Copper Coordination Complexes for Energy-Relevant Applications
Abstract
1. Introduction
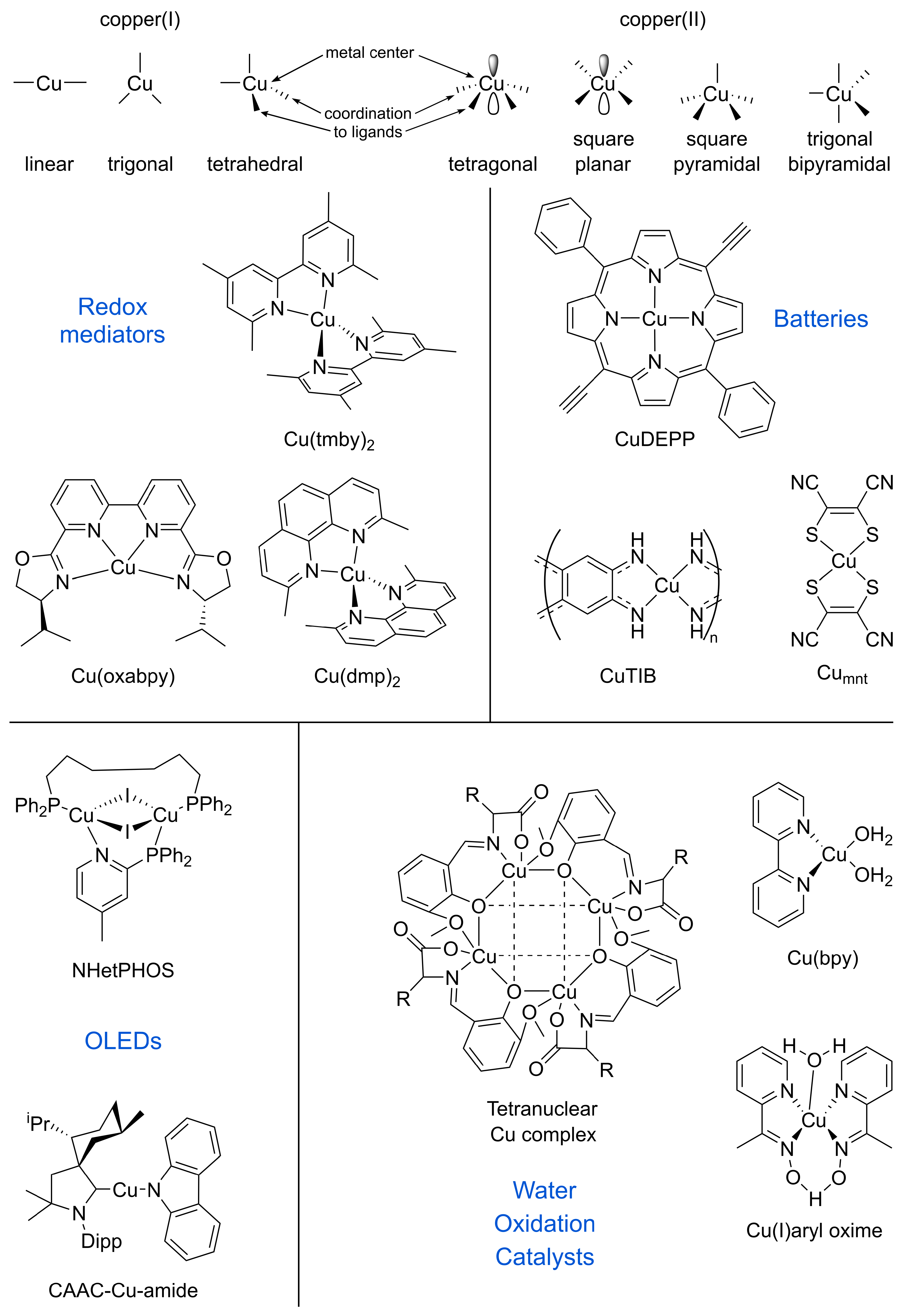
2. Fundamental Aspects of Copper Coordination Complexes
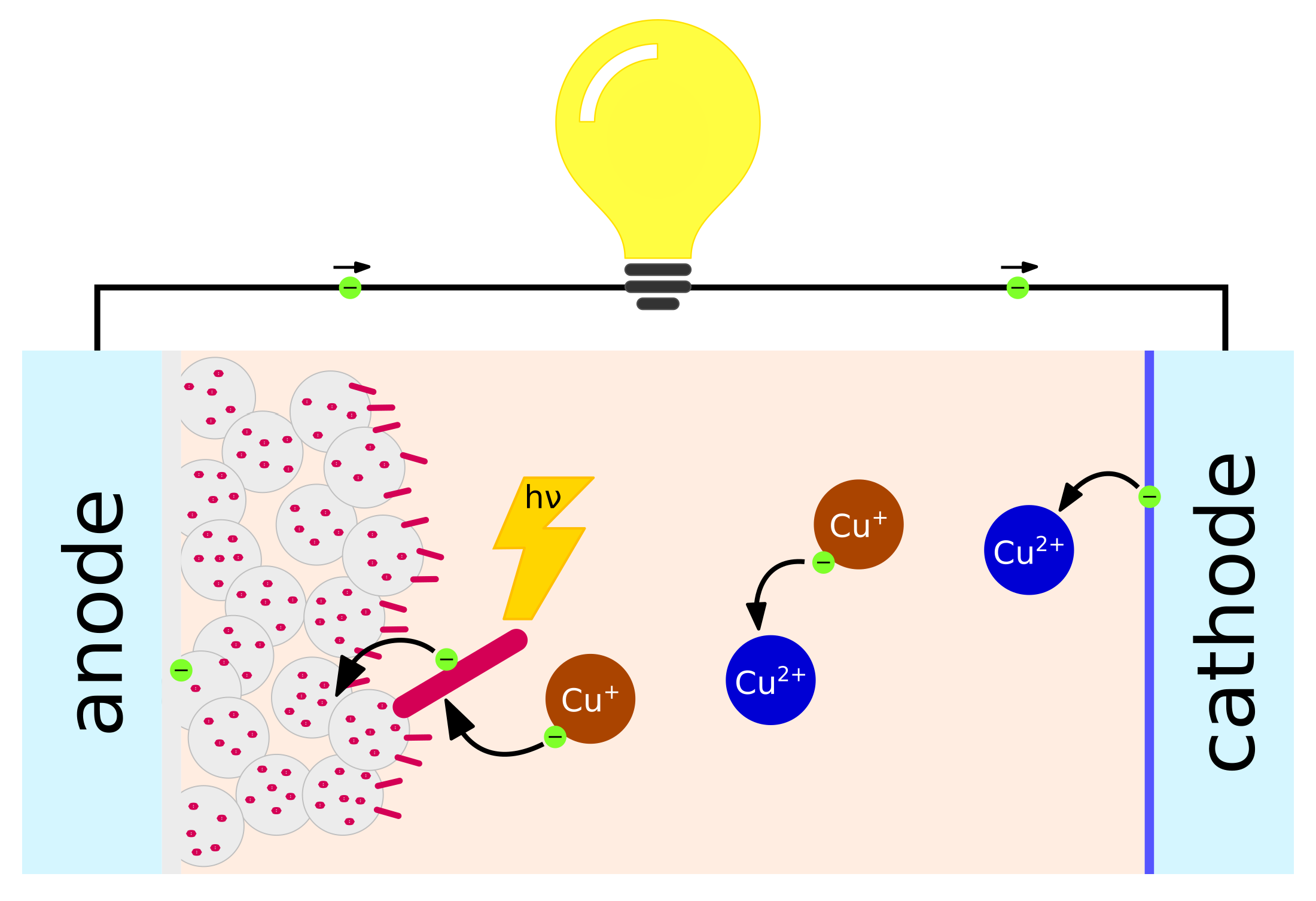
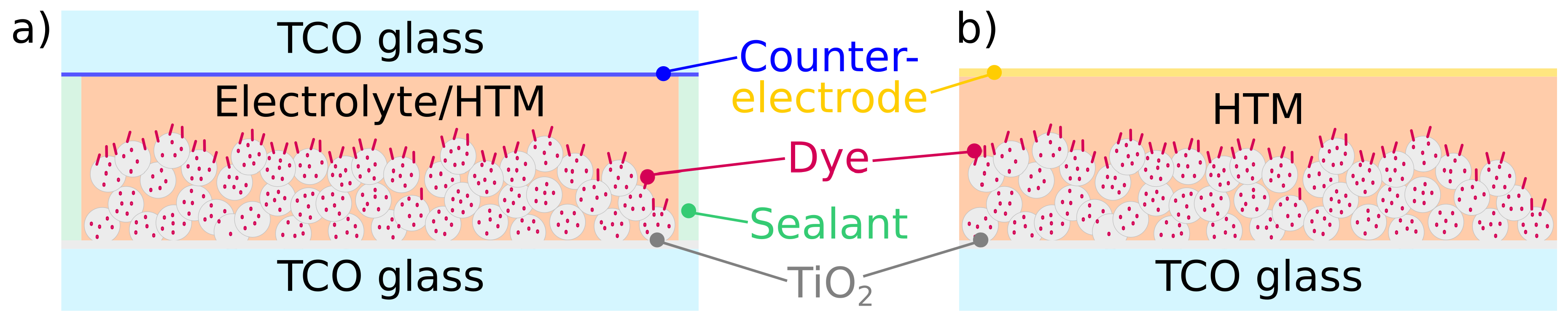
3. Dye-Sensitized Solar Cells
3.1. Redox Mediators and Hole Transporting Materials
3.2. Dyes
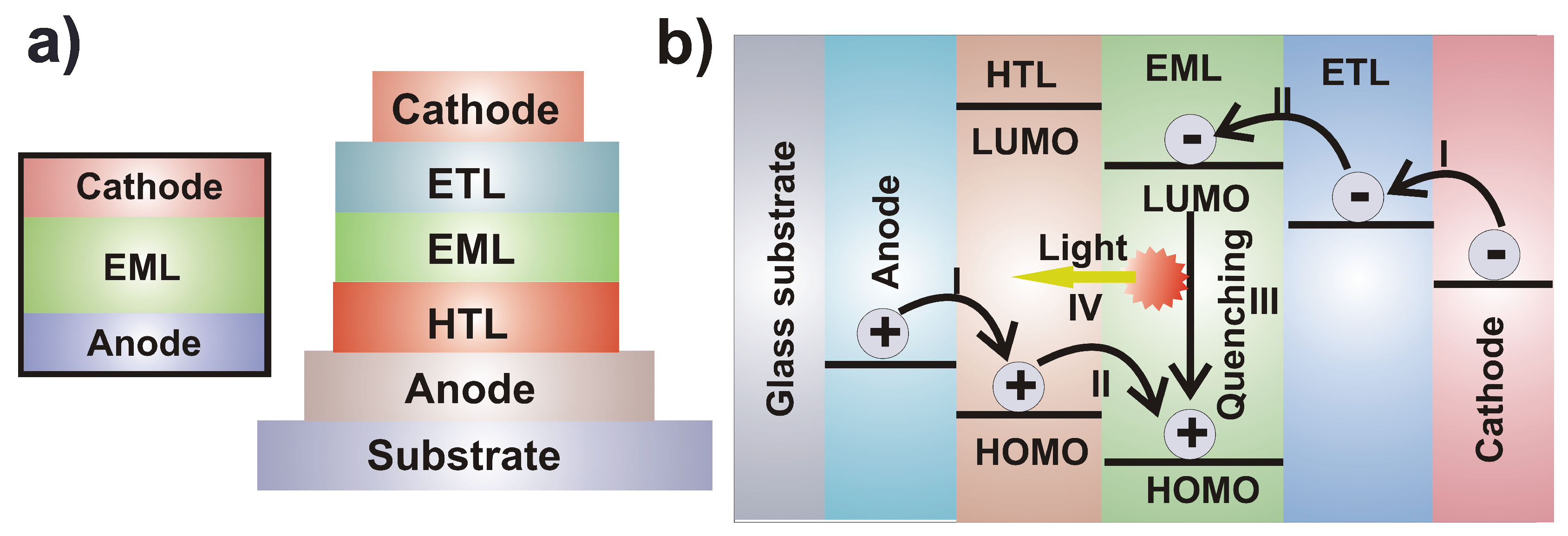
4. Organic Light-Emitting Diodes
Copper Complexes as Light Emitters in OLEDs
5. Batteries
5.1. Lithium Ion Batteries
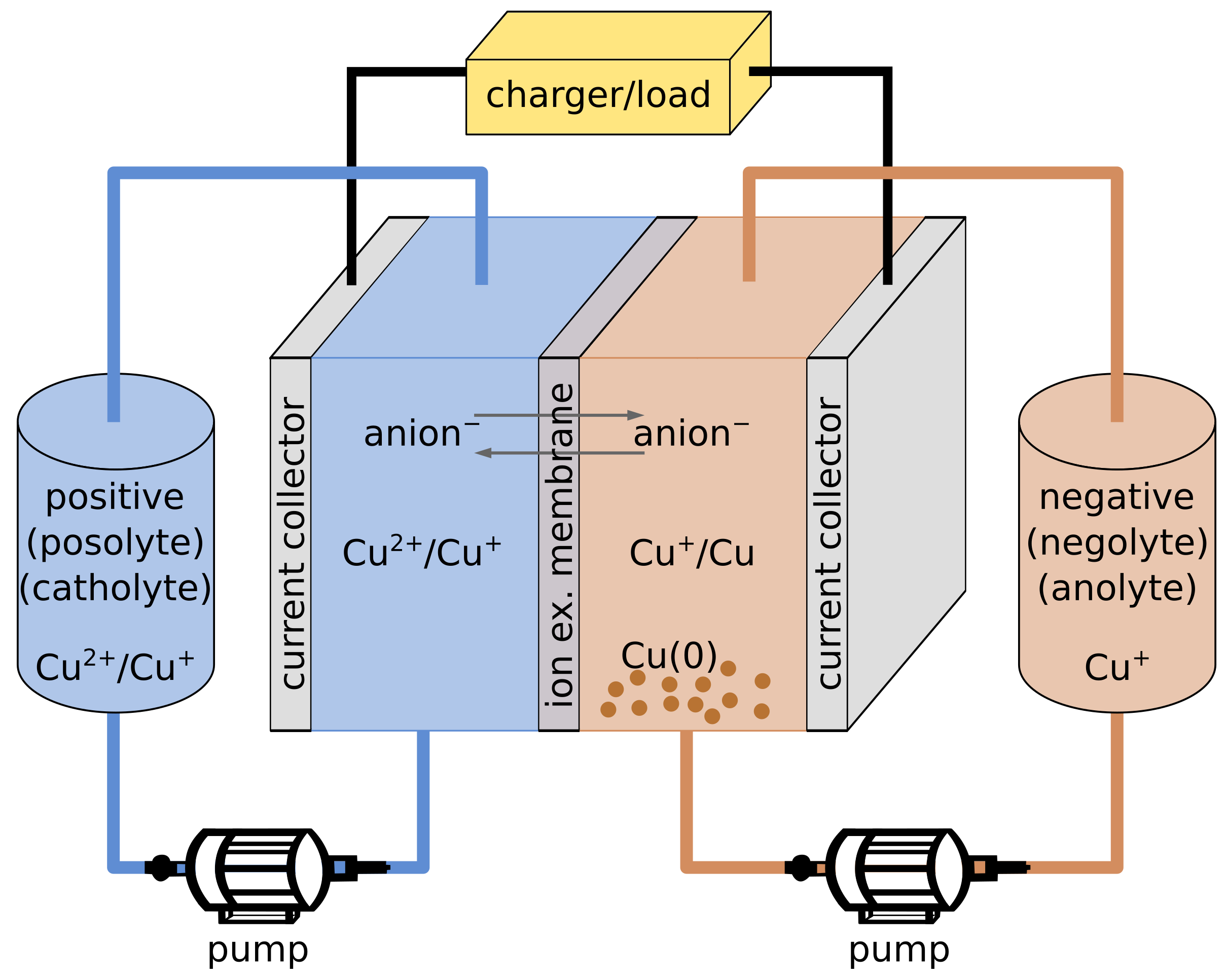
5.2. Redox Flow Batteries
6. Solar Fuels
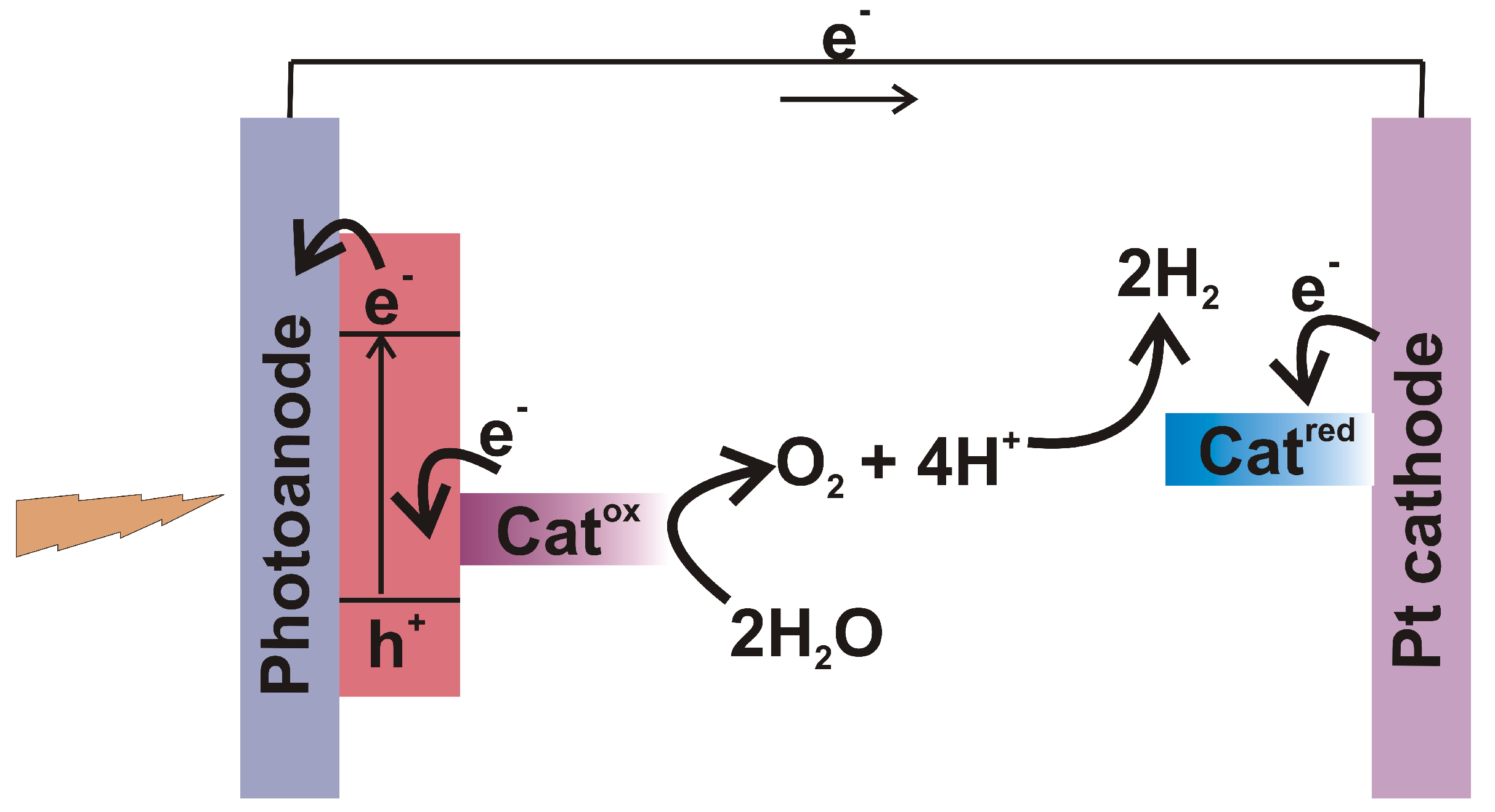
6.1. Working Principles of Solar Fuels
6.2. Copper Complexes as Water Oxidation Catalysts
7. Summary
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OLED | Organic light-emitting diode |
| LMMCT | Ligand-to-metal-to-metal charge transfer |
| DSC | Dye-sensitized solar cell |
| ssDSC | Solid-state dye-sensitized solar cell |
| HTM | Hole transporting material |
| IoT | Internet of things |
| TCO | Thin conductive oxide |
| VOC | Open-circuit voltage |
| PCE | Power conversion efficiency |
| EML | Emission layer |
| LUMO | Lowest unoccupied molecular orbital |
| HOMO | Highest occupied molecular orbital |
| TADF | Thermally-activated delayed fluorescence |
| EBL | Electron blocking layer |
| EQE | External quantum efficiency |
| LIB | Lithium ion battery |
| RFB | Redox flow battery |
| WOC | Water oxidation catalyst |
| TOF | Turn over frequency |
| OEC | Oxygen-evolving complex |
References
- Zanoni, K.P.S.; Coppo, R.L.; Amaral, R.C.; Murakami Iha, N.Y. Ir(III) complexes designed for light-emitting devices: Beyond the luminescence color array. Dalton Trans. 2015, 44, 14559–14573. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- He, L.; Qiao, J.; Duan, L.; Dong, G.; Zhang, D.; Wang, L.; Qiu, Y. Toward highly efficient solid-state white light-emitting electrochemical cells: Blue-green to red emitting cationic iridium complexes with imidazole-type ancillary ligands. Adv. Funct. Mater. 2009, 19, 2950–2960. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Tokito, S. Highly efficient, low-voltage phosphorescent organic light-emitting diodes using an iridium complex as the host material. Adv. Mater. 2007, 19, 276–280. [Google Scholar] [CrossRef]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet exciton confinement in green organic light-emitting diodes containing luminescent charge-transfer Cu(I) complexes. Adv. Funct. Mater. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Suttil, J.A.; Kucharyson, J.F.; Escalante-Garcia, I.L.; Cabrera, P.J.; James, B.R.; Savinell, R.F.; Sanford, M.S.; Thompson, L.T. Metal acetylacetonate complexes for high energy density non-aqueous redox flow batteries. J. Mater. Chem. A 2015, 3, 7929–7938. [Google Scholar] [CrossRef]
- Lloyd, D.; Vainikka, T.; Kontturi, K. The development of an all copper hybrid redox flow battery using deep eutectic solvents. Electrochim. Acta 2013, 100, 18–23. [Google Scholar] [CrossRef]
- Hogue, R.W.; Armstrong, C.G.; Toghill, K.E. Dithiolene Complexes of First-Row Transition Metals for Symmetric Nonaqueous Redox Flow Batteries. ChemSusChem 2019, 12, 4506–4515. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Pradhan, S.C.; Hagfeldt, A.; Soman, S. Resurgence of DSCs with copper electrolyte: A detailed investigation of interfacial charge dynamics with cobalt and iodine based electrolytes. J. Mater. Chem. A 2018, 6, 22204–22214. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wu, X.; Sun, L. Copper-based homogeneous and heterogeneous catalysts for electrochemical water oxidation. Nanoscale 2020, 12, 4187–4218. [Google Scholar] [CrossRef]
- Lei, H.; Li, X.; Meng, J.; Zheng, H.; Zhang, W.; Cao, R. Structure Effects of Metal Corroles on Energy-Related Small Molecule Activation Reactions. ACS Catal. 2019, 9, 4320–4344. [Google Scholar] [CrossRef]
- Barnett, S.M.; Goldberg, K.I.; Mayer, J.M. A soluble copper-bipyridine water-oxidation electrocatalyst. Nat. Chem. 2012, 4, 498–502. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Funes-Ardoiz, I.; Drouet, S.; Benet-Buchholz, J.; Maseras, F.; Llobet, A. Redox non-innocent ligand controls water oxidation overpotential in a new family of mononuclear cu-based efficient catalysts. J. Am. Chem. Soc. 2015, 137, 6758–6761. [Google Scholar] [CrossRef]
- Michaels, H.; Benesperi, I.; Edvinsson, T.; Muñoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 53. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Lee, J.M.; Taylor, N.S.; Cheema, H.; Chen, L.; Fortenberry, R.C.; Delcamp, J.H.; Jurss, J.W. Copper-based redox shuttles supported by preorganized tetradentate ligands for dye-sensitized solar cells. Dalton Trans. 2020, 49, 343–355. [Google Scholar] [CrossRef]
- Mathews, I.; Kantareddy, S.N.; Buonassisi, T.; Peters, I.M. Technology and Market Perspective for Indoor Photovoltaic Cells. Joule 2019, 3, 1415–1426. [Google Scholar] [CrossRef]
- Liu, Y.; Yiu, S.C.; Ho, C.L.; Wong, W.Y. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Fernández, M.R.; Casanova, E.Z.; Alonso, I.G. Review of Display Technologies Focusing on Power Consumption. Sustainability 2015, 7, 10854–10875. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Gerbaldi, C.; Viscardi, G. Cobalt-Based Electrolytes for Dye-Sensitized Solar Cells: Recent Advances towards Stable Devices. Energies 2016, 9, 384. [Google Scholar] [CrossRef]
- Sprengard, R.; Bonrad, K.; Daeubler, T.K.; Frank, T.; Hagemann, V.; Koehler, I.; Pommerehne, J.; Ottermann, C.R.; Voges, F.; Vingerling, B. OLED devices for signage applications: A review of recent advances and remaining challenges. In Organic Light-Emitting Materials and Devices VIII; International Society for Optics and Photonics: Bellingham, WA, USA, 2004; Volume 5519, pp. 173–183. [Google Scholar] [CrossRef]
- McMillin, D.R.; Kirchhoff, J.R.; Goodwin, K.V. Exciplex quenching of photo-excitd copper complexes. Coord. Chem. Rev. 1985, 64, 83–92. [Google Scholar] [CrossRef]
- Gushurst, A.K.I.; McMillin, D.R.; Dietrich-Buchecker, C.O.; Sauvage, J.P. Comparative studies of the photophysical properties of copper phenanthrolines: From Cu(dmp)2+ to the copper(I) catenates. Inorg. Chem. 1989, 28, 4070–4072. [Google Scholar] [CrossRef]
- Freitag, M.; Giordano, F.; Yang, W.; Pazoki, M.; Hao, Y.; Zietz, B.; Grätzel, M.; Hagfeldt, A.; Boschloo, G. Copper Phenanthroline as a Fast and High-Performance Redox Mediator for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 9595–9603. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Michaels, H.; Tiepelt, J.; Teuscher, J.; Massaro, A.; Pavone, M.; Giordano, F.; Zakeeruddin, S.M.; Boschloo, G.; et al. Effect of Coordination Sphere Geometry of Copper Redox Mediators on Regeneration and Recombination Behavior in Dye-Sensitized Solar Cell Applications. ACS Appl. Energy Mater. 2018, 1, 4950–4962. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in Regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The emergence of copper(i)-based dye sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the internet of things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjörnsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Bahng, H.W.; Cao, Y.; Yi, C.; Saygili, Y.; Luo, J.; Liu, Y.; Kavan, L.; Moser, J.E.; et al. Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells. Energy Environ. Sci. 2018, 11, 1779–1787. [Google Scholar] [CrossRef]
- Higashino, T.; Iiyama, H.; Nimura, S.; Kurumisawa, Y.; Imahori, H. Effect of Ligand Structures of Copper Redox Shuttles on Photovoltaic Performance of Dye-Sensitized Solar Cells. Inorg. Chem. 2020, 59, 452–459. [Google Scholar] [CrossRef]
- Fürer, S.O.; Bozic-Weber, B.; Neuburger, M.; Constable, E.C.; Housecroft, C.E. Heteroleptic copper(I) sensitizers with one versus two hole-transporting units in functionalized 2,9-dimethyl-1,10-phenanthroline ancillary ligands. RSC Adv. 2015, 5, 69430–69440. [Google Scholar] [CrossRef]
- Büttner, A.; Brauchli, S.Y.; Vogt, R.; Constable, E.C.; Housecroft, C.E. Combining phosphonic acid-functionalized anchoring ligands with asymmetric ancillary ligands in bis(diimine)copper(I) dyes for dye-sensitized solar cells. RSC Adv. 2016, 6, 5205–5213. [Google Scholar] [CrossRef]
- Klein, Y.M.; Willgert, M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Positional isomerism makes a difference: Phosphonic acid anchoring ligands with thienyl spacers in copper(I)-based dye-sensitized solar cells. Dalton Trans. 2016, 45, 4659–4672. [Google Scholar] [CrossRef]
- Fürer, S.O.; Luu, L.Y.N.; Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Improving performance of copper(I)-based dye sensitized solar cells through I3-/I- electrolyte manipulation. Dyes Pigments 2016, 132, 72–78. [Google Scholar] [CrossRef]
- Fürer, S.O.; Bozic-Weber, B.; Schefer, T.; Wobill, C.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Understanding why replacing I3-/I- by cobalt(II)/(III) electrolytes in bis(diimine)copper(I)-based dye-sensitized solar cells improves performance. J. Mater. Chem. A 2016, 4, 12995–13004. [Google Scholar] [CrossRef]
- Baumgartner, Y.; Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Cyanoacrylic- and (1-cyanovinyl)phosphonic acid anchoring ligands for application in copper-based dye-sensitized solar cells. RSC Adv. 2016, 6, 86220–86231. [Google Scholar] [CrossRef]
- Malzner, F.J.; Willgert, M.; Constable, E.C.; Housecroft, C.E. The way to panchromatic copper(I)-based dye-sensitized solar cells: Co-sensitization with the organic dye SQ2. J. Mater. Chem. A 2017, 5, 13717–13729. [Google Scholar] [CrossRef]
- Malzner, F.J.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Exploring simple ancillary ligands in copper-based dye-sensitized solar cells: Effects of a heteroatom switch and of co-sensitization. J. Mater. Chem. A 2017, 5, 4671–4685. [Google Scholar] [CrossRef]
- Stephens, A.J.; Malzner, F.J.; Constable, E.C.; Housecroft, C.E. The influence of phosphonic acid protonation state on the efficiency of bis(diimine)copper(I) dye-sensitized solar cells. Sustain. Energy Fuels 2018, 2, 786–794. [Google Scholar] [CrossRef]
- Büttner, A.; Brauchli, S.Y.; Constable, E.C.; Housecroft, C.E. Effects of Introducing Methoxy Groups into the Ancillary Ligands in Bis(diimine) Copper(I) Dyes for Dye-Sensitized Solar Cells. Inorganics 2018, 6, 40. [Google Scholar] [CrossRef]
- Malzner, F.J.; Housecroft, C.E.; Constable, E.C. The Versatile SALSAC Approach to Heteroleptic Copper(I) Dye Assembly in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 57. [Google Scholar] [CrossRef]
- Karpacheva, M.; Malzner, F.J.; Wobill, C.; Büttner, A.; Constable, E.C.; Housecroft, C.E. Cuprophilia: Dye-sensitized solar cells with copper(I) dyes and copper(I)/(II) redox shuttles. Dyes Pigments 2018, 156, 410–416. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Melchiorre, F.; Biagini, P.; Roberto, D. Coupling of a Copper Dye with a Copper Electrolyte: A Fascinating Springboard for Sustainable Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2018, 1, 751–756. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards efficient sustainable full-copper dye-sensitized solar cells. Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P. Improving the efficiency of copper-dye-sensitized solar cells by manipulating the electrolyte solution. Dalton Trans. 2019, 48, 9818–9823. [Google Scholar] [CrossRef]
- Alamin Ali, H.E.; Altındal, A.; Altun, S.; Odabaş, Z. Highly efficient dye-sensitized solar cells based on metal-free and copper(II) phthalocyanine bearing 2-phenylphenoxy moiety. Dyes Pigments 2016, 124, 180–187. [Google Scholar] [CrossRef]
- Solğun, D.G.; Horoz, S.; Ağırtaş, M.S. Synthesis of novel tetra (4-tritylphenoxy) substituted metallophthalocyanines and investigation of their aggregation, photovoltaic, solar cell properties. Inorg. Nano-Metal Chem. 2018, 48, 508–514. [Google Scholar] [CrossRef]
- Zanotti, G.; Angelini, N.; Mattioli, G.; Notarantonio, S.; Paoletti, A.M.; Pennesi, G.; Rossi, G.; Caschera, D.; De Marco, L.; Gigli, G. Modifications of an unsymmetrical phthalocyanine: Towards stable blue dyes for dye-sensitized solar cells. J. Porphyrins Phthalocyanines 2016, 20, 1207–1216. [Google Scholar] [CrossRef]
- Wills, K.A.; Mandujano-Ramírez, H.J.; Merino, G.; Oskam, G.; Cowper, P.; Jones, M.D.; Cameron, P.J.; Lewis, S.E. What difference does a thiophene make? Evaluation of a 4,4’-bis(thiophene) functionalised 2,2’-bipyridyl copper(I) complex in a dye-sensitized solar cell. Dyes Pigments 2016, 134, 419–426. [Google Scholar] [CrossRef]
- Yadav, R.; Waghadkar, Y.; Kociok-Köhn, G.; Kumar, A.; Rane, S.B.; Chauhan, R. Transition metal ferrocenyl dithiocarbamates functionalized dye-sensitized solar cells with hydroxy as an anchoring group. Opt. Mater. 2016, 62, 176–183. [Google Scholar] [CrossRef]
- Jayapal, M.; Haque, A.; Al-Busaidi, I.J.; Al-Rasbi, N.; Al-Suti, M.K.; Khan, M.S.; Al-Balushi, R.; Islam, S.M.; Xin, C.; Wu, W.; et al. Dicopper(I) Complexes Incorporating Acetylide-Functionalized Pyridinyl-Based Ligands: Synthesis, Structural, and Photovoltaic Studies. Inorg. Chem. 2018, 57, 12113–12124. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Y.; Liu, Y.; Zhu, C.; Chen, T.; Zhong, C. Dye sensitizers of polymer using the complex of Cd (II) or Cu (II) with imidazole as auxiliary electron acceptor for dye-sensitized solar cells. Dyes Pigments 2017, 139, 420–430. [Google Scholar] [CrossRef]
- Asatkar, A.K.; Bedi, A.; Zade, S.S. Metallo-organic conjugated systems for organic electronics. Isr. J. Chem. 2014, 54, 467–495. [Google Scholar] [CrossRef]
- Zink, D.M.; Volz, D.; Baumann, T.; Mydlak, M.; Flügge, H.; Friedrichs, J.; Nieger, M.; Bräse, S. Heteroleptic, dinuclear copper(i) complexes for application in organic light-emitting diodes. Chem. Mater. 2013, 25, 4471–4486. [Google Scholar] [CrossRef]
- Zink, D.M.; Grab, T.; Baumann, T.; Nieger, M.; Barnes, E.C.; Klopper, W.; Bräse, S. Experimental and theoretical study of novel luminescent Di-, Tri-, and tetranuclear copper triazole complexes. Organometallics 2011, 30, 3275–3283. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Su, Z. Realization of high-energy emission from [Cu(N-N)(P-P)] +complexes for organic light-emitting diode applications. J. Phys. Chem. C 2009, 113, 13968–13973. [Google Scholar] [CrossRef]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime. Science 2019, 363, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Chan, W.H.; Zhoub, X.M.; Che, C.M. Letter Light-emitting diode device from a luminescent organocopper(I) compound. New J. Chem. 1999, 23, 263–265. [Google Scholar] [CrossRef]
- Blaskie, M.W.; Mcmillin, D.R. Photostudies of Copper(I) Systems. 6. Room-Temperature Emission and Quenching Studies of [Cu(dmp)2]+. Inorg. Chem 1980, 19, 47907. [Google Scholar] [CrossRef]
- A Palmer, C.E.; McMillin, D.R.; Kirmaier, C.; Holten, D. Flash Photolysis and Quenching Studies of Copper(I) Systems in the Presence of Lewis Bases: Inorganic Exciplexes? Inorg. Chem 1987, 26, 3167–3170. [Google Scholar] [CrossRef]
- Buckner, M.T.; McMillin, D.R. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 17, 759–761. [Google Scholar] [CrossRef]
- Kuang, S.M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and structural characterization of Cu(I) and Ni(II) complexes that contain the bis[2-(diphenylphosphino)phenyl]ether ligand. Novel emission properties for the Cu(I) species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar] [CrossRef]
- Cuttell, D.G.; Kuang, S.M.; Fanwick, P.E.; McMillin, D.R.; Walton, R.A. Simple Cu(I) complexes with unprecedented excited-state lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. [Google Scholar] [CrossRef]
- Igawa, S.; Hashimoto, M.; Kawata, I.; Yashima, M.; Hoshino, M.; Osawa, M. Highly efficient green organic light-emitting diodes containing luminescent tetrahedral copper(i) complexes. J. Mater. Chem. C 2013, 1, 542–551. [Google Scholar] [CrossRef]
- Hashimoto, M.; Igawa, S.; Yashima, M.; Kawata, I.; Hoshino, M.; Osawa, M. Highly efficient green organic light-emitting diodes containing luminescent three-coordinate copper(I) complexes. J. Am. Chem. Soc. 2011, 133, 10348–10351. [Google Scholar] [CrossRef]
- Osawa, M.; Hoshino, M.; Hashimoto, M.; Kawata, I.; Igawa, S.; Yashima, M. Application of three-coordinate copper(i) complexes with halide ligands in organic light-emitting diodes that exhibit delayed fluorescence. Dalton Trans. 2015, 44, 8369–8378. [Google Scholar] [CrossRef]
- Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Fléchon, C.; Zink, D.M.; Friedrichs, J.; Flügge, H.; Steininger, R.; Göttlicher, J.; et al. Bridging the efficiency gap: Fully bridged dinuclear Cu(I)-complexes for singlet harvesting in high-efficiency OLEDs. Adv. Mater. 2015, 27, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, Z.; Zhao-Karger, Z.; Mueller, J.E.; Jung, C.; Klyatskaya, S.; Diemant, T.; Fuhr, O.; Jacob, T.; Behm, R.J.; et al. A Porphyrin Complex as a Self-Conditioned Electrode Material for High-Performance Energy Storage. Angew. Chem. Int. Ed. 2017, 56, 10341–10346. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Wu, D.Y.; Gao, P.; Chen, Z.; Ruben, M.; Fichtner, M. Monitoring the Electrochemical Energy Storage Processes of an Organic Full Rechargeable Battery via Operando Raman Spectroscopy: A Mechanistic Study. Chem. Mater. 2019, 31, 3239–3247. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, P.; Wang, W.; Klyatskaya, S.; Zhao-Karger, Z.; Wang, D.; Kübel, C.; Fuhr, O.; Fichtner, M.; Ruben, M. A Lithium-Free Energy-Storage Device Based on an Alkyne-Substituted-Porphyrin Complex. ChemSusChem 2019, 12, 3737–3741. [Google Scholar] [CrossRef]
- Wu, D.; Li, H.; Li, R.; Hu, Y.; Hu, X. In situ growth of copper rhodizonate complexes on reduced graphene oxide for high-performance organic lithium-ion batteries. Chem. Commun. 2018, 54, 11415–11418. [Google Scholar] [CrossRef]
- Kapaev, R.R.; Olthof, S.; Zhidkov, I.S.; Kurmaev, E.Z.; Stevenson, K.J.; Meerholz, K.; Troshin, P.A. Nickel(II) and Copper(II) Coordination Polymers Derived from 1,2,4,5-Tetraaminobenzene for Lithium-Ion Batteries. Chem. Mater. 2019, 31, 5197–5205. [Google Scholar] [CrossRef]
- Wang, H.G.; Wang, H.; Si, Z.; Li, Q.; Wu, Q.; Shao, Q.; Wu, L.; Liu, Y.; Wang, Y.; Song, S.; et al. A Bipolar and Self-Polymerized Phthalocyanine Complex for Fast and Tunable Energy Storage in Dual-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 10204–10208. [Google Scholar] [CrossRef]
- Peljo, P.; Lloyd, D.; Doan, N.; Majaneva, M.; Kontturi, K. Towards a thermally regenerative all-copper redox flow battery. Phys. Chem. Chem. Phys. 2014, 16, 2831–2835. [Google Scholar] [CrossRef]
- Li, Y.; Sniekers, J.; Malaquias, J.; Li, X.; Schaltin, S.; Stappers, L.; Binnemans, K.; Fransaer, J.; Vankelecom, I.F.J. A non-aqueous all-copper redox flow battery with highly soluble active species. Electrochim. Acta 2017, 236, 116–121. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Liu, S.; Wang, J.L.; Lin, W. A biomimetic copper water oxidation catalyst with low overpotential. J. Am. Chem. Soc. 2014, 136, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Stott, L.A.; Prosser, K.E.; Berdichevsky, E.K.; Walsby, C.J.; Warren, J.J. Lowering water oxidation overpotentials using the ionisable imidazole of copper(2-(2’-pyridyl)imidazole). Chem. Commun. 2017, 53, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Prevedello, A.; Bazzan, I.; Dalle Carbonare, N.; Giuliani, A.; Bhardwaj, S.; Africh, C.; Cepek, C.; Argazzi, R.; Bonchio, M.; Caramori, S.; et al. Heterogeneous and Homogeneous Routes in Water Oxidation Catalysis Starting from CuII Complexes with Tetraaza Macrocyclic Ligands. Chem. Asian J. 2016, 11, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, N.; Lei, H.; Guo, D.; Liu, H.; Zhang, Z.; Zhang, W.; Lai, W.; Cao, R. Electrocatalytic Water Oxidation by a Water-Soluble Copper(II) Complex with a Copper-Bound Carbonate Group Acting as a Potential Proton Shuttle. Inorg. Chem. 2017, 56, 13368–13375. [Google Scholar] [CrossRef]
- Kuilya, H.; Alam, N.; Sarma, D.; Choudhury, D.; Kalita, A. Ligand assisted electrocatalytic water oxidation by a copper(ii) complex in neutral phosphate buffer. Chem. Comm. 2019, 55, 5483–5486. [Google Scholar] [CrossRef]
- Zhang, M.T.; Chen, Z.; Kang, P.; Meyer, T.J. Electrocatalytic water oxidation with a copper(II) polypeptide complex. J. Am. Chem. Soc. 2013, 135, 2048–2051. [Google Scholar] [CrossRef]
- Pap, J.S.; Szyrwiel, Ł; Srankó, D.; Kerner, Z.; Setner, B.; Szewczuk, Z.; Malinka, W. Electrocatalytic water oxidation by CuII complexes with branched peptides. Chem. Comm. 2015, 51, 6322–6324. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Zhang, Z.; Zhang, W.; Lai, W.; Wang, Y.; Cao, R. Low overpotential water oxidation at neutral pH catalyzed by a copper(ii) porphyrin. Chem. Sci. 2019, 10, 2613–2622. [Google Scholar] [CrossRef]
- Fang, T.; Fu, L.Z.; Zhou, L.L.; Zhan, S.Z. A water-soluble dinuclear copper electrocatalyst, [Cu(oxpn)Cu(OH)2] for both water reduction and oxidation. Electrochim. Acta 2015, 161, 388–394. [Google Scholar] [CrossRef]
- Koepke, S.J.; Light, K.M.; Vannatta, P.E.; Wiley, K.M.; Kieber-Emmons, M.T. Electrocatalytic Water Oxidation by a Homogeneous Copper Catalyst Disfavors Single-Site Mechanisms. J. Am. Chem. Soc. 2017, 139, 8586–8600. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Yang, B.; Wei, X.Z.; Dong, B.W.; Kao, Y.; Huang, M.Y.; Tung, C.H.; Wu, L.Z. A Bio-inspired Cu4O4 Cubane: Effective Molecular Catalysts for Electrocatalytic Water Oxidation in Aqueous Solution. Angew. Chem. Int. Ed. 2018, 57, 7850–7854. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benesperi, I.; Singh, R.; Freitag, M. Copper Coordination Complexes for Energy-Relevant Applications. Energies 2020, 13, 2198. https://doi.org/10.3390/en13092198
Benesperi I, Singh R, Freitag M. Copper Coordination Complexes for Energy-Relevant Applications. Energies. 2020; 13(9):2198. https://doi.org/10.3390/en13092198
Chicago/Turabian StyleBenesperi, Iacopo, Reena Singh, and Marina Freitag. 2020. "Copper Coordination Complexes for Energy-Relevant Applications" Energies 13, no. 9: 2198. https://doi.org/10.3390/en13092198
APA StyleBenesperi, I., Singh, R., & Freitag, M. (2020). Copper Coordination Complexes for Energy-Relevant Applications. Energies, 13(9), 2198. https://doi.org/10.3390/en13092198




