Electrical Characterization of a New Crosslinked Copolymer Blend for DC Cable Insulation
Abstract
1. Introduction
2. Materials and Samples
3. Experimental Setup and Methods
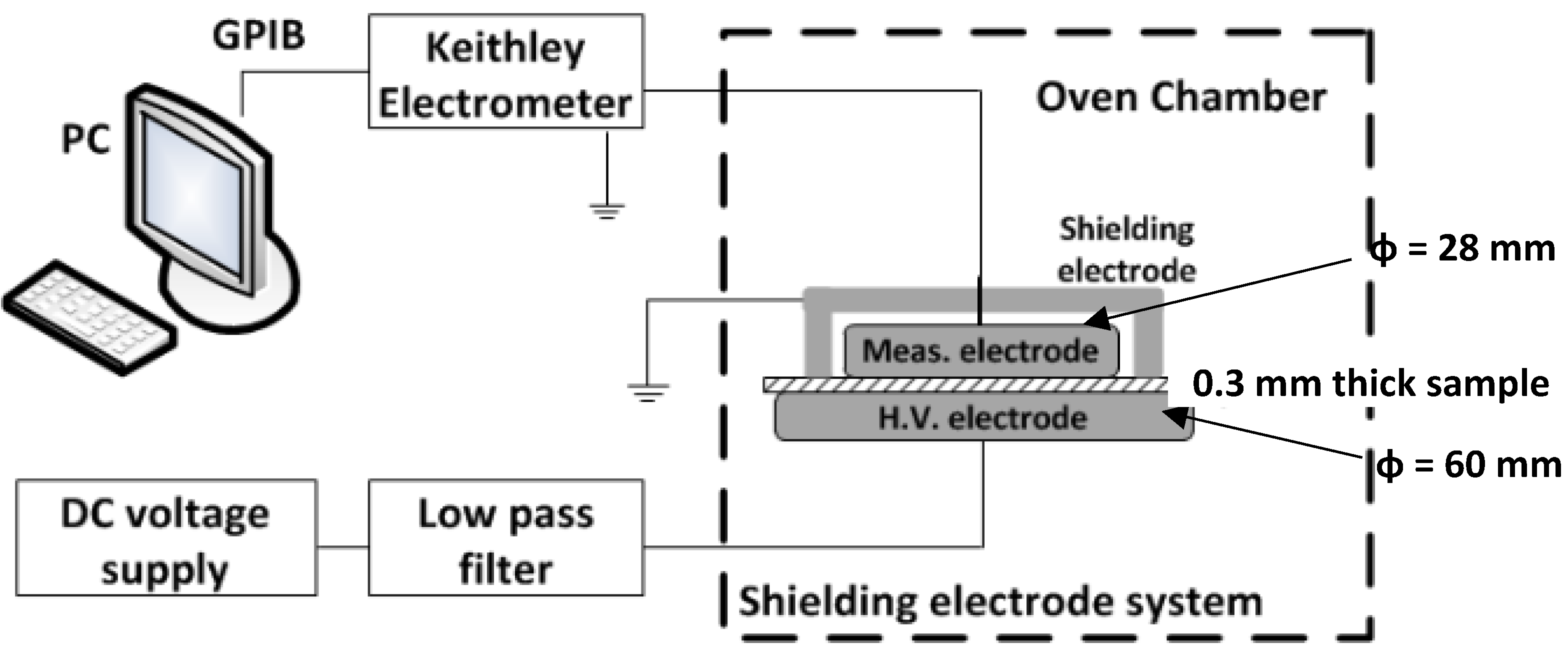
3.1. DC Conductivity Measurements
3.2. Dielectric Response Measurements
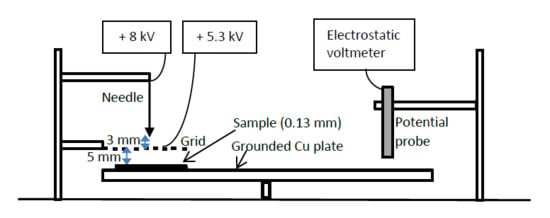
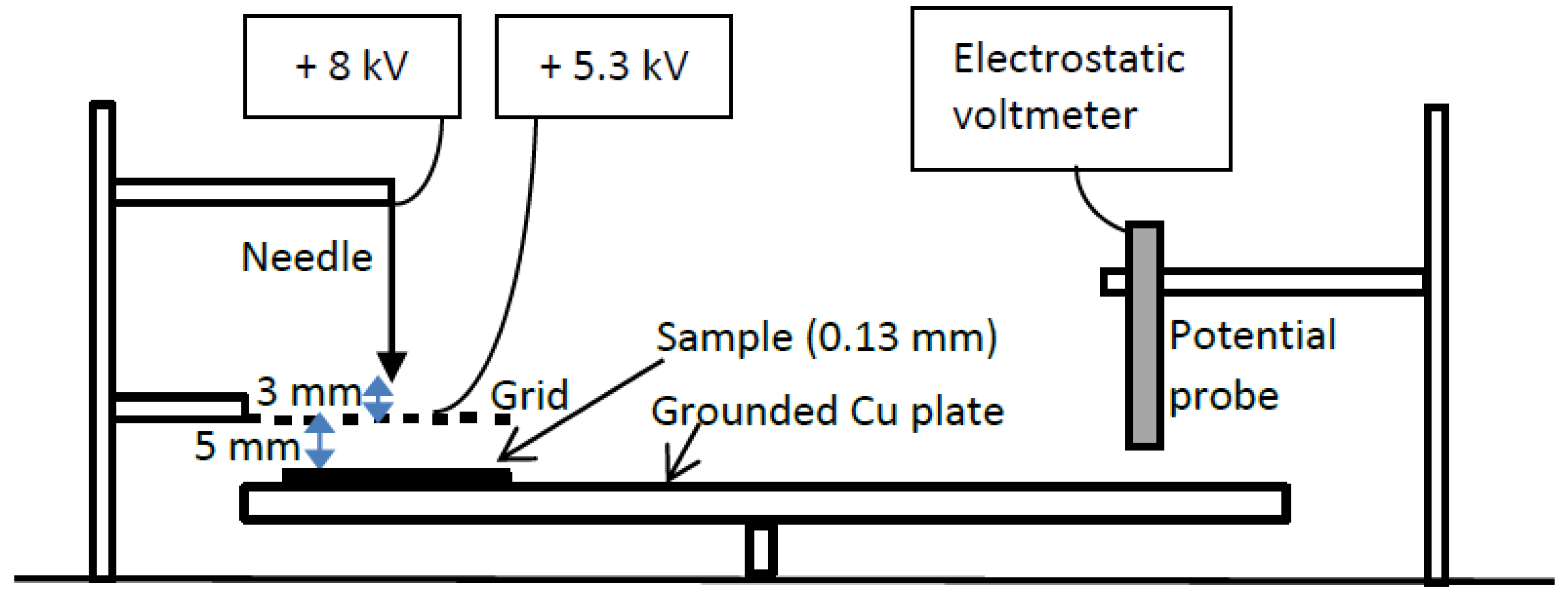
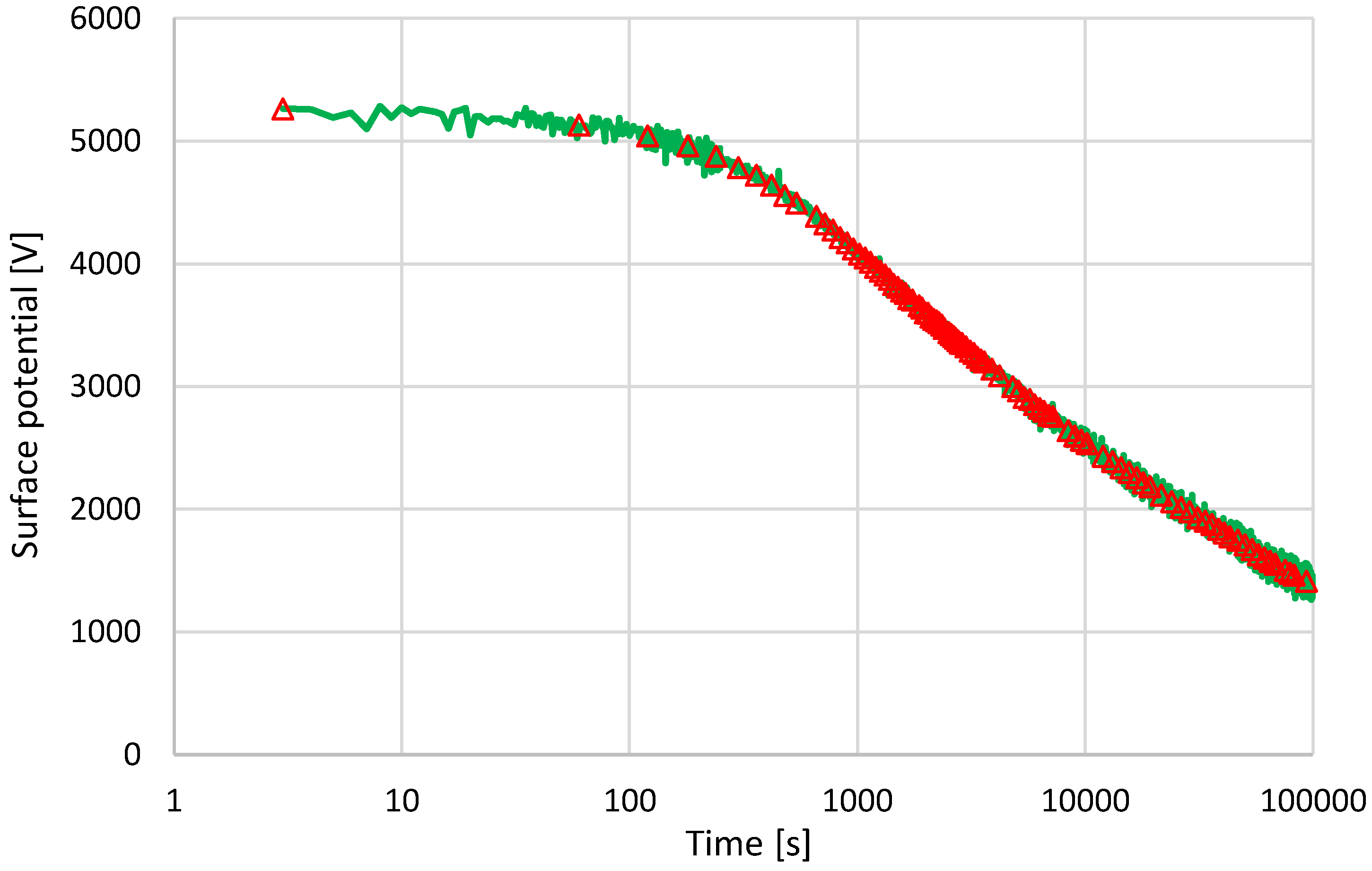
3.3. Surface Potential Decay Measurements
4. Results and Discussion
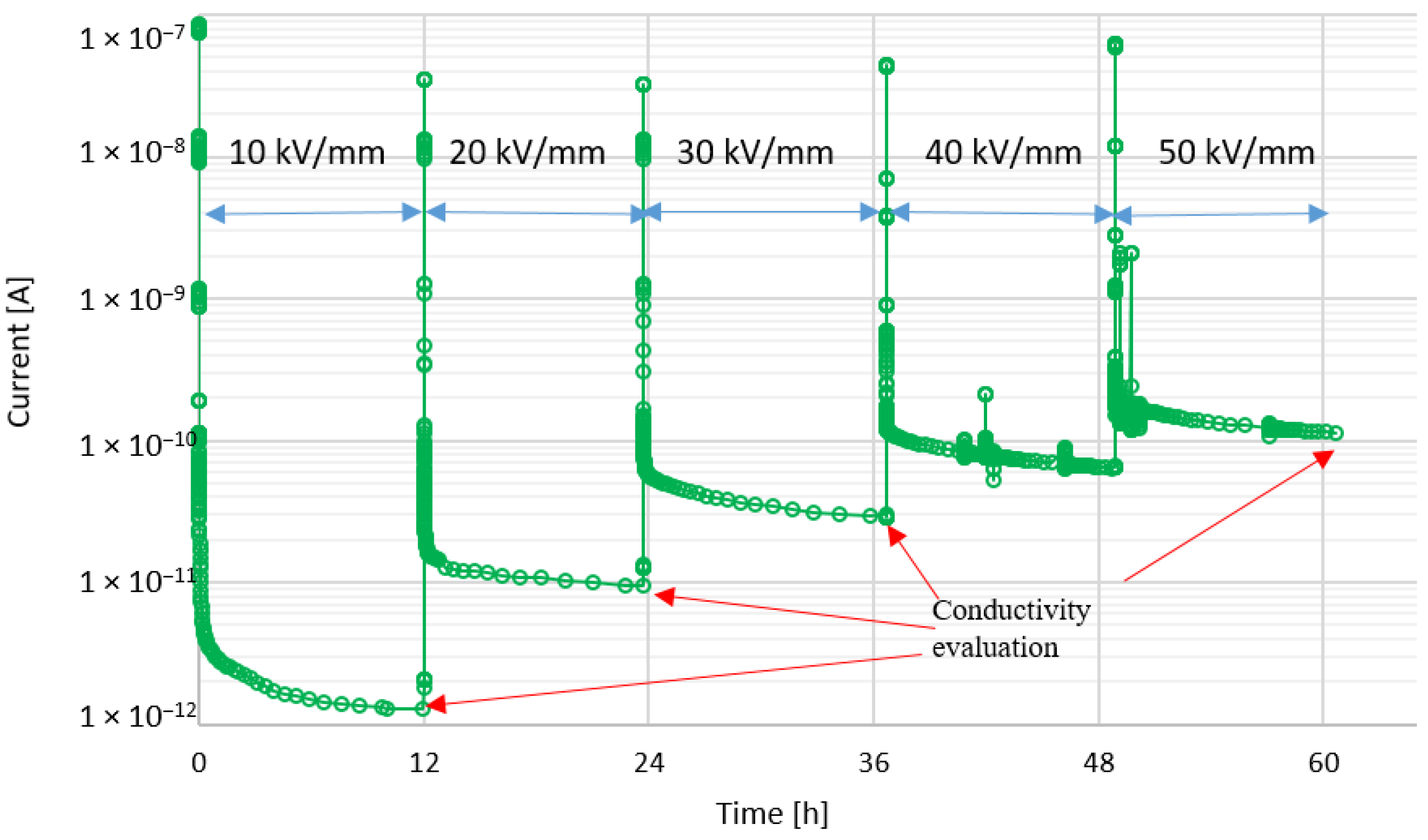
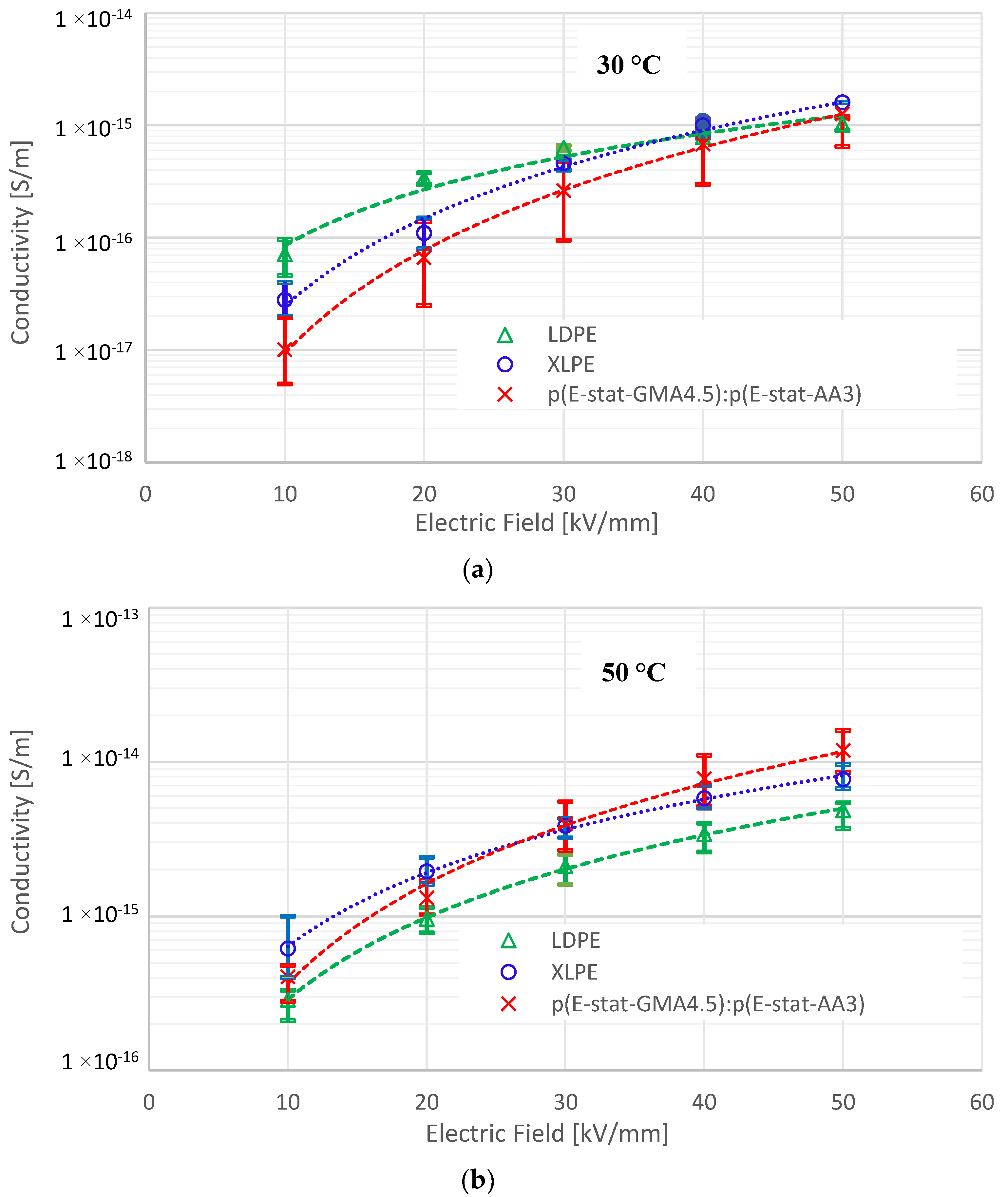
4.1. DC Conductivity
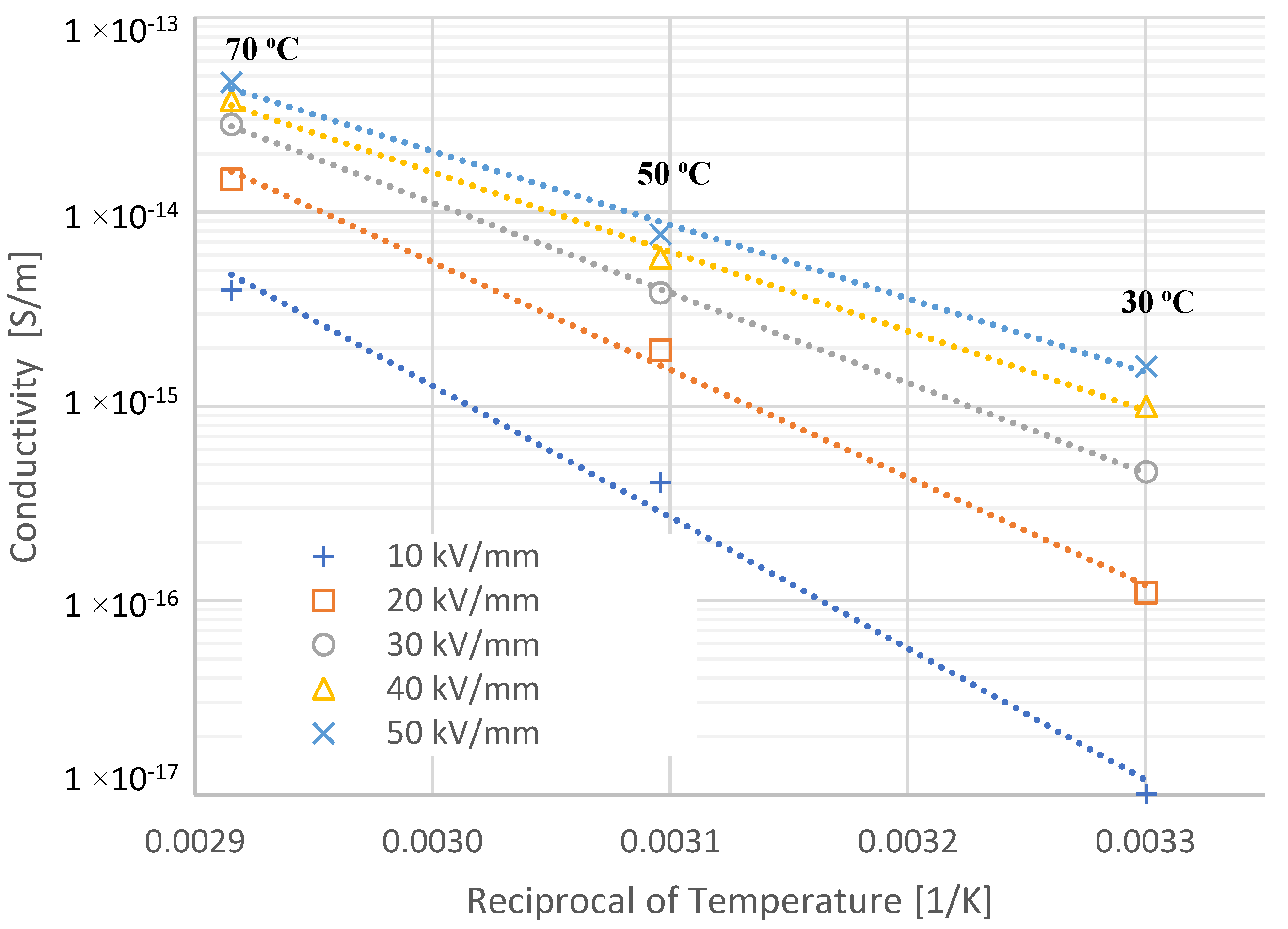
4.2. Activation Energy
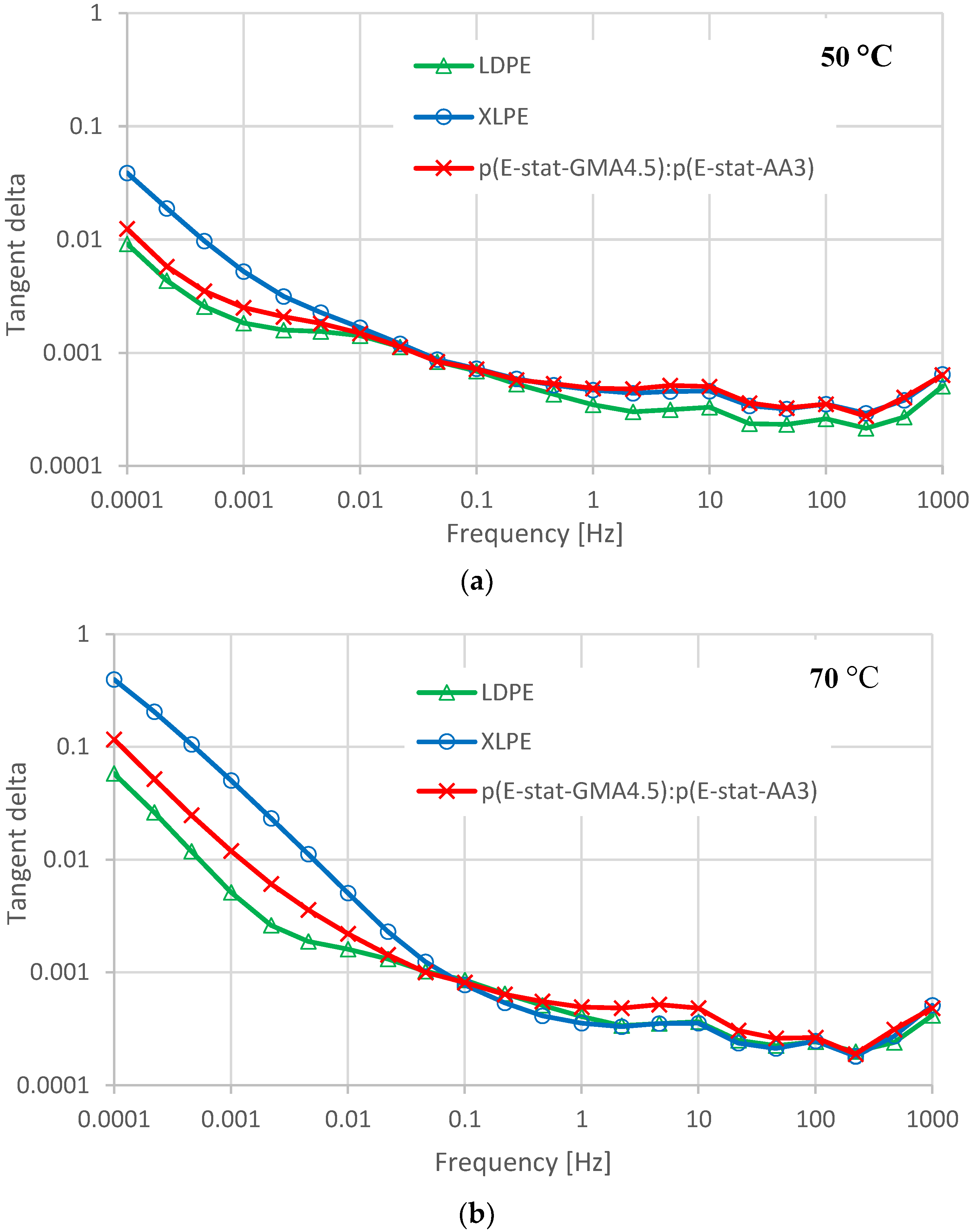
4.3. Dielectric Response and Losses
4.4. Field Dependent Conductivity
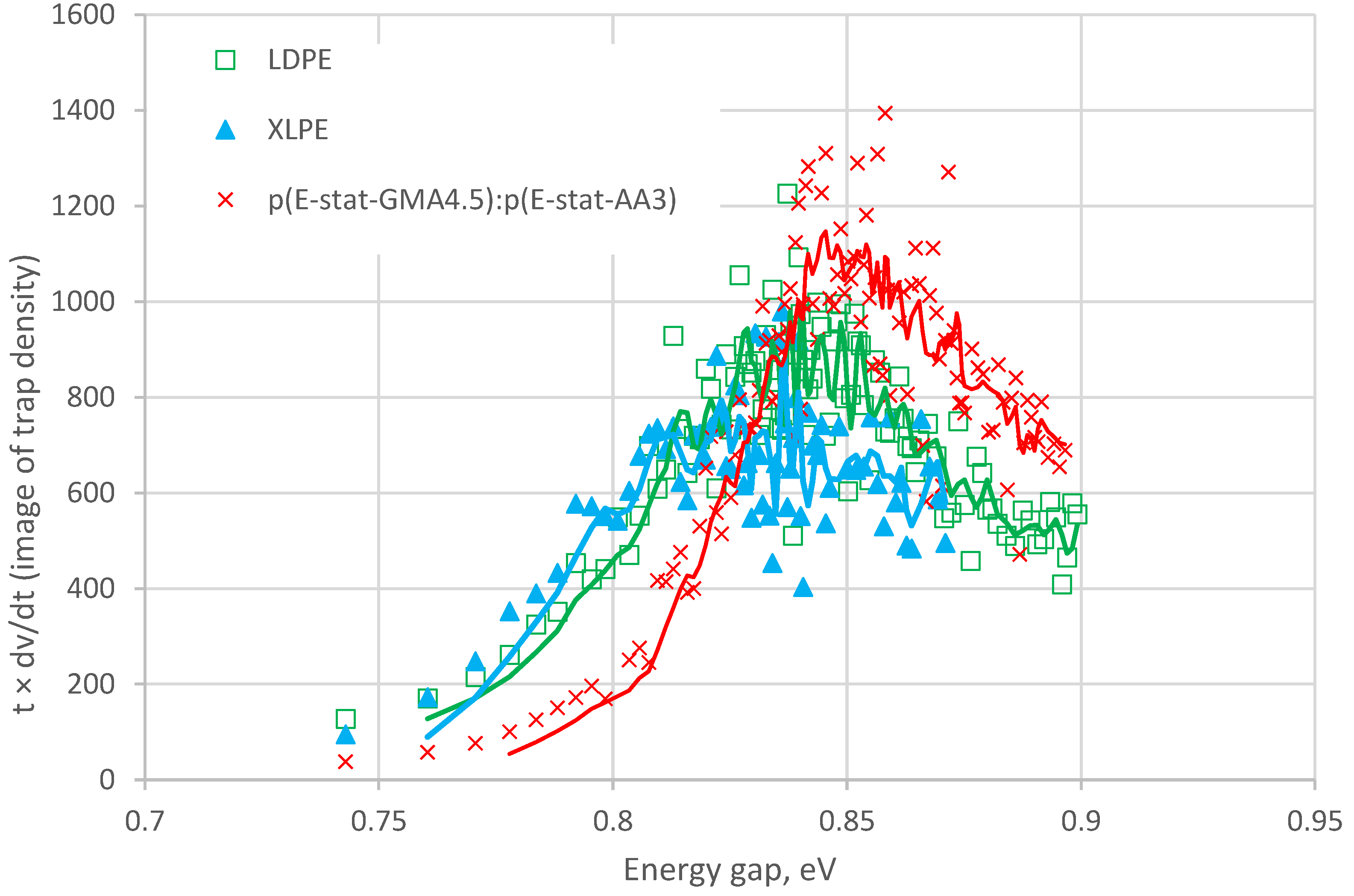
4.5. Trap Energy Distributions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Du, B. Polymeric insulation for high voltage DC extruded cables: Challenges and development directions. IEEE Electr. Insul. Mag. 2018, 34, 30–43. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.; Hu, J.; He, J. Polymeric insulation materials for HVDC cables: Developments, challenges and future perspectives. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1308–1318. [Google Scholar] [CrossRef]
- Gubanski, S.M. Insulating materials for next generations of HVAC and HVDC cables. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application (ICHVE), Chengdu, China, 19–22 September 2016. [Google Scholar]
- Montanari, G.C.; Morshuis, P.H.F.; Zhou, M.; Stevens, G.C.; Vaughan, A.S.; Han, Z.; Li, D. Criteria influencing the selection and design of HV and UHV DC cables in new network applications. High Volt. 2018, 3, 90–95. [Google Scholar] [CrossRef]
- Plesa, I.; Notingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlogl, S. Polyethylene nanocomposites for Power cable insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, M.; Li, Z.; Uehara, H.; Cao, Y. The correlation and balance of critical material properties for dc cable directrices. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Cancun, Mexico, 21–24 October 2018. [Google Scholar]
- Andrews, T.; Hampton, R.N.; Smedberg, A.; Wald, D.; Waschk, V.; Weissenberg, W. The role of degassing in XLPE power cable manufacture. IEEE Electr. Insul. Mag. 2006, 22, 5–16. [Google Scholar] [CrossRef]
- Chen, G.; Hao, M.; Xu, Z.; Vaughan, A.; Cao, J.; Wang, H. Review of high voltage direct current cable. CSEE J. Power Energy 2015, 1, 9–21. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Li, H.; Li, J.; Chen, Z. Improved DC performance of crosslinked polyethylene insulation depending on a higher purity. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1809–1817. [Google Scholar] [CrossRef]
- Farkas, A.; Olsson, C.; Dominguez, G.; Englund, V.; Hagstrand, P.; Nilsson, U. Development of high-performance polymeric materials for HVDC cables. In Proceedings of the 8th International Conference on Insulated Power Cables Jicable’11, Versailles, France, 19–23 June 2011. [Google Scholar]
- Jarvid, M.; Johansson, A.; Kroon, R.; Bjuggren, J.M.; Wutzel, H.; Englund, V.; Gubanski, S.; Andersson, M.R.; Müller, C. A new application area for fullerenes: Voltage stabilizers for power cable insulation. Adv. Mater. 2015, 27, 897–902. [Google Scholar] [CrossRef]
- Du, B.X.; Han, C.; Li, J.; Li, Z. Effect of voltage stabilizers on the space charge behavior of XLPE for HVDC cable application. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 34–42. [Google Scholar] [CrossRef]
- Tanka, T.; Imai, T. Advances in nanodielectric materials over the past 50 years. IEEE Electr. Insul. Mag. 2013, 29, 10–23. [Google Scholar] [CrossRef]
- Ohki, Y. Development of XLPE-insulated cable for high-voltage dc submarine transmission line (1). IEEE Electr. Insul. Mag. 2013, 29, 65–67. [Google Scholar] [CrossRef]
- Ohki, Y. Development of XLPE-insulated cable for high-voltage dc submarine transmission line (2). IEEE Electr. Insul. Mag. 2013, 29, 85–87. [Google Scholar] [CrossRef]
- Watanabe, C.; Itou, Y.; Sasaki, H.; Murata, Y.; Suizu, M.; Sakamaki, M.; Watanabe, M.; Katakai, S. Practical application of ±250 kV DC XLPE cable for Hokkaido-Honshu HVDC link. Electr. Eng. Jpn 2015, 191, 64–75. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Olsson, T.; Hedenqvist, M.S. The role of interfaces in polyethylene-metal-oxide nanocomposites for ultra high voltage insulating materials. Adv. Mater. 2018, 30, 1703624. [Google Scholar] [CrossRef]
- Hoang, A.T.; Pallon, L.; Liu, D.; Serdyuk, Y.V.; Gubanski, S.M.; Gedde, U.W. Charge transport in LDPE nanocomposites part I—experimental approach. Polymers 2016, 8, 173–198. [Google Scholar] [CrossRef]
- Meng, P.; Zhou, Y.; Yuan, C.; Li, Q.; Liu, J.; Wang, H.; Hu, J.; He, J. Comparisons of different polypropylene copolymers as potential recyclable HVDC cable insulation materials. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 674–680. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Dang, B.; He, J. Mechanism of highly improved electrical properties in polypropylene by chemical modification of grafting maleic anhydride. J. Phys. D Appl. Phys. 2016, 49, 415301. [Google Scholar] [CrossRef]
- Dang, B.; He, J.L.; Hu, J.; Zhou, Y. Large improvement in trap level and space charge distribution of polypropylene by enhancing the crystalline-amorphous interfaces effect in blends. Polym. Int. 2016, 65, 371–379. [Google Scholar] [CrossRef]
- Huang, X.Y.; Fan, Y.Y.; Zhang, J.; Jiang, P.K. Polypropylene based thermoplastic polymers for potential recyclable HVDC cable insulation applications. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1446–1456. [Google Scholar] [CrossRef]
- Tefferi, M.; Li, Z.; Cao, Y.; Uehara, H.; Chen, Q. Novel EPR-Insulated DC cables for future multi-terminal MVDC integration. IEEE Electr. Insul. Mag. 2019, 35, 20–27. [Google Scholar] [CrossRef]
- Sellick, R.L.; Sullivan, J.S.; Chen, Q.; Calebrese, C. Future improvements to HVDC cables through new cable insulation materials. In Proceedings of the 13th IET International Conference on AC and DC Power Transmission (ACDC), Manchester, UK, 14–16 February 2017. [Google Scholar]
- Hosier, I.L.; Vaughan, A.S.; Pye, A.; Stevens, C. Polymer Blends for HVDC Applications. In Proceedings of the IEEE International Conference on Dielectrics (ICD), Budapest, Hungary, 1–5 July 2018. [Google Scholar]
- Chen, X.; Jiang, C.; Hou, Y.; Dai, C.; Yu, L.; Wei, Z.; Zhou, H.; Tanaka, Y. Polyethylene blends with/without graphene for potential recyclable HVDC cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 851–858. [Google Scholar] [CrossRef]
- Andersson, M.G.; Hynynen, J.; Andersson, M.R.; Englund, V.; Hagstrand, P.O.; Gkourmpis, T.; Müller, C. Highly insulating polyethylene blends for high-voltage direct-current power cables. ACS Macro Lett. 2017, 6, 78–82. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Hoang, A.T.; Liu, D.; Pallon, L.K.H.; Gubanski, S.M.; Olsson, R.T.; Gedde, U.W.; Hedenqvist, M.S. Polyethylene and ZnO nano/hierarchical particles: A novel approach toward ultralow electrical conductivity insulations. Adv. Mater. 2016, 28, 8651–8657. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.C.; Thomas, J.L.; Pye, A.; Vaughan, A.S.; Hosier, I.L.; Denizet, I. Thermoplastic blend balanced property developments for sustainable high-performance power cables. In Proceedings of the IEEE International Conference on Dielectrics (ICD), Budapest, Hungary, 1–5 July 2018. [Google Scholar]
- Mauri, M.; Tran, N.; Preto, O.; Hjertberg, T.; Müller, C. Crosslinking of an ethylene-glycidyl methacrylate copolymer with amine click chemistry. Polymer 2017, 111, 27–35. [Google Scholar] [CrossRef]
- Mauri, M.; Svenningsson, L.; Hjertberg, T.; Nordstierna, L.; Preto, O.; Müller, C. Orange is the new white: Rapid curing of an ethylene-glycidyl methacrylate copolymer with a Ti-bisphenolate type catalyst. Polym. Chem. 2018, 9, 1710–1718. [Google Scholar] [CrossRef]
- Mauri, M.; Peterson, A.; Senol, A.; Elamin, K.; Gitsaa, A.; Hjertberg, T.; Matic, A.; Gkourmpis, T.; Preto, O.; Müller, C. Byproduct free curing of a highly insulating polyethylene copolymer blend: An alternative to peroxide crosslinking. J. Mater. Chem. C 2018, 6, 11292–11302. [Google Scholar] [CrossRef]
- Mauri, M.; Hofmann, A.I.; Heincke, D.G.; Kumara, S.; Pourrahimi, A.M.; Ouyang, Y.; Hagstrand, P.O.; Gkourmpis, T.; Xu, X.; Prieto, O.; et al. Click chemistry type crosslinking of a low-conductivity polyethylene copolymer ternary blend for power cable insulation. Polym. Int. 2020, 69, 404–412. [Google Scholar] [CrossRef]
- Xu, X.; Gaska, K.; Karlsson, M.; Hillborg, H.; Gedde, U.W. Precision electric characterization of LDPE specimens made by different manufacturing processes. Proceedings of IEEE International Conference on High Voltage Engineering and Application (ICHVE), Athens, Greece, 10–13 September 2018. [Google Scholar]
- Xu, X.; Karlsson, M.; Gaska, K.; Gubanski, S.M.; Hillborg, H.; Gedde, U.W. Robust measurement of electric conductivity in polyethylene-based materials: Measurement setup, data processing and impact of sample preparation. Proceedings of Nordic Insulation Symposium (Nord-IS), Vasteras, Sweden, 19–21 June 2017. [Google Scholar]
- Ghorbani, H.; Christen, T.; Edin, H. Role of thermal and electrical relaxations for the long-term conduction current in polyethylene. In Proceedings of the IEEE International Conference on Dielectrics (ICD), Montpellier, France, 3–7 July 2016. [Google Scholar]
- IDAX User’s Manual; Megger AB: Taby, Sweden, 2009.
- Kumara, J.R.S.S.; Serdyuk, Y.V.; Gubanski, S.M. Surface charge decay on LDPE and LDPE/Al2O3 nano composites: Measurements and modeling. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3466–3475. [Google Scholar] [CrossRef]
- Logakis, E.; Herrmann, L.; Christen, T. Electrical characterization of LDPE films with TSC and dielectric spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Fothergill, J.C.; Dodd, S.J.; Dissado, L.A.; Liu, T.; Nilsson, U.H. The measurement of very low conductivity and dielectric loss in XLPE cables: A possible method to detect degradation due to thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1544–1553. [Google Scholar] [CrossRef]
- Raju, G.G. Dielectric in Electric Fields, 1st ed.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 167–172. [Google Scholar]
- Molinie, P. A review of mechanisms and models accounting for surface potential decay. IEEE Trans. Plasma Sci. 2012, 40, 167–175. [Google Scholar] [CrossRef]
- Kumara, S.; Serdyuk, Y.V.; Gubanski, S.M. Surface charge decay on polymeric materials under different neutralization modes in air. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1779–1788. [Google Scholar] [CrossRef]
- Simmons, J.G.; Tam, M.C. Theory of isothermal currents and the direct determination of trap parameters in semiconductors and insulators containing arbitrary trap distributions. Phys. Rev. B 1973, 7, 3706–3713. [Google Scholar] [CrossRef]
- Watson, P.K.; Schmidlin, F.W.; Ladonna, R.V. The trapping of electrons in polystyrene. IEEE Trans. Electr. Insul. 1992, 27, 680–686. [Google Scholar] [CrossRef]
- Shen, W.W.; Mua, H.B.; Zhang, G.J.; Deng, J.B.; Tu, D.M. Identification of electron and hole trap based on isothermal surface potential decay model. J. Appl. Phys. 2013, 113, 083706. [Google Scholar] [CrossRef]
- Llovera, P.; Molinie, P. New Methodology for Surface Potential Decay Measurements: Application to Study Charge Injection Dynamics on Polypropylene Films. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 1049–1056. [Google Scholar] [CrossRef]
- Kumara, S.; Ma, B.; Serdyuk, Y.V.; Gubanski, S.M. Surface charge decay on HTV silicone rubber: Effect of material treatment by corona discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 2189–2195. [Google Scholar] [CrossRef]
- Lewis, T.J. Nanometric dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 812–825. [Google Scholar] [CrossRef]
- Dai, C.; Yu, L.; Jiang, C.; Chen, X.; Zhou, H.; Tanaka, Y. Effect of Thermal Ageing on Space Charge Behavior of HVDC XLPE Materials. In Proceedings of the International Conference on Electrical Materials and Power Equipment (ICEMPE), Guangzhou, China, 7–10 April 2019. [Google Scholar]



| Material | Temperature [°C] | Loss Tangent at 6.66 kV/mm, 0.1 mHz | Conductivity from FDS at 6.66 kV/mm [fS/m] | Measured Average DC Conductivity at 10 kV/mm [fS/m] |
|---|---|---|---|---|
| LDPE | 50 | 0.01 | 0.08 | 0.29 |
| 70 | 0.06 | 0.52 | 1.80 | |
| XLPE | 50 | 0.04 | 0.37 | 0.62 |
| 70 | 0.40 | 3.96 | 4.42 | |
| p(E-stat-GMA4.5) | 50 | 0.01 | 0.13 | 0.40 |
| p(E-stat-AA3) | 70 | 0.12 | 1.19 | 3.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumara, S.; Xu, X.; Hammarström, T.; Ouyang, Y.; Pourrahimi, A.M.; Müller, C.; Serdyuk, Y.V. Electrical Characterization of a New Crosslinked Copolymer Blend for DC Cable Insulation. Energies 2020, 13, 1434. https://doi.org/10.3390/en13061434
Kumara S, Xu X, Hammarström T, Ouyang Y, Pourrahimi AM, Müller C, Serdyuk YV. Electrical Characterization of a New Crosslinked Copolymer Blend for DC Cable Insulation. Energies. 2020; 13(6):1434. https://doi.org/10.3390/en13061434
Chicago/Turabian StyleKumara, Sarath, Xiangdong Xu, Thomas Hammarström, Yingwei Ouyang, Amir Masoud Pourrahimi, Christian Müller, and Yuriy V. Serdyuk. 2020. "Electrical Characterization of a New Crosslinked Copolymer Blend for DC Cable Insulation" Energies 13, no. 6: 1434. https://doi.org/10.3390/en13061434
APA StyleKumara, S., Xu, X., Hammarström, T., Ouyang, Y., Pourrahimi, A. M., Müller, C., & Serdyuk, Y. V. (2020). Electrical Characterization of a New Crosslinked Copolymer Blend for DC Cable Insulation. Energies, 13(6), 1434. https://doi.org/10.3390/en13061434