Abstract
The present work focuses on the impact of dielectric barrier discharge (DBD) plasma actuators (PAs) on non-premixed lifted flame stabilization in a methane CH4-air Bunsen burner. Two coaxial DBD-PA configurations are considered. They are composed of a copper corona, installed on the outer surface of a quartz tube and powered with a high voltage sinusoidal signal, and a grounded needle installed along the burner axis. The two configurations differ in the standoff distance value, which indicates the positioning of the high frequency/high voltage (HV) electrode’s upper edge with respect to the needle tip. Experimental results highlight that flame reattachment is obtained at a lower dissipated power when using a negative standoff distance (i.e., placing the needle upstream with respect to the corona). At 11 kV peak-to-peak voltage and 20 kHz frequency, plasma actuation allowed for reattaching the flame with a very low dissipated power (of about 0.05 W). Numerical simulations of the electrostatic field confirmed that this negative standoff configuration has a beneficial effect on the momentum sources, which oppose the flow and show that the highest electric field extends into the inner quartz tube, as confirmed by experimental visualization close to the needle tip. The modeling predicted an increase in the gas temperature of about 21.8 °C and a slight modification of the fuel composition at the burner exit. This impacts the flame speed with a 10% increase close to the stoichiometric conditions with respect to the clean configuration.
1. Introduction
Nowadays, the aeroengine industries have addressed the development of low NOx combustion technologies in many ambitious national and international research programs. Along these research paths, the “lean burn” has been considered for mid- to long-term targets, pointing to the design of new low NOx emission combustors. Lean burning combustors include low-temperature flames of lean fuel mixture as one of the most promising solutions [,]. However, lean flames are usually affected by efficiency penalty as well as strong flame instabilities that increase the risk of flame quenching (i.e., the flame blow-off, as leaner conditions are approached). The incipience of the blow-off condition is usually anticipated by the lifting of the flame, whose behavior has not been deeply understood yet. It is worth underlining that the lean burn is defined by the lean flammability limit (LFL) expressed as percentage by volume of the fuel into the mixture; lean combustion usually occurs below the LFL [].
In cases concerning premixed or partially premixed flames, the conventional way to address flame stabilization involves adding a pilot flame for safe operation []. However, this pilot flame may generate a significant level of unwanted NOx emissions. In addition to a pilot flame, passive control systems are of common use in the field of aeroengine combustor design. They are based on the modification of the air injection geometry (such as swirlers) and flame holders, which promote the flame stability by means of the establishment of large-scale vortexes. These flow structures permit turbulent mixing with recirculation of hot gases and extend the mixture flammability range. In this regard, an experimental investigation concerning the stabilization of turbulent non-premixed flames was presented by Cha and Chung [], Fokaides et al. [] provided, instead, an experimental characterization of the mixing evolution, the flow pattern, and the temperature distribution of a lifted-stabilized swirl flame near lean blowout conditions.
In the case of non-premixed diffusive lifted flames, flame stabilization is strongly affected by the velocity gradient and the burnt gas expansion on edge flame propagation, as determined by Chen []. In a different study, Chen and colleagues [] also found that in presence of lifted diffusion jet, the flame stability is influenced by local flame extinction in the flame area where the outer mixing layer merges with the central flame front.
A different and complementary active control approach is the use of plasma assisted combustion (PAC) technology []. PAC has the potential to enhance combustion by acting as a source of heat, radicals, excited species, electrons/ions, and fuel fragments, simultaneously [,]. Furthermore, plasma actuators also produce strong electric fields; they introduce significant electro-hydrodynamic momentum sources which affect the electrically charged flow (ion wind) resulting from both combustion and plasma ionization reactions. Recently, several experimental investigations were conducted to evaluate the impact of an external electric field into the combustion region on non-premixed methane-air diffusive flames by means of an ion current [,,,]. Based on the experimental observation that chemi-ions modify flame behavior in the presence of an applied electric field, Lopez-Camara et al. [] performed a numerical investigation on the potential relationship between peak location and magnitude of ions and excited species. Previously, Belhi et al. [] carried out numerical simulations which demonstrated the capability of both direct current (DC) and alternating current (AC) external electric fields in stabilizing a lifted diffusion methane-air flame. In particular, plasma actuators are active, electrically-driven devices able to provide a high frequency/high voltage (HV) signal. When applied to combustion enhancement, they provide high flexibility since they can be combined with other passive control devices such as swirlers. Furthermore, they are characterized by an extremely short response time when compared to other active control systems, which allows for a rapid adjustment of control parameters according to real-time operation conditions.
In the field of electrical discharge plasmas, non-thermal plasmas (NTPs), sometimes called non-equilibrium or ‘cold’ plasmas, were recently investigated. As underlined by Rosocha, Kim, and Stange [], in NTPs, electrons and ions/neutral gas are not in thermal equilibrium: the former have high energy and “hot” temperature, whereas the latter are near ambient temperature (“cool”). This results in little waste enthalpy (heat) deposited in a process gas. Consequently, NTPs provide a small gas temperature rise in combination with high electrical-to-fluidic energy conversion efficiency, namely the ratio between the power delivered to the fluid and the electrical power consumption. One of the most promising non-thermal plasma devices is the dielectric barrier discharge plasma actuator (DBD-PA). As demonstrated by Kim and colleagues [] and Rosocha and co-workers [], in such plasmas, electron temperatures lower than 12 eV can break down the fuel species producing free radicals. To date, several DBD-PAs have been investigated to improve the fuel/air mixtures ignition [,,], increase flame propagation [,], or enhance flame stabilization by extending flammability limits [,,,,,]. Concerning this last aspect, combustion enhancement has been observed when applying a DBD-PA for fuel/oxidizer decomposition [,]. Among all configurations, coaxial DBD-PAs have been widely investigated as fuel reforming reactors with promising results when applied to combustion systems [,,].
In this context, the present work provides an investigation of plasma actuation in a coaxial DBD configuration for a non-premixed lean lifted flame stabilization. Experiments were carried out in a Bunsen-type burner with an inner methane jet surrounded by an outer air jet (LFL equal to 0.05, corresponding to a lean burn equivalence ratio Φ of about 0.485), confined by two coaxial quartz tubes. The coaxial DBD was composed of a high-voltage corona electrode mounted on an outer quartz tube, coupled with an axially-placed grounded needle electrode. Based on the relative position of the needle electrode with respect to the corona electrode, two coaxial DBD configurations were investigated. Hence, the analysis of the experimental results is supported by simulations of the electrostatic field, in combination with a one-dimensional spatio-temporal analysis of the plasma chemistry inside the methane tube, aiming to decouple thermal and kinetics effects.
2. Experimental Set-Up and Methods
The experimental setup consisted of a coaxial Bunsen burner equipped with a plasma actuator in a ring-needle configuration, as shown in Figure 1. Dedicated gas lines fed air and methane into two coaxial quartz tubes, the inner one for CH4 and the outer one for air. The inner quartz tube (methane line) had an external diameter of 10 mm and a thickness of 1 mm, while the coaxial outer tube (air line) had an external diameter of 30 mm and a thickness of 2 mm.
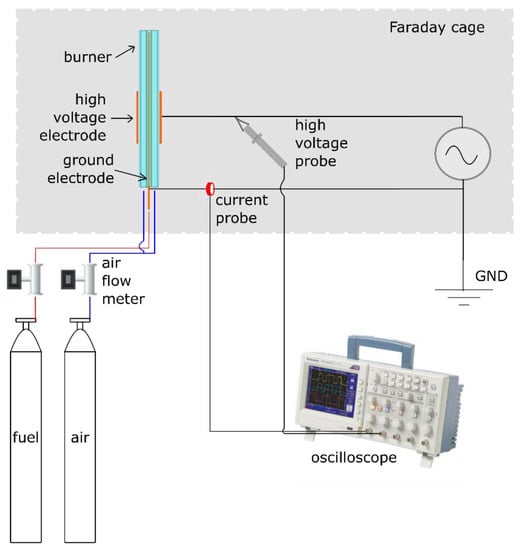
Figure 1.
Diagram of the experimental electrical setup.
The final coaxial flow configuration resulted in a normal diffusive flame (NDF), which was characterized by an inner fuel jet surrounded by an outer oxidizer jet. The top ends of the two coaxial quartz tubes were aligned as shown in Figure 2; thus, the flame established at the exit of the burner.
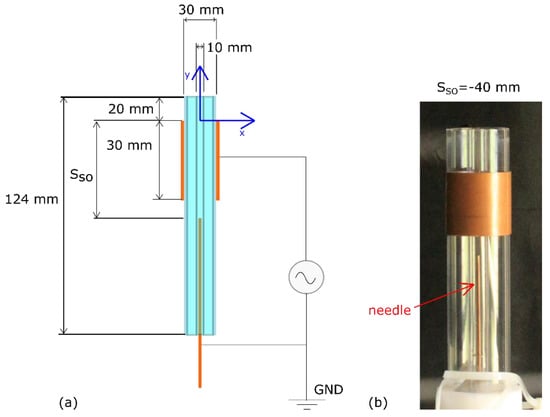
Figure 2.
Coaxial dielectric barrier discharge plasma actuator (DBD-PA): (a) geometry and dimensions; (b) picture of the actual setup at stand-off distance SSO = −40 mm.
The air and methane flow rates were recorded using flow meters, with an accuracy of ±3% of reading and ±0.3% full scale value for the air flow, and ±0.8% of reading and ±0.2% full scale value for the methane flow.
The electrical setup was composed of a HV generator, a HV probe, and a current transformer, which allowed for the measurement of the electrical power. A copper ring 30 mm in length, 1 mm in thickness, and 30 mm in inner diameter, was fastened by screws onto the external surface of the outer quartz tube and connected to the HV (herein referred as HV electrode). The grounded electrode consisted of a copper needle having a blunt tip with a 90° angle, and a diameter of 1 mm, which was axially placed into the inner quartz tube. Concerning the electrical feeding, a sinusoidal high frequency/HV signal was provided to the HV electrode by means of a HV generator (the PVM500 Plasma Resonant and Dielectric Barrier Corona Driver). The applied voltage signal was acquired using the HV probe Tektronix P6015A (accuracy of ±3% of reading). The current transformer Bergoz mod. CT-C1.0-BNC was used, instead, to measure the current flowing in the circuit with an accuracy of ±0.5% of reading.
Both the HV probe and the current transformer signals were simultaneously acquired by means of the oscilloscope model Tektronix TDS2024C (accuracy of ±3% of reading). The acquisition sample rate was set to 25 MHz and each measurement point was given by the average of 128 samples. Based on voltage and current measurements, the electrical power consumption of the DBD was computed as follows:
where is the period of the applied voltage and I(t) and V(t) are the acquired current and the voltage signals, respectively.
Table 1 shows the experimental test matrix, where SSO is the standoff distance defined as the axial distance between the HV electrode upper edge and the needle tip (see Figure 2a). All experiments were conducted by fixing both the air and the fuel flow rates at 1.12 ± 0.04 g/s and 0.0023 ± 0.0002 g/s respectively, which corresponded to the characteristic axial velocities of fuel and air flows at the burner exit of about uf = 0.074 ms−1 and ua = 2.3 ms−1, and a global equivalence ratio [] of 0.035, which is defined as , where is the mass flow rate, the subscripts f and a denote the fuel and the air respectively, while the subscript st refers to the stoichiometric conditions with a stoichiometric fuel-to-air ratio equal to 0.0559. It is worth observing that the local equivalence ratio differs from the global one: it varies into the flame region due to the diffusive nature of the flame, approaching unity in the proximity of the flame surface []. The LFL was equal to 0.05, corresponding to a lean burn equivalence ratio ΦLFL = 0.485. Regarding the plasma actuation, the peak-to-peak (Vpp) value of the sinusoidal HV was changed at a fixed actuation frequency of about 20 kHz.

Table 1.
Test matrix of experiments and operating conditions. Plasma actuation frequency of 20 kHz. Characteristic axial velocities: fuel flow, uf = 0.074 ms−1; air flow, ua = 2.3 ms−1.
For each test case, several snapshots of the flame appearance were captured using a Canon EOS 700D camera, equipped with a Canon EFS 55-250 mm lens with resolution of 2912 pixels × 5184 pixels. The image pixel gray level value was discretized using 8 bits, ranging from 0 to 255; they defined the full dark condition (ultra-low luminosity) and the full bright condition (ultra-high luminosity). The toolbox Matlab® was used to process the flame images, which were cropped in order to select the region of interest (ROI). Then, the image grayscale was converted to the image luminance I ranging between 0 and 1. Finally, a proper false-color map ranging between 0 and 0.7 was chosen in order to highlight the flame structure depicted into each raw image.
3. Numerical Modeling
3.1. Electrostatic Field Simulation
In order to provide further insights for the analysis of the experimental findings, two-dimensional axisymmetric numerical computations of the electric field inside the burner were performed. The prediction of the electric field aims to identify the ionization area, where the presence of body force induced by the plasma discharge would be relevant. Furthermore, it also permits the evaluation of the direction of this body force. Due to the negligible influence of the magnetic induction and the absence of a relevant external magnetic field, electric field simulations were performed using the electric potential method provided by the magnetohydrodynamic (MHD) module of the toolbox Ansys® Fluent 2019 R1. This allowed to solve the electric field into the computational domain without further addition of user-defined functions and scalars. The set of the governing equations was thus defined as follows:
where represents the electric potential, is the electric conductivity in a solid zone or the ionic conductivity in a fluid zone, and is the electric current density. Equations (2)–(4) are reduced to the Laplacian of the electric potential, namely:
Both coaxial DBD configurations were investigated, as reported in Table 2.

Table 2.
Test matrix of electrostatic field simulations.
A suitable computational grid was defined for each based on the same geometrical domain (see Figure 3a), but different location of the axial needle. The geometrical domain extended about 0.16 mm along the burner tubes, while the external region extended 3Roqt along the axial direction and 2Roqt along the radial direction, where Roqt is the outer radius of the outer quartz tube. The resulting mesh had 125,280 cells. Dielectric surfaces, such as the quartz tubes, were treated as an insulating boundary, and a zero flux of electrons was set on them, as well as the far-field external boundaries. A zero-voltage condition was set instead on the conductive walls of the grounded electrode, and a specific fixed voltage was imposed on the conductive walls of the HV electrode. Coupled walls defined the interfaces between fluid and solid. Both air and methane temperatures were set at 300 K. Based on the experimental mass flow rates, the methane jet velocity at the inner quartz tube inlet was fixed at 0.074 ms−1, while the air velocity at the entrance of the outer quartz tube was set at 2.3 ms−1.
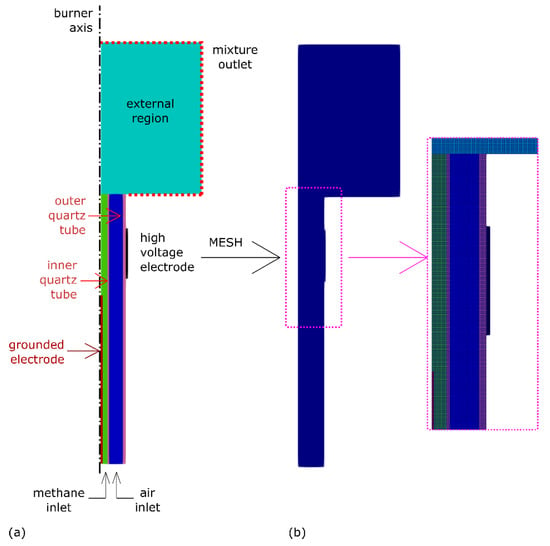
Figure 3.
Two-dimensional (2D) computational domain for electrostatic field simulations: (a) geometry; (b) mesh.
Computations were performed using a pressure-based steady-state solver in combination with the coupled scheme for the pressure-velocity coupling, a Green–Gauss cell-based scheme for the gradient operator, a second-order scheme for the pressure, and a second-order upwind scheme for all the rest of the spatial discretization variables. Simulations were stopped when all residuals were below 10−6.
3.2. Plasma Modeling of the Methane Kinetics and Reactor
By coupling electrostatic field computation insights with experimental observations, ionization was supposed to occur only in the methane tube meant to work as a plasma reactor, while negligible ionization was considered to occur in the air coaxial tube. Therefore, a numerical analysis of the methane plasma chemistry was performed in order to evaluate the thermo-chemistry effect of plasma actuation on the flame behavior which was experimentally observed. To this purpose, the configuration with SSO = −40 mm was considered (test case 2), since it resulted as the most performing.
The electron-impact excitation, dissociation, and ionization reactions were integrated into the reduced gas-phase radicals reaction scheme shown in Table 3. Similar to a study by Poinsot and Veynante [], the zero-dimensional plasma kinetics solver ZDPlasKin [] was coupled with the Chemkin-Pro [] package for the spatial solution of the plasma chemistry along the methane quartz tube and for the flame analysis.

Table 3.
Overall reactions scheme. Electron-impact reaction rates were calculated at Telec = 1.7 eV. Reaction rate coefficients . Units: , cm·molecules·s·K; , dimensionless; , K.
In particular, the ZDPlasKin model implemented a Boltzmann equation solver [] for the electron-impact reactions, and computed the corresponding reaction rate constants, based on the input list of collision cross sections. The last one was built using the database LXCat []. A non-equilibrium system with an electron temperature much higher than gas temperature was set, whereas ion temperature was not considered. During the discharge process, the time evolution of each species was computed using the following equation:
where the subscripts i and j refer to the species i and the reaction j, respectively, N represents the species number density, Qij is the source rates of the species i corresponding to the reaction j, and imax and jmax are the total number of species and reactions considered in the model.
ZDPlasKin computations were performed at constant atmospheric pressure and gas temperature of 300 K. The gap length was set equal to the inner radius of the methane tube, while the ionization region was supposed to be semi-spherical with the same radius. A constant applied voltage equal to the root mean square of the experimental voltage signal (i.e., Vapp = (Vpp/2)/√2 = 3.76 kV), was used for the estimation of the average reduced electric field established in the ionization region. The time step was set to 10−5 s, and computations were stopped after 300 s, which ensured the steady-state solution. The electrical behavior of the plasma discharge was considered by tuning a resistance R based on comparison between the experimental current peak with the one predicted by the ZDPlasKin model. As a result, R was set at 1000 kΩ.
Computations estimated a reduced electric field E/N of about 30 Td in accordance with Pitchford and colleagues []. Concerning the plasma kinetics, in accordance with Sun and co-workers [], the electron-impact reaction scheme involved 11 species (CH4, CH3, CH2, CH, H2, H, , , , and the electron e), and consisted in three electron-impact reaction groups, namely the ionization reactions (Equations (E1)–(E9) in Table 3), the recombination reactions (Equations (E10)–(E13) in Table 3), and the dissociation reactions (Equations (E14)–(E18) in Table 3). The solution provided by ZDPlasKin estimated the reaction rate constants of all electron-impact reactions, corresponding to an electron temperature Telec = 1.7 eV.
Therefore, based on the reaction path analysis provided by Masi and colleagues [], underlining that the plasma zone would mainly consist of hydrogen radicals with trace amounts of CHx radicals, the reaction scheme was enlarged. In particular, 12 more reversible gas-phase reactions (Equations (R1)–(R13) in Table 3) involving five new species (i.e., C2H2, C2H3, C2H4, C2H5, and C2H6) were considered. The reaction rate constants of the gas-phase reactions were taken from the GRI-Mech 3.0 kinetic scheme [] and the thermodynamic properties of all 16 species were retrieved from the Burcat database [].
The overall plasma kinetics were then spatially solved by means of Chemkin-Pro. To this purpose, the plasma plug flow reactor (PFR) model was used.
The chemical reactor is represented in the model as a long tube with a constant cross section. Standard assumption of the plug flow model is that the fluid is perfectly mixed in the direction perpendicular to the axis. Furthermore, it is assumed that radial and angular variations of the flow variables (as species fraction, axial velocity, and temperature) are negligible. Perfect mixing also implies strong friction between species, such that they all have the same axial velocity.
It is worth observing that some discrepancies between numerical predictions and experimental data can be retrieved, owing to the simplified treatment of the heat transfer inside the reactor body, the simplified treatment of the heat and mass transfer in the radial direction (due to averaging) and the lack of heat and mass transfer (plug flow assumption) in the axial direction (due to the Lagrangian approach). Despite these limitations, the implemented two-step modeling approach is valuable for process engineering applications involving design, optimization, and verification of plasma reactors by using much less computational resources than a two-dimensional model.
Based on one-dimensional (1D) approximation in relation to the axisymmetric geometry of the reactor, it solved the following species, momentum, and energy balance equations:
where A represents the cross-sectional area of the reactor, F is the drag force, P is pressure, T is the temperature, h is the specific enthalpy, u is axial velocity, and ρ is density. Also, W is the molecular weight, Y is the molar fraction, is molar production rate, is the mean heat capacity per mass, is the plasma power deposition per unit length, is the inner surface area per unit length, and is the heat flux from the surroundings to the reactor tube.
PFR computations estimated the plasma-induced modification of the fuel composition at the methane tube exit section. The zero axial position was set at the tip of the needle and the reactor diameter was set equal to the inner diameter of the methane quartz tube. A pressure value equal to 101,325 Pa was set at the inlet section, together with a 0.99 molar fraction of CH4, a 0.001 molar fraction of H2, and an electron number density of about 108 molecules/cm3. Computations were performed by fixing the gas temperature at 300 K. The plasma power deposition profile, shown in Figure 4, was provided in order to reproduce the plasma reactor behavior.
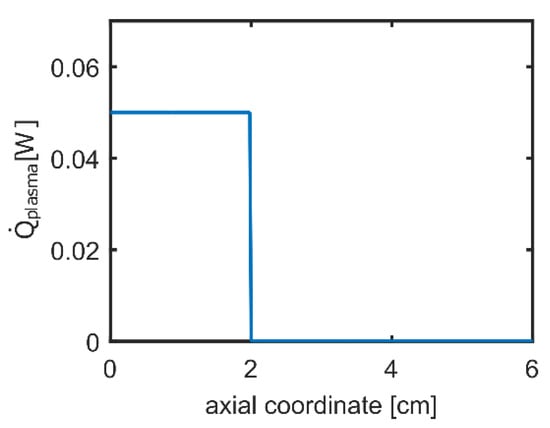
Figure 4.
Plasma power deposition used for plug flow reactor (PFR) computations.
The amplitude of the plasma power was set equal to the experimental dissipated power (i.e., 0.05 W). The heat exchange between the gas and the surroundings was neglected based on cold-plasma assumption.
Finally, the modification of the flame speed as a function of the local equivalence ratio Φ value in the presence of plasma actuation was analyzed using the laminar premixed flame speed (LPFS) model. It solves the mass, momentum, species, and energy balance equations of a premixed flow based on on*10-dimensional approximation. The temperature and the modified fuel composition (estimated at the exit of the burner by means of PFR computations) were used for flame speed predictions, which were then compared with the ones predicted in the absence of plasma actuation, namely at ambient temperature and pure methane.
4. Results
Figure 5 shows the effect of the standoff distance on the flame anchoring. When applying a peak-to-peak voltage of about 11 kV, the effectiveness of the coaxial DBD configuration is estimated. In particular, the flame reattached to the lips of the inner quartz tube when SSO = −40 mm (test case 2), while for SSO = 20 mm (test case 3), flame reattachment was not experienced, and no differences were exhibited with respect to the clean case (test case 1). This result increased its relevance when the dissipated power results were taken in consideration: its value for SSO = −40 mm was about 18% less than the one measured for SSO = 20 mm. When using the actuator configuration with SSO = 20 mm, a partially reattached flame condition was observed when increasing the supplied voltage, and the complete flame reattachment was obtained for Vpp = 18.4 kV (test case 4). As a result, when considering the plasma power required for flame reattachment, the DBD configuration with SSO = 20 mm revealed to be less efficient since it was 23 times more power-consuming than the configuration with SSO = −40 mm.
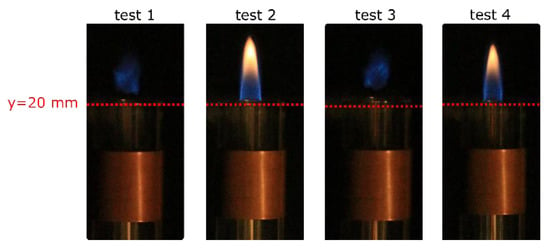
Figure 5.
Impact of the stand-off distance (SSO) on the flame at fixed Vpp in comparison with the clean case (test 1). Test 2 at SSO = −40 mm; tests 3 and 4 at SSO = 20 mm.
In particular, the comparison between the clean case (test case 1) and the plasma actuated case with negative stand-off (test case 2) figured out that the overall plasma-induced combustion enhancement allowed to overcome the strong confinement effect produced by the outer air flow, which caused a lifted flame when in the absence of plasma actuation. To this regard, the rate of flame propagation, or burning velocity, is of primary importance in determining the flame stability, which occurs when the burning velocity balances the local fuel-air mixture velocity at the flame surface.
Figure 6 shows the comparison between two experimental snapshots of the flame appearance, as previously described in Section 2: on the left side the flame is lifting, as observed when operating without plasma actuation (test case 1); on the right side, the flame is anchored to the Bunsen lips as a consequence of the plasma-induced flame reattachment (test case 2). The black dashed curves represent the iso-line of the image luminance at I = 0.4, and they qualitatively denote the flame surface, along which the stabilization point is located at the most upstream point. As a result, the plasma actuation modified the local flow structure close to the stabilization point that moved towards the burner exit. This allowed for the extension of the flame region until complete flame reattachment was achieved, owing to the flame propagation rate enhancement.
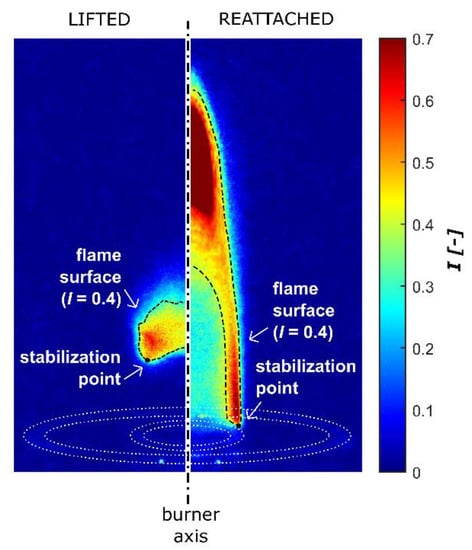
Figure 6.
Flame structure modification: lifted flame condition without plasma actuation (test 1) on the left; reattached flame condition in presence of plasma actuation (test 2) on the right. The white dotted curves represent the burner lips at the exit section.
This could be due to three main coupled effects:
- momentum effect which led to the slowing down of the flow into the quartz tube;
- plasma chemistry effect with fuel conversion;
- thermal effects with gas temperature rise.
Therefore, a numerical investigation was performed in order to decouple these effects. As first, it was considered that the DBD configuration with SSO = −40 mm mostly differs from the one with SSO = 20 mm due to the presence of stronger electric field edge effects at the needle tip. Consequently, simulations of the electrostatic field under steady-state conditions were performed in order to estimate the electric field strength in both DBD configurations. Results are shown in Figure 7, where the contour plot of the electric field in the radial direction (Ex) and the axial direction (Ey) are compared. It is worth mentioning that the color map has been properly scaled in order to better figure out the extension and the shape of the electric field. Furthermore, a negative value of Ey represents a negative component of the axial-induced body force on the flow, pointing towards the inlets of the Bunsen. As expected, simulations determined that the maxima of the electric field were placed in the region of the electrode corners. In particular, when considering the axial electric field, the extension of the negative Ey for SSO = −40 mm was larger than the one computed for SSO = 20 mm. Conversely, a strong positive electric field was established just above the needle tip when SSO = 20 mm. Consequently, the DBD configuration with SSO = −40 mm introduced momentum sources opposed to the flow, slowing it down with the consequent reduction of the mean residence times. On the contrary, the DBD configuration with SSO = 20 mm led to a momentum increase in the direction of the flow, and the flow velocity rose in such a way that the flame reattachment effect was damped. Moreover, when SSO = 20 mm, the positive and the negative maxima of Ey were of the same order of magnitude while, for SSO = −40 mm, the negative part was highly prevailing on the positive one.
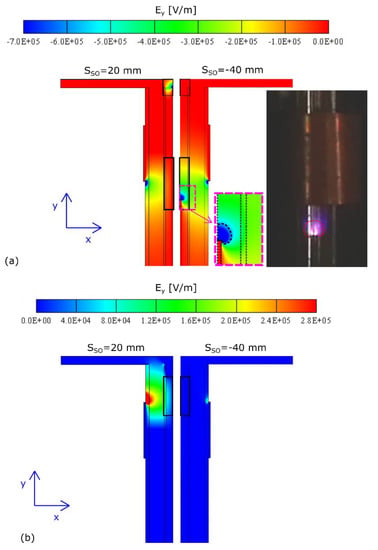
Figure 7.
Numerical prediction of the electric field inside the burner for both stand-off configurations under steady-state conditions: (a) Ey < 0; (b) Ey > 0. An experimental image of the plasma discharge is also shown in (a). SSO = -40 mm (SIM 1) vs SSO = 20 mm (SIM 2).
Methane Plasma-Reactor Model Results
Numerical simulations showed that when using the DBD configuration with SSO = −40 mm, the highest electric field extended into the inner quartz tube, as confirmed by experimental visualization close to the needle tip, as shown in Figure 7a. This impacted the plasma chemistry and methane dissociation, leading to the activation of electron-impact and radical reactions, as confirmed by the reaction rates shown in Figure 8 resulting from the zero-dimensional chemistry analysis on ZDPlasKin.
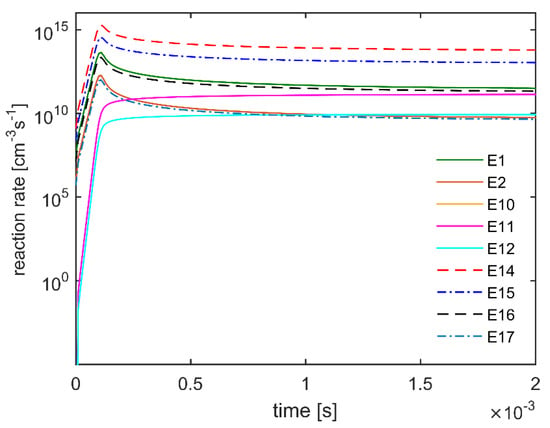
Figure 8.
Reaction rates predicted by ZDPlasKin.
Therefore, based on experimental conditions of test 2, the solution of the plasma flow into the inner quartz tube, modeled as a PFR, predicted both the fuel composition and gas temperature at the entrance of the flame region. Figure 9 shows the axial profiles of the concentration of the main species in terms of number density. Results indicate that at the burner exit, CH4 consumption, due to plasma-induced dissociation, was about 6%. On the other hand, plasma discharge promoted the production of new species (hydrocarbons like ethane C2H6 and ethylene C2H4), radicals (e.g., methyl), and ions. It is worth underlining the prevailing production of the methyl radical CH3, which plays a crucial role in DBD and low-energy density discharges. As experimentally retrieved in studies by Burcat and Ruscic [] and Nozaki and Okazaki [], electron impact reactions drive the reactivity in such systems, leading to CH3 radical formation in the discharge and, consequently, to ethane and ethylene production owing to CH3 radical recombination reactions at low temperatures.
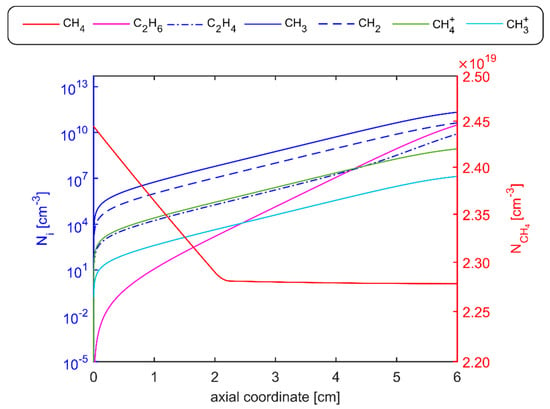
Figure 9.
Axial profile of the number density predicted by the PFR model: production of C2H6, C2H4, CH3, CH2, and on the left axis; CH4 consumption on the right axis.
Plasma actuation also led to a slight increase of gas temperature at about 21.8 °C, as highlighted in Figure 10.
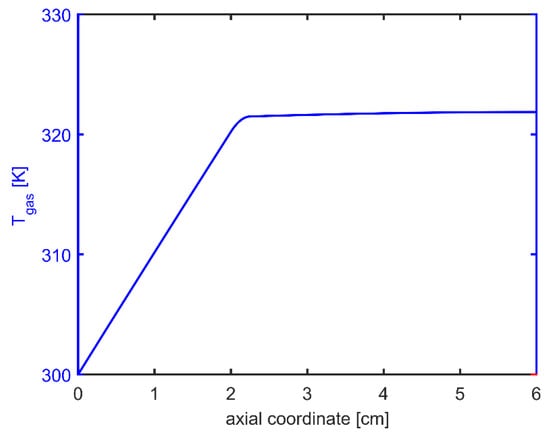
Figure 10.
Axial profiles of temperature predicted by PFR computations.
Therefore, the analysis of the effect of the plasma discharge was extended to the flame region. To this purpose, the comparison between the clean configuration (test case 1) and the plasma actuated one (test case 2) was performed in terms of flame speed and neat heat production, which were estimated by means of LPFS computations. The clean configuration was set at ambient temperature and pure methane, while the plasma actuated case was initialized using the fuel composition and temperature at the burner exit predicted by PFR computations. Figure 11 shows the comparison of the predicted flame speed as a function of the local equivalence ratio.
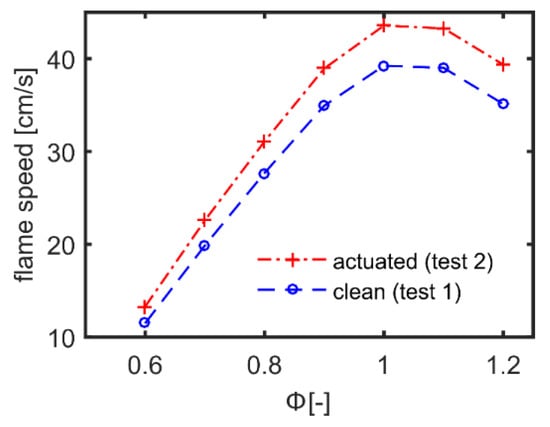
Figure 11.
Numerical prediction of the flame speed as a function of the local equivalence ratio Φ.
As a result, the establishment of weak ionization process into the burner was estimated with no relevant modification of the fuel composition. However, it coupled with the temperature rise of 21.8 °C which significantly impacted the laminar flame speed. In fact, an increase of about 10% was retrieved near stoichiometric conditions. This implies that the chemical characteristic time reduced, and the combustion process became faster in the presence of plasma actuation with respect to the clean configuration.
This finding is confirmed by the chemical heat release versus gas temperature plot shown in Figure 12. In particular, the comparison highlights that when in presence of plasma actuation, the chemical heat release increased at lower temperature because of the additional energy that was coupled into the system by the plasma. Consequently, the elevated level of heat release in the flame accelerated all reactions, which impacted the structure of the flame and enhanced its propagation rate.
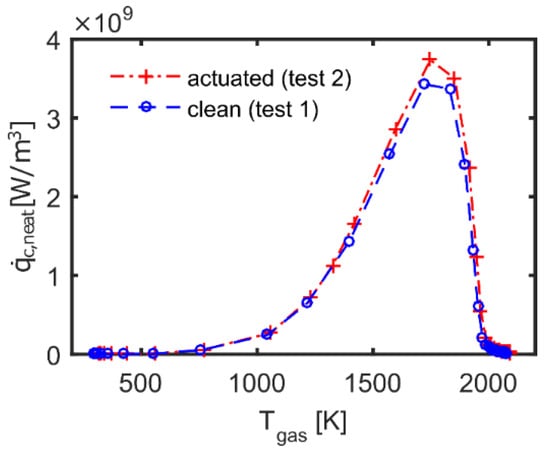
Figure 12.
Chemical heat release versus temperature.
In conclusion, the plasma reactor/laminar premixed flame analysis demonstrated that the flame reattachment experimentally observed could be related to flame speed enhancement due to plasma actuation.
5. Conclusions
In the present work, the effect of a sinusoidal plasma actuation on lifted flames was investigated. At this purpose, a lean non-premixed CH4/air flame in a non-premixed Bunsen-type burner with annular fuel jet was considered. Experiments focused on investigating the effect of the actuator standoff distance SSO on the flame lift-off. To this purpose, two different coaxial DBD configurations were considered, namely SSO = −40 mm and SSO = 20 mm.
Experimental results showed that the flame reattached to the burner lips when operating at Vpp voltage of about 11 kV and using the configuration with SSO = −40 mm. On the contrary, the configuration with SSO = 20 mm did not work for flame reattachment. Moreover, a reduction of the dissipated power of about 18% was also retrieved when using the configuration with SSO = −40 mm. In order to reattach the flame with SSO = 20 mm configuration, the applied voltage had to increase up to Vpp = 18 kV, with a power consumption about 23 times higher than the one measured with SSO = −40 mm at Vpp = 11 kV.
Furthermore, in order to decouple the effect of the plasma actuation in terms of momentum, plasma chemistry, and thermal effects, a numerical analysis was performed. Steady-state electrostatic field simulations were combined with computations of the plasma-chemistry into the methane quartz tube modeled as a plasma reactor. Finally, the impact of the predicted fuel composition and temperature when in the presence of plasma actuation was investigated in terms of flame speed modification.
As predicted by the electric field computations, the DBD configuration with SSO = −40 mm exhibited an electric field opposed to the flow. Therefore, due to the proportional relation between the electric field and the plasma-induced body forces, the DBD configuration with a negative SSO value allowed to introduce momentum sources, with the consequent reduction of the mean residence times. Furthermore, the highest electric field extended into the inner quartz tube, as confirmed by experimental visualization close to the needle tip.
The methane plasma reactor model predicted the activation of the electron-impact reactions which led to the production of CH3; this enabled the gas-phase reaction for C2H6 production and, hence, C2H4 production by ethane dehydrogenation. The plasma discharge also led to the increase of gas temperature of about 21.8 °C. Consequently, the flame speed increased in the measure of about 10% when close to the stoichiometric conditions, in comparison with the absence of plasma actuation. Therefore, the flame reattachment experimentally observed can be related to the enhancement of the flame speed due to reduced chemical characteristic times and a faster combustion process when in the presence of plasma actuation.
Author Contributions
Conceptualization, M.G.D.G.; methodology, E.P.; software, D.F.; validation, E.P. and A.S.; formal analysis, D.F.; investigation, D.F.; data curation, D.F.; writing—original draft preparation, D.F.; writing—review and editing, M.G.D.G.; visualization, E.P. and A.S.; supervision, A.F. and M.G.D.G.; funding acquisition, A.F. and M.G.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the Clean Sky 2 Joint Undertaking (JU) under grant agreement No. 831881. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and the Clean Sky 2 JU members other than the Union.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| Symbols | ||
| A | cross-section area of the reactor | (m2) |
| inner surface area per unit length | (m) | |
| mean heat capacity per mass | (J/kgK) | |
| electric field | (V/m) | |
| E/N | reduced electric field | (Td=10−17 V cm2) |
| F | drag force | (N) |
| h | specific enthalpy | (J/kg) |
| I(t) | electrical current | (A) |
| electrical current density | (A/m2) | |
| mass flow rate | (g/s) | |
| number density of the species i | (1/m3) | |
| P | pressure force | (N/m2) |
| electrical power | (W) | |
| Qij | source rate of the species i and reaction j | (1/m3/s) |
| external heat flux | (W/m2) | |
| plasma power deposition per unit length | (W/m) | |
| R | electrical resistance | (Ω) |
| stand-off distance | (mm) | |
| temperature | (K) | |
| time | (s) | |
| period of the applied voltage | (s) | |
| u | axial velocity | (m/s) |
| V(t) | voltage | (V) |
| W | molecular weight | (kg/mol) |
| Y | molar fraction | (-) |
| Greek symbols | ||
| Φ | equivalence ratio | (-) |
| electric potential | (V) | |
| ρ | density | (kg/m3) |
| electric or ionic conductivity | (S/m) | |
| molar production rate | (mol/s) | |
| Chemical symbols | ||
| C2H2 | acetylene | |
| C2H3 | vinyl radical | |
| C2H4 | ethylene | |
| C2H5 | ethyl radical | |
| C2H6 | ethane | |
| CH4 | methane | |
| methane ion | ||
| CH3 | methyl radical | |
| methyl ion | ||
| CH2 | methylene radical | |
| CH | methylidyne radical | |
| methylidyne ion | ||
| e | electron | |
| H2 | hydrogen | |
| H | atomic hydrogen radical | |
| Subscripts | ||
| a | refers to the air | |
| app | refers to the applied voltage | |
| elec | refers to electrons | |
| f | refers to the fuel | |
| i | refers to the generic specie | |
| in | refers to the inlet condition | |
| j | refers to the generic reaction | |
| pp | refers to the peak-to-peak voltage | |
| st | refers to the stochiometric condition | |
| Acronyms | ||
| AC | alternate current | |
| DBD | dielectric barrier discharge | |
| HV | high voltage | |
| LFL | lean flammability limit | |
| LPFS | laminar premixed flame speed | |
| NDF | normal diffusive flame | |
| NTP | non-thermal plasma | |
| PAC | plasma assisted combustion | |
| PFR | plug flow reactor | |
References
- Oh, J.; Noh, D. Flame characteristics of a non-premixed oxy-fuel jet in a lab-scale furnace. Energy 2015, 81, 328–343. [Google Scholar] [CrossRef]
- Lee, S.; Padilla, R.; Dunn-Rankin, D.; Pham, T.; Kwon, O.C. Extinction limits and structure of counterflow nonpremixed H2O-laden CH4/air flames. Energy 2015, 93, 442–450. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Holley, A.T.; Law, C.K. An assessment of the lean flammability limits of CH4/air and C3H8/air mixtures at engin*10-like conditions. Proc. Combust. Inst. 2007, 31, 3015–3022. [Google Scholar] [CrossRef]
- Baigmohammadi, M.; Tabejamaat, S.; Zarvandi, J. Numerical study of the behavior of methan*10-hydrogen/air pr*10-mixed flame in a micro reactor equipped with catalytic segmented bluff body. Energy 2015, 85, 117–144. [Google Scholar] [CrossRef]
- Cha, M.S.; Chung, S.H. Characteristics of lifted flames in non-premixed turbulent confined jets. Symp. Int. Combust. 1996, 26, 121–128. [Google Scholar] [CrossRef]
- Fokaides, P.; Zarzalis, N. Lean blowout dynamics of a lifted stabilized, non-premixed swirl flame. Eur. Combust. Meet. 2007, 7, 2. [Google Scholar]
- Chung, S.H. Stabilization, propagation and instability of tribrachial triple flames. Proc. Combust. Inst. 2007, 31, 877–892. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, C.-C.; Pan, K.-L.; Yang, J.-T. Flame Lift-off and Stabilization Mechanisms of Nonpremixed Jet Flames on a Bluff-body Burner. Combust. Flame 1998, 115, 51–65. [Google Scholar] [CrossRef]
- Ju, Y.; Sun, W. Plasma assisted combustion: Dynamics and chemistry. Prog. Energy Combust. Sci. 2015, 48, 21–83. [Google Scholar] [CrossRef]
- Pescini, E.; Sciolti, A.; Francioso, L.; Ficarella, A.; De Giorgi, M.G. Effect of a micro dielectric barrier discharge plasma actuator on quiescent flow. IET Sci. Meas. Technol. 2014, 8, 135–142. [Google Scholar] [CrossRef]
- Pescini, E.; Martínez, D.; De Giorgi, M.G.; Ficarella, A. Characterization of the effects of a dielectric barrier discharge plasma actuator on a coaxial jet in a Bunsen burner. Exp. Therm. Fluid Sci. 2018, 91, 292–305. [Google Scholar] [CrossRef]
- Karnani, S.; Dunn-Rankin, D. Detailed characterization of DC electric field effects on small non-premixed flames. Combust. Flame 2015, 162, 2865–2872. [Google Scholar] [CrossRef]
- Tinajero, J.; Bernard, G.; Autef, L.; Dunn-Rankin, D. Characterizing I-V Curves for Non-Premixed Methane Flames Stabilized on Different Burner Configurations. Combust. Sci. Technol. 2017, 189, 1739–1750. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Dunn-Rankin, D. Electric Field Induced Changes of a Diffusion Flame and Heat Transfer near an Impinging Surface. Energies 2018, 11, 1235. [Google Scholar] [CrossRef]
- Tinajero, J.; Dunn-Rankin, D. Non-premixed axisymmetric flames driven by ion currents. Combust. Flame 2019, 199, 365–376. [Google Scholar] [CrossRef]
- López-Cámara, C.F.; Éplénier, G.; Tinajero, J.; Dunn-Rankin, D. Numerical Simulation of Methane/Air Flames Including Ions and Excited Species; Combustion Institute: Provo, UT, USA, 2015. [Google Scholar]
- Belhi, M.; Domingo, P.; Vervisch, P. Modelling of the effect of DC and AC electric fields on the stability of a lifted diffusion methane/air flame. Combust. Theory Model. 2013, 17, 749–787. [Google Scholar] [CrossRef]
- Rosocha, L.A.; Kim, Y.; Stange, S. Application of a Non-Thermal Plasma to Combustion Enhancement; No. LA-UR-04-6890; Los Alamos National Laboratory: Tokyo, Japan, 2004. [Google Scholar]
- Kim, Y.; Stange, S.M.; Rosocha, L.A.; Ferreri, V.W. Enhancement of Propane Flame Stability by Dielectric Barrier Discharges. J. Adv. Oxid. Technol. 2005, 8, 188–192. [Google Scholar] [CrossRef]
- Rosocha, L.A.; Kim, Y.; Anderson, G.K.; Abbate, S. Combustion enhancement using silent electrical discharges. Int. J. Environ. Sci. Technol. 2007, 1, 8–13. [Google Scholar]
- Klimov, A.; Bityurin, V. External and Internal Plasma-Assisted Combustion. In Proceedings of the 41st Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 5–8 January 2003. [Google Scholar]
- Bao, A.; Utkin, Y.G.; Keshav, S.; Lou, G.; Adamovich, I.V. Ignition of Ethylene–Air and Methane–Air Flows by Low-Temperature Repetitively Pulsed Nanosecond Discharge Plasma. IEEE Trans. Plasma Sci. 2007, 35, 1628–1638. [Google Scholar] [CrossRef]
- Mao, X.; Rousso, A.; Chen, Q.; Ju, Y. Numerical modeling of ignition enhancement of CH4/O2/He mixtures using a hybrid repetitive nanosecond and DC discharge. Proc. Combust. Inst. 2019, 37, 5545–5552. [Google Scholar] [CrossRef]
- Ombrello, T.; Won, S.H.; Ju, Y.; Williams, S. Flame propagation enhancement by plasma excitation of oxygen. Part I: Effects of O3. Combust. Flame 2010, 157, 1906–1915. [Google Scholar] [CrossRef]
- Ombrello, T.; Won, S.H.; Ju, Y.; Williams, S. Flame propagation enhancement by plasma excitation of oxygen. Part II: Effects of O2(a1Δg). Combust. Flame 2010, 157, 1916–1928. [Google Scholar] [CrossRef]
- Starikovskii, A.Y.; Anikin, N.B.; Kosarev, I.; Mintoussov, E.I.; Starikovskaia, S.M.; Zhukov, V.P. Plasma-assisted combustion. Pure Appl. Chem. 2006, 78, 1265–1298. [Google Scholar] [CrossRef]
- Starikovskiy, A.; Anikin, N.B.; Kosarev, I.; Mintoussov, E.I.; Nudnova, M.M.; Rakitin, A.E.; Roupassov, D.V.; Starikovskaia, S.M.; Zhukov, V.P.; Anikin, N.B. Nanosecond-Pulsed Discharges for Plasma-Assisted Combustion and Aerodynamics. J. Propuls. Power 2008, 24, 1182–1197. [Google Scholar] [CrossRef]
- Kim, Y.; Ferreri, V.W.; Rosocha, L.A.; Anderson, G.K.; Abbate, S.; Kim, K.-T. Effect of Plasma Chemistry on Activated Propane/Air Flames. IEEE Trans. Plasma Sci. 2006, 34, 2532–2536. [Google Scholar] [CrossRef]
- Pilla, G.; Galley, D.; Lacoste, D.; Lacas, F.; Veynante, D.; Laux, C. Stabilization of a Turbulent Premixed Flame Using a Nanosecond Repetitively Pulsed Plasma. IEEE Trans. Plasma Sci. 2006, 34, 2471–2477. [Google Scholar] [CrossRef]
- Pilla, G.L.; Lacoste, D.; Veynante, D.; Laux, C. Stabilization of a Swirled Propane–Air Flame Using a Nanosecond Repetitively Pulsed Plasma. IEEE Trans. Plasma Sci. 2008, 36, 940–941. [Google Scholar] [CrossRef]
- Won, S.H.; Cha, M.S.; Park, C.S.; Chung, S.H. Effect of electric fields on reattachment and propagation speed of tribrachial flames in laminar co-flow jets. Proc. Combust. Inst. 2007, 31, 963e70. [Google Scholar] [CrossRef]
- Sheng, Z.; Kameshima, S.; Sakata, K.; Nozaki, T. Plasma-Enabled Dry Methane Reforming. In Plasma Chemistry and Gas Conversion; IntechOpen: London, UK, 2018. [Google Scholar]
- Kim, W.; Mungal, M.G.; Cappelli, M.A. The role of in situ reforming in plasma enhanced ultra lean premixed methane/air flames. Combust. Flame 2010, 157, 374–383. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yang, L.; Li, B.; Li, Z.S.; Sun, Z.W.; Aldén, M.; Konnov, A.A. Investigation of combustion enhancement by ozone additive in CH4/air flames using direct laminar burning velocity measurements and kinetic simulations. Combust. Flame 2012, 159, 120–129. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Z.; Weng, W.; Zhou, Z.; Huang, Z.; Zhou, J.; Cen, K. Study of ozon*10-enhanced combustion in H2/CO/N2/air premixed flames by laminar burning velocity measurements and kinetic modeling. Int. J. Hydrog. Energy 2013, 38, 1177–1188. [Google Scholar] [CrossRef]
- Poinsot, T.J.; Veynante, D.P. Combustion. Encyclopedia of Computational Mechanics, 2nd ed.; 2018; pp. 1–30. [Google Scholar]
- Lefkowitz, J.K.; Guo, P.; Rousso, A.; Ju, Y. Species and temperature measurements of methane oxidation in a nanosecond repetitively pulsed discharge. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140333. [Google Scholar] [CrossRef] [PubMed]
- ArrayExpress—A Database of Functional Genomics Experiments. Available online: http://www.ebi.ac.uk/arrayexpress/ (accessed on 12 November 2012).
- Lutz, A.E.; Kee, R.J.; Miller, J.A. Senkin: A Fortran Program for Predicting Homogeneous Gas Phase Chemical Kinetics with Sensitivity Analysis; Report SAND87-8248; Sandia National Laboratories: Albuquerque, NM, USA, 1988. [Google Scholar]
- Hagelaar, G.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Pitchford, L.C.; Alves, L.L.; Bartschat, K.; Biagi, S.F.; Bordage, M.-C.; Bray, I.; Brion, C.E.; Brunger, M.J.; Campbell, L.; Chachereau, A.; et al. LXCat: An Open-Access, Web-Based Platform for Data Needed for Modeling Low Temperature Plasmas. Plasma Process. Polym. 2016, 14, 1600098. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.; Yang, X.; E Koel, B. Effects of non-equilibrium excitation on methane oxidation in a low-temperature RF discharge. J. Phys. D Appl. Phys. 2019, 53, 064001. [Google Scholar] [CrossRef]
- Masi, M.; Cavallotti, C.; Carrà, S. Different approaches for methane plasmas modeling. Chem. Eng. Sci. 1998, 53, 3875–3886. [Google Scholar] [CrossRef]
- Kheirollahivash, M.; Rashidi, F.; Moshrefi, M.M. Hydrogen Production from Methane Decomposition Using a Mobile and Elongating Arc Plasma Reactor. Plasma Chem. Plasma Process. 2019, 39, 445–459. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H.; Goldenberg, M.; Smith, G.P.; Golden, D.M. GRI-MECH: An Optimized Detailed Chemical Reaction Mechanism for Methane Combustion; SRI International: Menlo Park, CA, USA, 1995. [Google Scholar]
- Burcat, A.; Ruscic, B. Third Millennium Ideal Gas and Condensed Phase Thermochemical Database for Combustion with updates from Active Thermochemical Tables ANL-05/20 and TAE 960; Aerospace Engineering, and Argonne National Laboratory, Chemistry Division: Argonne, IL, USA, 2005. [Google Scholar]
- Nozaki, T.; Okazaki, K. Non-thermal plasma catalysis of methane: Principles, energy efficiency, and applications. Catal. Today 2013, 211, 29–38. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).