Experimental Realization of Heavily p-doped Half-Heusler CoVSn Compound
Abstract
1. Introduction
2. Materials and Methods
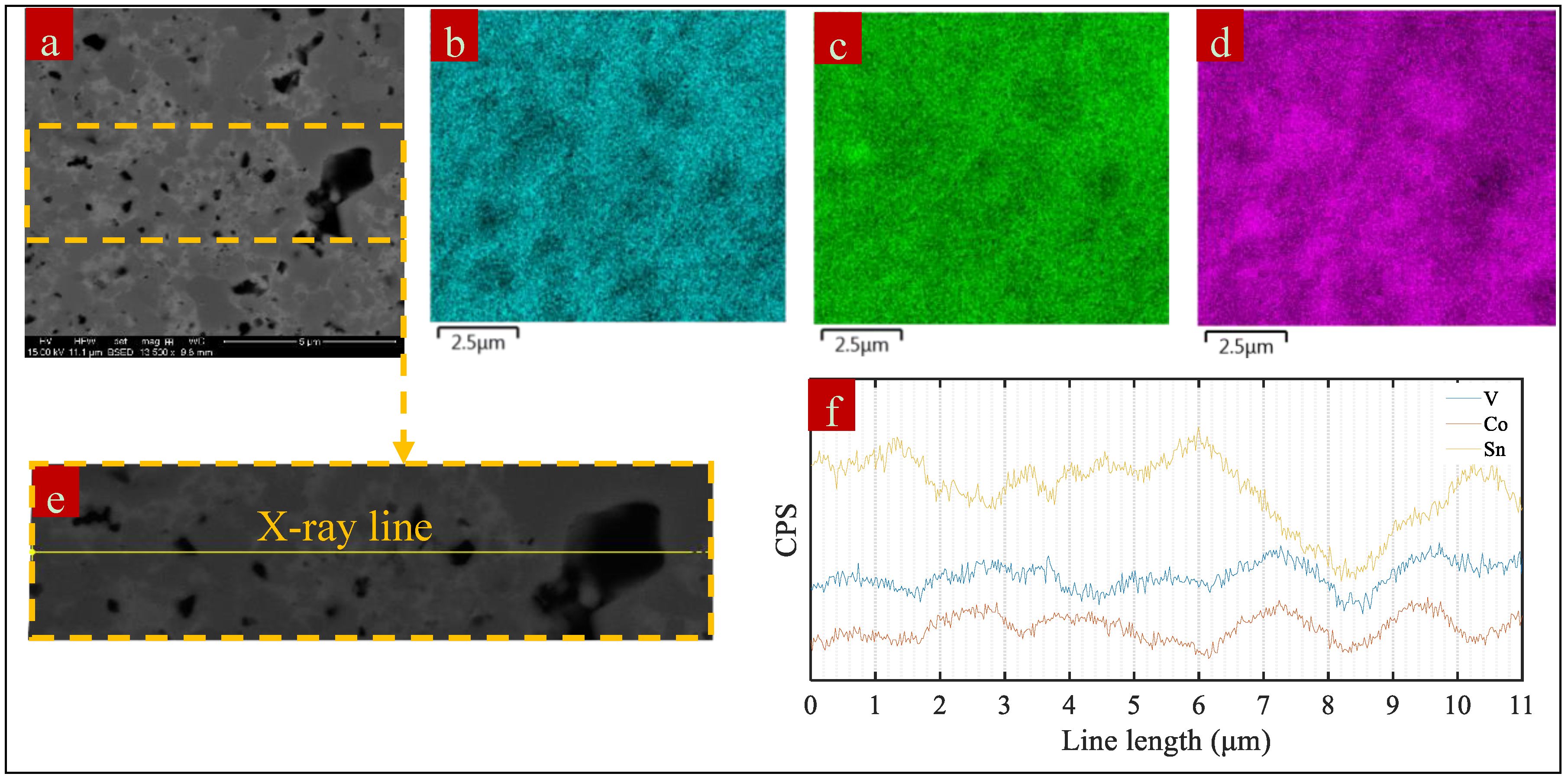
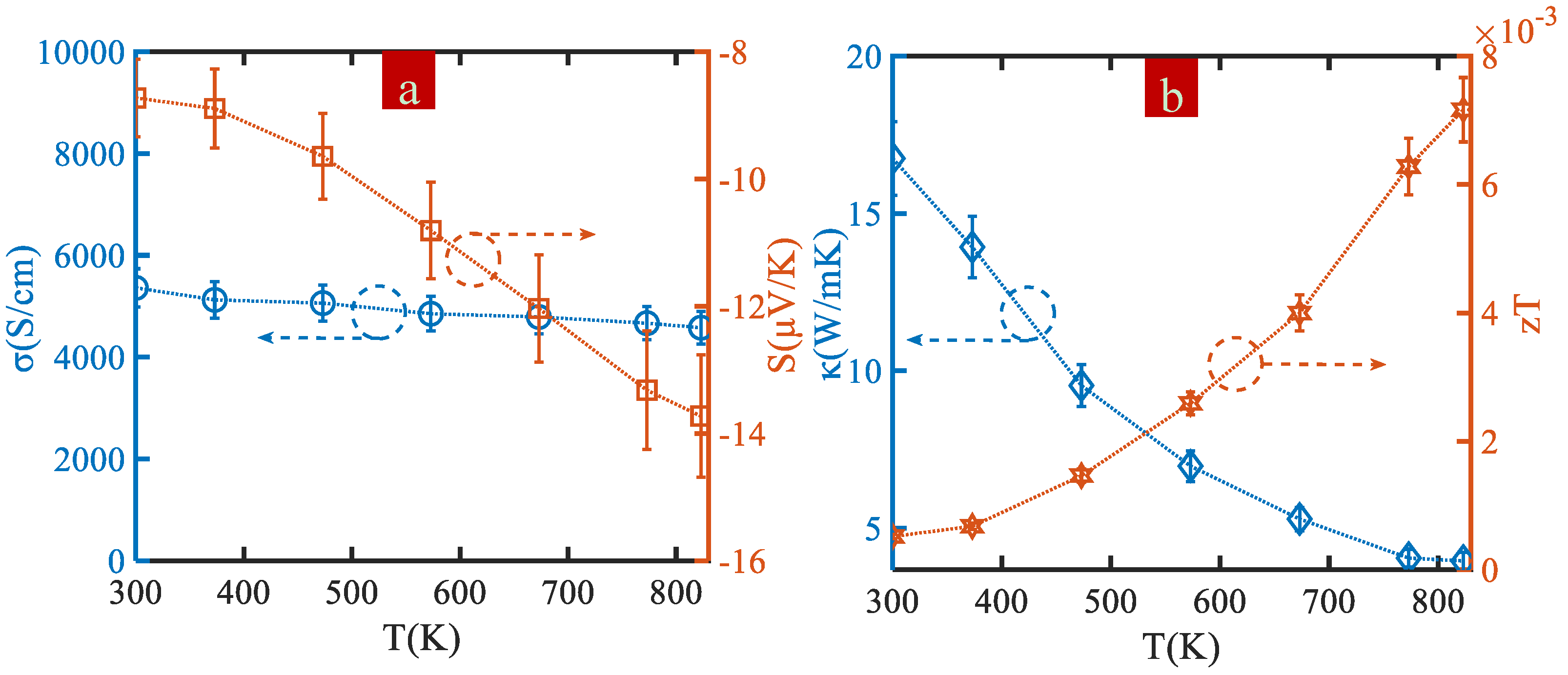
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shafai, C. Optimization of Bi2Te3 thin films for microintegrated Peltier heat pumps. J. Vac. Sci. Technol. A 1997, 15, 2798–2801. [Google Scholar] [CrossRef]
- Scoville, A.N.; Bajgar, C.; Vandersande, J.; Fleurial, J. High figure-of-merit n-type SiGe/GaP alloys, IECEC 91. In Proceedings of the 26th Intersociety Energy Conversion Engineering Conference, Boston, MA, USA, 4–9 August 1991; American Nuclear Society: La Grange Park, IL, USA, 1991; pp. 224–229. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Martín-González, M.S.; Beekman, M.; Balke, B.; Cerretti, G.; Tremel, W.; et al. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R: Rep. 2019, 138, 100501. [Google Scholar] [CrossRef]
- Hermann, R.P.; Jin, R.; Schweika, W.; Grandjean, F.; Mandrus, D.G.; Sales, B.C.; Long, G.J. Einstein Oscillators in Thallium Filled Antimony Skutterudites. Phys. Rev. Lett. 2003, 90, 135505. [Google Scholar] [CrossRef]
- Bentien, A.; Christensen, M.; Bryan, J.D.; Sanchez, A.; Paschen, S.; Steglich, F.; Stucky, G.D.; Iversen, B.B. Thermal conductivity of thermoelectric clathrates. Phys. Rev. B 2004, 69, 045107. [Google Scholar] [CrossRef]
- Anand, S.; Wood, M.; Wolverton, C.; Snyder, G.J. An Enormous Class of Double Half-Heusler Compounds with Low Thermal Conductivity; Cornell University: Ithaca, NY, USA, 2019. [Google Scholar]
- Jain, A.; Shin, Y.; Persson, K.A. Computational predictions of energy materials using density functional theory. Nat. Rev. Mater. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Fu, C.; Bai, S.; Liu, Y.; Tang, Y.; Chen, L.; Zhao, X.; Zhu, T. Realizing high figure of merit in heavy-band p-type half-Heusler thermoelectric materials. Nat. Commun. 2015, 6, 8144. [Google Scholar] [CrossRef]
- Galanakis, I. Theory of Heusler and Full-Heusler Compounds; Springer: Cham, Switzerland, 2015; Volume 222, pp. 3–36. [Google Scholar]
- Shutohs, S.S.N. Thermoelectric properties of the TiX (Zr0.5Hf0.5)1-XNiSn half-Heusler compounds. J. Alloys Compd. 2005, 389, 204–208. [Google Scholar] [CrossRef]
- Huang, L.; He, R.; Chen, S.; Zhang, H.; Dahal, K.; Zhou, H.; Wang, H.; Zhang, Q.; Ren, Z. A new n-type half-Heusler thermoelectric material NbCoSb. Mater. Res. Bull. 2015, 70, 773–778. [Google Scholar] [CrossRef]
- Zhou, M.; Feng, C.; Chen, L.; Huang, X. Effects of partial substitution of Co by Ni on the high-temperature thermoelectric properties of TiCoSb-based half-Heusler compounds. J. Alloys Compd. 2005, 391, 194–197. [Google Scholar] [CrossRef]
- Chai, Y.W.; Oniki, T.; Kimura, Y. Microstructure and thermoelectric properties of a ZrNi1.1Sn half-Heusler alloy. Acta Mater. 2015, 85, 290–300. [Google Scholar] [CrossRef]
- Sakurada, S.; Shutoh, N. Effect of Ti substitution on the thermoelectric properties of (Zr,Hf) NiSn half-Heusler compounds. Appl. Phys. Lett. 2005, 86, 82105. [Google Scholar] [CrossRef]
- Misra, D.K.; Rajput, A.; Bhardwaj, A.; Chauhan, N.S.; Singh, S. Enhanced power factor and reduced thermal conductivity of a half-Heusler derivative Ti9Ni7Sn8: A bulk nanocomposite thermoelectric material. Appl. Phys. Lett. 2015, 106, 103901. [Google Scholar] [CrossRef]
- Lee, P.-J.; Chao, L.-S. High-temperature thermoelectric properties of Ti0.5 (ZrHf) 0.5−xNbxNi0.9Pd0.1Sn0.98Sb0.02 half-Heusler alloys. J. Alloys Compd. 2010, 504, 192–196. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Huang, L.; Chen, S.; Dahal, H.; Wang, D.; Ren, Z. Synthesis and thermoelectric properties of n-type half-Heusler compound VCoSb with valence electron count of 19. J. Alloys Compd. 2016, 654, 321–326. [Google Scholar] [CrossRef]
- Katsuyama, S.; Kobayashi, T. Effect of mechanical milling on thermoelectric properties of half-Heusler ZrNiSn0.98Sb0.02 intermetallic compound. Mater. Sci. Eng. B 2010, 166, 99–103. [Google Scholar] [CrossRef]
- Gürth, M.; Rogl, G.; Romaka, V.V.; Grytsiv, A.; Bauer, E.; Rogls, P. Thermoelectric high ZT half-Heusler alloys Ti 1−x−y Zr x Hf y NiSn (0 ≤ x ≤ 1; 0 ≤ y ≤ 1). Acta Mater. 2016, 104, 210–222. [Google Scholar] [CrossRef]
- Zhu, T.J.; Xiao, K.; Yu, C.; Shen, J.J.; Yang, S.H.; Zhou, A.J.; Zhao, X.B.; He, J. Effects of yttrium doping on the thermoelectric properties of Hf 0.6 Zr 0.4 NiSn 0.98 Sb 0.02 half-Heusler alloys. J. Appl. Phys. 2010, 108, 044903. [Google Scholar]
- Zhang, H.; Wang, Y.; Dahal, K.; Mao, J.; Huang, L.; Zhang, Q.; Ren, Z. Thermoelectric properties of n-type half-Heusler compounds (Hf0.25Zr0.75)1–Nb NiSn. Acta Mater. 2016, 113, 41–47. [Google Scholar] [CrossRef]
- Joshi, G.; Yan, X.; Wang, H.; Liu, W.; Chen, G.; Ren, Z. Enhancement in Thermoelectric Figure-Of-Merit of an N-Type Half-Heusler Compound by the Nanocomposite Approach. Adv. Energy Mater. 2011, 1, 643–647. [Google Scholar] [CrossRef]
- Berry, T.; Fu, C.; Auffermann, G.; Fecher, G.H.; Schnelle, W.; Serrano-Sanchez, F.; Yue, Y.; Liang, H.; Felser, C. Enhancing Thermoelectric Performance of TiNiSn Half-Heusler Compounds via Modulation Doping. Chem. Mater. 2017, 29, 7042–7048. [Google Scholar] [CrossRef]
- Hazama, H.; Matsubara, M.; Asahi, R. Thermoelectric Properties of Off-Stoichiometric Ti-Ni-Sn Half-Heusler Systems. J. Electron. Mater. 2012, 41, 1730–1734. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Q.; Wang, Y.; He, R.; Shuai, J.; Zhang, J.; Wang, C.; Ren, Z. The effect of Sn doping on thermoelectric performance of n-type half-Heusler NbCoSb. Phys. Chem. Chem. Phys. 2017, 19, 25683–25690. [Google Scholar] [CrossRef] [PubMed]
- Rausch, E.; Balke, B.; Ouardi, S.; Felser, C. Enhanced thermoelectric performance in the p-type half-Heusler (Ti/Zr/Hf)CoSb0.8Sn0.2 system via phase separation. Phys. Chem. Chem. Phys. 2014, 16, 25258–25262. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhu, H.; Sun, J.; Mao, J.; Reith, H.; Chen, S.; Schierning, G.; Nielsch, K.; Ren, Z. Improved thermoelectric performance of n-type half-Heusler MCo1-xNixSb (M = Hf, Zr). Mater. Today Phys. 2017, 1, 24–30. [Google Scholar] [CrossRef]
- Gałązka, K.; Populoh, S.; Xie, W.; Yoon, S.; Saucke, G.; Hulliger, J.; Weidenkaff, A.; Büttner, G. Improved thermoelectric performance of (Zr0.3Hf0.7)NiSn half-Heusler compounds by Ta substitution. J. Appl. Phys. 2014, 115, 183704. [Google Scholar]
- Zhao, D.; Zuo, M.; Bo, L.; Wang, Y. Synthesis and Thermoelectric Properties of Pd-Doped ZrCoBi Half-Heusler Compounds. Materials 2018, 11, 728. [Google Scholar] [CrossRef]
- Geng, H.; Zhang, H. Effects of phase separation on the thermoelectric properties of (Ti, Zr, Hf) NiSn half-Heusler alloys. J. Appl. Phys. 2014, 116, 33708. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Martin, L.H.J.; Ivas, T.; Leinenbach, C.; Anand, S.; Peters, M.; Snyder, G.J.; Battaglia, C.; Cuervo-Reyes, E. Impact of Ni content on the thermoelectric properties of half-Heusler TiNiSn. Energy Environ. Sci. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Xie, H.; Wang, H.; Fu, C.; Liu, Y.; Snyder, G.J.; Zhao, X.; Zhu, T. The intrinsic disorder related alloy scattering in ZrNiSn half-Heusler thermoelectric materials. Sci. Rep. 2014, 4, 6888. [Google Scholar] [CrossRef]
- Xia, K.; Liu, Y.; Anand, S.; Snyder, G.J.; Xin, J.; Yu, J.; Zhao, X.; Zhus, T. Enhanced Thermoelectric Performance in 18-Electron Nb0.8CoSb Half-Heusler Compound with Intrinsic Nb Vacancies. Adv. Funct. Mater. 2018, 28, 1705845. [Google Scholar] [CrossRef]
- Zeeshan, M.; Brink, J.V.D.; Kandpal, H.C. Hole-doped cobalt-based Heusler phases as prospective high-performance high-temperature thermoelectrics. Phys. Rev. Mater. 2017, 1, 74401. [Google Scholar] [CrossRef]
- Öğüt, S.; Rabe, K.M. Band gap and stability in the ternary intermetallic compounds NiSn M (M = Ti, Zr, Hf): A first-principles study. Phys. Rev. B 1995, 51, 10443–10453. [Google Scholar] [CrossRef] [PubMed]
- Hichour, M.; Rached, D.; Khenata, R.; Rabah, M.; Merabet, M.; Reshak, A.; Bin-Omran, S.; Ahmed, R. Theoretical investigations of NiTiSn and CoVSn compounds. J. Phys. Chem. Solids 2012, 73, 975–981. [Google Scholar] [CrossRef]
- Shi, H.; Ming, W.; Parker, D.S.; Du, M.H.; Singh, D.J. Prospective high thermoelectric performance of the heavily p-doped half-Heusler compound CoVSn. Phys. Rev. B 2017, 95, 195207. [Google Scholar] [CrossRef]
- Zeeshan, M.; Singh, H.K.; Brink, J.V.D.; Kandpal, H.C. Ab initio design of new cobalt-based half-Heusler materials for thermoelectric applications. Phys. Rev. Mater. 2017, 1, 075407. [Google Scholar] [CrossRef]
- Schroeder, D.V.; Gould, H. An Introduction to Thermal Physics; Addison-Wesley: Reading, MA, USA, 2000; pp. 149–220. [Google Scholar]
- Tobola, J.; Kaprzyk, S.; Skolozdra, R.V.; A Kouacou, M.; Pierre, J. Crossover from semiconductor to magnetic metal in semi-Heusler phases as a function of valence electron concentration. J. Phys.: Condens. Matter 1998, 10, 1013–1032. [Google Scholar] [CrossRef]
- Villars, P. Inorganic Solid Phases, Springer Materials (Online Database); Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Brillson, L. Metal-semiconductor interfaces. Surf. Sci. 1994, 299, 909–927. [Google Scholar] [CrossRef]
- Batra, I.; Tekman, E.; Ciraci, S. Theory of Schottky barrier and metallization. Prog. Surf. Sci. 1991, 36, 289–361. [Google Scholar] [CrossRef]
- Lue, C.; Öner, Y.; Naugle, D.; Ross, J.J.H. Magnetism of new semi-Heusler compounds FeVSn and CoVSn. IEEE Trans. Magn. 2001, 37, 2138–2140. [Google Scholar] [CrossRef]
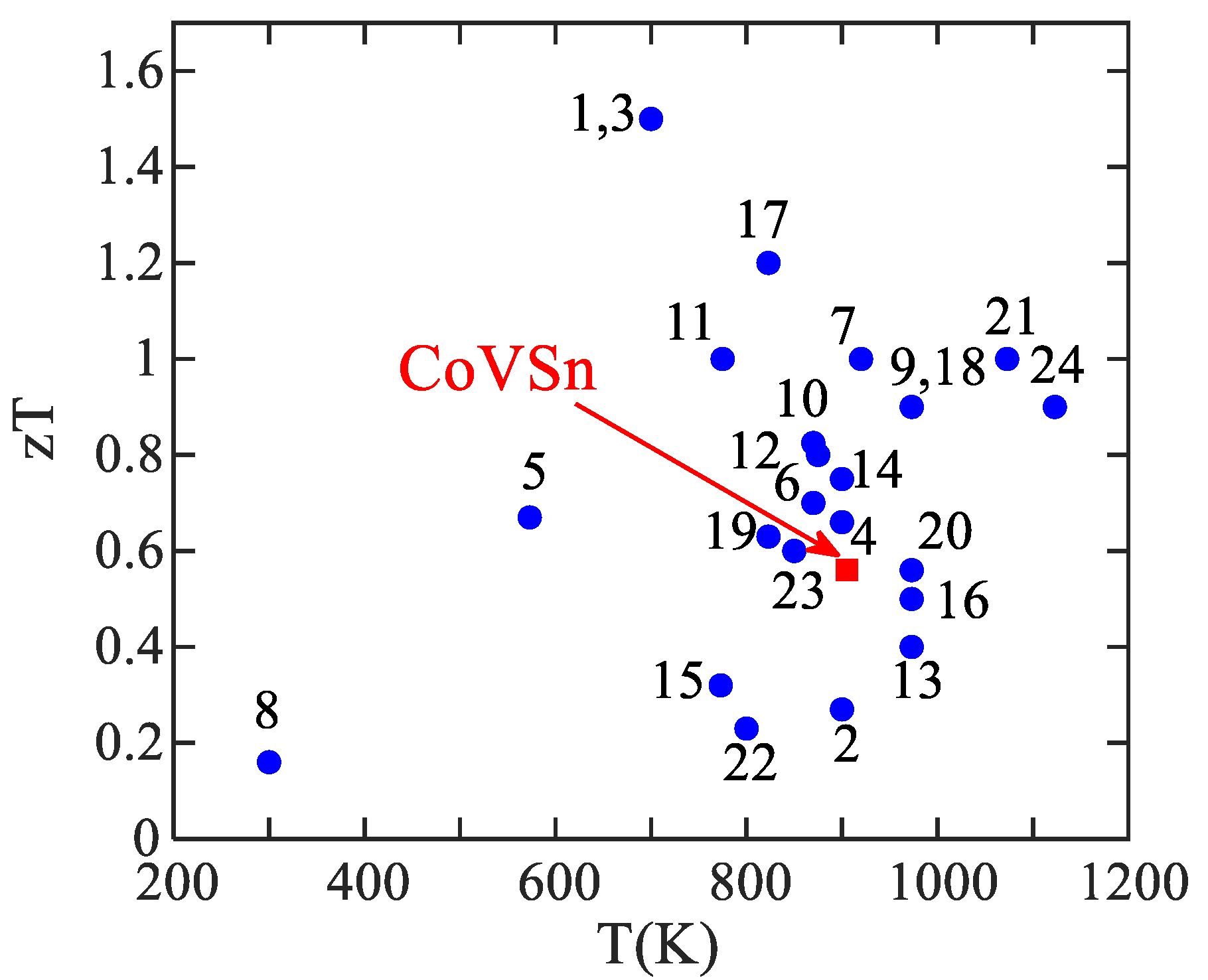
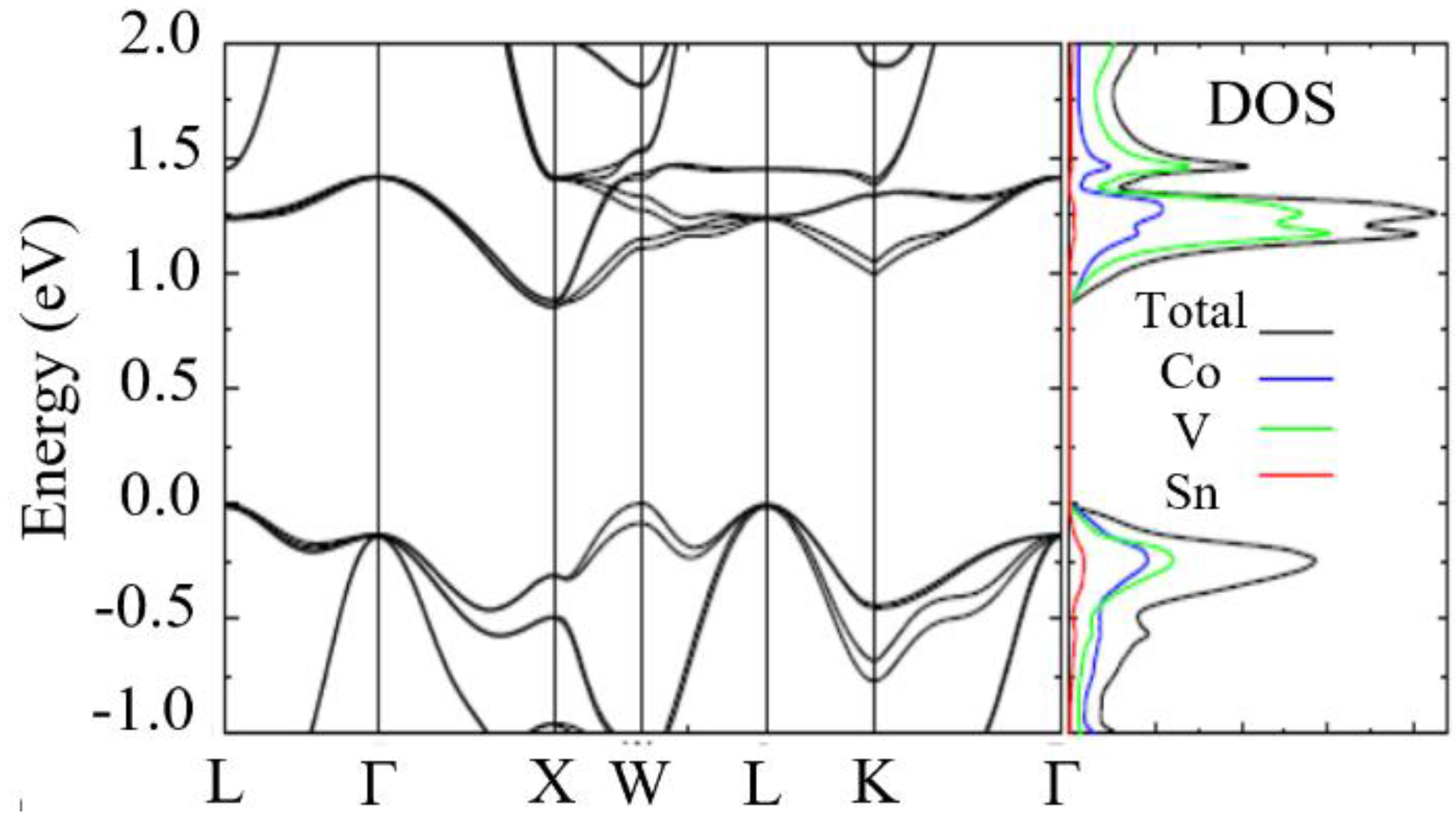
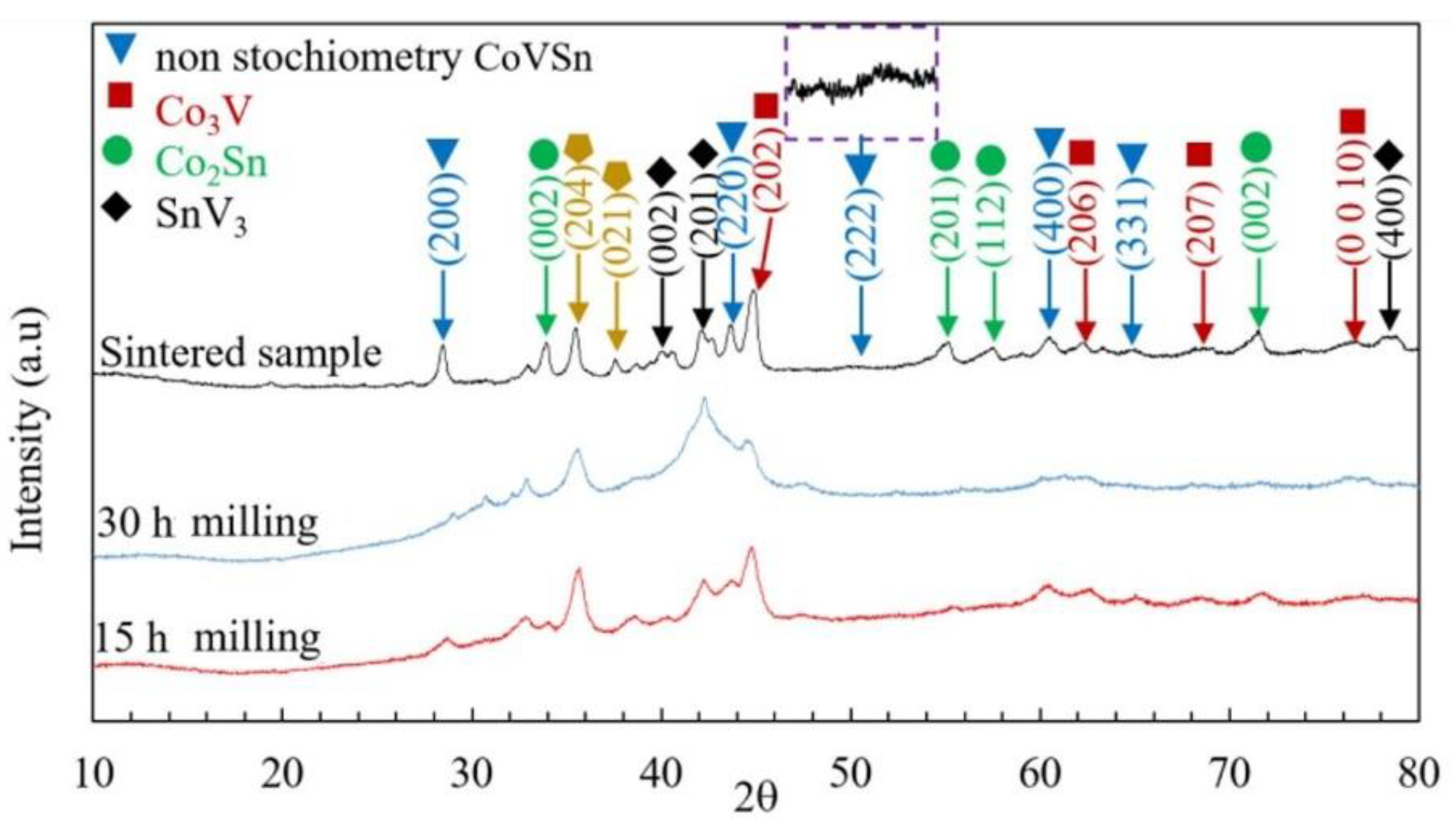


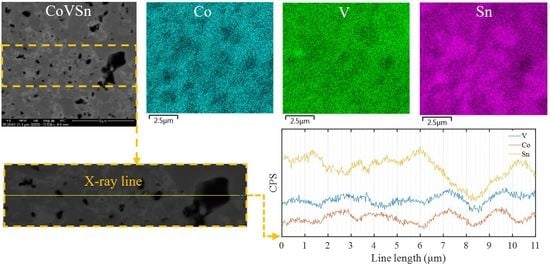
| Item | Compound | Ref. | Item | Compound | Ref. |
|---|---|---|---|---|---|
| 1 | Ti0.5(Zr0.5Hf0.5)0.5NiSn0.998Sb0.002 | [11] | 13 | NbCoSb | [12] |
| 2 | TiCo0.95Ni0.05Sb | [13] | 14 | Zr,Ni,Sn | [14] |
| 3 | (Zr0.5Hf0.5)0.5Ti0.5NiSn0.998Sb0.002 | [15] | 15 | Ti,Ni,Sn | [16] |
| 4 | Ti0.5(ZrHf)0.49Nb0.01Ni0.9Pd0.1Sn0.98Sb0.02 | [17] | 16 | VCoSb | [18] |
| 5 | ZrNiSn0.98Sb0.02 | [19] | 17 | Ti0.5Zr0.5NiSn0.98Sb0.02 | [20] |
| 6 | (Hf0.6Zr0.4)0.99Y0.01NiSn0.98Sb0.02 | [21] | 18 | (Hf0.25Zr0.75)0.995Nb0.005NiSn | [22] |
| 7 | Hf0.75Zr0.25NiSn0.99Sb0.01 | [23] | 19 | (TiNiSn)0.95 + (MnNiSb)0.05 | [24] |
| 8 | 1.5% Y-Sb-doped Ti-Ni-Sn | [25] | 20 | NbCoSb0.8Sn0.2 | [26] |
| 9 | TiCoSb0.8Sn0.2 | [27] | 21 | Zr0.5Hf0.5Co0.9Ni0.1Sb | [28] |
| 10 | (Zr0.3Hf0.65Ta0.05)NiSn | [29] | 22 | ZrCo0.97Pd0.03Bi | [30] |
| 11 | (Ti0.4(Zr0.5Hf0.5)0.6)0.99Ta0.01NiSn | [31] | 23 | TiNi1.06Sn0.81Sb0.17 | [32] |
| 12 | ZrNiSn0.99Sb0.01 | [33] | 24 | Nb0.83CoSb | [34] |
| Predicted TE factors of CoVSn compound, κ = 4.1 W/mK, S = 175 μV K−1, zT = 0.53 at 900 K. | [35] | ||||
| Temperature (°C) | Label | Phase (s) |
|---|---|---|
| 25 | a2 | Co3Sn2_A + HCP_A3 + HCP_ORD |
| b2 | Co3Sn2_A + CoSn + HCP_ORD | |
| c2 | CoSn + HCP_ORD + Sn3V2 | |
| d2 | CoSn + CoSn2 + Sn3V2 | |
| e2 | BCT_A5 + CoSn2 + Sn3V2 | |
| f2 | HCP_ORD + Sn3V2 + SnV3 | |
| g2 | CoV3_A15 + HCP_ORD + SnV3 | |
| h2 | BCC_B2 + CoV3_A15 + SnV3 | |
| i2 | BCC_B2 + CoV3_A15 | |
| 600 | a6 | Co3Sn2_A + FCC_L12 |
| b6 | Co3Sn2_B + FCC_L12 + HCP_ORD | |
| c6 | Co3Sn2_B + HCP_ORD | |
| d6 | Co3Sn2_B + CoSn + HCP_ORD | |
| e6 | ALTA_SIGMA (V,Co) + CoSn + HCP_ORD | |
| f6 | ALTA_SIGMA (V, Co) + CoSn | |
| g6 | ALTA_SIGMA(V, Co) + CoSn+ SnV3 | |
| h6 | CoSn + Sn3V2 + SnV3 | |
| i6 | LIQUID + CoSn + Sn3V2 | |
| j6 | LIQUID + Sn3V2 | |
| k6 | ALTA_SIGMA(V, Co) + SnV3 | |
| l6 | ALTA_SIGMA(V, Co) + CoV3_A15 + SnV3 | |
| h2 | BCC_B2 + CoV3_A15 + SnV3 | |
| i2 | BCC_B2 + CoV3_A15 | |
| o6 | BCC_B2 | |
| p6 | BCC_B2 + SnV3 | |
| 900 | a9 | FCC_L12 |
| b9 | Co3Sn2_B + FCC_L12 | |
| c9 | Co3Sn2_B + FCC_L12 + HCP_ORD | |
| d9 | Co3Sn2_B + HCP_ORD | |
| e9 | ALTA_SIGMA(V, Co) + Co3Sn2_B + HCP_ORD | |
| f9 | ALTA_SIGMA(V, Co) + Co3Sn2_B | |
| g9 | Co3Sn2_B+BCC_B2+CoSn | |
| h9 | ALTA_SIGMA(V, Co) + Co3Sn2_B + BCC_B2 | |
| i9 | BCC_B2 + Co3Sn2_B | |
| j9 | LIQUID + BCC_B2 + CoSn | |
| k9 | ALTA_SIGMA (V, Co) + CoV3_A15 + BCC_B2 | |
| l9 | BCC_B2 + CoSn | |
| i2 | BCC_B2 + CoV3_A15 | |
| o6 | BCC_B2 | |
| o9 | LIQUID + SnV3 | |
| p9 | LIQUID + BCC_B2 + SnV3 | |
| p6 | BCC_B2 + SnV3 | |
| r9 | LIQUID + BCC_B2 | |
| s9 | ALTA_SIGMA(V, Co) + BCC_B2 | |
| 1100 | a9 | FCC_L12 |
| b9 | Co3Sn2_B + FCC_L12 | |
| c11 | LIQUID + Co3Sn2_B + FCC_L12 | |
| d11 | LIQUID + FCC_L12 | |
| e11 | LIQUID + ALTA_SIGMA | |
| f11 | LIQUID + ALTA_SIGMA (V, Co) + FCC_L12 | |
| g11 | LIQUID | |
| h11 | LIQUID + ALTA_SIGMA(V, Co) + BCC_B2 | |
| i11 | ALTA_SIGMA (V, Co) + BCC_B2 | |
| o6 | BCC_B2 | |
| p6 | BCC_B2 + SnV3 | |
| l11 | LIQUID + BCC_B2 | |
| m11 | LIQUID + LIQUID #2 + SnV3 | |
| n11 | LIQUID + LIQUID #2 + BCC_B2 | |
| l11 | LIQUID + BCC_B2 | |
| o9 | LIQUID + SnV3 | |
| q11 | LIQUID + LIQUID #2 |
| T (°C) | Phase/Crystal Structure/Elements |
|---|---|
| 25 | CoSn, HCP_ORD (Co, V) and Sn3V2 |
| 100 | SnV3, HCP_ORD (Co, V) and Sn3V2 |
| 200 | SnV3, HCP_ORD (Co, V) and Sn3V2 |
| 300 | CoSn, HCP_ORD (Co, V) and Sn3V2 |
| 400 | CoSn, HCP_ORD (Co, V) and Sn3V2 |
| 500 | CoSn, HCP_ORD (Co, V) and Sn3V2 |
| 600 | CoSn, SnV3, ALTA_SIGMA (V, CO) |
| 700 | SnV3, BCC_B2 (Co,V,Sn), ALTA_SIGMA (V, Co) |
| 800 | Equilibrium line between two areas of (CoSn, BCC_B2 (Co,V,Sn)) and (ALTA_SIGMA (V, Co), CoSn, BCC_B2 (Co,V,Sn)) |
| 900 | Equilibrium line between two areas of (CoSn, BCC_B2 (Co,V,Sn)) and (LIQUID, CoSn, BCC_B2 (Co,V,Sn)) |
| 1000 | LIQUID, BCC_B2 (Co,V,Sn) |
| 1100 | LIQUID |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooshmand Zaferani, S.; Darebaghi, A.; Hong, S.-J.; Vashaee, D.; Ghomashchi, R. Experimental Realization of Heavily p-doped Half-Heusler CoVSn Compound. Energies 2020, 13, 1459. https://doi.org/10.3390/en13061459
Hooshmand Zaferani S, Darebaghi A, Hong S-J, Vashaee D, Ghomashchi R. Experimental Realization of Heavily p-doped Half-Heusler CoVSn Compound. Energies. 2020; 13(6):1459. https://doi.org/10.3390/en13061459
Chicago/Turabian StyleHooshmand Zaferani, Sadeq, Alireza Darebaghi, Soon-Jik Hong, Daryoosh Vashaee, and Reza Ghomashchi. 2020. "Experimental Realization of Heavily p-doped Half-Heusler CoVSn Compound" Energies 13, no. 6: 1459. https://doi.org/10.3390/en13061459
APA StyleHooshmand Zaferani, S., Darebaghi, A., Hong, S.-J., Vashaee, D., & Ghomashchi, R. (2020). Experimental Realization of Heavily p-doped Half-Heusler CoVSn Compound. Energies, 13(6), 1459. https://doi.org/10.3390/en13061459