Preparation and Application of Magnetic Nano-Solid Acid Catalyst Fe3O4-PDA-SO3H
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe3O4 Nanoparticles
2.3. Preparation of Fe3O4-PDA Nanoparticles
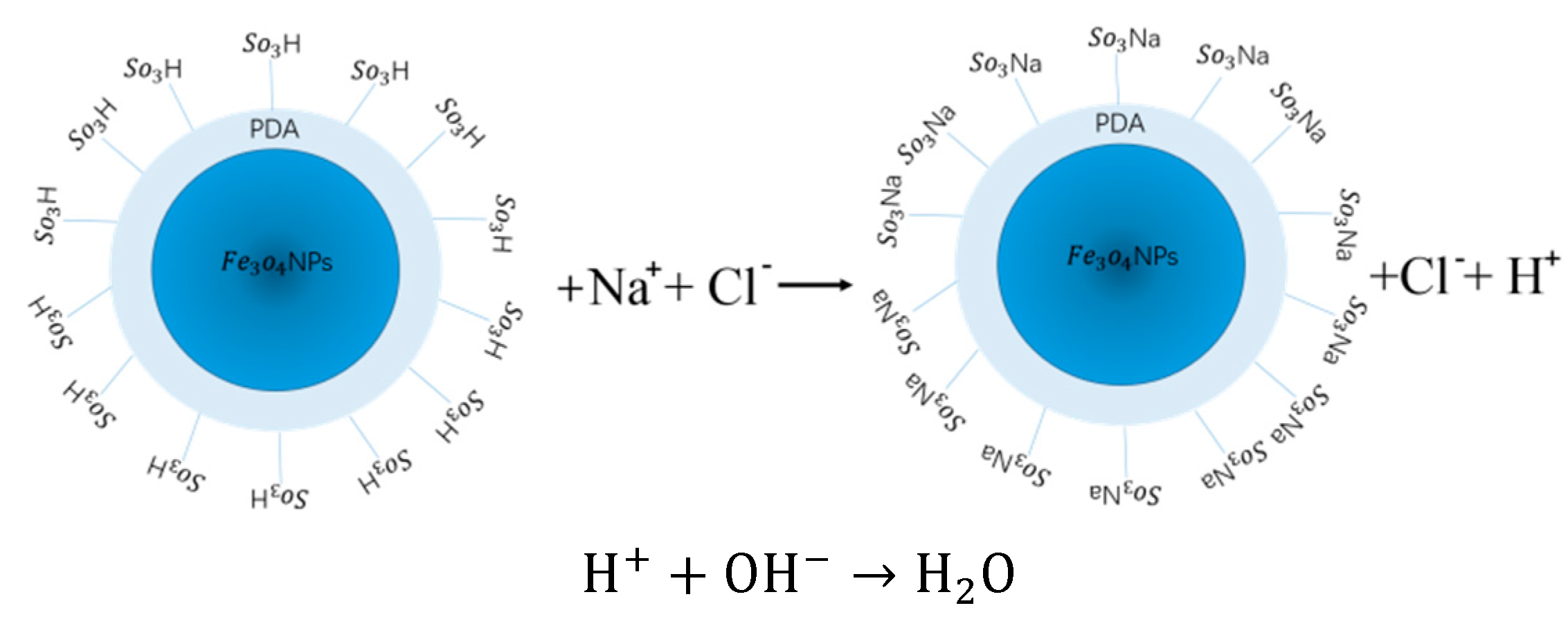
2.4. Preparation of Fe3O4-PDA-SO3H Nanoparticles
2.5. Characterization
2.6. Determination of Acid Density
2.7. Typical Experimental Produre for Esterification Between LA with Different Alcohols
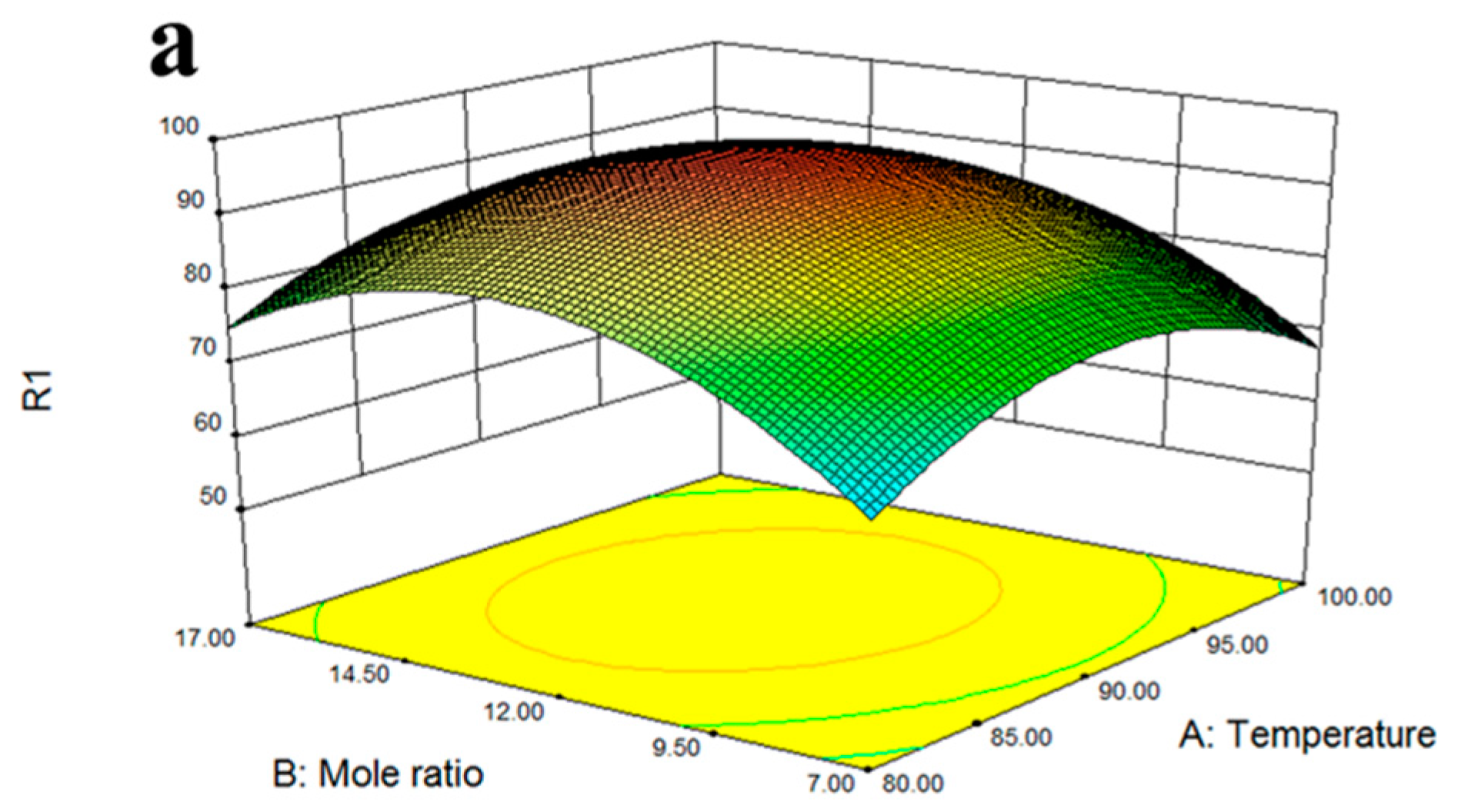
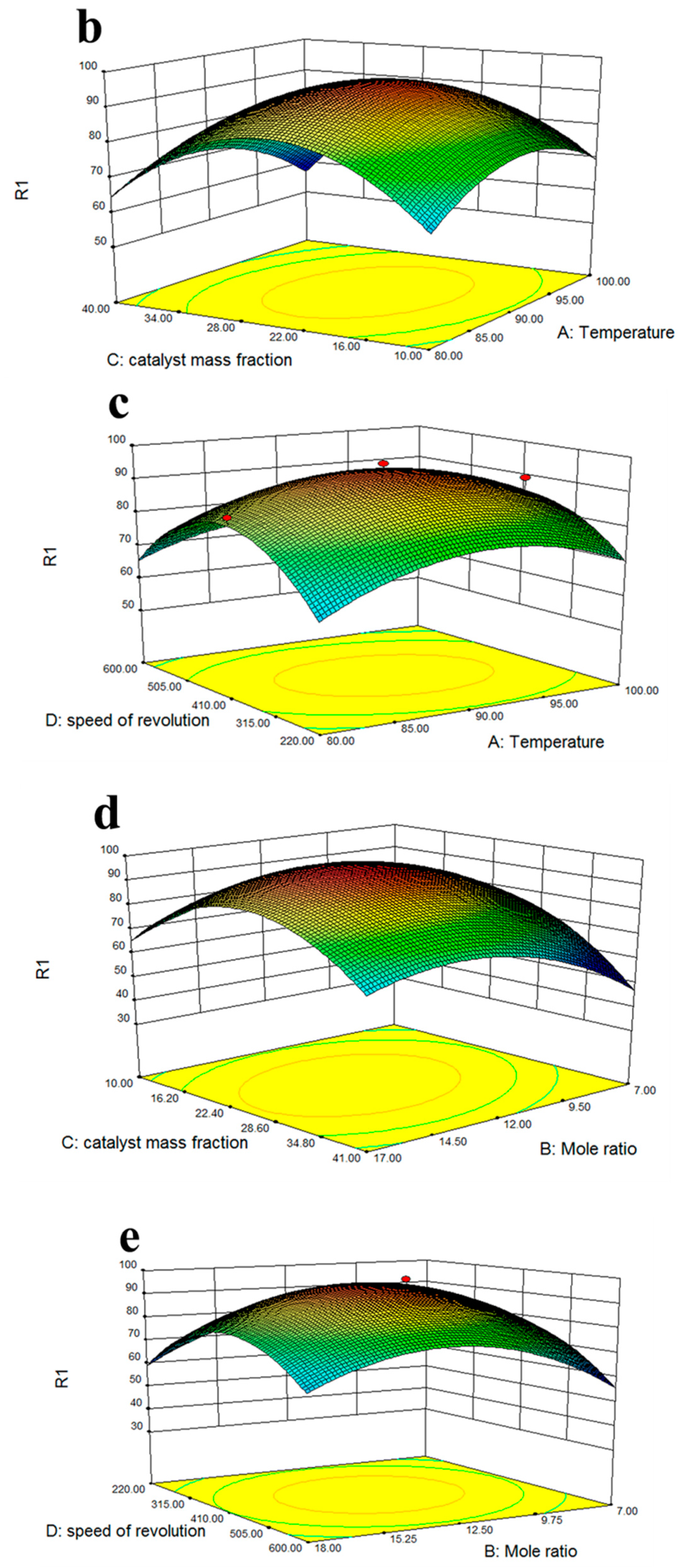
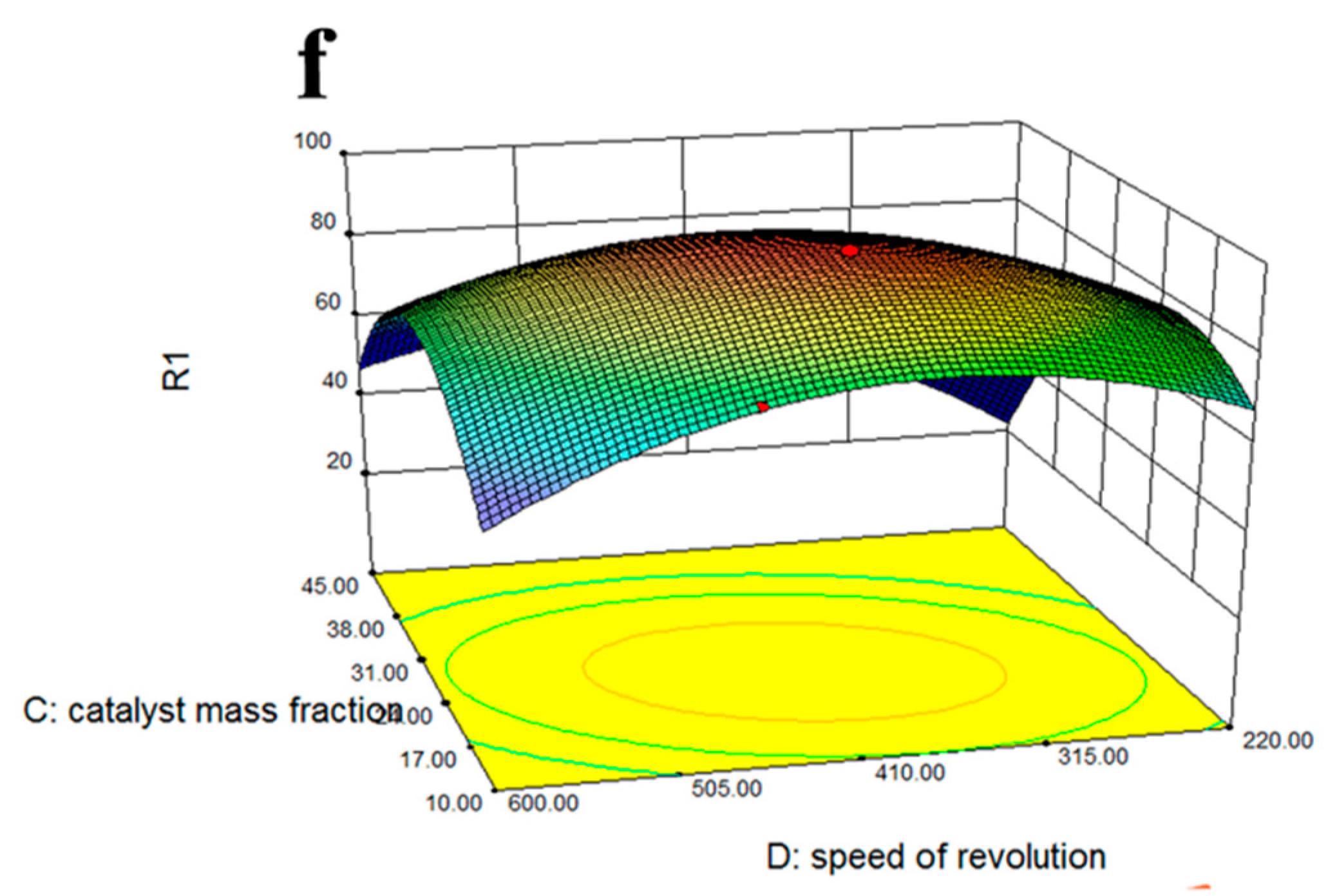
2.8. Response Surface Optimization
3. Results and Discussion
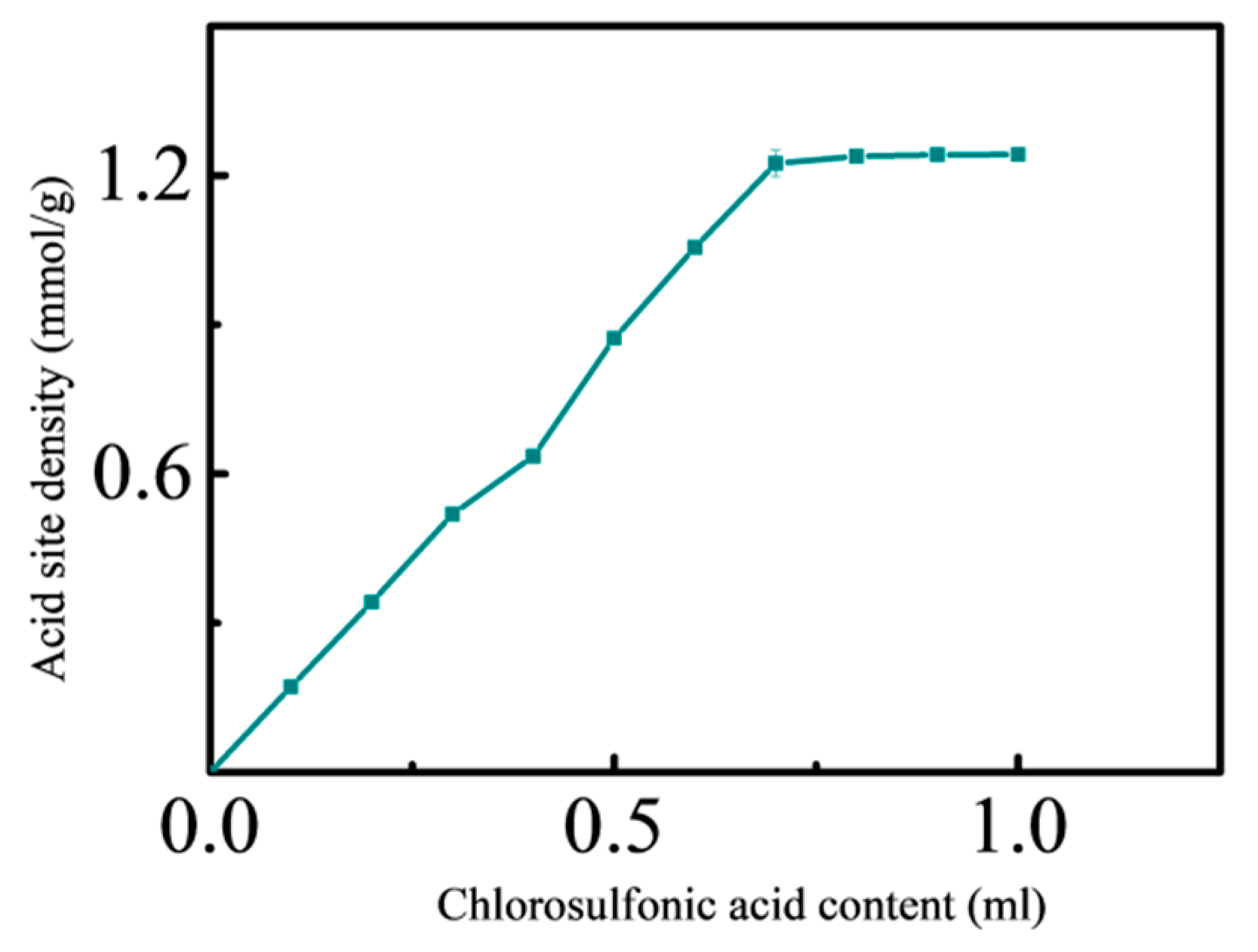
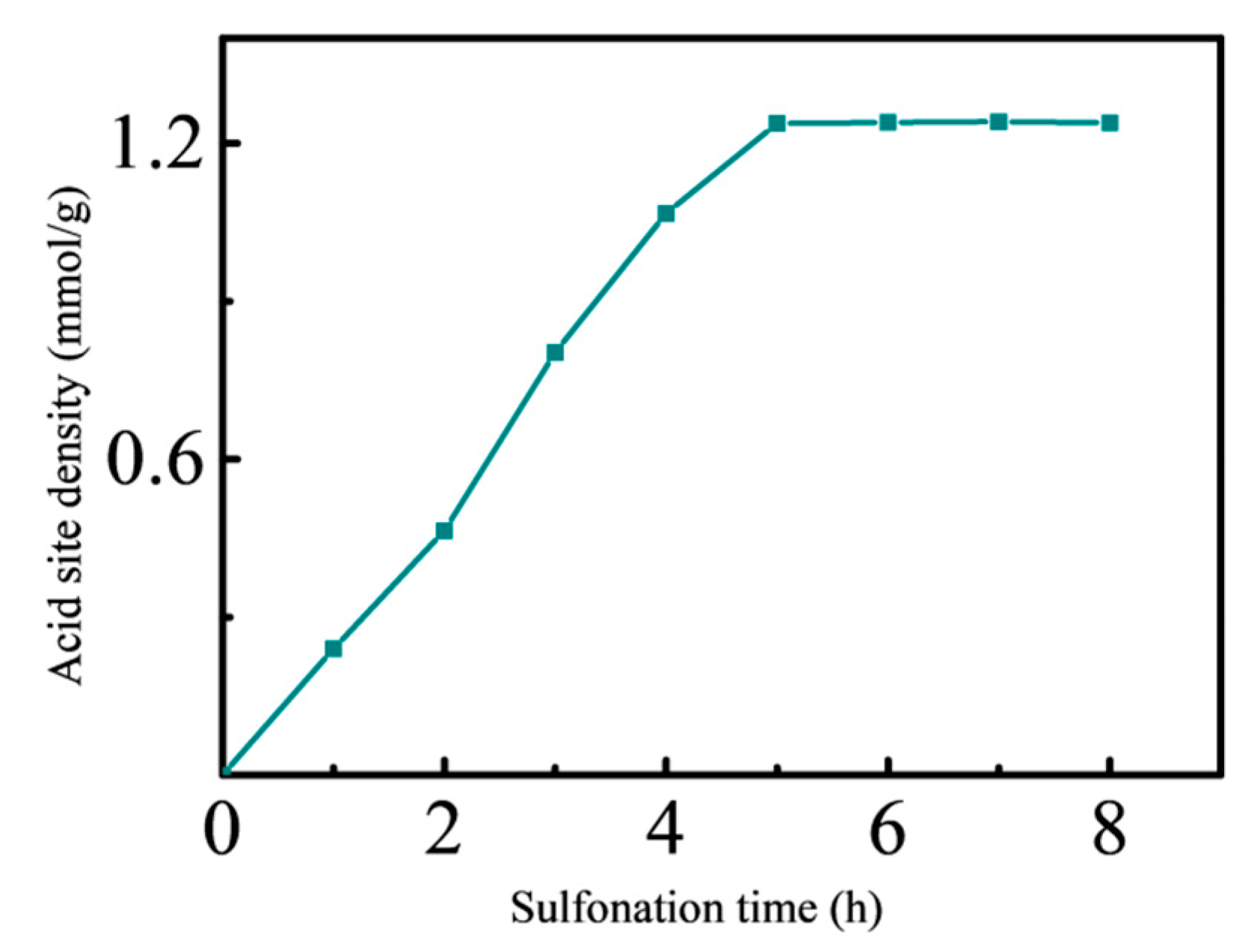
3.1. Effect of Sulfonation of Chlorosulfonic Acid
3.1.1. Effect of Chlorosulfonic Acid on Magnetic Nanosolid Acid Catalyst
3.1.2. Effect of Sulfonation Time on Solid Acid Density
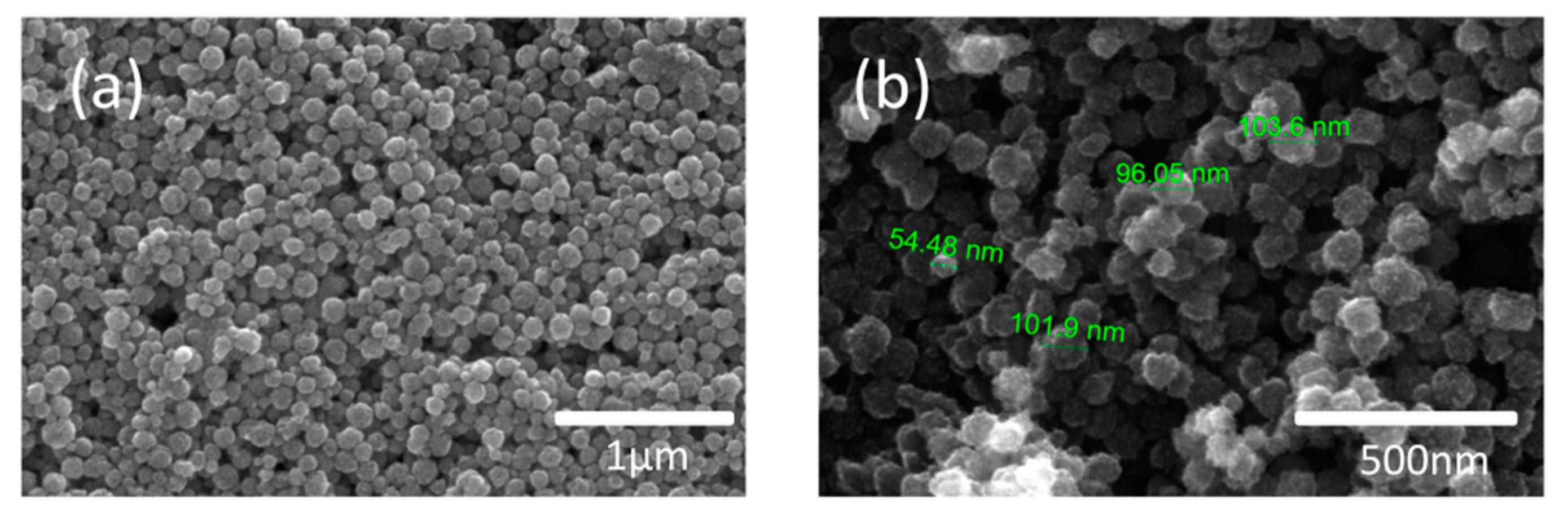
3.2. Catalyst Characterization
3.2.1. SEM Characterization
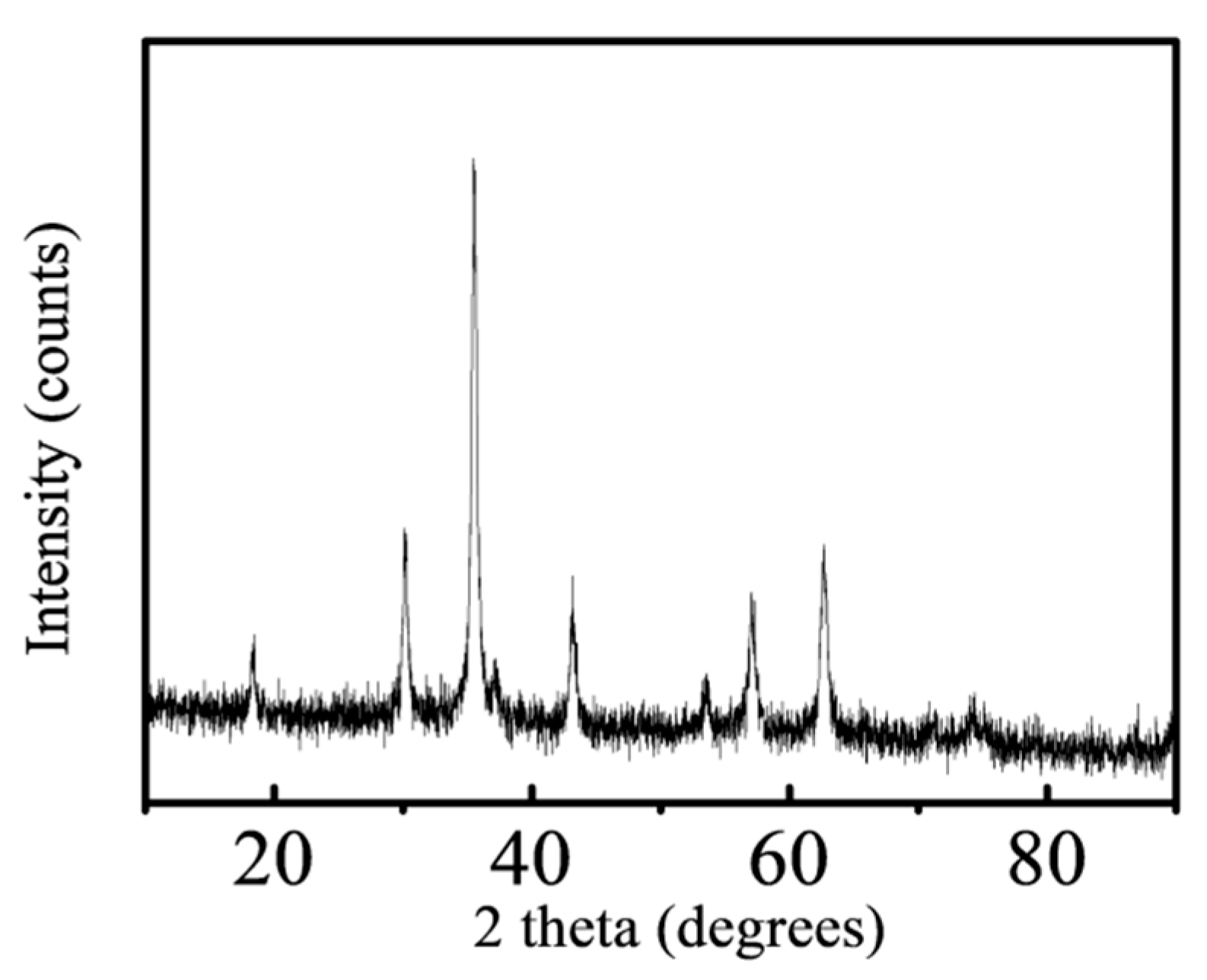
3.2.2. XRD Characterization
3.2.3. EDX Characterization
3.2.4. FT-IR Characterization
3.3. Optimum Reaction Conditions for Fe3O4-PDA-SO3H Magnetic Nano-Solid Acid
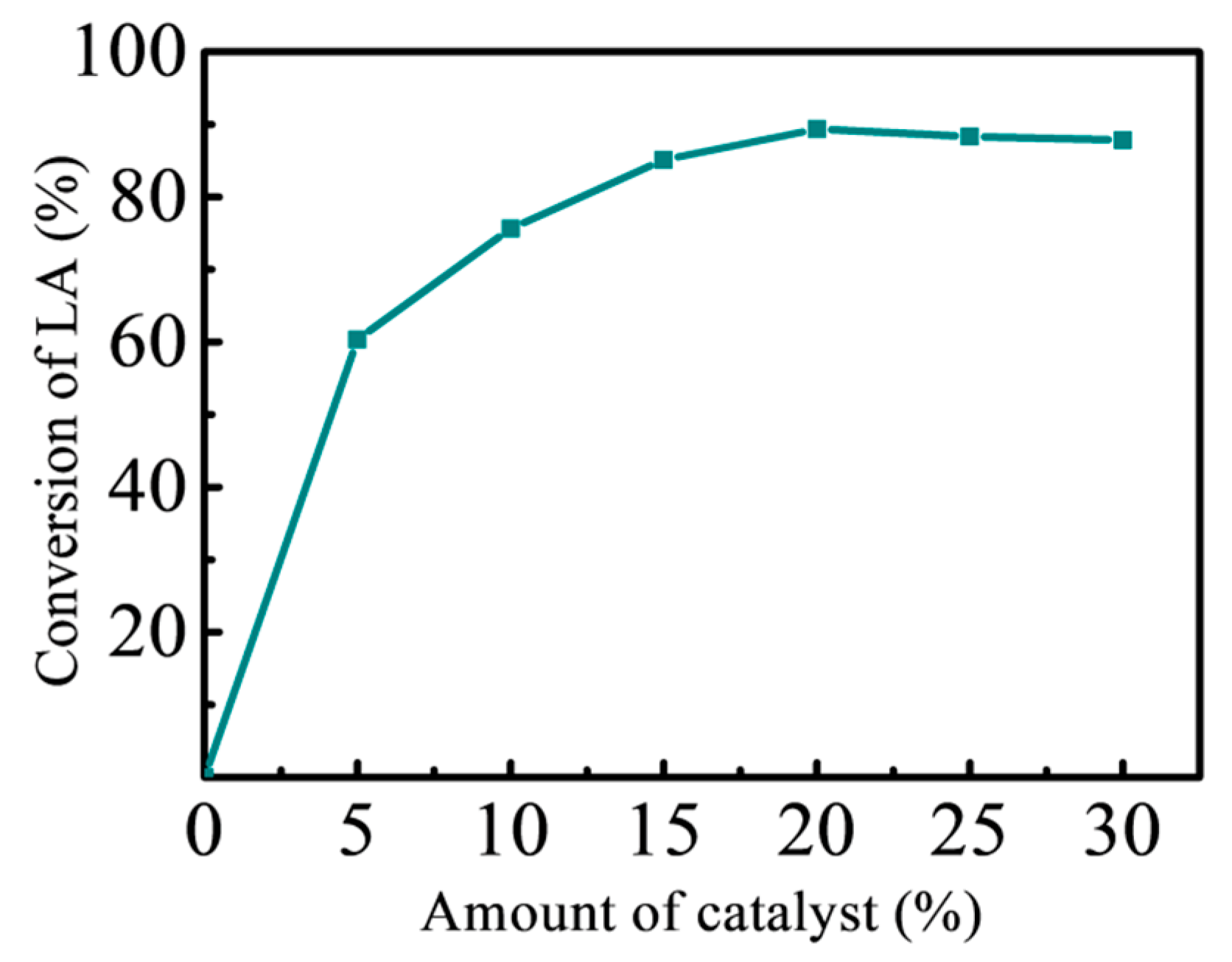
3.3.1. Effect of Catalyst Dosage on Esterification Reaction
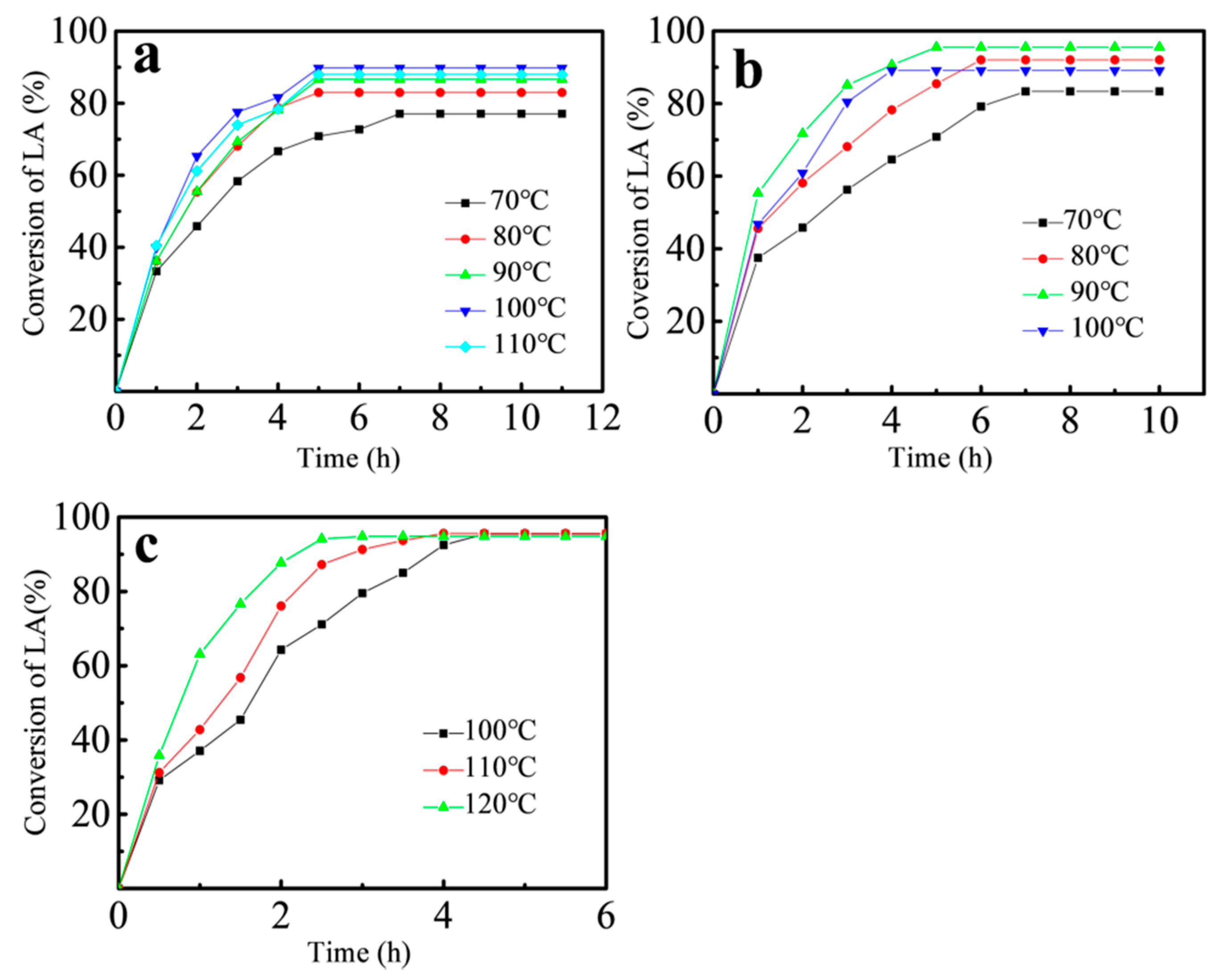
3.3.2. Effect of Reaction Temperature
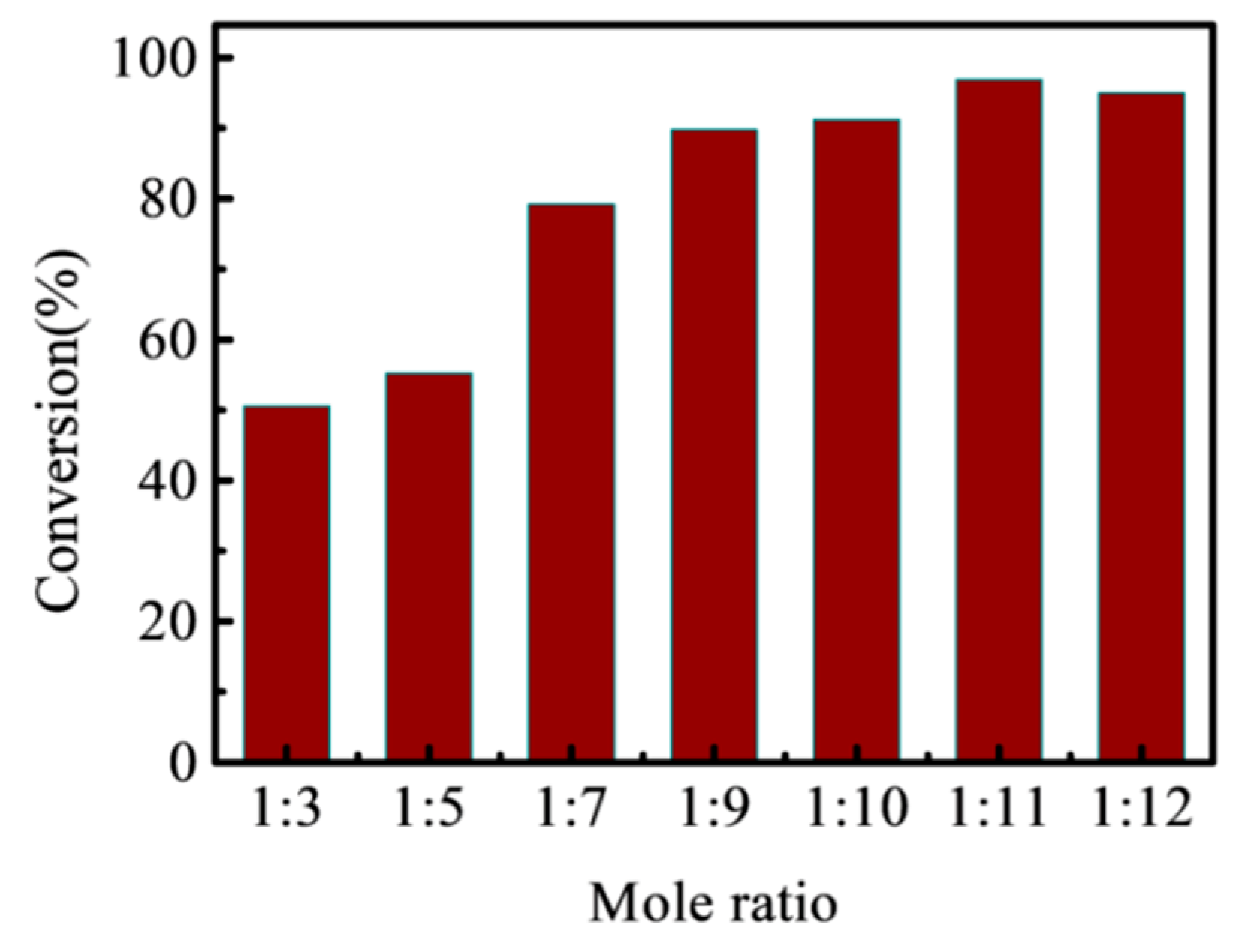
3.3.3. Effect of Acid: Alcohol Mole Ratio
3.4. Optimization of Esterification Conditions of Solid Acid Catalyst Fe3O4-PDA-SO3H
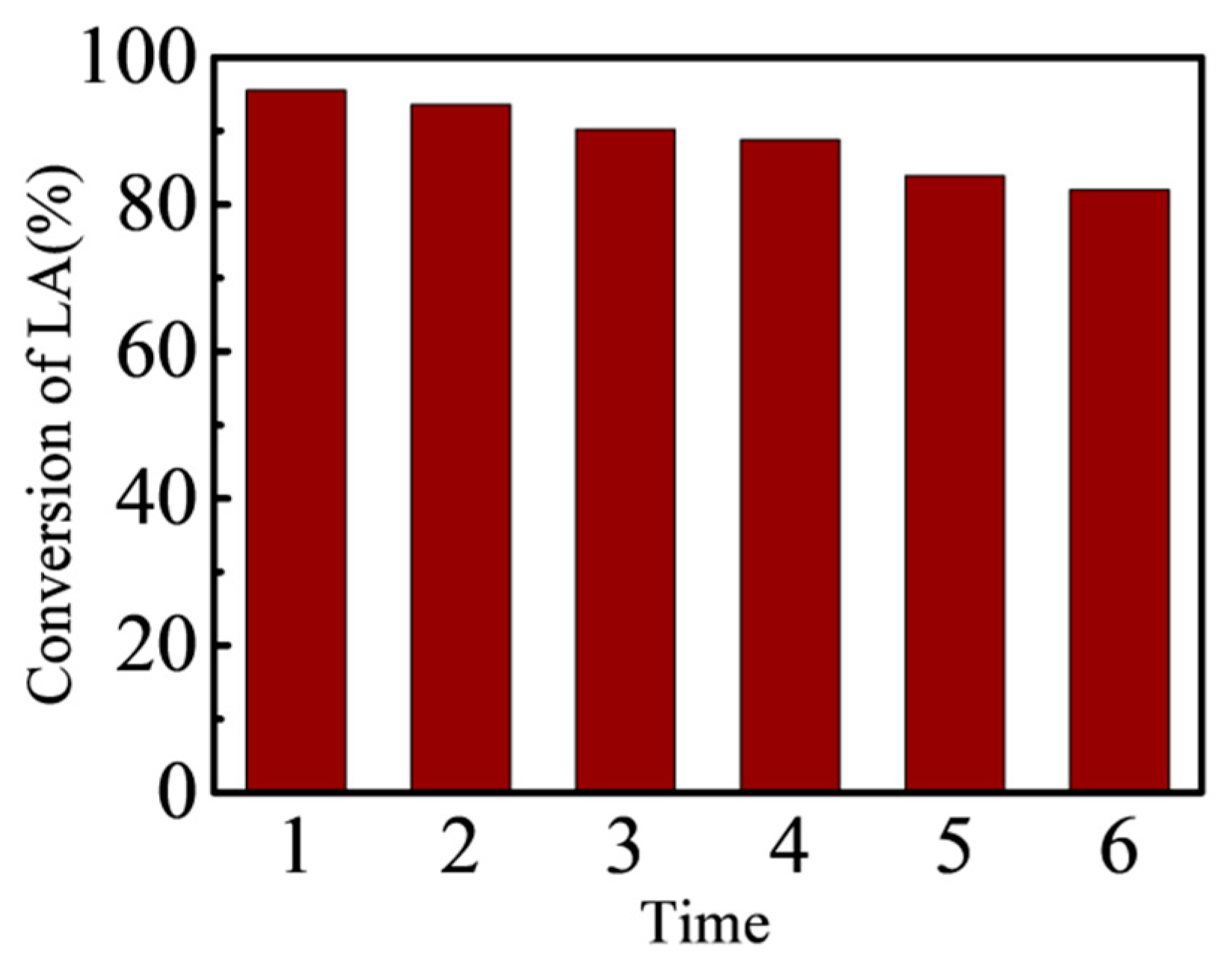
3.5. Recyclability of Catalyst
3.6. Comparison with other Typical Solid Acids
4. Conclusions
- (1)
- FT-IR and EDX demonstrated successful loading of polydopamine and sulfonic groups. SEM and XRD showed that the catalyst showed a complete and uniform core-shell structure, and the loading of chlorosulfonic acid would not affect the crystal form of Fe3O4.
- (2)
- The optimum reaction conditions for catalytic reaction of Fe3O4-PDA-SO3H were obtained by single factor and response surface optimization. The optimum experimental reaction conditions were the reaction temperature is 90.55 °C, the molar ratio of acid to alcohol is 1:12.94, optimal amount of catalyst is 23.99% of the mass of acetylpropionic acid and the stirring rate is 401 r/min. The conversion of acetylpropionic acid was 95.87% under this condition.
- (3)
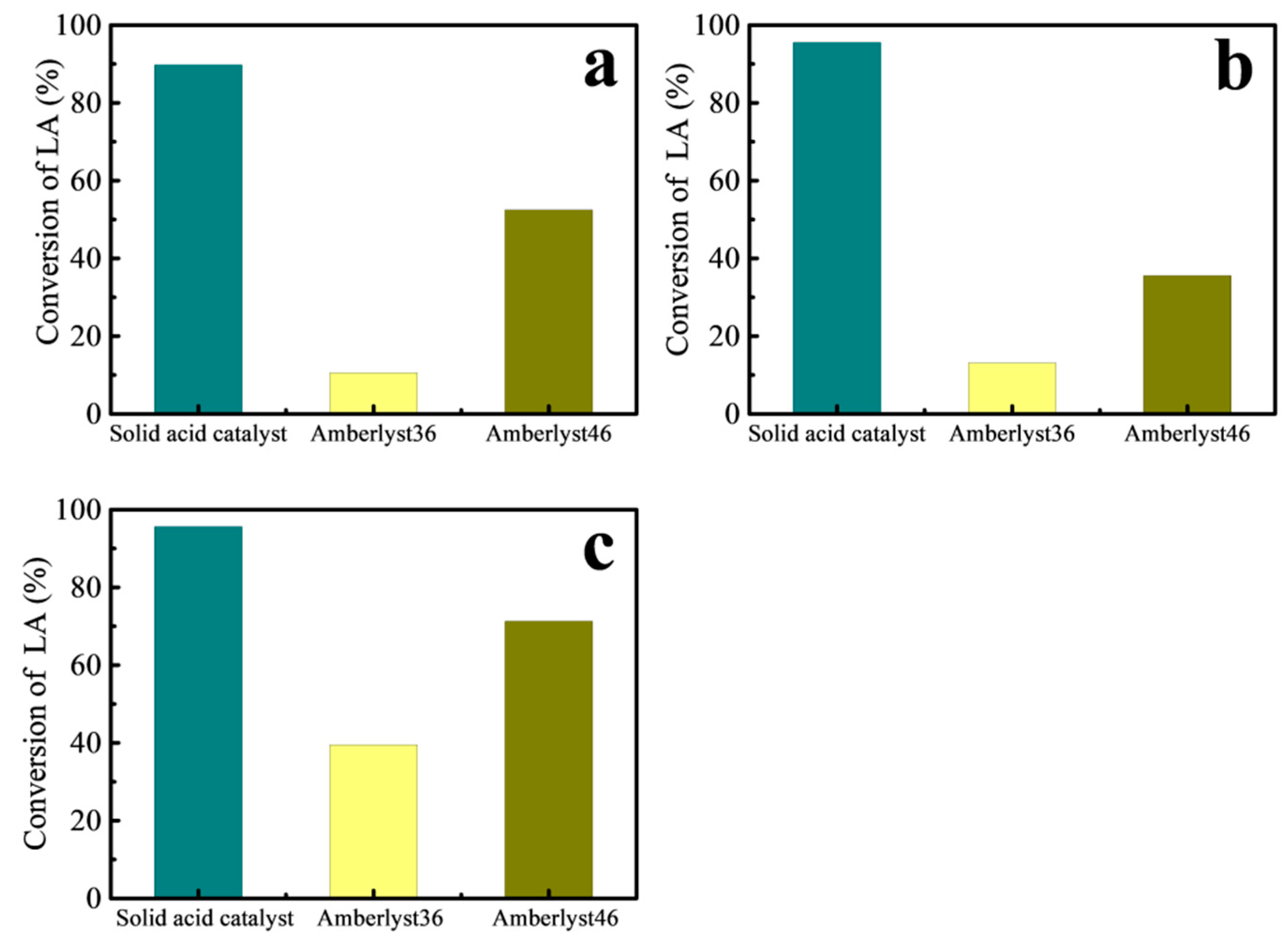
- The results of LA with different alcohol esterification can be proved that this solid acid catalyst has highly efficient and good reusability. Compared with the common cation exchange resin Amberlyst 36 and Amberlyst 46, the results showed that the effect was better under the same reaction conditions.
Author Contributions
Funding
Conflicts of Interest
References
- Maheria, K.C.; Kozinski, J.; Dalai, A. Esterification of Levulinic Acid to n-Butyl Levulinate Over Various Acidic Zeolites. Catal. Lett. 2013, 143, 1220–1225. [Google Scholar] [CrossRef]
- Bala, D.D.; Misra, M.; Chidambaram, D. Solid-acid catalyzed biodiesel production, part I: Biodiesel synthesis from low quality feedstock. J. Clean. Prod. 2017, 142, 4169–4177. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Hou, Y.; Ma, J.; Wang, T.; Fu, Q. Phosphotungstic acid supported on magnetic core–shell nanoparticles with high photocatalytic activity. Mater. Sci. Semicond. Process. 2015, 39, 229–234. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Nemoto, K.; Fujitani, T. Esterification of levulinic acid with ethanol over sulfated Si-doped ZrO2 solid acid catalyst: Study of the structure–activity relationships. Appl. Catal. A Gen. 2014, 476, 186–196. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Tabassum, M.; Coutts, S.; Titirici, M.-M. Esterification of levulinic acid into ethyl levulinate catalysed by sulfonated hydrothermal carbons. Chin. J. Catal. 2014, 35, 929–936. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Mai, E.F.; Mota, C.J.A.; Teixeira da Silva, V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A Gen. 2012, 425, 199–204. [Google Scholar] [CrossRef]
- Tejero, M.A.; Ramírez, E.; Fité, C.; Tejero, J.; Cunill, F. Esterification of levulinic acid with butanol over ion exchange resins. Appl. Catal. A Gen. 2016, 517, 56–66. [Google Scholar] [CrossRef]
- Lange, J.P.; van de Graaf, W.D.; Haan, R.J. Conversion of furfuryl alcohol into ethyl levulinate using solid acid catalysts. ChemSusChem 2009, 2, 437–441. [Google Scholar] [CrossRef]
- Xu, Z.X.; Cheng, J.H.; He, Z.X.; Wang, Q.; Shao, Y.W.; Hu, X. Hydrothermal liquefaction of cellulose in ammonia/water. Bioresour. Technol. 2019, 278, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Yadava, S.K.; Bokade, V.V. Production of octyl levulinate biolubricant over modified H-ZSM-5: Optimization by response surface methodology. J. Energy Chem. 2014, 23, 535–541. [Google Scholar] [CrossRef]
- Hegner, J.; Pereira, K.C.; DeBoef, B.; Lucht, B.L. Conversion of cellulose to glucose and levulinic acid via solid-supported acid catalysis. Tetrahedron Lett. 2010, 51, 2356–2358. [Google Scholar] [CrossRef]
- Siva Sankar, E.; Mohan, V.; Suresh, M.; Saidulu, G.; David Raju, B.; Rama Rao, K.S. Vapor phase esterification of levulinic acid over ZrO2/SBA-15 catalyst. Catal. Commun. 2016, 75, 1–5. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; González, H.; Gutiérrez-Alejandre, A.; Rico, J.L.; Huirache-Acuña, R.; Maya-Yescas, R.; del Río, R.E. Heterogeneous acid conversion of a tricaprylin-palmitic acid mixture over Al-SBA-15 catalysts: Reaction study for biodiesel synthesis. Catal. Today 2017, 282, 195–203. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabrés i Xamena, F.X. Conversion of levulinic acid into chemicals: Synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Popova, M.; Shestakova, P.; Lazarova, H.; Dimitrov, M.; Kovacheva, D.; Szegedi, A.; Mali, G.; Dasireddy, V.; Likozar, B.; Wilde, N.; et al. Efficient solid acid catalysts based on sulfated tin oxides for liquid phase esterification of levulinic acid with ethanol. Appl. Catal. A Gen. 2018, 560, 119–131. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Dong, M.; Wu, Y.; Zheng, G.; Huang, J.; Guan, X.; Zheng, X. MCM-41 immobilized 12-silicotungstic acid mesoporous materials: Structural and catalytic properties for esterification of levulinic acid and oleic acid. J. Taiwan Inst. Chem. Eng. 2016, 61, 147–155. [Google Scholar] [CrossRef]
- Song, D.; An, S.; Sun, Y.; Zhang, P.; Guo, Y.; Zhou, D. Ethane-Bridged Organosilica Nanotubes Functionalized with Arenesulfonic Acid and Phenyl Groups for the Efficient Conversion of Levulinic Acid or Furfuryl Alcohol to Ethyl Levulinate. ChemCatChem 2016, 8, 2037–2048. [Google Scholar] [CrossRef]
- Najafi Chermahini, A.; Nazeri, M. Esterification of the levulinic acid with n-butyl and isobutyl alcohols over aluminum-containing MCM-41. Fuel Process. Technol. 2017, 167, 442–450. [Google Scholar] [CrossRef]
- Salimi, Z.; Hosseini, S.A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 2019, 239, 1204–1212. [Google Scholar] [CrossRef]
- Veisi, H.; Taheri, S.; Hemmati, S. Preparation of polydopamine sulfamic acid-functionalized magnetic Fe3O4 nanoparticles with a core/shell nanostructure as heterogeneous and recyclable nanocatalysts for the acetylation of alcohols, phenols, amines and thiols under solvent-free conditions. Green Chem. 2016, 18, 6337–6348. [Google Scholar] [CrossRef]
- Mirhosseyni, M.S.; Nemati, F.; Elhampour, A. Hollow Fe3O4@DA-SO3H: An efficient and reusable heterogeneous nano-magnetic acid catalyst for synthesis of dihydropyridine and dioxodecahydroacridine derivatives. J. Iran. Chem. Soc. 2016, 14, 791–801. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, D.; Liu, X.; Chen, Y.; Yin, D. Titanate nanotubes-bonded organosulfonic acid as solid acid catalyst for synthesis of butyl levulinate. RSC Adv. 2018, 8, 3657–3662. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernández, B.; Penedo, S. Efficient conversion of levulinic acid into alkyl levulinates catalyzed by sulfonic mesostructured silicas. Appl. Catal. A Gen. 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Dharne, S.; Bokade, V.V. Esterification of levulinic acid to n-butyl levulinate over heteropolyacid supported on acid-treated clay. J. Nat. Gas Chem. 2011, 20, 18–24. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Bokade, V.V. Environmentally benign catalytic process for esterification of renewable levulinic acid to various alkyl levulinates biodiesel. Environ. Prog. Sustain. Energy 2015, 34, 795–801. [Google Scholar] [CrossRef]
- Lee, A.; Chaibakhsh, N.; Rahman, M.B.A.; Basri, M.; Tejo, B.A. Optimized enzymatic synthesis of levulinate ester in solvent-free system. Ind. Crops Prod. 2010, 32, 246–251. [Google Scholar] [CrossRef]


| Experimental Factors | Level | |
|---|---|---|
| Low | High | |
| Temperature of reaction (°C) | 80 | 100 |
| mole ratio | 1:7 | 1:15 |
| Amount of catalyst (wt%) | 10 | 25 |
| Speed of revolution (r/min) | 320 | 466 |
| Type of Catalyst | Acid Density (mmol/g) | LA Conversion with Ethanol (%) | LA Conversion with n-Butanol (%) | LA Conversion with n-Octanol (%) |
|---|---|---|---|---|
| Fe3O4-PDA-SO3H | 1.238 | 89.8 | 95.55 | 95.65 |
| Amberlyst 36 | 5.4 | 10.59 | 13.14 | 39.53 |
| Amberlyst 46 | 3.1 | 52.5 | 35.56 | 71.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lu, Y.; Liu, H.; Yin, Y.; Liang, J. Preparation and Application of Magnetic Nano-Solid Acid Catalyst Fe3O4-PDA-SO3H. Energies 2020, 13, 1484. https://doi.org/10.3390/en13061484
Wang H, Lu Y, Liu H, Yin Y, Liang J. Preparation and Application of Magnetic Nano-Solid Acid Catalyst Fe3O4-PDA-SO3H. Energies. 2020; 13(6):1484. https://doi.org/10.3390/en13061484
Chicago/Turabian StyleWang, Honghai, Yifan Lu, Hongli Liu, Yi Yin, and Jun Liang. 2020. "Preparation and Application of Magnetic Nano-Solid Acid Catalyst Fe3O4-PDA-SO3H" Energies 13, no. 6: 1484. https://doi.org/10.3390/en13061484
APA StyleWang, H., Lu, Y., Liu, H., Yin, Y., & Liang, J. (2020). Preparation and Application of Magnetic Nano-Solid Acid Catalyst Fe3O4-PDA-SO3H. Energies, 13(6), 1484. https://doi.org/10.3390/en13061484





