Ruthenium (Ru) Doped Titanium Dioxide (P25) Electrode for Dye Sensitized Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
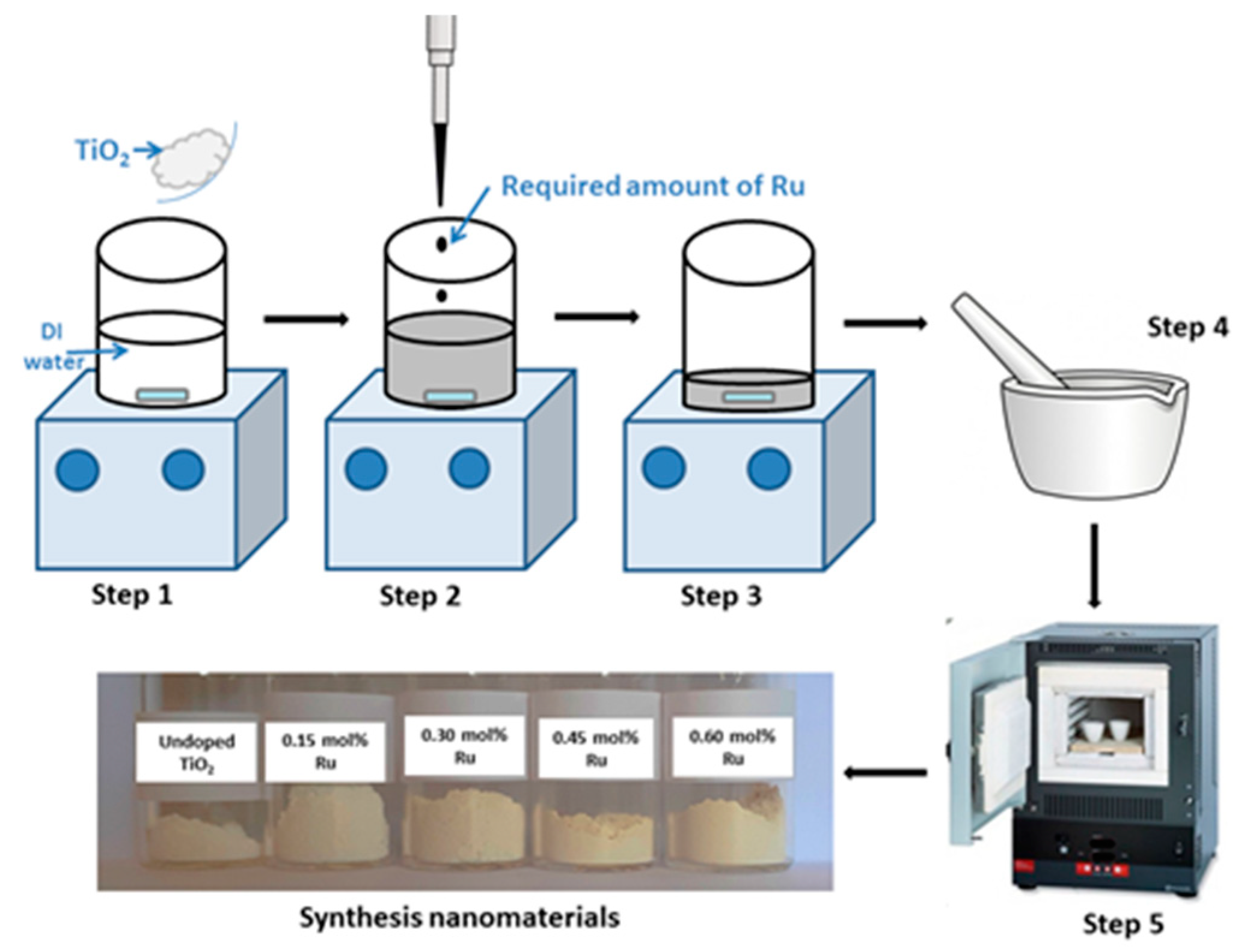
2.2.1. Preparation of Ru-Doped TiO2 Nanomaterials
2.2.2. Fabrication of DSSCs
2.2.3. Characterization
3. Results and Discussion
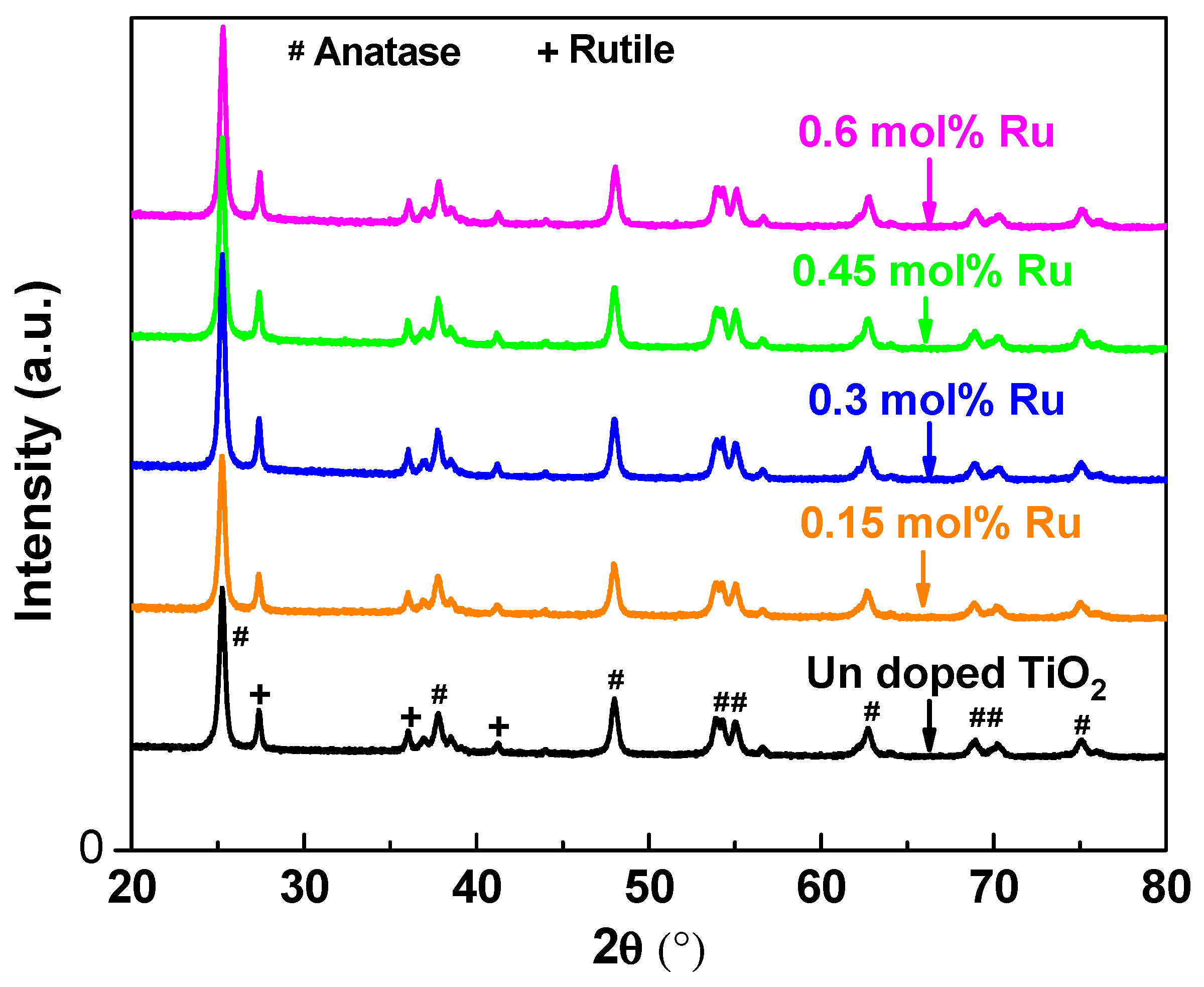
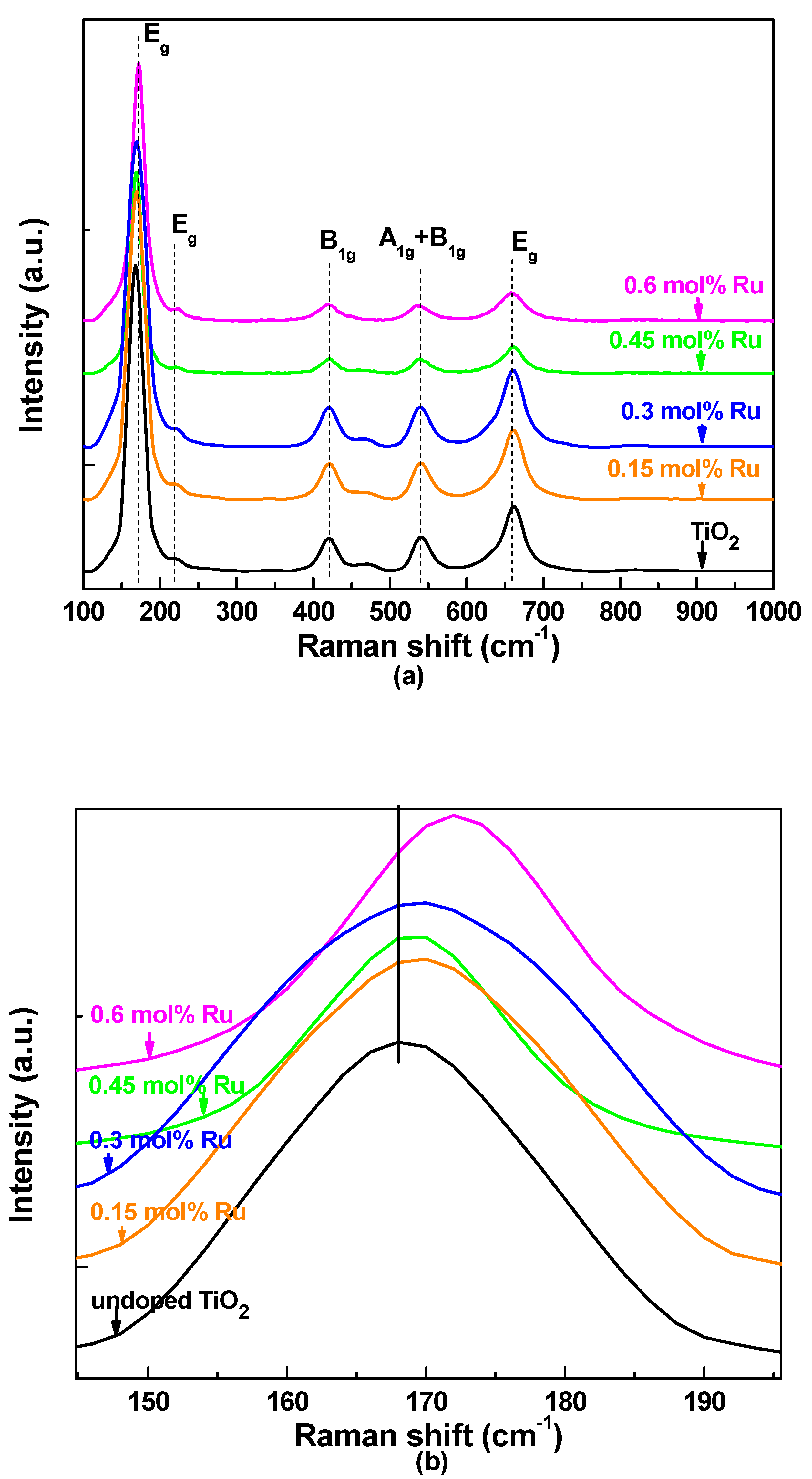
3.1. X-ray Siffraction and Raman Spectroscopy
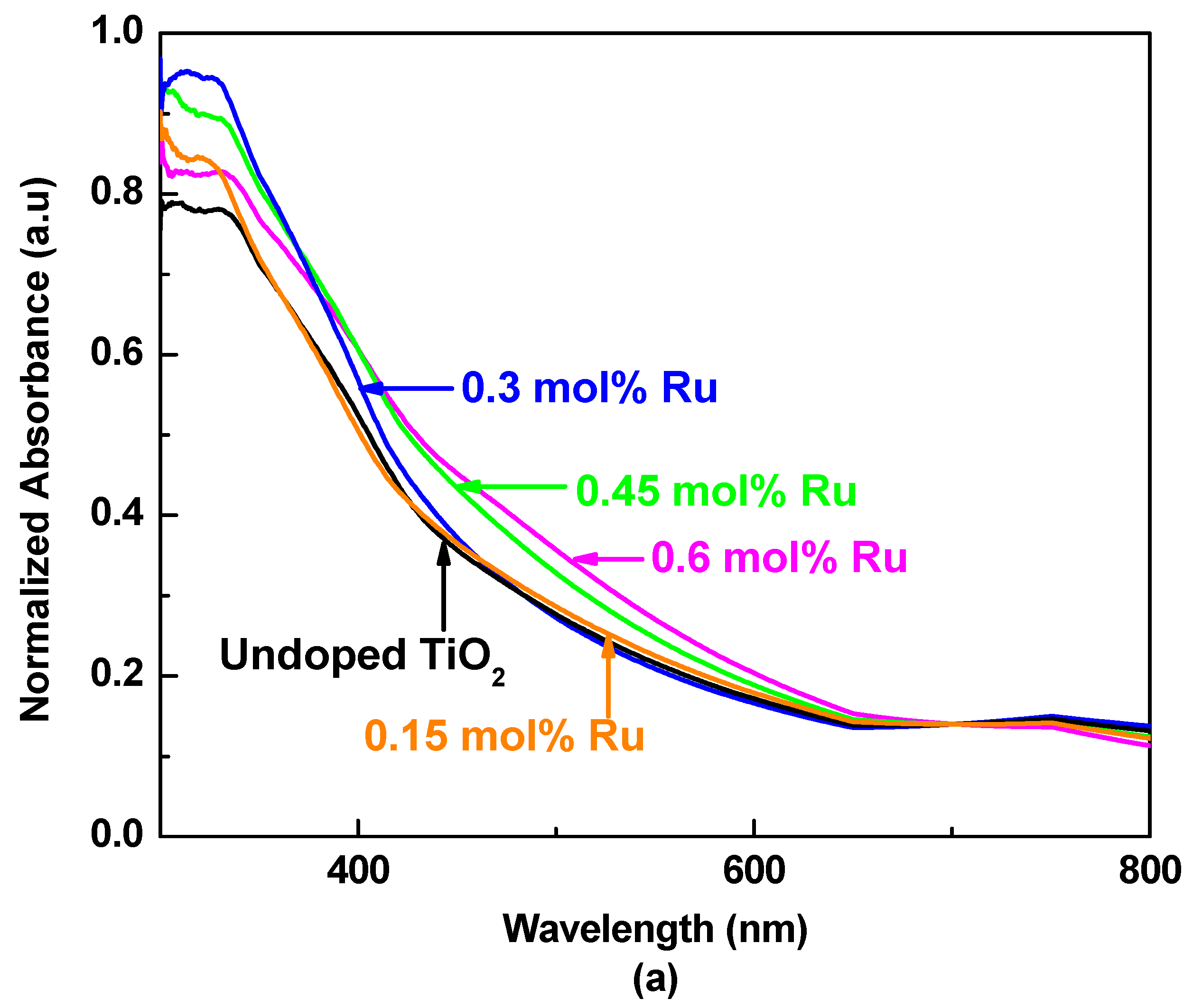
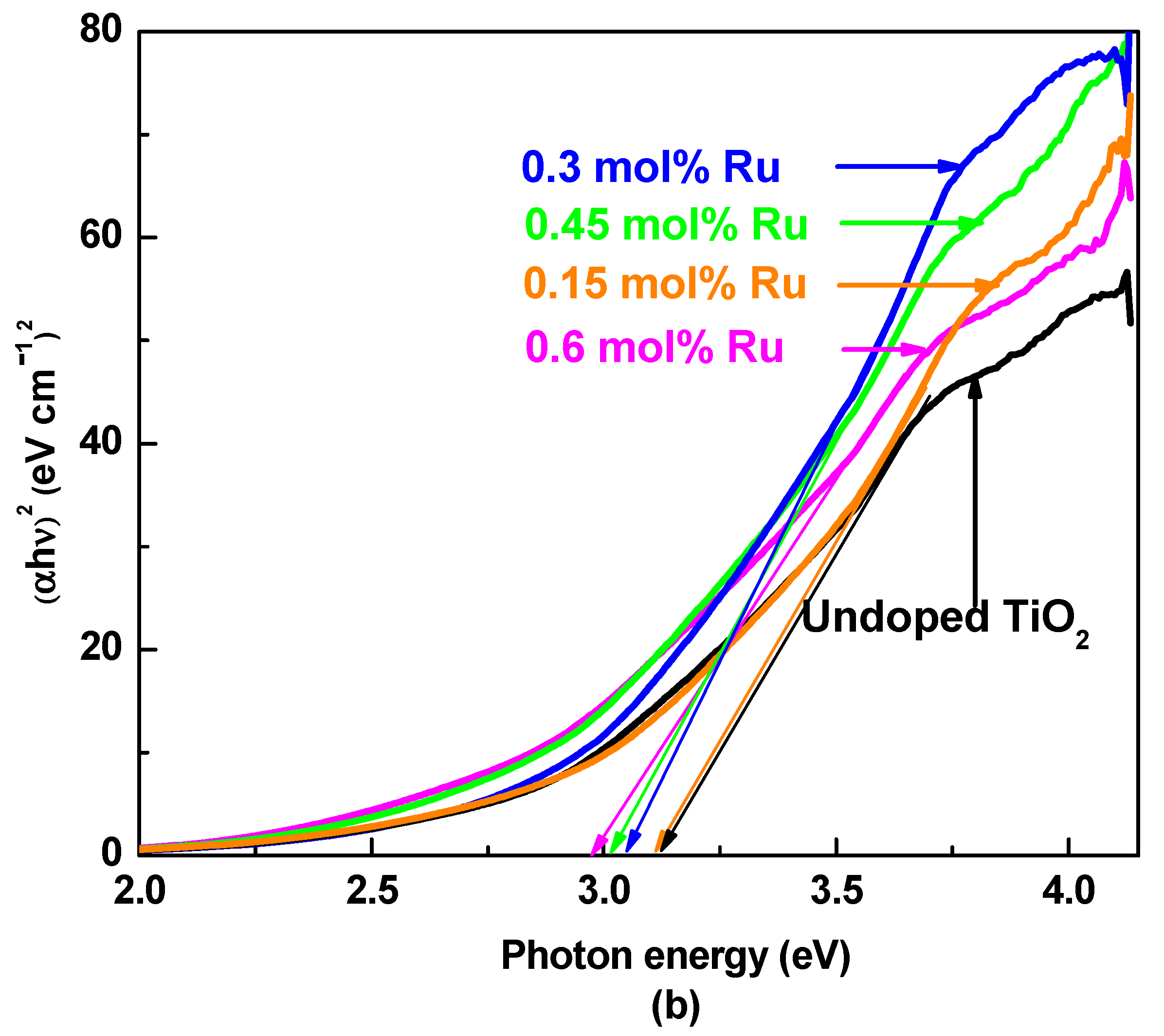
3.2. UV–Visible Absorption Spectroscopy
3.3. Energy-Dispersive X-ray Spectroscopy
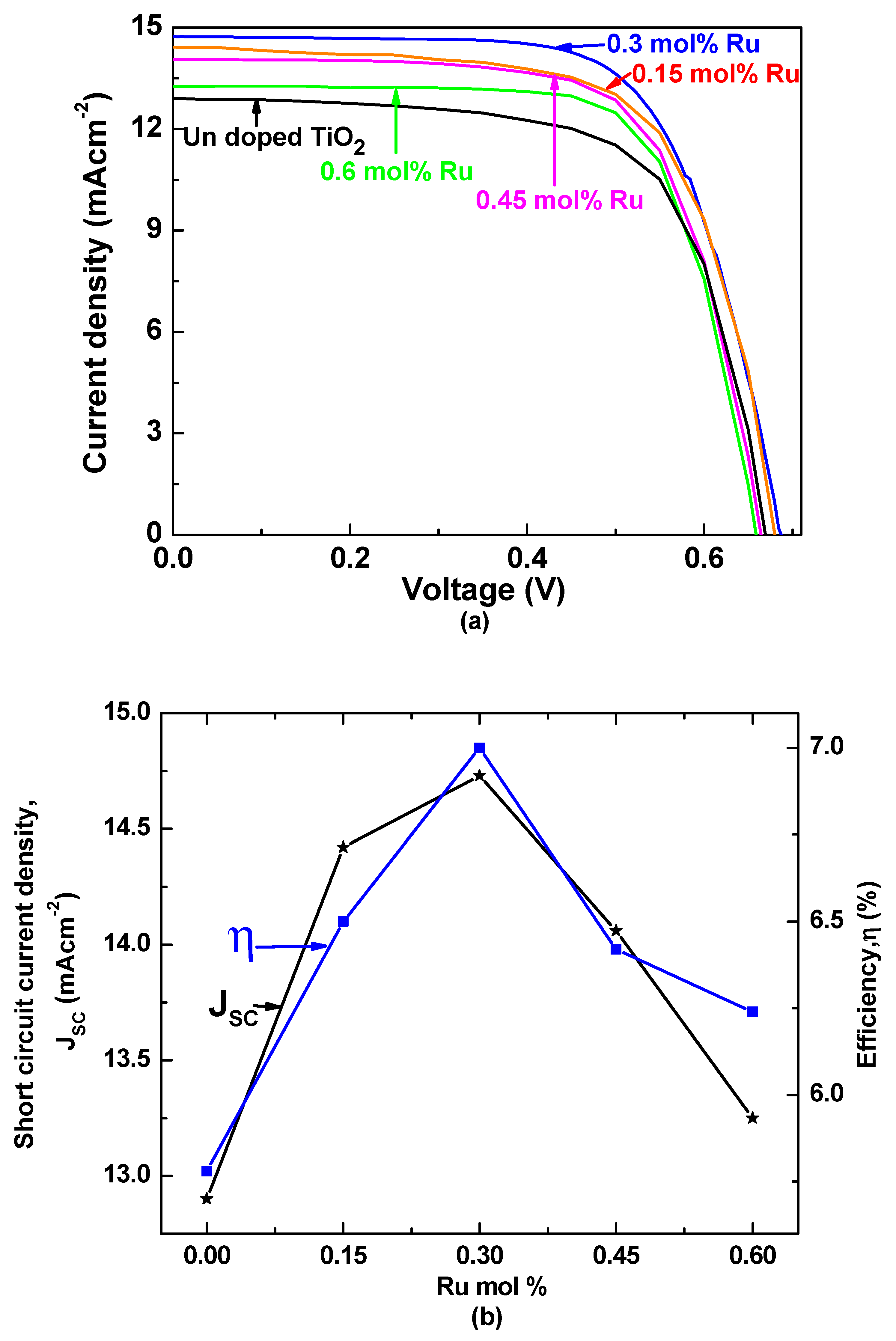
3.4. J–V Characteristics
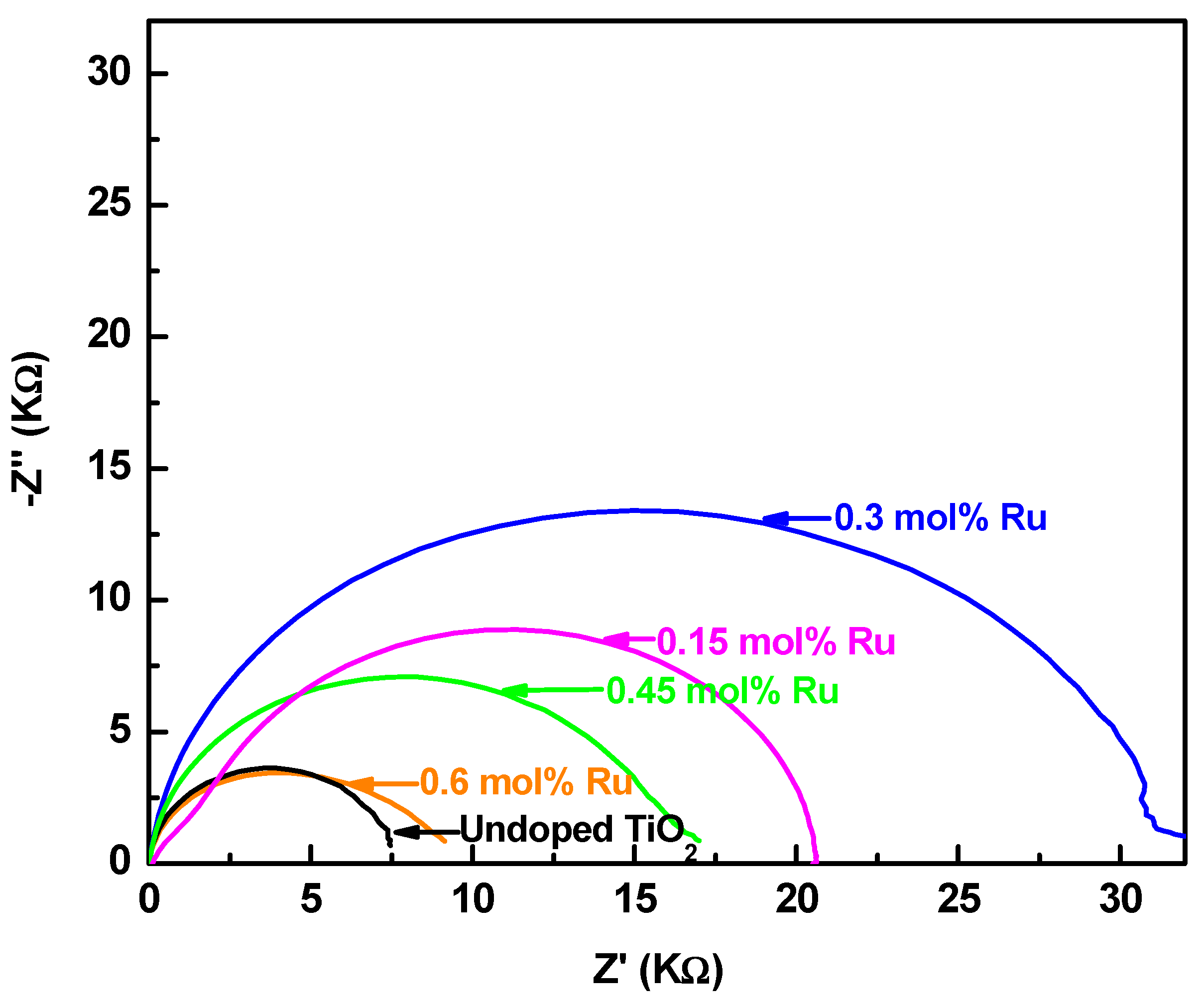
3.5. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kay, A.; Grätzel, M. Dye-sensitized core−shell nanocrystals: Improved efficiency of mesoporous tin oxide electrodes coated with a thin layer of an insulating oxide. Chem. Mater. 2002, 14, 2930–2935. [Google Scholar] [CrossRef]
- Burnside, S.; Moser, J.-E.; Brooks, K.; Grätzel, M.; Cahen, D. Nanocrystalline mesoporous strontium titanate as photoelectrode material for photosensitized solar devices: Increasing photovoltage through flatband potential engineering. J. Phys. Chem. B 1999, 103, 9328–9332. [Google Scholar] [CrossRef]
- Sayama, K.; Sugihara, H.; Arakawa, H. Photoelectrochemical properties of a porous Nb2O5 electrode sensitized by a ruthenium dye. Chem. Mater. 1998, 10, 3825–3832. [Google Scholar] [CrossRef]
- Saito, M.; Fujihara, S. Large photocurrent generation in dye-sensitized ZnO solar cells. Energy Environ. Sci. 2008, 1, 280–283. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Gokilamani, N.; Muthukumarasamy, N.; Thambidurai, M.; Ranjitha, A.; Velauthapillai, D. Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J. Sol-Gel Sci. Technol. 2013, 66, 212–219. [Google Scholar] [CrossRef]
- Prabavathy, N.; Balasundaraprabhu, R.; Balaji, G.; Malikaramage, A.; Prasanna, S.; Sivakumaran, K.; Kumara, G.; Rajapakse, R.; Velauthapillai, D. Investigations on the photo catalytic activity of calcium doped TiO2 photo electrode for enhanced efficiency of anthocyanins based dye sensitized solar cells. J. Photochem. Photobiol. A Chem. 2019, 377, 43–57. [Google Scholar] [CrossRef]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Muthukumarasamy, N. Enhancement in the photostability of natural dyes for dye-sensitized solar cell (DSSC) applications: A review. Int. J. Energy Res. 2017, 41, 1372–1396. [Google Scholar] [CrossRef]
- Senthil, T.; Muthukumarasamy, N.; Velauthapillai, D.; Agilan, S.; Thambidurai, M.; Balasundaraprabhu, R. Natural dye (cyanidin 3-O-glucoside) sensitized nanocrystalline TiO2 solar cell fabricated using liquid electrolyte/quasi-solid-state polymer electrolyte. Renew. Energy 2011, 36, 2484–2488. [Google Scholar] [CrossRef]
- Tennakone, K.; Hewaparakkrama, K.; Dewasurendra, M.; Jayatissa, A.; Weerasena, L. Dye-sensitised solid-state photovoltaic cells. Semicond. Sci. Technol. 1988, 3, 382. [Google Scholar] [CrossRef]
- Memarian, N.; Concina, I.; Braga, A.; Rozati, S.M.; Vomiero, A.; Sberveglieri, G. Hierarchically assembled ZnO nanocrystallites for high-efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011, 50, 12321–12325. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef]
- Tanyi, A.R.; Rafieh, A.I.; Ekaneyaka, P.; Tan, A.L.; Young, D.J.; Zheng, Z.; Chellappan, V.; Subramanian, G.S.; Chandrakanthi, R. Enhanced efficiency of dye-sensitized solar cells based on Mg and La co-doped TiO2 photoanodes. Electrochim. Acta 2015, 178, 240–248. [Google Scholar] [CrossRef]
- Mehnane, H.F.; Wang, C.; Kondamareddy, K.K.; Yu, W.; Sun, W.; Liu, H.; Bai, S.; Liu, W.; Guo, S.; Zhao, X.-Z. Hydrothermal synthesis of TiO2 nanoparticles doped with trace amounts of strontium, and their application as working electrodes for dye sensitized solar cells: Tunable electrical properties & enhanced photo-conversion performance. RSC Adv. 2017, 7, 2358–2364. [Google Scholar]
- Yang, S.; Guo, S.; Xu, D.; Xue, H.; Kou, H.; Wang, J.; Zhu, G. Improved efficiency of dye-sensitized solar cells applied with F-doped TiO2 electrodes. J. Fluor. Chem. 2013, 150, 78–84. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Wang, P.; Zheng, J.; Zhang, X.; Cui, Y.; Feng, J.; Zhu, Y. Sulfur-doped TiO2 nanocrystalline photoanodes for dye-sensitized solar cells. J. Renew. Sustain. Energy 2012, 4, 023104. [Google Scholar] [CrossRef]
- Xing, G.; Zhang, Z.; Qi, S.; Zhou, G.; Zhang, K.; Cui, Z.; Feng, Y.; Shan, Z.; Meng, S. Effect of cerium ion modifications on the photoelectrochemical properties of TiO2-based dye-sensitized solar cells. Opt. Mater. 2018, 75, 102–108. [Google Scholar] [CrossRef]
- Sakthivel, T.; Kumar, K.A.; Ramanathan, R.; Senthilselvan, J.; Jagannathan, K. Silver doped TiO2 nano crystallites for dye-sensitized solar cell (DSSC) applications. Mater. Res. Express 2017, 4, 126310. [Google Scholar] [CrossRef]
- Ranjitha, A.; Muthukumarasamy, N.; Thambidurai, M.; Velauthapillai, D. Enhanced photovoltaic performance of quantum dot sensitized solar cells with Ag-doped TiO2 nanocrystalline thin films. J. Mater. Sci. Mater. Electron. 2014, 25, 2724–2729. [Google Scholar] [CrossRef]
- Yacoubi, B.; Samet, L.; Bennaceur, J.; Lamouchi, A.; Chtourou, R. Properties of transition metal doped-titania electrodes: Impact on efficiency of amorphous and nanocrystalline dye-sensitized solar cells. Mater. Sci. Semicond. Process. 2015, 30, 361–367. [Google Scholar] [CrossRef]
- Jin, E.M.; Jeong, S.M.; Kang, H.-C.; Gu, H.-B. Photovoltaic effect of metal-doped TiO2 nanoparticles for dye-sensitized solar cells. ECS J. Solid State Sci. Technol. 2016, 5, Q109–Q114. [Google Scholar] [CrossRef]
- Asemi, M.; Maleki, S.; Ghanaatshoar, M. Cr-doped TiO2-based dye-sensitized solar cells with Cr-doped TiO2 blocking layer. J. Sol-Gel Sci. Technol. 2017, 81, 645–651. [Google Scholar] [CrossRef]
- Lü, X.; Mou, X.; Wu, J.; Zhang, D.; Zhang, L.; Huang, F.; Xu, F.; Huang, S. Improved-performance dye-sensitized solar cells using Nb-doped TiO2 electrodes: Efficient electron injection and transfer. Adv. Funct. Mater. 2010, 20, 509–515. [Google Scholar] [CrossRef]
- Tong, Z.; Peng, T.; Sun, W.; Liu, W.; Guo, S.; Zhao, X.-Z. Introducing an intermediate band into dye-sensitized solar cells by W6+ doping into TiO2 nanocrystalline photoanodes. J. Phys. Chem. C 2014, 118, 16892–16895. [Google Scholar] [CrossRef]
- Wijayarathna, T.; Aponsu, G.; Ariyasinghe, Y.; Premalal, E.; Kumara, G.; Tennakone, K. A high efficiency indoline-sensitized solar cell based on a nanocrystalline TiO2 surface doped with copper. Nanotechnology 2008, 19, 485703. [Google Scholar] [CrossRef]
- Qu, X.; Hou, Y.; Liu, M.; Shi, L.; Zhang, M.; Song, H.; Du, F. Yttrium doped TiO2 porous film photoanode for dye-sensitized solar cells with enhanced photovoltaic performance. Results Phys. 2016, 6, 1051–1058. [Google Scholar] [CrossRef]
- Latini, A.; Cavallo, C.; Aldibaja, F.K.; Gozzi, D.; Carta, D.; Corrias, A.; Lazzarini, L.; Salviati, G. Efficiency improvement of DSSC photoanode by scandium doping of mesoporous titania beads. J. Phys. Chem. C 2013, 117, 25276–25289. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhu, Y.; Ma, Z.; Miao, X.; Ma, R.; Wang, C.-Y. Enhanced performance of TiO2-based perovskite solar cells with Ru-doped TiO2 electron transport layer. Sol. Energy 2018, 169, 335–342. [Google Scholar] [CrossRef]
- Houšková, V.; Štengl, V.; Bakardjieva, S.; Murafa, N.; Tyrpekl, V. Efficient gas phase photodecomposition of acetone by Ru-doped Titania. Appl. Catal. B Environ. 2009, 89, 613–619. [Google Scholar] [CrossRef]
- Kong, D.; Jin, X.; Sun, W.; Du, J.; Tong, J.; Chen, C.; Yang, X.; Cheng, Y.; Li, Q. Ruthenium cation substitutional doping for efficient charge carrier transfer in organic/inorganic hybrid solar cells. J. Power Sources 2015, 274, 701–708. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ru-doped TiO2 nanotubes: Improved performance in dye-sensitized solar cells. Phys. Status Solidi (RRL) Rapid Res. Lett. 2012, 6, 169–171. [Google Scholar] [CrossRef]
- Lu, W.-H.; Chou, C.-S.; Chen, C.-Y.; Wu, P. Micro-and electronic structure optimization of Ru-doped TiO2 electrodes for efficient dye-sensitized solar cells. Sol. Energy 2016, 139, 318–327. [Google Scholar] [CrossRef]
- Ohno, T.; Tanigawa, F.; Fujihara, K.; Izumi, S.; Matsumura, M. Photocatalytic oxidation of water by visible light using ruthenium-doped titanium dioxide powder. J. Photochem. Photobiol. A Chem. 1999, 127, 107–110. [Google Scholar] [CrossRef]
- Senthilnanthan, M.; Ho, D.; Vigneswaran, S.; Ngo, H.; Shon, H. Visible light responsive ruthenium-doped titanium dioxide for the removal of metsulfuron-methyl herbcide in aqueous phase. Sep. Purif. Technol. 2010, 75, 415–419. [Google Scholar] [CrossRef]
- Upadhyay, P.; Srivastava, V. Synthesis of ruthenium metal doped titanium dioxide nanoparticles for CO2 hydrogenation. AIP Conf. Proc. 2016, 1724, 020074. [Google Scholar]
- Lim, S.P.; Pandikumar, A.; Lim, H.N.; Ramaraj, R.; Huang, N.M. Boosting photovoltaic performance of dye-sensitized solar cells using silver nanoparticle-decorated N, S-Co-doped-TiO2 photoanode. Sci. Rep. 2015, 5, 11922. [Google Scholar] [CrossRef]
- Thambidurai, M.; Foo, S.; Salim, K.M.; Harikesh, P.; Bruno, A.; Jamaludin, N.F.; Lie, S.; Mathews, N.; Dang, C. Improved photovoltaic performance of triple-cation mixed-halide perovskite solar cells with binary trivalent metals incorporated into the titanium dioxide electron transport layer. J. Mater. Chem. C 2019, 7, 5028–5036. [Google Scholar] [CrossRef]
- Loheeswaran, S.; Thanihaichelvan, M.; Ravirajan, P.; Nelson, J. Controlling recombination kinetics of hybrid poly-3-hexylthiophene (P3HT)/titanium dioxide solar cells by self-assembled monolayers. J. Mater. Sci. Mater. Electron. 2017, 28, 4732–4737. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Sockiah, K.; Balashangar, K.; Ravirajan, P. Cadmium sulfide interface layer for improving the performance of titanium dioxide/poly (3-hexylthiophene) solar cells by extending the spectral response. J. Mater. Sci. Mater. Electron. 2015, 26, 3558–3563. [Google Scholar] [CrossRef]
- Ismael, M. High effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Elezović, N.; Babić, B.; Radmilovic, V.; Vračar, L.M.; Krstajić, N. Novel Pt catalyst on ruthenium doped TiO2 support for oxygen reduction reaction. Appl. Catal. B Environ. 2013, 140, 206–212. [Google Scholar] [CrossRef]
- Madurai, V.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2 nanoparticles by modi fi ed solvothermal method towards e ff ective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar]
- Thambidurai, M.; Shini, F.; Harikesh, P.; Mathews, N.; Dang, C. Highly stable and efficient planar perovskite solar cells using ternary metal oxide electron transport layers. J. Power Sources 2020, 448, 227362. [Google Scholar] [CrossRef]
- Shini, F.; Thambidurai, M.; Harikesh, P.; Mathews, N.; Huang, Y.; Dang, C. Heterogeneous electron transporting layer for reproducible, efficient and stable planar perovskite solar cells. J. Power Sources 2019, 437, 226907. [Google Scholar] [CrossRef]
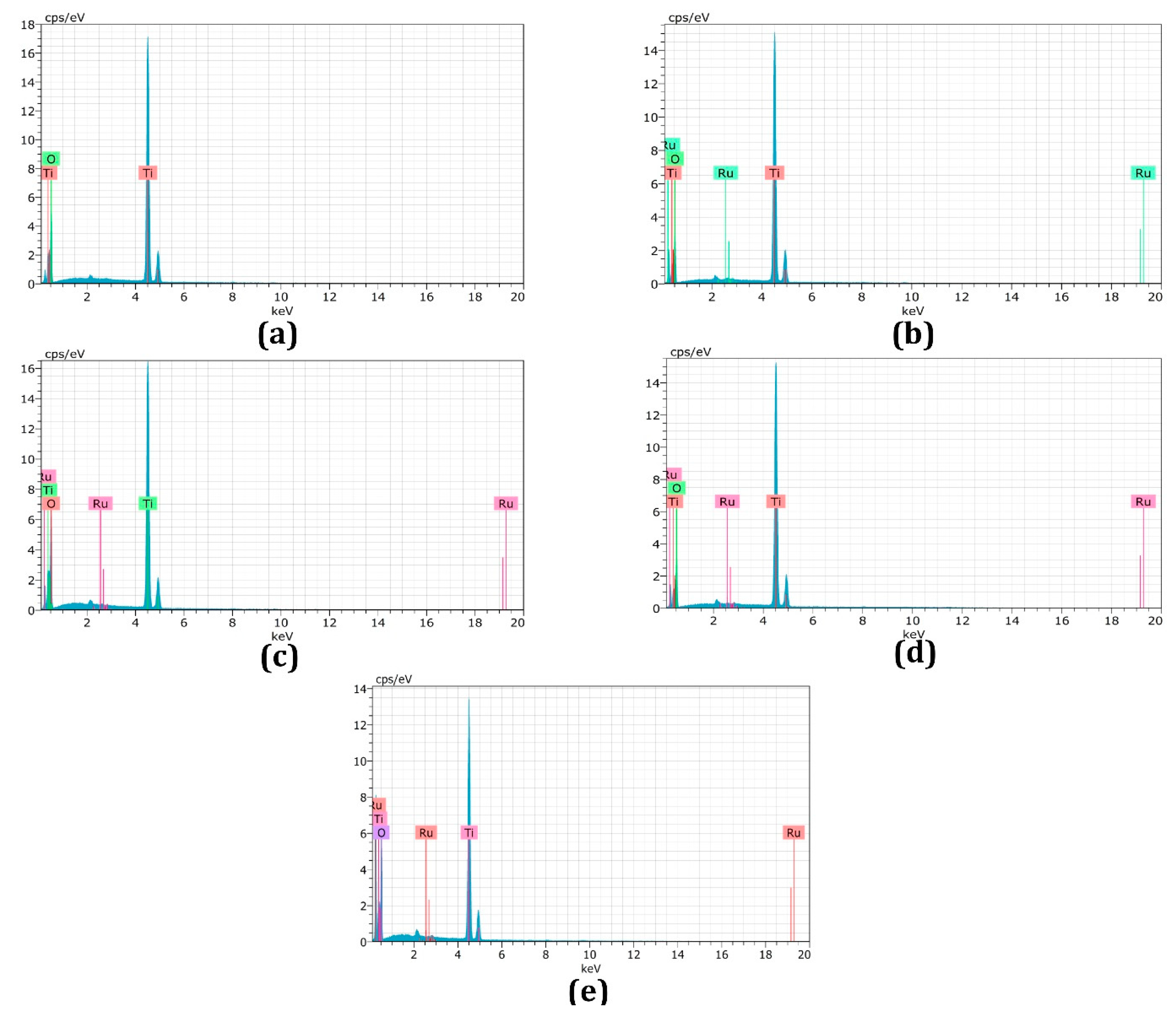
| Samples | EDX Result-Ru at. % |
|---|---|
| Undoped TiO2 (a) | 0 |
| 0.15 mol% Ru (b) | 0.10 |
| 0.30 mol% Ru (c) | 0.25 |
| 0.45 mol% Ru (d) | 0.46 |
| 0.60 mol% Ru( e) | 0.75 |
| mol% of Ru in TiO2 | JSC (mA/cm2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|
| 0.6 | 13.25 | 0.66 | 0.70 | 6.24 |
| 0.45 | 14.06 | 0.66 | 0.68 | 6.42 |
| 0.3 | 14.73 | 0.69 | 0.68 | 7.00 |
| 0.15 | 14.42 | 0.67 | 0.67 | 6.50 |
| 0 | 12.90 | 0.66 | 0.66 | 5.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaramanan, T.; Natarajan, M.; Ravirajan, P.; Senthilnanthanan, M.; Velauthapillai, D. Ruthenium (Ru) Doped Titanium Dioxide (P25) Electrode for Dye Sensitized Solar Cells. Energies 2020, 13, 1532. https://doi.org/10.3390/en13071532
Rajaramanan T, Natarajan M, Ravirajan P, Senthilnanthanan M, Velauthapillai D. Ruthenium (Ru) Doped Titanium Dioxide (P25) Electrode for Dye Sensitized Solar Cells. Energies. 2020; 13(7):1532. https://doi.org/10.3390/en13071532
Chicago/Turabian StyleRajaramanan, Tharmakularasa, Muthukumarasamy Natarajan, Punniamoorthy Ravirajan, Meena Senthilnanthanan, and Dhayalan Velauthapillai. 2020. "Ruthenium (Ru) Doped Titanium Dioxide (P25) Electrode for Dye Sensitized Solar Cells" Energies 13, no. 7: 1532. https://doi.org/10.3390/en13071532
APA StyleRajaramanan, T., Natarajan, M., Ravirajan, P., Senthilnanthanan, M., & Velauthapillai, D. (2020). Ruthenium (Ru) Doped Titanium Dioxide (P25) Electrode for Dye Sensitized Solar Cells. Energies, 13(7), 1532. https://doi.org/10.3390/en13071532







