. Sol. Cells 2012, 107, 28–36. [Google Scholar] [CrossRef]
- Solé, A.; Miró, L.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Review of the T -history method to determine thermophysical properties of phase change materials (PCM). Renew. Sustain. Energy Rev. 2013, 26, 425–436. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Microencapsulated PCM slurries’ dynamic viscosity experimental investigation and temperature-dependent prediction model. Int. J. Heat Mass Transf. 2019, 145. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B.; Białko, B. The experimental investigation of mPCM slurries density at phase change temperature. Int. J. Heat Mass Transf. 2020, 159. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowski, K.; Kruzel, M.; Zajączkowski, B. Determining the Heat of Fusion and Specific Heat of Microencapsulated Phase Change Material Slurry by Thermal Delay Method. Energies 2021, 14, 179. https://doi.org/10.3390/en14010179
Dutkowski K, Kruzel M, Zajączkowski B. Determining the Heat of Fusion and Specific Heat of Microencapsulated Phase Change Material Slurry by Thermal Delay Method. Energies. 2021; 14(1):179. https://doi.org/10.3390/en14010179
Chicago/Turabian StyleDutkowski, Krzysztof, Marcin Kruzel, and Bartosz Zajączkowski. 2021. "Determining the Heat of Fusion and Specific Heat of Microencapsulated Phase Change Material Slurry by Thermal Delay Method" Energies 14, no. 1: 179. https://doi.org/10.3390/en14010179
APA StyleDutkowski, K., Kruzel, M., & Zajączkowski, B. (2021). Determining the Heat of Fusion and Specific Heat of Microencapsulated Phase Change Material Slurry by Thermal Delay Method. Energies, 14(1), 179. https://doi.org/10.3390/en14010179





