Optimization of Perfluoropolyether-Based Gas Diffusion Media Preparation for PEM Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
3. Results
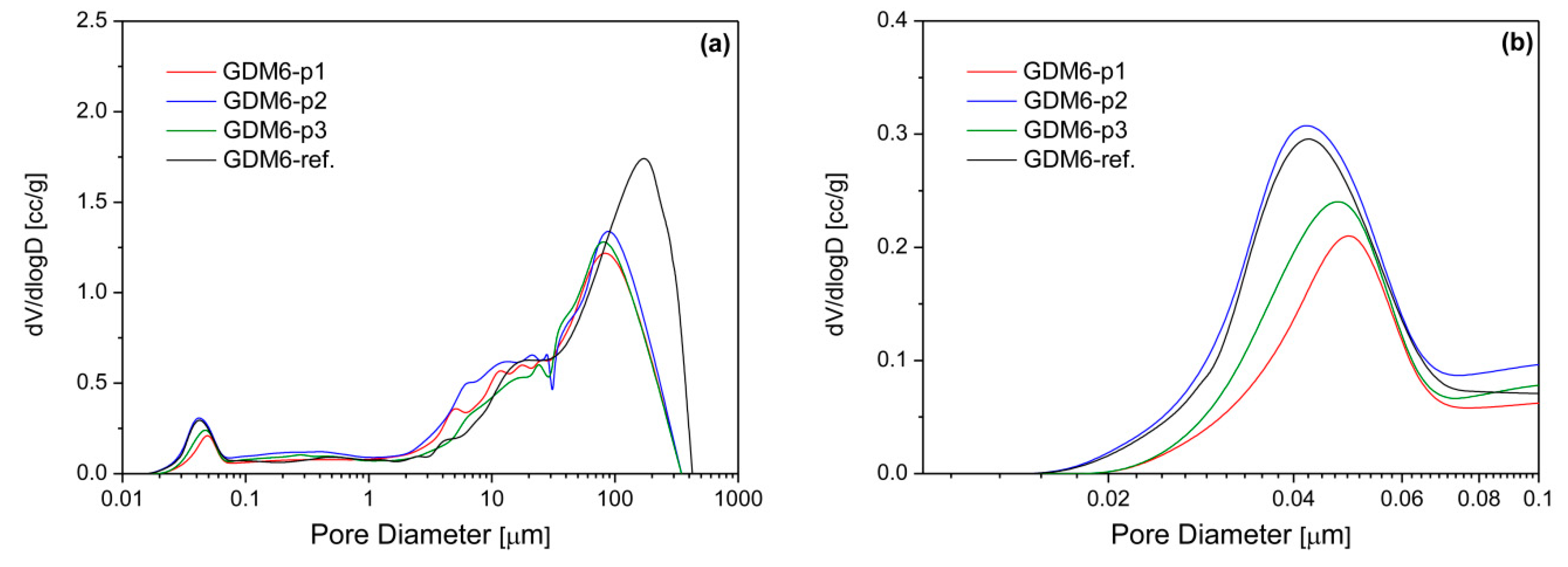
3.1. Physical Characterization
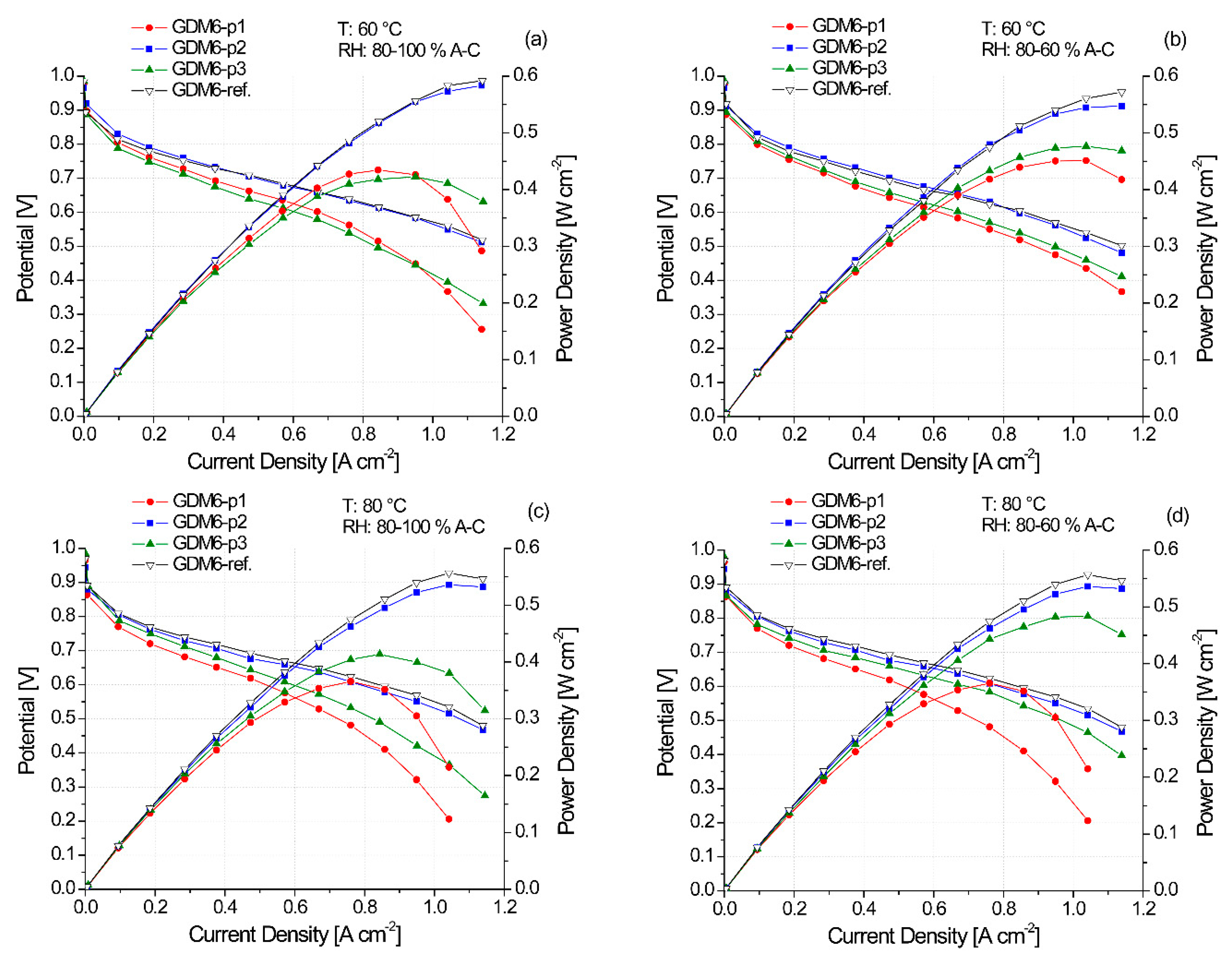
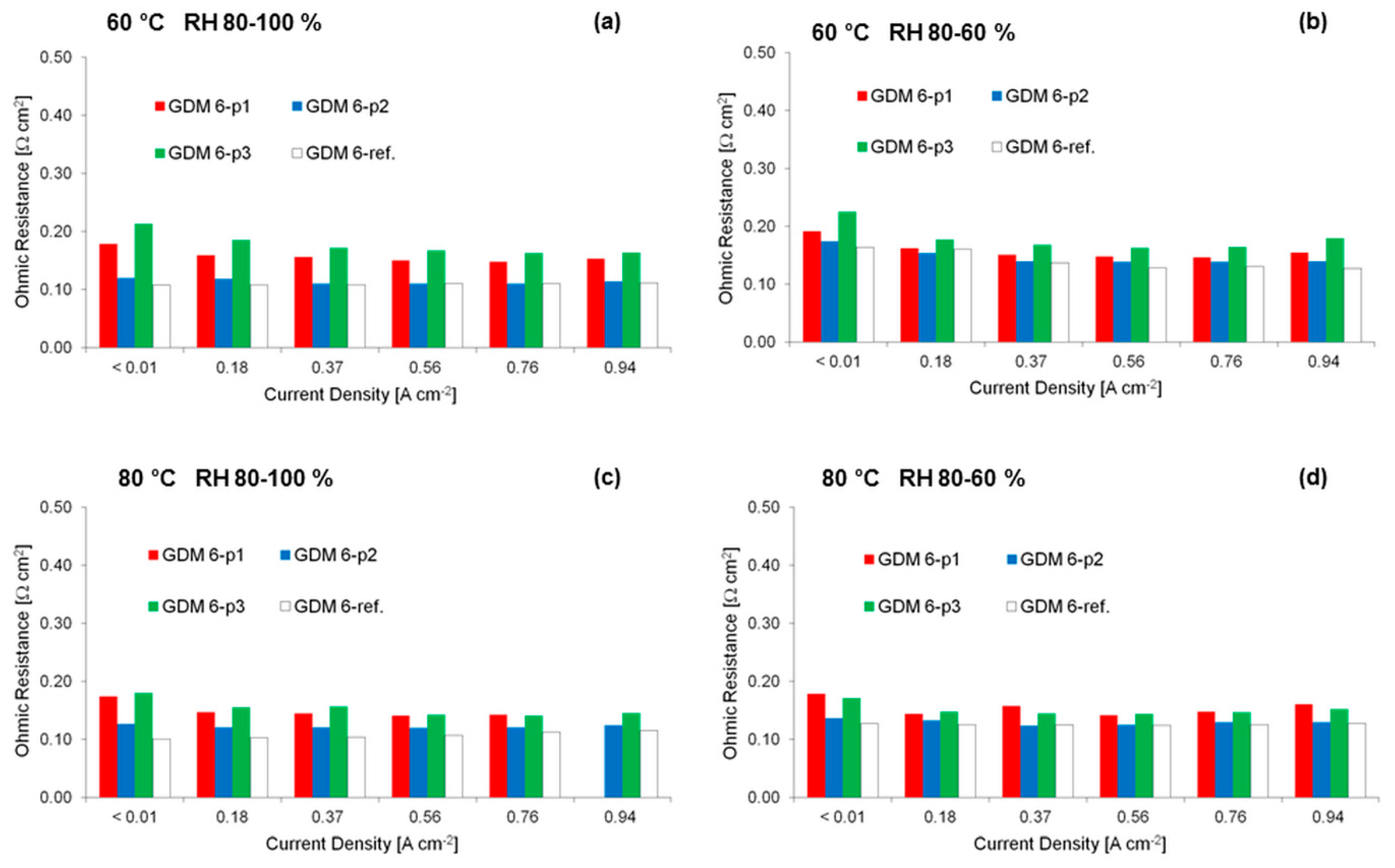
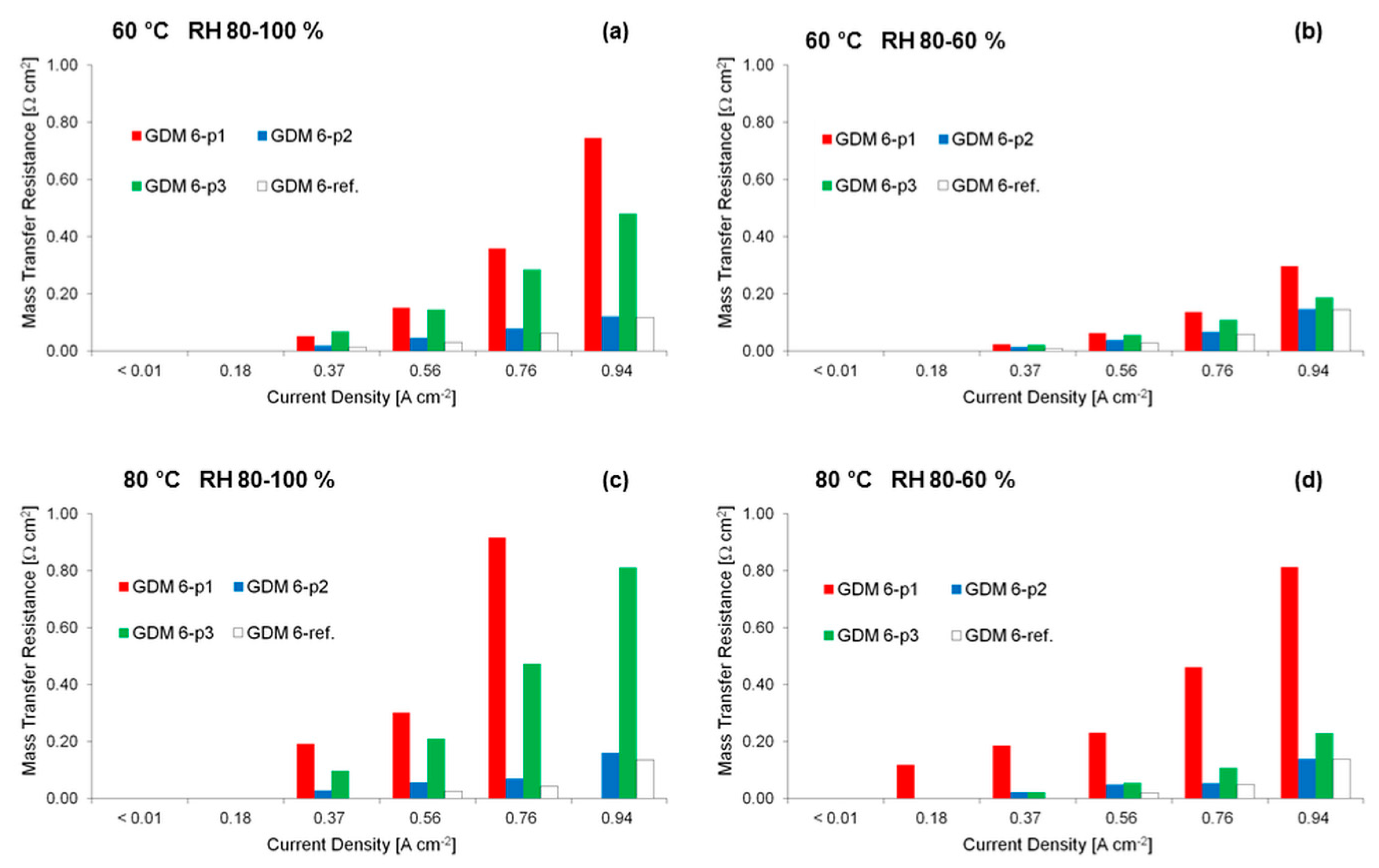
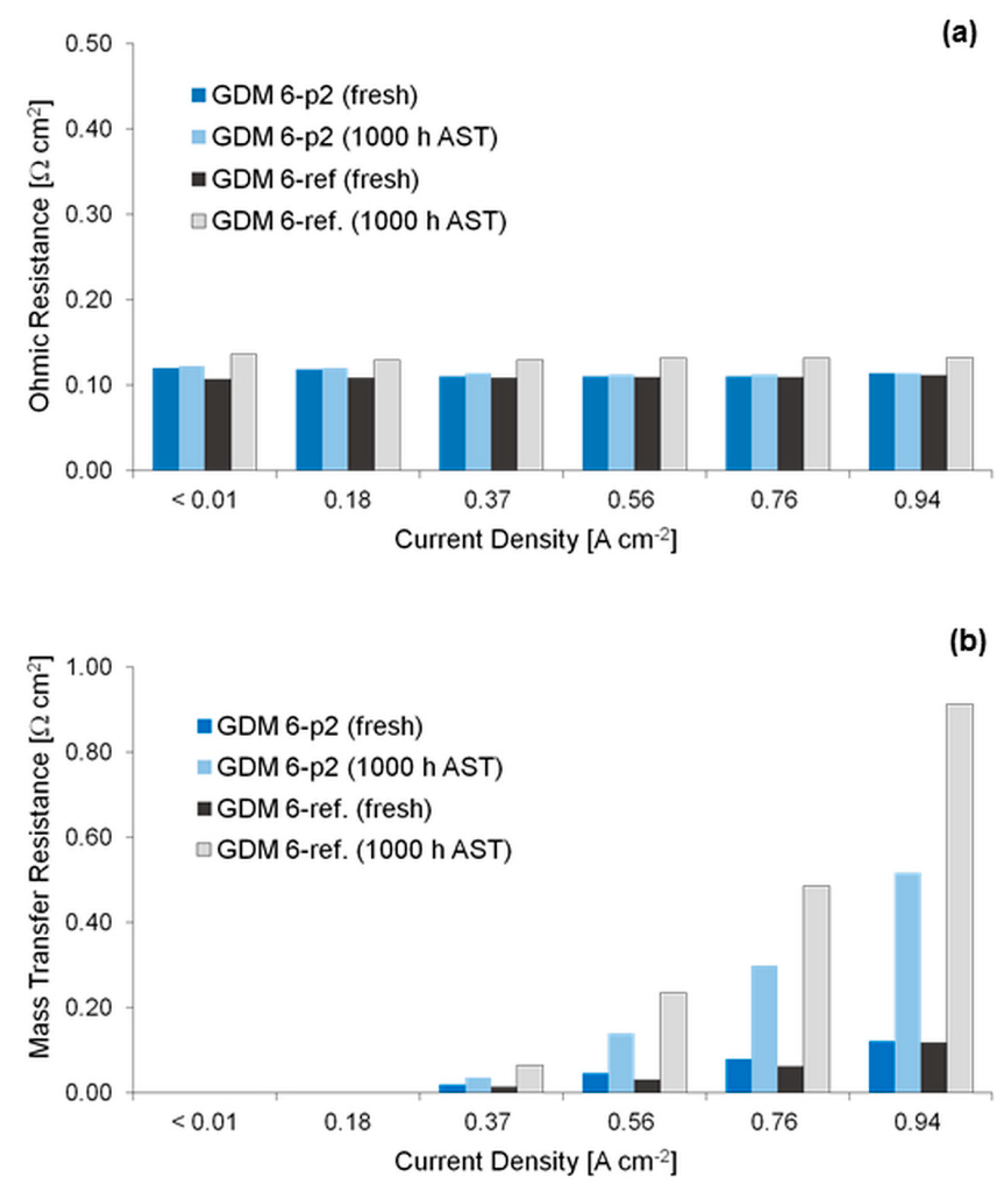
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Omrani, R.; Shabani, B. Review of gas diffusion layer for proton exchange membrane-based technologies with a focus on unitised regenerative fuel cells. Int. J. Hydrog. Energy 2019, 44, 3834–3860. [Google Scholar] [CrossRef]
- Ehteshami, S.M.M.; Chan, S.H. The role of hydrogen and fuel cells to store renewable energy in the future energy network—Potentials and challenges. Energy Policy 2014, 73, 103–109. [Google Scholar] [CrossRef]
- Garland, N.L.; Papageorgopoulos, D.C.; Stanford, J.M. Hydrogen and fuel cell technology: Progress, challenges, and future directions. Enrgy Proced 2012, 28, 2–11. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice, 2nd ed.; Academic Press: London, UK, 2013; p. 444. [Google Scholar]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Wan, Z.M.; Chang, H.W.; Shu, S.M.; Wang, Y.X.; Tang, H.L. A Review on Cold Start of Proton Exchange Membrane Fuel Cells. Energies 2014, 7, 3179–3203. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. Gas diffusion layer modifications and treatments for improving the performance of proton exchange membrane fuel cells and electrolysers: A review. Int. J. Hydrog. Energy 2017, 42, 28515–28536. [Google Scholar] [CrossRef]
- Leeuwner, M.J.; Patra, A.; Wilkinson, D.P.; Gyenge, E.L. Graphene and reduced graphene oxide based microporous layers for high-performance proton-exchange membrane fuel cells under varied humidity operation. J. Power Sources 2019, 423, 192–202. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.W.; Popov, B.N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrog. Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Morgan, J.M.; Datta, R. Understanding the gas diffusion layer in proton exchange membrane fuel cells. I. How its structural characteristics affect diffusion and performance. J. Power Sources 2014, 251, 269–278. [Google Scholar] [CrossRef]
- Mariani, M.; Latorrata, S.; Stampino, P.G.; Dotelli, G. Evaluation of Graphene Nanoplatelets as a Microporous Layer Material for PEMFC: Performance and Durability Analysis. Fuel Cells 2019, 19, 685–694. [Google Scholar] [CrossRef]
- Chang, H.M.; Lin, C.W.; Chang, M.H.; Shiu, H.R.; Chang, W.C.; Tsau, F.H. Optimization of polytetrafluoroethylene content in cathode gas diffusion layer by the evaluation of compression effect on the performance of a proton exchange membrane fuel cell. J. Power Sources 2011, 196, 3773–3780. [Google Scholar] [CrossRef]
- Gurau, V.; Bluemle, M.J.; De Castro, E.S.; Tsou, Y.M.; Mann, J.A.; Zawodzinski, T.A. Characterization of transport properties in gas diffusion layers for proton exchange membrane fuel cells—1. Wettability (internal contact angle to water and surface energy of GDL fibers). J. Power Sources 2006, 160, 1156–1162. [Google Scholar] [CrossRef]
- Gurau, V.; Bluemle, M.J.; De Castro, E.S.; Tsou, Y.M.; Zawodzinski, T.A.; Mann, J.A. Characterization of transport properties in gas diffusion layers for proton exchange membrane fuel cells 2. Absolute permeability. J. Power Sources 2007, 165, 793–802. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.Y.; Chen, K.S. Elucidating differences between carbon paper and carbon cloth in polymer electrolyte fuel cells. Electrochim. Acta 2007, 52, 3965–3975. [Google Scholar] [CrossRef]
- Ozden, A.; Shahgaldi, S.; Li, X.G.; Hamdullahpur, F. A graphene-based microporous layer for proton exchange membrane fuel cells: Characterization and performance comparison. Renew Energy 2018, 126, 485–494. [Google Scholar] [CrossRef]
- Alink, R.; Gerteisen, D. Modeling the Liquid Water Transport in the Gas Diffusion Layer for Polymer Electrolyte Membrane Fuel Cells Using a Water Path Network. Energies 2013, 6, 4508–4530. [Google Scholar] [CrossRef]
- Lee, J.; Liu, H.; George, M.G.; Banerjee, R.; Ge, N.; Chevalier, S.; Kotaka, T.; Tabuchi, Y.; Bazylak, A. Microporous layer to carbon fibre substrate interface impact on polymer electrolyte membrane fuel cell performance. J. Power Sources 2019, 422, 113–121. [Google Scholar] [CrossRef]
- Latorrata, S.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Performance Evaluation and Durability Enhancement of FEP-Based Gas Diffusion Media for PEM Fuel Cells. Energies 2017, 10, 2063. [Google Scholar] [CrossRef]
- Kitahara, T.; Nakajima, H.; Mori, K. Hydrophilic and hydrophobic double microporous layer coated gas diffusion layer for enhancing performance of polymer electrolyte fuel cells under no-humidification at the cathode. J. Power Sources 2012, 199, 29–36. [Google Scholar] [CrossRef]
- Leeuwner, M.J.; Wilkinson, D.P.; Gyenge, E.L. Novel Graphene Foam Microporous Layers for PEM Fuel Cells: Interfacial Characteristics and Comparative Performance. Fuel Cells 2015, 15, 790–801. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Effects of microporous layers in polymer electrolyte fuel cells. J. Electrochem. Soc. 2005, 152, A677–A688. [Google Scholar] [CrossRef]
- Park, S.; Popov, B.N. Effect of hydrophobicity and pore geometry in cathode GDL on PEM fuel cell performance. Electrochim. Acta 2009, 54, 3473–3479. [Google Scholar] [CrossRef]
- Wong, A.K.C.; Ge, N.; Shrestha, P.; Liu, H.; Fahy, K.; Bazylak, A. Polytetrafluoroethylene content in standalone microporous layers: Tradeoff between membrane hydration and mass transport losses in polymer electrolyte membrane fuel cells. Appl. Energy 2019, 240, 549–560. [Google Scholar] [CrossRef]
- Shrestha, P.; Ouellette, D.; Lee, J.; Ge, N.; Kai, A.; Wong, C.; Muirhead, D.; Liu, H.; Banerjee, R.; Bazylak, A. Graded Microporous Layers for Enhanced Capillary-Driven Liquid Water Removal in Polymer Electrolyte Membrane Fuel Cells. Adv. Mater. Interfaces 2019, 6, 1901157. [Google Scholar] [CrossRef]
- Balzarotti, R.; Latorrata, S.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Development and Characterization of Non-Conventional Micro-Porous Layers for PEM Fuel Cells. Energies 2015, 8, 7070–7083. [Google Scholar] [CrossRef]
- Latorrata, S.; Balzarotti, R.; Stampino, P.G.; Cristiani, C.; Dotelli, G.; Guilizzoni, M. Design of properties and performances of innovative gas diffusion media for polymer electrolyte membrane fuel cells. Prog. Org. Coat. 2015, 78, 517–525. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells: Fundamentals and Applications; Springer: London, UK, 2010; pp. 1–420. [Google Scholar]
- Latorrata, S.; Pelosato, R.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Use of Electrochemical Impedance Spectroscopy for the Evaluation of Performance of PEM Fuel Cells Based on Carbon Cloth Gas Diffusion Electrodes. J. Spectrosc. 2018, 2018, 3254375. [Google Scholar] [CrossRef]
- Asghari, S.; Mokmeli, A.; Samavati, M. Study of PEM fuel cell performance by electrochemical impedance spectroscopy. Int. J. Hydrog. Energy 2010, 35, 9283–9290. [Google Scholar] [CrossRef]
- Wagner, N. Characterization of membrane electrode assemblies in polymer electrolyte fuel cells using a.c. impedance spectroscopy. J. Appl. Electrochem. 2002, 32, 859–863. [Google Scholar] [CrossRef]
- Dhirde, A.M.; Dale, N.V.; Salehfar, H.; Mann, M.D.; Han, T.H. Equivalent Electric Circuit Modeling and Performance Analysis of a PEM Fuel Cell Stack Using Impedance Spectroscopy. IEEE Trans. Energy Convers. 2010, 25, 778–786. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; Kumbur, E.C.; Mench, M.M.; Liu, W.; Moore, D.; Murthy, M. Investigation of macro- and micro-porous layer interaction in polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2008, 33, 3351–3367. [Google Scholar] [CrossRef]
- Latorrata, S.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Development of an optimal gas diffusion medium for polymer electrolyte membrane fuel cells and assessment of its degradation mechanisms. Int. J. Hydrog. Energy 2015, 40, 14596–14608. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, H.M.; Zhang, J.L.; Xu, H.F.; Zhu, X.B.; Chen, J.; Yi, B.L. A bi-functional micro-porous layer with composite carbon black for PEM fuel cells. J. Power Sources 2006, 162, 474–479. [Google Scholar] [CrossRef]



| Sample | GDL | MPL | Production Process | ||
|---|---|---|---|---|---|
| Hydrophobic Agent | Content [wt. %] | Hydrophobic Agent | Content [wt. %] | ||
| GDM6-p1 | PFPE | 1 | PFPE | 6 | Path 1 |
| GDM6-p2 | PFPE | 1 | PFPE | 6 | Path 2 |
| GDM6-p3 | PFPE | 1 | PFPE | 6 | Path 3 |
| GDM6-ref. 1 | PTFE | 12 | PFPE | 6 | reference 1 |
| Sample | C.A. BCT [°] | C.A. ACT [°] | Loss ACT [wt. %] | |
|---|---|---|---|---|
| Anode | Cathode | |||
| GDM6-p1 | 152 ± 3 | 124 ± 9 | 152 ± 4 | 9.2 |
| GDM6-p2 | 151 ± 2 | 149 ± 5 | 149 ± 3 | 0.9 |
| GDM6-p3 | 150 ± 3 | 153 ± 3 | 153 ± 4 | 5.7 |
| GDM6-ref. [27] | 146 ± 5 | 154 ± 3 | 145 ± 2 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzarotti, R.; Latorrata, S.; Mariani, M.; Gallo Stampino, P.; Dotelli, G. Optimization of Perfluoropolyether-Based Gas Diffusion Media Preparation for PEM Fuel Cells. Energies 2020, 13, 1831. https://doi.org/10.3390/en13071831
Balzarotti R, Latorrata S, Mariani M, Gallo Stampino P, Dotelli G. Optimization of Perfluoropolyether-Based Gas Diffusion Media Preparation for PEM Fuel Cells. Energies. 2020; 13(7):1831. https://doi.org/10.3390/en13071831
Chicago/Turabian StyleBalzarotti, Riccardo, Saverio Latorrata, Marco Mariani, Paola Gallo Stampino, and Giovanni Dotelli. 2020. "Optimization of Perfluoropolyether-Based Gas Diffusion Media Preparation for PEM Fuel Cells" Energies 13, no. 7: 1831. https://doi.org/10.3390/en13071831
APA StyleBalzarotti, R., Latorrata, S., Mariani, M., Gallo Stampino, P., & Dotelli, G. (2020). Optimization of Perfluoropolyether-Based Gas Diffusion Media Preparation for PEM Fuel Cells. Energies, 13(7), 1831. https://doi.org/10.3390/en13071831









