Study of How Photoelectrodes Modified by TiO2/Ag Nanofibers in Various Structures Enhance the Efficiency of Dye-Sensitized Solar Cells under Low Illumination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Prepaiation of the Electrospun Nanofibers
2.3. Preparation of the TiO2–Ag Composited Photoelectrode
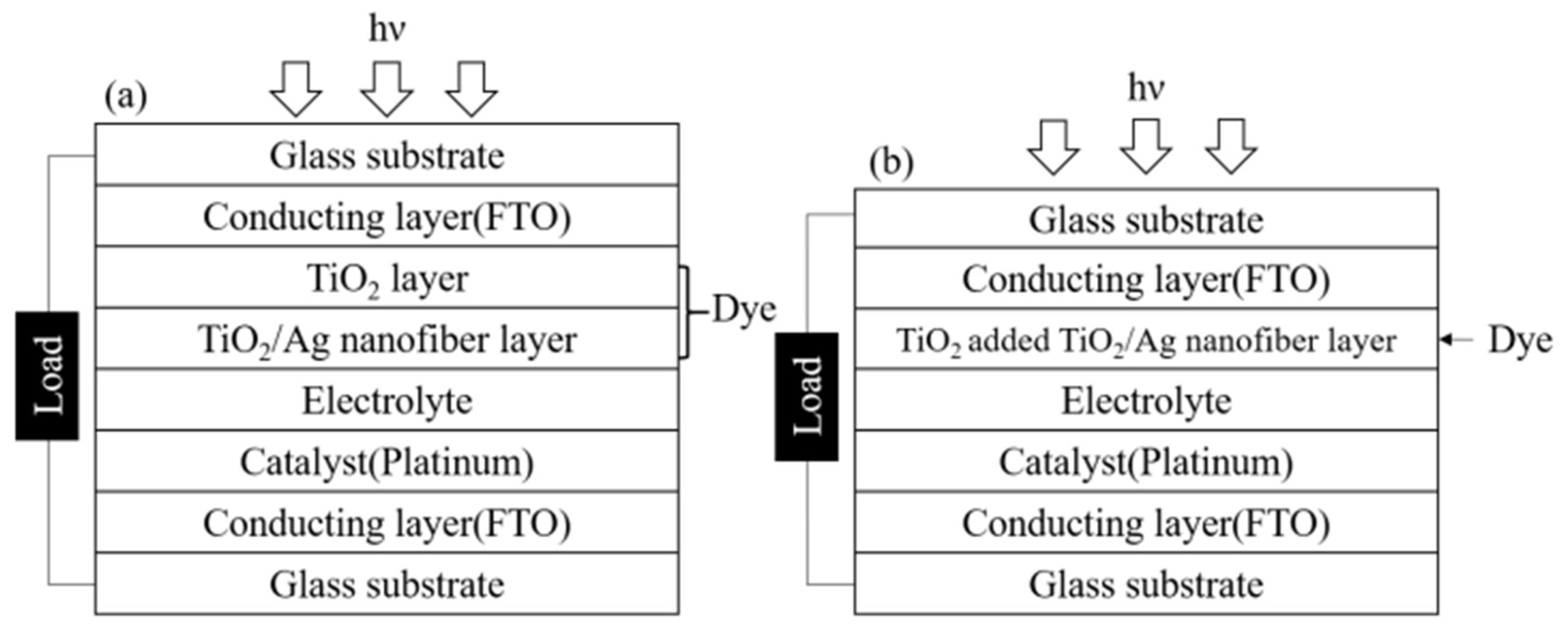
2.4. Fabrication of the Dye-Sensitized Solar Cell
2.5. Measurement System
3. Results and Discussion
3.1. Morphology and Characterization of Ag/ TiO2 Nanofiber
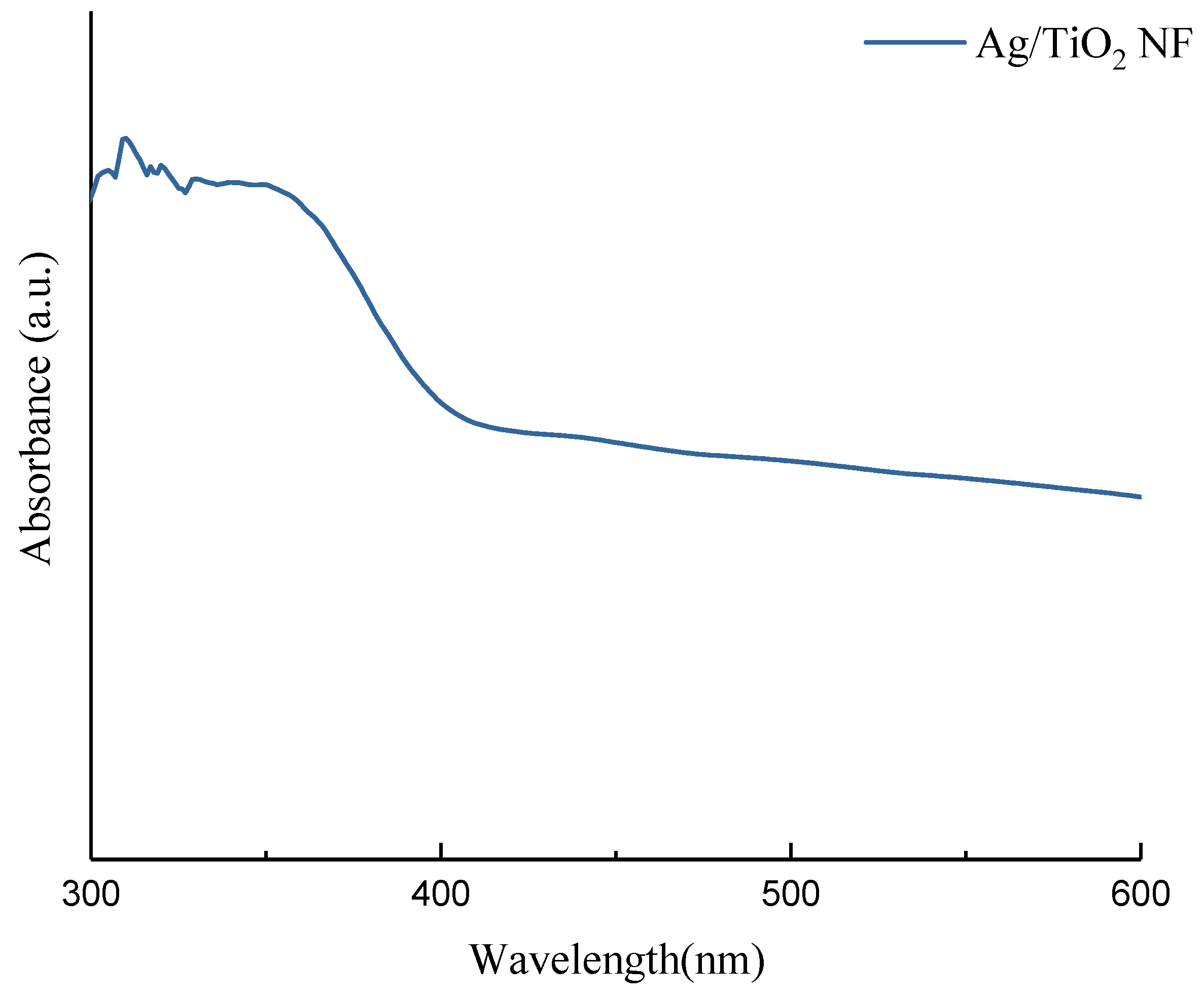
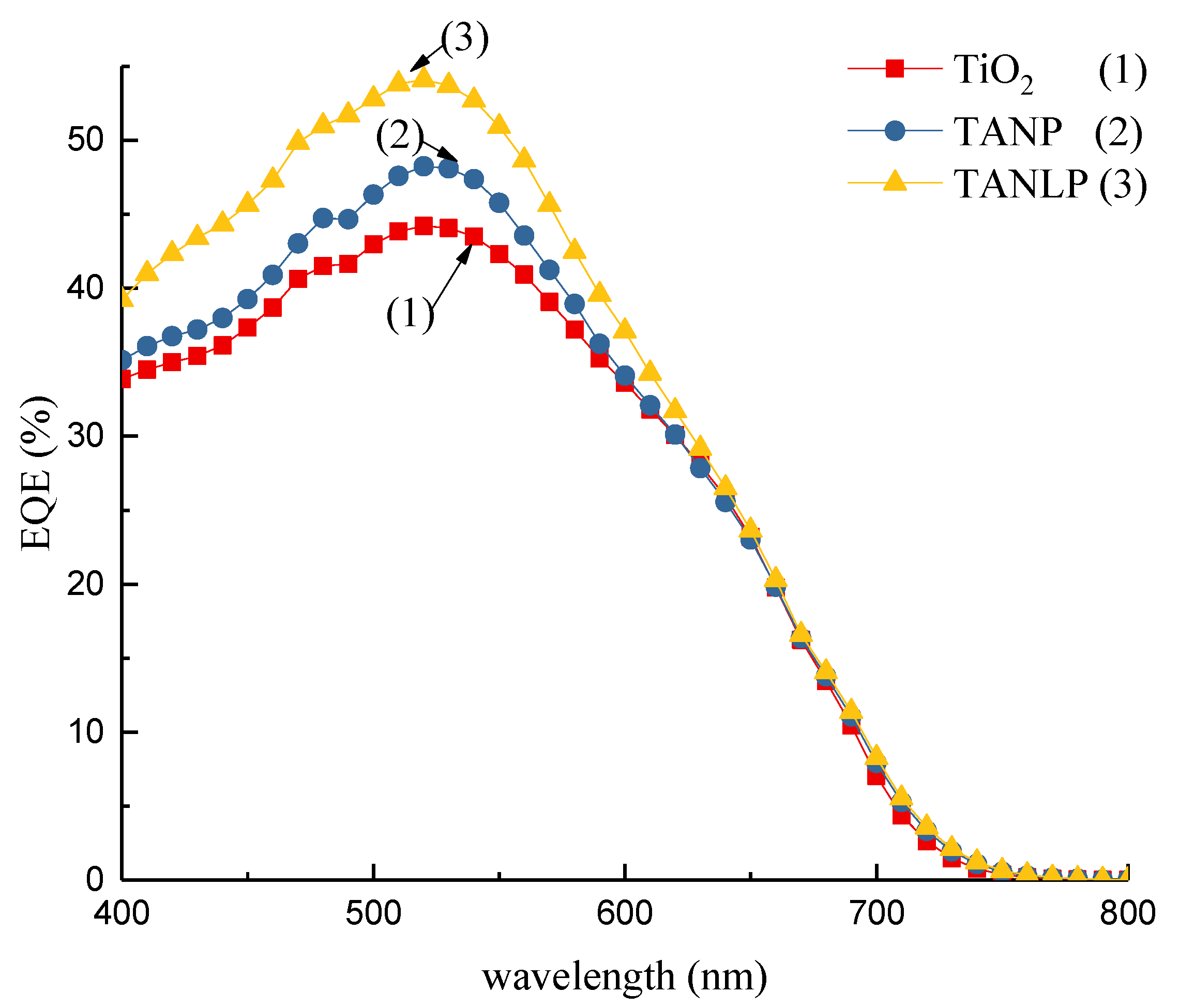
3.2. Ultraviolet–Visible Spectroscopy
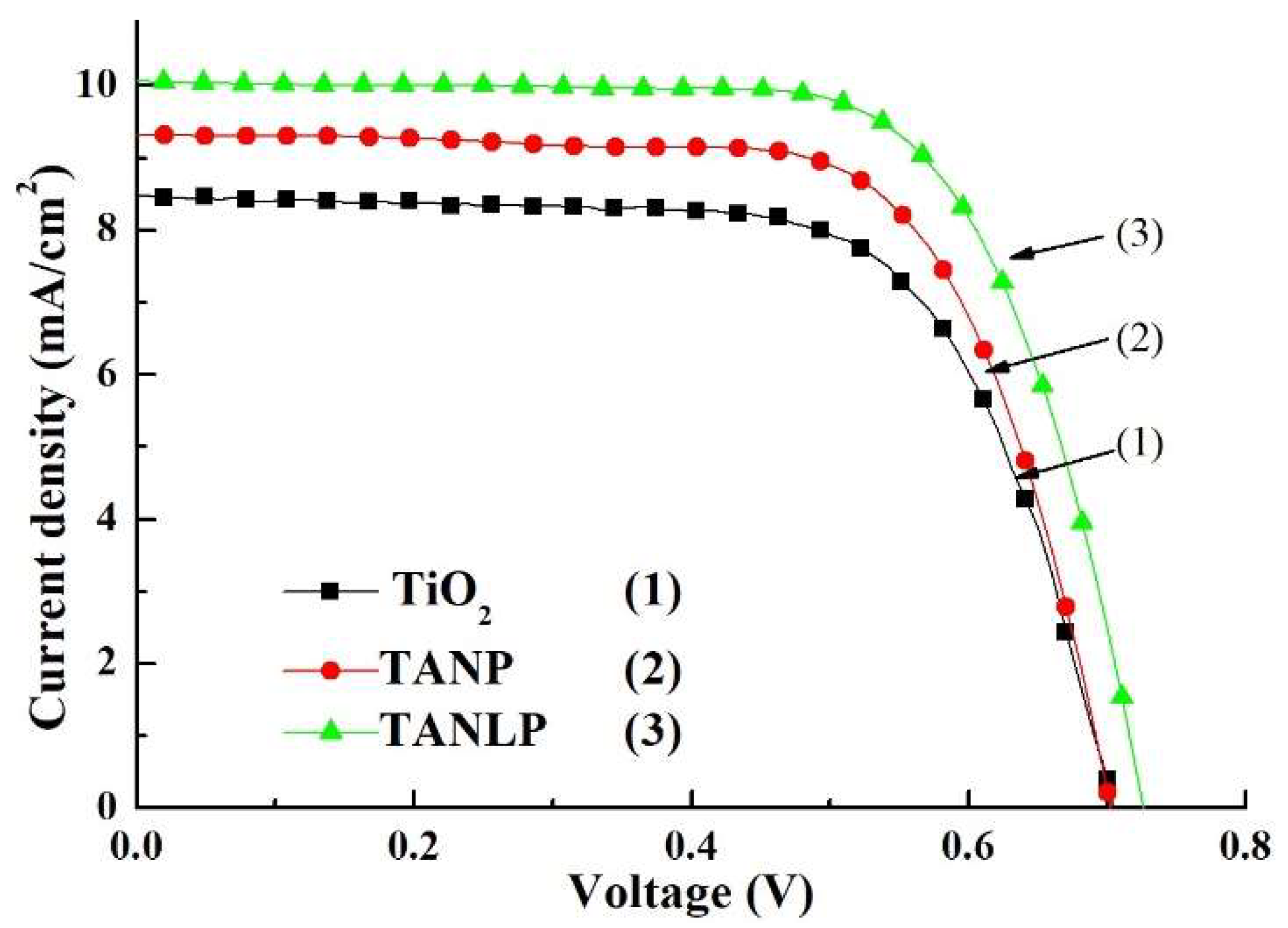
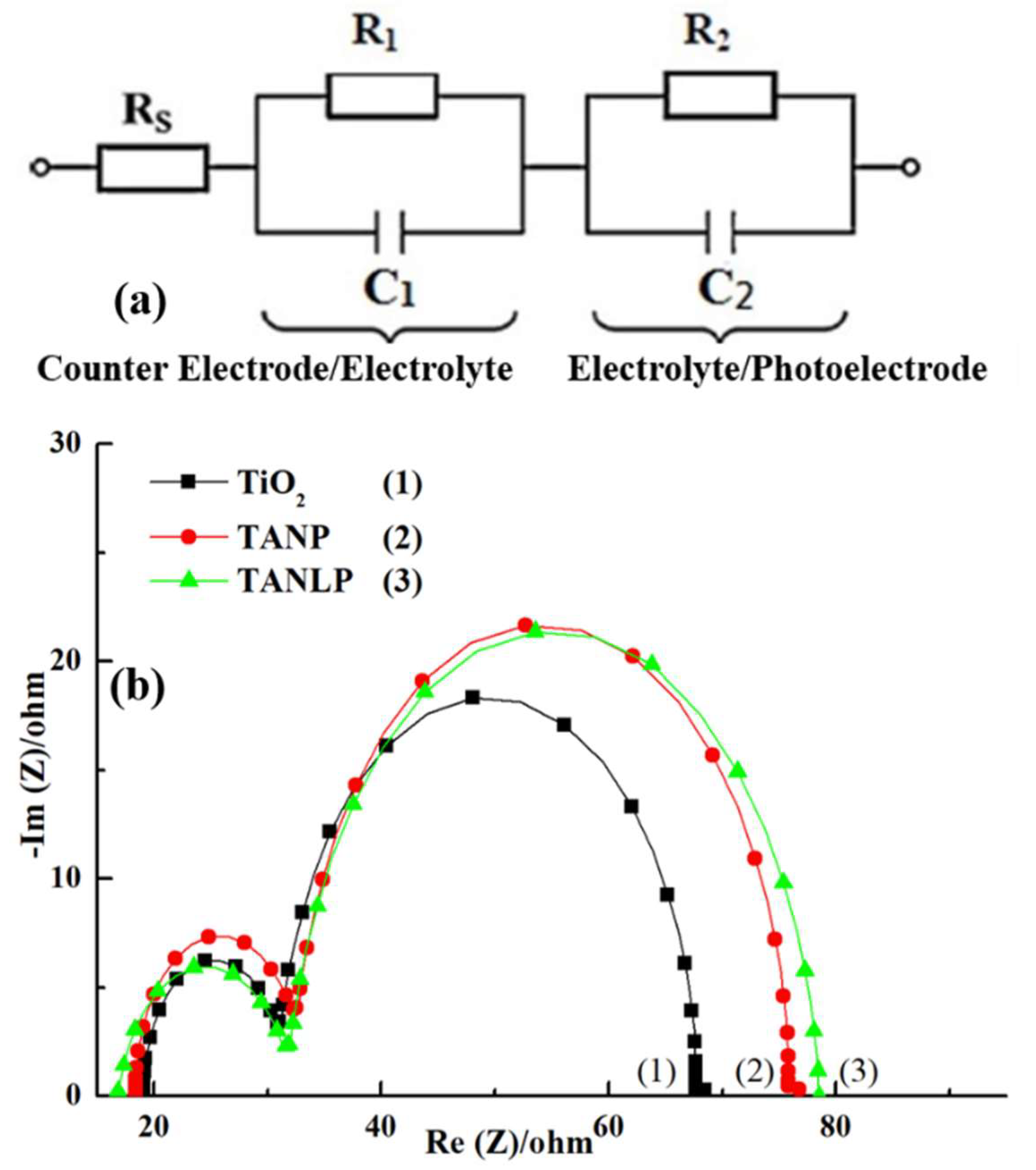
3.3. Measurement for the Photovoltaic Parameters of the DSSC
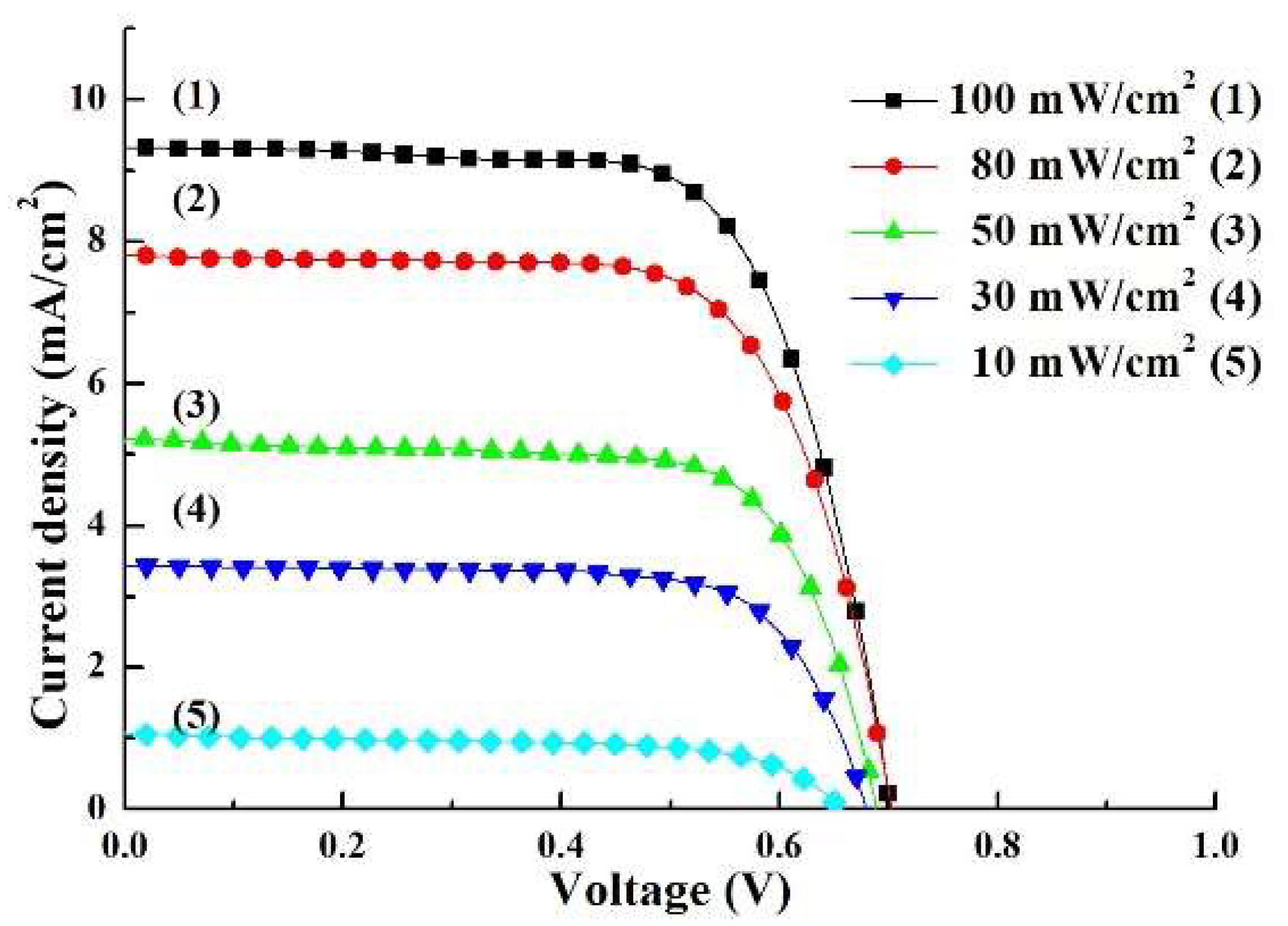
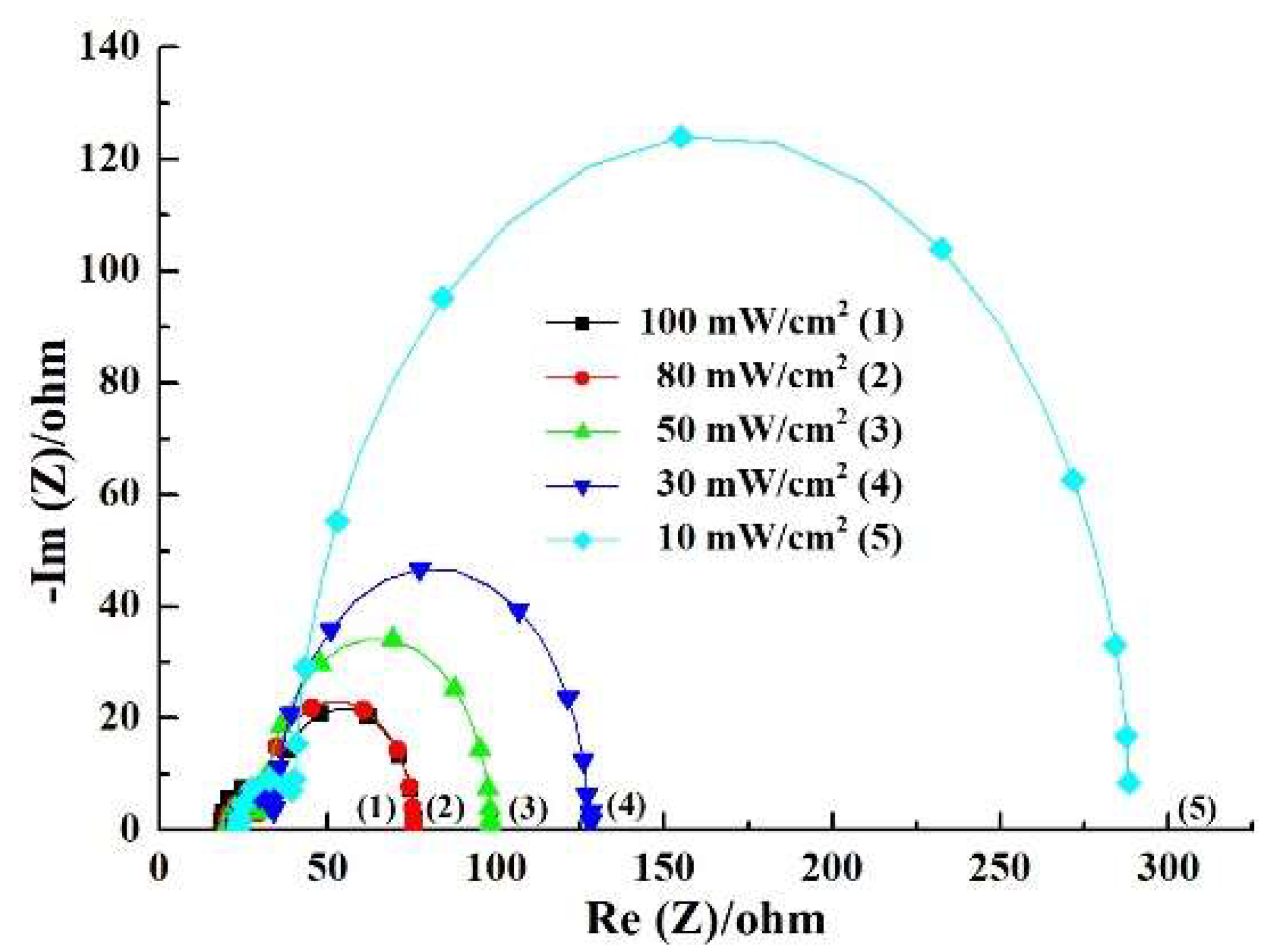
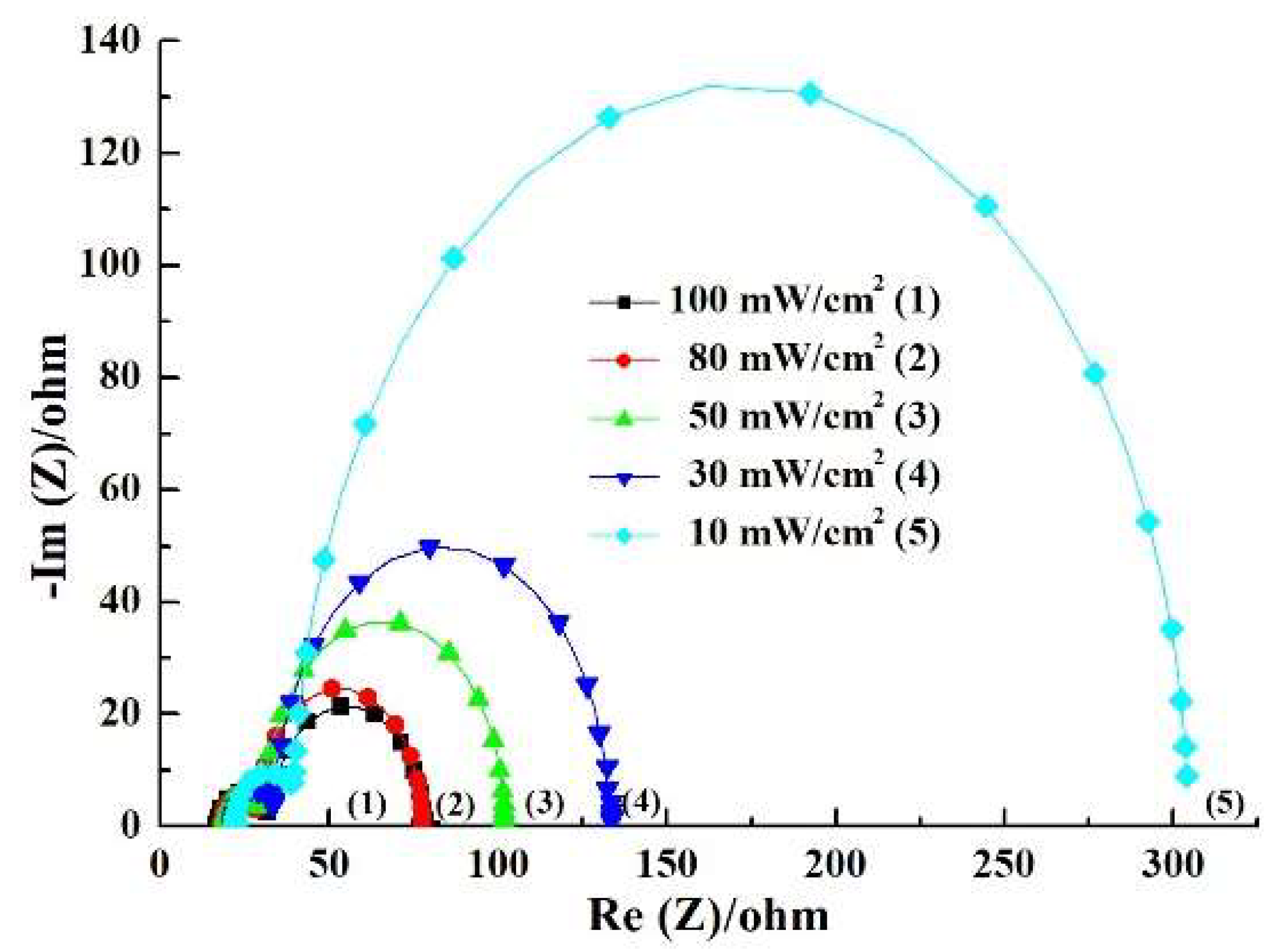
3.4. Performance of the DSSC Under Different Illuminations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal tio 2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (ii/iii)–based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, M.; Kang, M.G.; Jun, Y. Improvement of dye-sensitized solar cells toward the broader light harvesting of the solar spectrum. Chem. Commun. 2013, 49, 1471–1487. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, M.; Wang, X. Electrodeposition of hierarchical zno nanorod-nanosheet structures and their applications in dye-sensitized solar cells. ACS Appl. Mat. Interfaces 2011, 3, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M.J.C.S. Dye-sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the internet of things. R. Soc. Chem. 2020, 11, 2895–2906. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Sonigara, K.K.; Suresh, L.; Panahandeh-Fard, M.; Soni, S.S.; Tan, S.C. Efficient power generating devices utilizing low intensity indoor lights via non-radiative energy transfer mechanism from organic ionic redox couples. Nano Energy 2019, 60, 457–466. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.J.N.P. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372. [Google Scholar] [CrossRef]
- Chou, J.; Kuo, C.; Kuo, P.; Lai, C.; Nien, Y.; Liao, Y.; Ko, C.; Yang, C.; Wu, C. The retardation structure for improvement of photovoltaic performances of dye-sensitized solar cell under low illumination. IEEE J. Photovoltaics 2019, 9, 926–933. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Chandrashekar, B.N.; Jayaprakash, G.K.; Cheng, C.; Srikanta Swamy, S.; Byrappa, K. Electronically semitransparent zno nanorods with superior electron transport ability for dsscs and solar photocatalysis. Ceram. Int. 2018, 44, 7202–7208. [Google Scholar] [CrossRef]
- Kilic, B. Produce of carbon nanotube/zno nanowires hybrid photoelectrode for efficient dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 3482–3487. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Lu, N.L. Nanocomposite structures related to electrospun nanofibers for highly efficient and cost-effective dye-sensitized solar cells. In Multifunctional Nanocomposites for Energy and Environmental Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 113–133. [Google Scholar]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R. Electrochemical synthesis of one-dimensional zno nanostructures on zno seed layer for dssc applications. Appl. Surf. Sci. 2018, 428, 385–394. [Google Scholar] [CrossRef]
- Devulapally, K.; Vardhaman, A.K.; Katakam, R.; Upadhyaya, H.M.; Rajeswari, R.; Islavath, N.; Giribabu, L. One-dimensional hollow metal-complex as catalytic electrode for dye-sensitized solar cells. Sol. Energy 2018, 174, 502–507. [Google Scholar] [CrossRef]
- Gao, X.; Kong, C.-P.; Jia, R.; Jian, W.; Wang, J.; Bai, F.-Q.; Zhang, H.-X. Influence of one-dimensional TiO2 nanotube on interfacial electron transfer in dye-sensitized solar cells: Insights from theoretical investigation. Sol. Energy 2018, 176, 545–555. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.; Davoux, D.; Zhu, Z.; Galipeau, D.; Fong, H.; Qiao, Q. Composite of TiO2 nanofibers and nanoparticles for dye-sensitized solar cells with significantly improved efficiency. Energy Environ. Sci. 2010, 3, 1507–1510. [Google Scholar] [CrossRef]
- Madhavan, A.; Kalluri, S.; Chacko, D.; Arun, T.A.; Nagarajan, S.; Subramanian, K.R.V.; Nair, S.; Nair, S.; Balakrishnan, A. Electrical and optical properties of electrospun TiO2-graphene composite nanofibers and its application as dssc photo-anodes. RSC Adv. 2012, 2, 2012. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, Z. Preparation of the au@TiO2 nanofibers by one-step electrospinning for the composite photoanode of dye-sensitized solar cells. Mater. Chem. Phys. 2018, 208, 35–40. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Hsu, C.-F.; Wu, M.-J.; Chen, C.-N.; Huang, J.-J. Ag–TiO2 composite photoelectrode for dye-sensitized solar cell. Appl. Phys. A 2017, 123, 357. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Sonigara, K.K.; Fadadu, K.B.; Soni, S.S. Hybrid agnp–TiO2 thin film based photoanode for dye sensitized solar cell. Perspect. Sci. 2016, 8, 46–49. [Google Scholar] [CrossRef][Green Version]
- Nien, Y.-H.; Chen, H.-H.; Hsu, H.-H.; Kuo, P.-Y.; Chou, J.-C.; Lai, C.-H.; Hu, G.-M.; Kuo, C.-H.; Ko, C.-C. Enhanced photovoltaic conversion efficiency in dye-sensitized solar cells based on photoanode consisting of TiO2/go/ag nanofibers. Vacuum 2019, 167, 47–53. [Google Scholar] [CrossRef]
- Hussein, A.M.; Iefanova, A.V.; Koodali, R.T.; Logue, B.A.; Shende, R.V. Interconnected ZRO2 doped zno/ TiO2 network photoanode for dye-sensitized solar cells. Energy Rep. 2018, 4, 56–64. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B.J.K.J. Phase characterization of TiO2 powder by xrd and tem. IJSRST 2008, 42, 357–361. [Google Scholar]
- Philip, D. Biosynthesis of au, ag and au–ag nanoparticles using edible mushroom extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.N.; Riyazuddin; Babji, P.; Ahmad, N.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; Husain, F.M. Green synthesis and structural classification of acacia nilotica mediated-silver doped titanium oxide (ag/TiO2 spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J. Biol. Sci. 2019, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Abbad, S.; Guergouri, K.; Gazaout, S.; Djebabra, S.; Zertal, A.; Barille, R.; Zaabat, M. Effect of silver doping on the photocatalytic activity of TiO2 nanopowders synthesized by the sol-gel route. J. Environ. Chem. Eng. 2020, 8, 103718. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, Y.; Zhang, S. Photocatalysis for environmental applications. Front. Chem. 2019, 7, 303. [Google Scholar] [CrossRef]
- Tunc, I.; Bruns, M.; Gliemann, H.; Grunze, M.; Koelsch, P.J.S.; Analysis, I. Bandgap determination and charge separation in ag@TiO2 core shell nanoparticle films. Surf. Interface Anal. 2010, 42, 835–841. [Google Scholar] [CrossRef]
- Yun, J.; Hwang, S.H.; Jang, J. Fabrication of au@ag core/shell nanoparticles decorated TiO2 hollow structure for efficient light-harvesting in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 2055–2063. [Google Scholar] [CrossRef]
- Gosnell, J.D.; Weiss, S.M. Light scattering by white-emitting cdse nanocrystals and traditional yag: Ce3+ phosphor particles. Mater. Res. Soc. Symp. Proc. 2009, 1148. [Google Scholar]
- Ramamurthy, V.; Schanze, K.S. Semiconductor Photochemistry and Photophysics/Volume ten; CRC Press: Boca Raton, FL, USA, 2003; Volume 10. [Google Scholar]
- Saud, P.S.; Pant, B.; Twari, A.P.; Ghouri, Z.K.; Park, M.; Kim, H.-Y. Effective photocatalytic efficacy of hydrothermally synthesized silver phosphate decorated titanium dioxide nanocomposite fibers. J. Colloid Interface Sci. 2016, 465, 225–232. [Google Scholar] [CrossRef]
- Wolf, M.; Rauschenbach, H. Series resistance effects on solar cell measurements. Adv. Energy Convers. 1963, 3, 455–479. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Fernández Pulido, Y.; Blanco, C.; Anseán, D.; García, V.M.; Ferrero, F.; Valledor, M. Determination of suitable parameters for battery analysis by electrochemical impedance spectroscopy. Measurement 2017, 106, 1–11. [Google Scholar] [CrossRef]
- Zhai, P.; Lee, H.; Huang, Y.-T.; Wei, T.-C.; Feng, S.-P. Study on the blocking effect of a quantum-dot TiO2 compact layer in dye-sensitized solar cells with ionic liquid electrolyte under low-intensity illumination. J. Power Sources 2016, 329, 502–509. [Google Scholar] [CrossRef]
- Pal, A.; Jana, A.; Bhattacharya, S.; Datta, J. Spr effect of agnps decorated TiO2 in dssc using tpmpi in the electrolyte: Approach towards low light trapping. Electrochim. Acta 2017, 243, 33–43. [Google Scholar] [CrossRef]
- Salvador, P.; Hidalgo, M.G.; Zaban, A.; Bisquert, J. Illumination intensity dependence of the photovoltage in nanostructured TiO2 dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 15915–15926. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Ranjbar, M.; Kouhestanian, E.; Salar Amoli, H.; Armanmehr, M.H. An investigation on the effect of electrodeposited nanostructured zno on the electron transfer process efficiency of TiO2 based dssc. Mat. Sci. Semicond. Process. 2015, 40, 285–292. [Google Scholar] [CrossRef]







| Photoanode | Dye-Loading of N3 (nmol/cm2) |
|---|---|
| TiO2 | 25.63 |
| TANP | 36.89 |
| TANLP | 67.76 |
| Intensity (mW/cm2) | RS (Ω) | R1 (Ω) | R2 (Ω) | JSC (mA/cm2) | VOC (V) | F. F. (%) | η (%) |
|---|---|---|---|---|---|---|---|
| 100 | 19.09 | 7.88 | 36.91 | 8.36 ± 0.03 | 0.70 ± 0.02 | 68.12 ± 0.18 | 3.99 ± 0.15 |
| 80 | 19.49 | 8.45 | 40.49 | 6.94 ± 0.03 | 0.69 ± 0.02 | 69.91 ± 0.14 | 4.24 ± 0.13 |
| 50 | 20.09 | 9.00 | 59.35 | 4.69 ± 0.02 | 0.68 ± 0.01 | 71.02 ± 0.16 | 4.53 ± 0.11 |
| 30 | 22.17 | 9.71 | 82.55 | 3.01 ± 0.02 | 0.67 ± 0.02 | 71.95 ± 0.15 | 4.84 ± 0.12 |
| 10 | 22.78 | 13.38 | 218.70 | 0.91 ± 0.01 | 0.66 ± 0.01 | 70.51 ± 0.13 | 4.22 ± 0.14 |
| Intensity (mW/cm2) | RS (Ω) | R1 (Ω) | R2 (Ω) | JSC (mA/cm2) | VOC (V) | F. F. (%) | η (%) |
|---|---|---|---|---|---|---|---|
| 100 | 18.37 | 9.11 | 42.72 | 9.31 ± 0.03 | 0.71 ± 0.01 | 69.68 ± 0.53 | 4.61 ± 0.15 |
| 80 | 18.84 | 9.74 | 46.94 | 7.80 ± 0.02 | 0.70 ± 0.02 | 70.46 ± 0.49 | 4.81 ± 0.13 |
| 50 | 19.54 | 10.42 | 68.69 | 5.22 ± 0.03 | 0.69 ± 0.02 | 71.52 ± 0.53 | 5.16 ± 0.12 |
| 30 | 22.00 | 11.34 | 95.48 | 3.41 ± 0.01 | 0.68 ± 0.01 | 72.43 ± 0.51 | 5.60 ± 0.14 |
| 10 | 22.72 | 15.96 | 252.46 | 1.04 ± 0.01 | 0.66 ± 0.02 | 70.67 ± 0.53 | 4.83 ± 0.12 |
| Intensity (mW/cm2) | RS (Ω) | R1 (Ω) | R2 (Ω) | JSC (mA/cm2) | VOC (V) | F. F. (%) | η (%) |
|---|---|---|---|---|---|---|---|
| 100 | 17.69 | 9.70 | 43.40 | 10.05 ± 0.09 | 0.73 ± 0.01 | 69.92 ± 0.51 | 5.13 ± 0.16 |
| 80 | 18.17 | 10.22 | 49.30 | 8.29 ± 0.12 | 0.72 ± 0.02 | 70.75 ± 0.47 | 5.28 ± 0.14 |
| 50 | 18.89 | 10.94 | 72.13 | 5.60 ± 0.14 | 0.71 ± 0.01 | 71.84 ± 0.48 | 5.71 ± 0.12 |
| 30 | 20.41 | 12.84 | 100.28 | 3.67 ± 0.13 | 0.70 ± 0.01 | 72.66 ± 0.45 | 6.23 ± 0.16 |
| 10 | 21.15 | 17.05 | 265.13 | 1.08 ± 0.13 | 0.69 ± 0.02 | 70.83 ± 0.46 | 5.31 ± 0.13 |
| Photoanode | Electron Lifetime under 100 mW/cm2(ms) | Electron Lifetime under 80 mW/cm2(ms) | Electron Lifetime under 50 mW/cm2(ms) | Electron Lifetime under 30 mW/cm2(ms) | Electron Lifetime under 10 mW/cm2(ms) |
|---|---|---|---|---|---|
| TiO2 | 27.14 | 27.53 | 34.46 | 43.00 | 68.32 |
| TANP | 22.62 | 23.54 | 27.32 | 31.59 | 45.24 |
| TANLP | 26.34 | 27.70 | 28.46 | 30.75 | 49.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nien, Y.-H.; Chen, H.-H.; Hsu, H.-H.; Rangasamy, M.; Hu, G.-M.; Yong, Z.-R.; Kuo, P.-Y.; Chou, J.-C.; Lai, C.-H.; Ko, C.-C.; et al. Study of How Photoelectrodes Modified by TiO2/Ag Nanofibers in Various Structures Enhance the Efficiency of Dye-Sensitized Solar Cells under Low Illumination. Energies 2020, 13, 2248. https://doi.org/10.3390/en13092248
Nien Y-H, Chen H-H, Hsu H-H, Rangasamy M, Hu G-M, Yong Z-R, Kuo P-Y, Chou J-C, Lai C-H, Ko C-C, et al. Study of How Photoelectrodes Modified by TiO2/Ag Nanofibers in Various Structures Enhance the Efficiency of Dye-Sensitized Solar Cells under Low Illumination. Energies. 2020; 13(9):2248. https://doi.org/10.3390/en13092248
Chicago/Turabian StyleNien, Yu-Hsun, Huang-Hua Chen, Hui-Hsuan Hsu, Manjunath Rangasamy, Geng-Ming Hu, Zhen-Rong Yong, Po-Yu Kuo, Jung-Chuan Chou, Chih-Hsien Lai, Cheng-Chu Ko, and et al. 2020. "Study of How Photoelectrodes Modified by TiO2/Ag Nanofibers in Various Structures Enhance the Efficiency of Dye-Sensitized Solar Cells under Low Illumination" Energies 13, no. 9: 2248. https://doi.org/10.3390/en13092248
APA StyleNien, Y.-H., Chen, H.-H., Hsu, H.-H., Rangasamy, M., Hu, G.-M., Yong, Z.-R., Kuo, P.-Y., Chou, J.-C., Lai, C.-H., Ko, C.-C., & Chang, J.-X. (2020). Study of How Photoelectrodes Modified by TiO2/Ag Nanofibers in Various Structures Enhance the Efficiency of Dye-Sensitized Solar Cells under Low Illumination. Energies, 13(9), 2248. https://doi.org/10.3390/en13092248








