Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Samples
2.2. Microorganism
2.3. Reactor for Hemicellulose Extraction
2.4. Other Materials
2.5. Hemicellulose Extraction from Ground Wood Chips
2.6. Microorganism Cultivation
2.7. Analytical Methods
3. Results
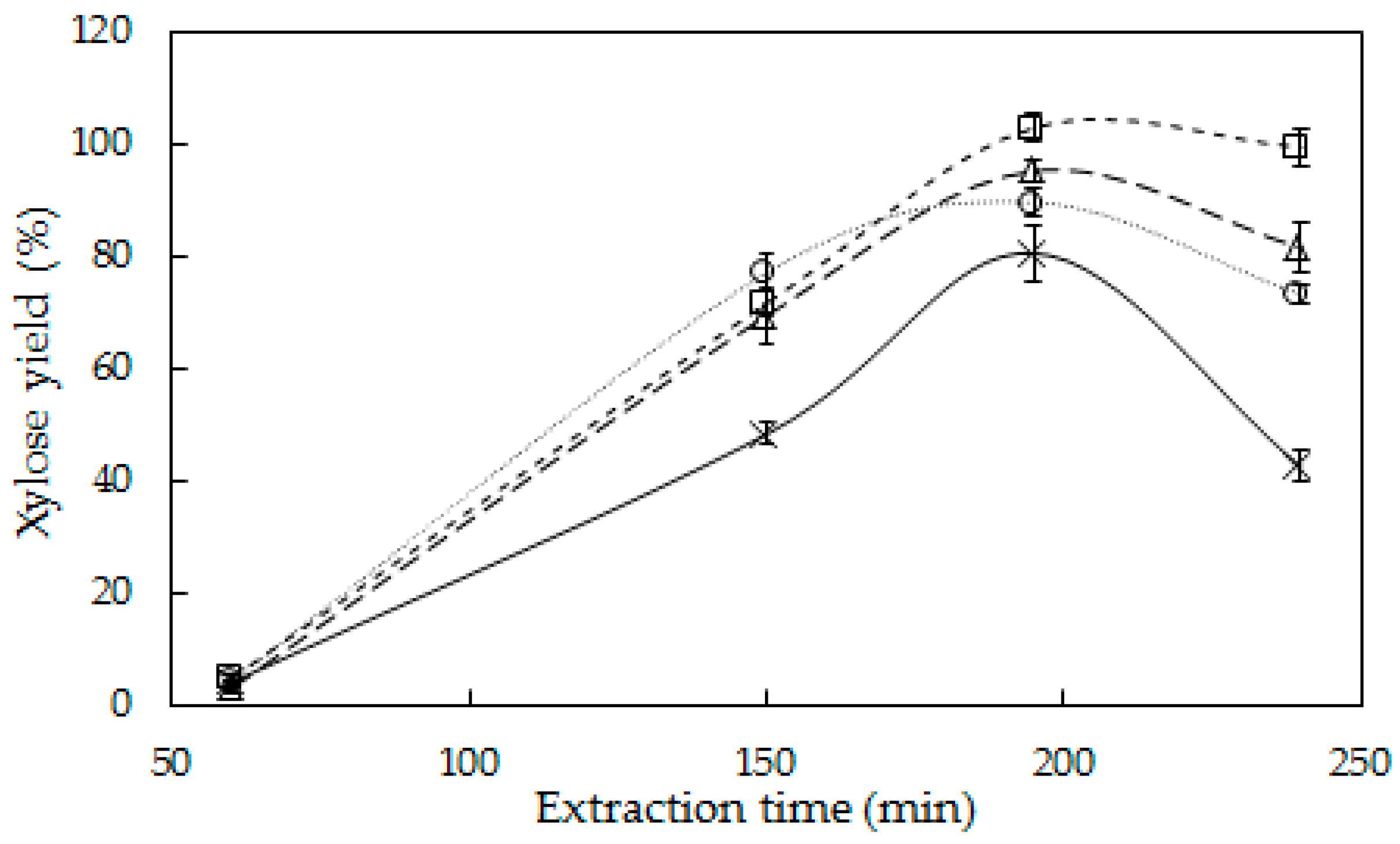
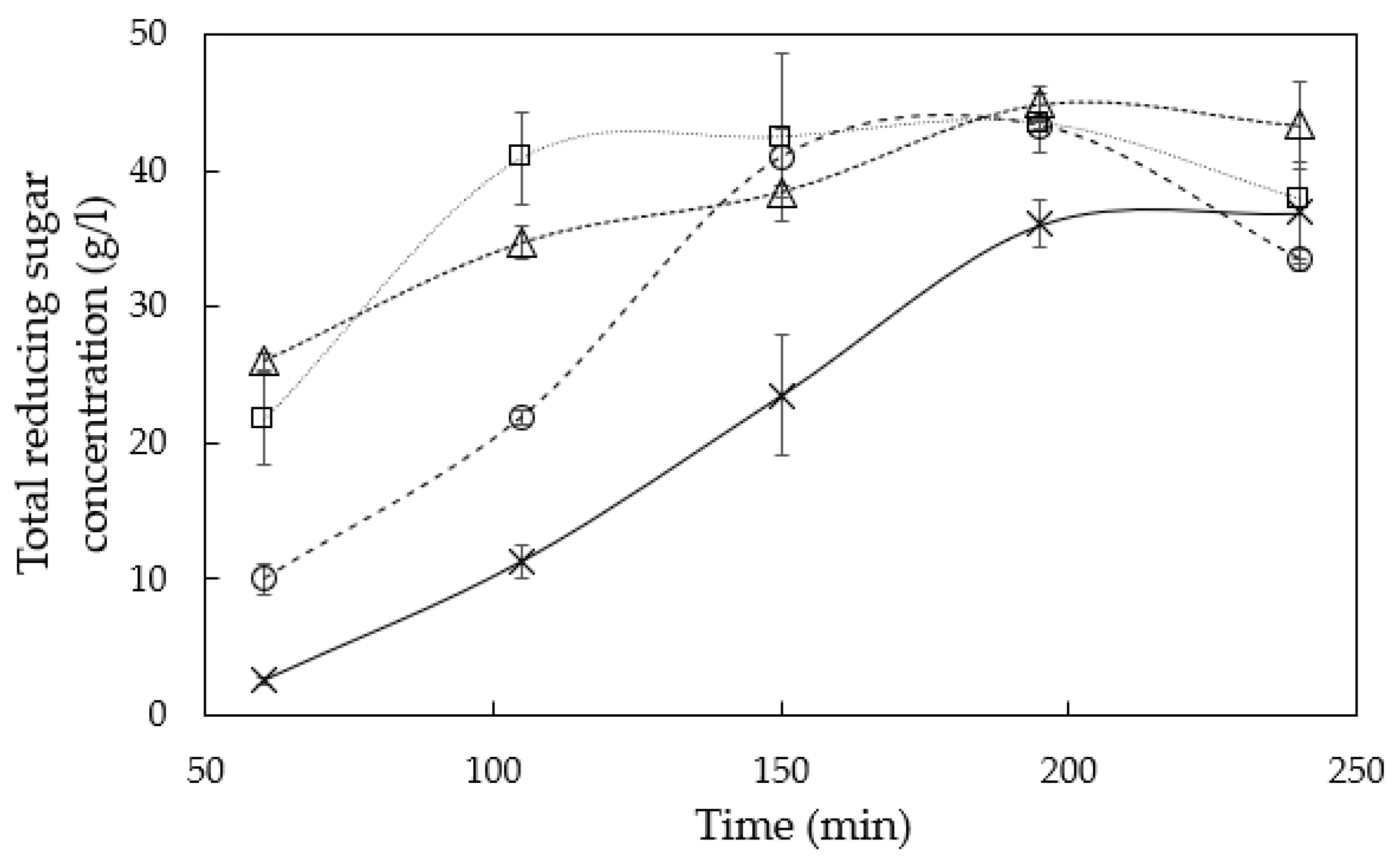
3.1. Hemicellulose Extraction
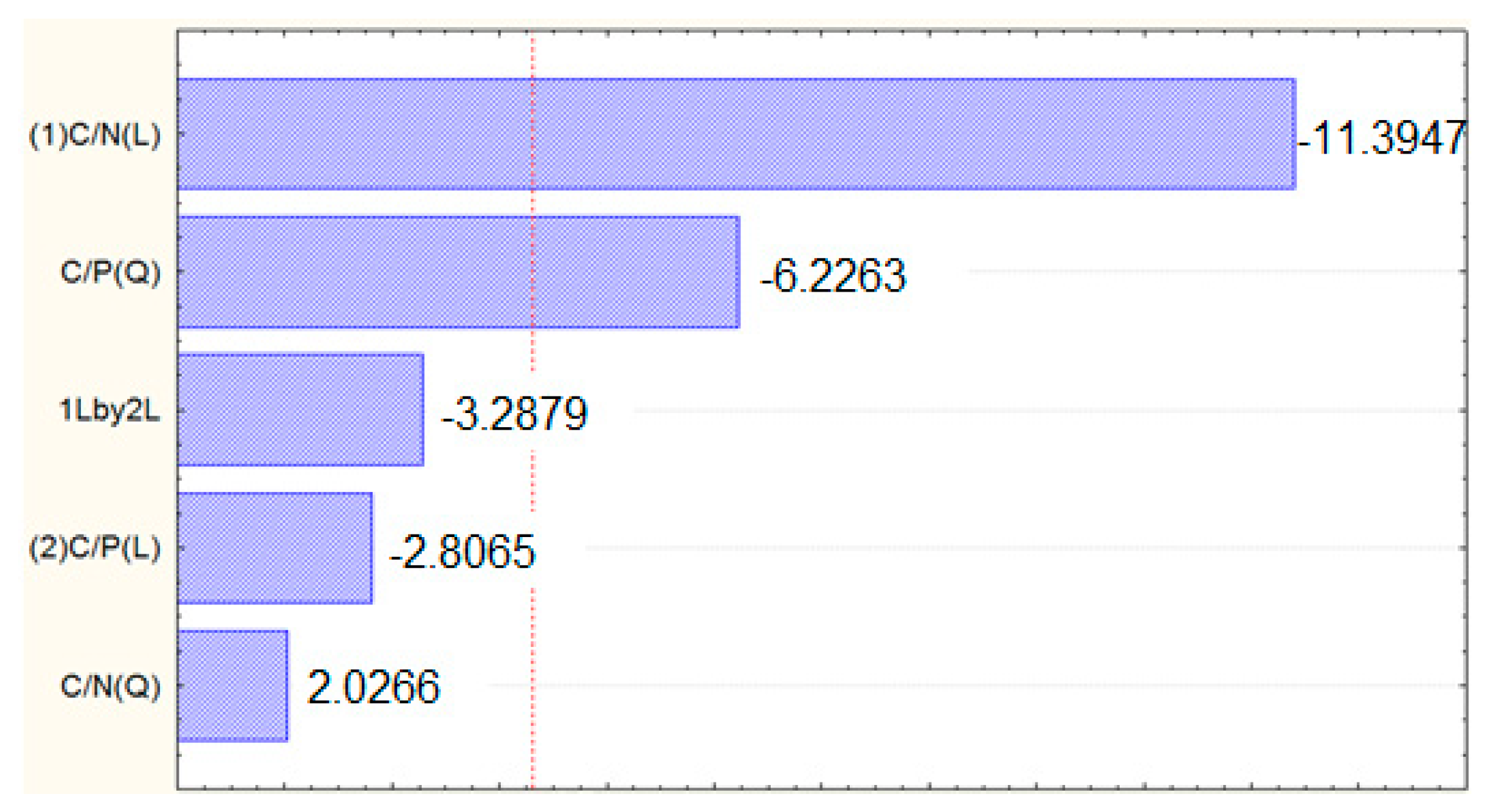
3.2. R. toruloides Cultivation and Lipid Production
4. Discussion
4.1. Hemicellulose Extraction
4.2. R. toruloides Cultivation and Lipid Production
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Outlook 2019. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 4 September 2018).
- OECD/FAO Agrucultural Outlook—Biofuels. Available online: http://dx.doi.org/10.1787/agr_outlook-2016-en (accessed on 24 September 2018).
- Azócar, L.; Ciudad, G.; Heipieper, H.J.; Navia, R. Biotechnological processes for biodiesel production using alternative oils. Appl. Microbiol. Biotechnol. 2010, 88, 621–636. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.C.; Strub, C.; Schorr-Galindo, S. Single cell oils (SCOs) from oleaginous yeasts and moulds: Production and genetics. Biomass Bioenergy 2014, 68, 135–150. [Google Scholar] [CrossRef]
- Park, Y.-K.; Nicaud, J.-M.; Ledesma-Amaro, R. The Engineering Potential of Rhodosporidium toruloides as a Workhorse for Biotechnological Applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.K.; Bai, F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzym. Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Schädel, C.; Richter, A.; Blöchl, A.; Hoch, G. Hemicellulose concentration and composition in plant cell walls under extreme carbon source-sink imbalances. Physiol. Plant. 2010, 139, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Erdocia, X.; Hernández-Ramos, F.; Alriols, M.G.; Labidi, J. Lignin Separation and Fractionation by Ultrafiltration. In Separation of Functional Molecules in Food by Membrane Technology; Academic Press: London, UK, 2019; pp. 229–265. [Google Scholar]
- Vila, C.; Romero, J.; Francisco, J.L.; Garrote, G.; Parajó, J.C. Extracting value from Eucalyptus wood before kraft pulping: Effects of hemicelluloses solubilization on pulp properties. Bioresour. Technol. 2011, 102, 5251–5254. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, A.; van Walsum, G. Adding Value to the Integrated Forest Biorefinery with Co-Products from Hemicellulose-Rich Pre-Pulping Extract. In Biorefinery Co-Products: Phytochemicals, Primary Metabolites and Value-Added Biomass Processing; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 287–310. [Google Scholar]
- Osorio-González, C.S.; Hegde, K.; Ferreira, P.; Brar, S.K.; Kermanshahipour, A.; Soccol, C.R.; Avalos-Ramírez, A. Lipid production in Rhodosporidium toruloides using C-6 and C-5 wood hydrolysate: A comparative study. Biomass Bioenergy 2019, 130, 105355. [Google Scholar] [CrossRef]
- Brandenburg, J.; Blomqvist, J.; Pickova, J.; Bonturi, N.; Sandgren, M.; Passoth, V. Lipid production from hemicellulose with Lipomyces starkeyi in a pH regulated fed-batch cultivation. Yeast 2016, 33, 451–462. [Google Scholar] [CrossRef]
- Matsakas, L.; Novak, K.; Enman, J.; Christakopoulos, P.; Rova, U. Acetate-detoxification of wood hydrolysates with alkali tolerant Bacillus sp. as a strategy to enhance the lipid production from Rhodosporidium toruloides. Bioresour. Technol. 2017, 242, 287–294. [Google Scholar] [CrossRef]
- Silva, S.S.; Felipe, M.G.A.; Silva, J.B.A.; Prata, A.M.R. Acid hydrolysis of Eucalyptus grandis chips for microbial production of xylitol. Process Biochem. 1998, 33, 63–67. [Google Scholar] [CrossRef]
- Converti, A.; Domínguez, J.M.; Perego, P.; da Silva, S.S.; Zilli, M. Wood Hydrolysis and Hydrolyzate Detoxification for Subsequent Xylitol Production. Chem. Eng. Technol. 2000, 23, 1013–1020. [Google Scholar] [CrossRef]
- Canettieri, E.V.; Silva, J.B.A.E.; Felipe, M.G. Application of factorial design to the study of xylitol production from eucalyptus hemicellulosic hydrolysate. Appl. Biochem. Biotechnol. 2001, 94, 159–168. [Google Scholar] [CrossRef]
- Diz, J.; Cruz Freire, J.M.; Domínguez, H.; Parajó, J.C. Xylitol Production from Eucalyptus Wood Hydrolysates in Low-Cost Fermentation Media. Food Technol. Biotechnol. 2002, 40, 191–197. [Google Scholar]
- Instituto Brasileiro de Árvores. Dados do Relatório Ibá 2018. 2017. Available online: https://iba.org/images/shared/Biblioteca/IBA_RelatorioAnual2017.pdf (accessed on 14 September 2018).
- Santos, S.A.O.; Villaverde, J.J.; Freire, C.S.R.; Domingues, M.R.M.; Neto, C.P.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis (E. grandis × E. urophylla) and E. maidenii bark extracts. Ind. Crop Prod. 2012, 39, 120–127. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Ataíde, C.H. Valorization of Eucalyptus urograndis wood via carbonization: Product yields and characterization. Energy 2019, 172, 509–516. [Google Scholar] [CrossRef]
- Bonturi, N.; Crucello, A.; Viana, J.A.C.; Miranda, E.A. Microbial oil production in sugarcane bagasse hemicellulosic hydrolysate without nutrient supplementation by a Rhodosporidium toruloides adapted strain. Process Biochem. 2017, 57, 16–25. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; NREL Technical Report: NREL/TP-510-42623; National Renewable Energy Laboratory: Lakewood, CA, USA, 2008. [Google Scholar]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Santucci, B.S.; Maziero, P.; Rabelo, S.C.; Curvelo, A.A.S.; Pimenta, M.T.B. Autohydrolysis of Hemicelluloses from Sugarcane Bagasse During Hydrothermal Pretreatment: A Kinetic Assessment. BioEnergy Res. 2015, 8, 1778–1787. [Google Scholar] [CrossRef]
- Zumdahl, S.S.; Zumdahl, S.A. Chemistry, 7th ed.; Stratton, R., Ed.; Houghton Mifflin Company: Boston, MA, USA, 2007; pp. 629–630. [Google Scholar]
- Liu, S. Woody biomass: Niche position as a source of sustainable renewable chemicals and energy and kinetics of hot-water extraction/hydrolysis. Biotechnol. Adv. 2010, 28, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuelsbioprod. Biorefin. Innov. A Sustain. Econ. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Jun, A.; Tschirner, U.W.; Tauer, Z. Hemicellulose extraction from aspen chips prior to kraft pulping utilizing kraft white liquor. Biomass Bioenergy 2012, 37, 229–236. [Google Scholar] [CrossRef]
- Sun, S.-N.; Cao, X.-F.; Li, H.-Y.; Xu, F.; Sun, R.-C. Structural characterization of residual hemicelluloses from hydrothermal pretreated Eucalyptus fiber. Int. J. Biol. Macromol. 2014, 69, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gallina, G.; Cabeza, Á.; Biasi, P.; García-Serna, J. Optimal conditions for hemicelluloses extraction from Eucalyptus globulus wood: Hydrothermal treatment in a semi-continuous reactor. Fuel Process. Technol. 2016, 148, 350–360. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Design of process parameters for the production of xylose from wood sawdust. Chem. Eng. Res. Des. 2012, 90, 1307–1312. [Google Scholar] [CrossRef]
- van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Zheng, Y.; Chi, Z.; Ahring, B.K.; Chen, S. Oleaginous yeast Cryptococcus curvatus for biofuel production: Ammonia’s effect. Biomass Bioenergy 2012, 37, 114–121. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Ramos, L.R.; Silva, E.L. Co-Fermentation of Cheese Whey and Crude Glycerol in EGSB Reactor as a Strategy to Enhance Continuous Hydrogen and Propionic Acid Production. Appl. Biochem. Biotechnol. 2017, 183, 712–728. [Google Scholar] [CrossRef]
- Veeramalini, J.B.; Selvakumari, I.A.E.; Park, S.; Jayamuthunagai, J.; Bharathiraja, B. Continuous production of biohydrogen from brewery effluent using co-culture of mutated Rhodobacter M 19 and Enterobacter aerogenes. Bioresour. Technol. 2019, 286, 121402. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Liu, J.-N.; Lu, L.-J.; Peng, K.-M.; Yang, G.-X.; Liu, J. Culture strategies for lipid production using acetic acid as sole carbon source by Rhodosporidium toruloides. Bioresour. Technol. 2016, 206, 141–149. [Google Scholar] [CrossRef] [PubMed]

| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| T (°C) | 120 | 140 | 160 |
| t (min) | 60 | 105 | 150 |
| S/L ratio | 1/4 | 1/6 | 1/8 |
| T (°C) | S/L Ratio (g/mL) | Time (min) | Total Reducing Sugar (g) |
|---|---|---|---|
| 120 | 1/4 | 60 | 0.04 |
| 120 | 1/8 | 60 | 0.03 |
| 120 | 1/4 | 150 | 0.11 |
| 120 | 1/8 | 150 | 0.08 |
| 140 | 1/6 | 105 | 0.19 |
| 140 | 1/6 | 105 | 0.19 |
| 140 | 1/6 | 105 | 0.16 |
| 160 | 1/4 | 60 | 0.13 |
| 160 | 1/8 | 60 | 0.25 |
| 160 | 1/4 | 150 | 1.60 |
| 160 | 1/8 | 150 | 1.56 |
| T (°C) | t (min) | Volume (L) | Compound (g/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Glucose | Xylose | Arabinose | Acetic Acid | HMF | Furfural | |||
| 145 | 60 | 0.115 | 0.215 | 0.472 | 0.052 | 0.094 | 0.001 | 0.013 |
| 150 | 0.135 | 0.326 | 4.668 | 0.073 | 1.137 | 0.017 | 0.249 | |
| 195 | 0.135 | 0.387 | 7.781 | n.d. 1 | 1.885 | 0.021 | 0.424 | |
| 240 | 0.13 | 0.293 | 4.264 | 0.081 | 1.075 | 0.000 | 0.212 | |
| 150 | 60 | 0.105 | 0.153 | 0.541 | 0.045 | 0.094 | 0.000 | 0.017 |
| 150 | 0.135 | 0.372 | 7.447 | 0.054 | 1.767 | 0.022 | 0.371 | |
| 195 | 0.135 | 0.419 | 8.656 | n.d. 1 | 2.129 | 0.023 | 0.516 | |
| 240 | 0.137 | 0.348 | 6.963 | n.d. 1 | 1.727 | 0.020 | 0.341 | |
| 155 | 60 | 0.115 | 0.177 | 0.357 | 0.059 | 0.071 | 0.003 | 0.007 |
| 150 | 0.133 | 0.344 | 6.809 | 0.052 | 1.649 | 0.017 | 0.334 | |
| 195 | 0.132 | 0.532 | 9.552 | n.d. 1 | 2.337 | 0.012 | 0.652 | |
| 240 | 0.130 | 0.390 | 8.177 | n.d. 1 | 2.023 | 0.007 | 0.452 | |
| 160 | 60 | 0.112 | 0.187 | 0.573 | 0.002 | 1.750 | 0.003 | 0.410 |
| 150 | 0.130 | 0.320 | 7.790 | 0.001 | 1.840 | 0.018 | 0.492 | |
| 195 | 0.115 | 0.421 | 12.196 | n.d. 1 | 2.412 | 0.025 | 0.587 | |
| 240 | 0.110 | 0.489 | 12.352 | n.d. 1 | 2.348 | 0.021 | 0.644 | |
| Factor | Level | ||||
|---|---|---|---|---|---|
| –1.41 | –1 | 0 | 1 | +1.41 | |
| C/N | 38.5 | 100 | 250 | 400 | 461.5 |
| C/P | 50.8 | 100 | 220 | 340 | 389.2 |
| Sample | C/N | C/P | Biomass (g/L) | Lipid * (g/L) | Lipid ** (%) | Productivity [mg/(L·h)] |
|---|---|---|---|---|---|---|
| 1 | 100 | 100 | 5.11 | 1.35 | 26.42 | 14.00 |
| 2 | 100 | 340 | 6.52 | 1.45 | 22.24 | 15.00 |
| 3 | 400 | 100 | 3.05 | 0.69 | 22.62 | 7.00 |
| 4 | 400 | 340 | 3.02 | 0.39 | 12.91 | 4.00 |
| 5 | 38.5 | 220 | 5.96 | 0.81 | 13.59 | 8.00 |
| 6 | 461.5 | 220 | 3.16 | 0.64 | 20.25 | 7.00 |
| 7 | 250 | 50.8 | 4.81 | 1.04 | 21.62 | 11.00 |
| 8 | 250 | 389.2 | 3.88 | 0.84 | 21.65 | 9.00 |
| 9 | 250 | 220 | 5.08 | 0.76 | 14.96 | 8.00 |
| 10 | 250 | 220 | 3.25 | 0.66 | 20.31 | 7.00 |
| 11 | 250 | 220 | 3.75 | 0.65 | 17.33 | 7.00 |
| Overliming | Concentration (g/L) | ||||
|---|---|---|---|---|---|
| Xylose | Acetic Acid | Glucose | Arabinose | HMF | |
| Before | 47.810 | 14.092 | 1.965 | 1.458 | 0.160 |
| After * | 37.399 | 9.884 | 1.810 | 0.950 | 0.119 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, H.J.S.; Bonturi, N.; Miranda, E.A. Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies 2020, 13, 795. https://doi.org/10.3390/en13040795
Lopes HJS, Bonturi N, Miranda EA. Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies. 2020; 13(4):795. https://doi.org/10.3390/en13040795
Chicago/Turabian StyleLopes, Helberth Júnnior Santos, Nemailla Bonturi, and Everson Alves Miranda. 2020. "Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source" Energies 13, no. 4: 795. https://doi.org/10.3390/en13040795
APA StyleLopes, H. J. S., Bonturi, N., & Miranda, E. A. (2020). Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies, 13(4), 795. https://doi.org/10.3390/en13040795






