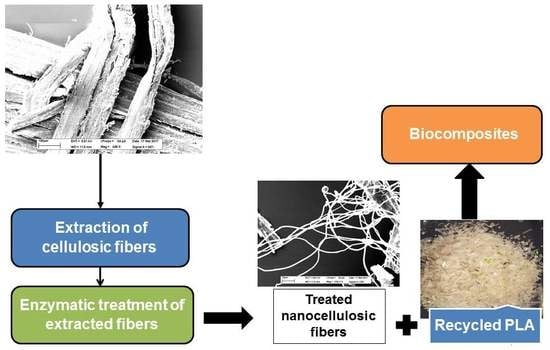
Biocomposite Fabrication from Enzymatically Treated Nanocellulosic Fibers and Recycled Polylactic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enzyme Production
2.3. Enzyme Extraction
2.4. Enzyme Estimation
2.5. Extraction of Cellulosic Fibers
2.6. Enzymatic Treatment
2.7. Fabrication of Biocomposite
2.8. Analysis of the Modified Fibers and the Formulated Biocomposites
3. Results and Discussion
3.1. Enzyme Production by Tra. versicolor and Tri. reesei
3.2. Effect of Chemical Extraction and Enzymatic Treatment of Fibers
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
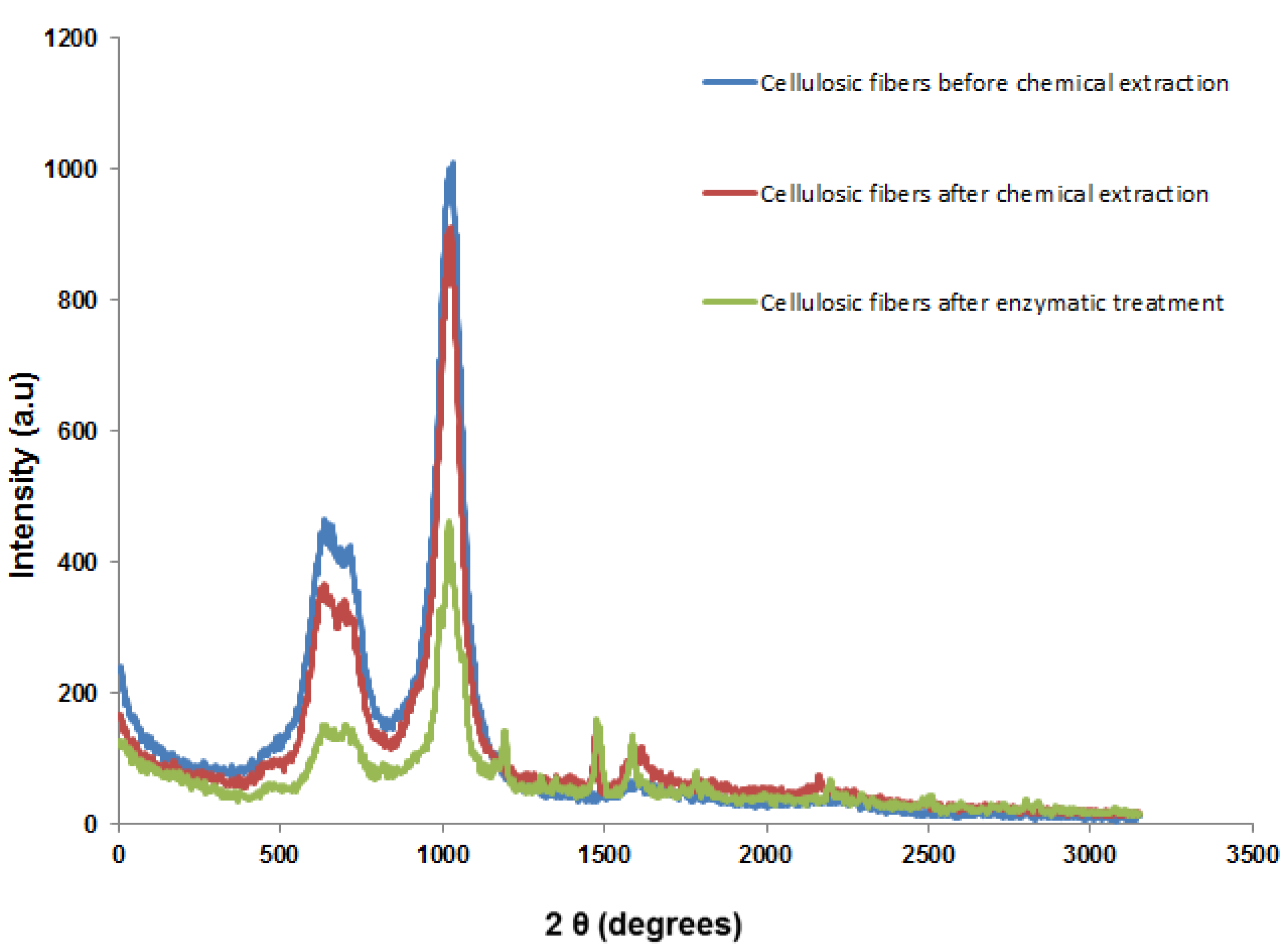
3.4. X-ray Diffraction (XRD) Analysis
3.5. Thermogravimetric (TGA) Analysis
3.6. Mechanical Properties of the Fabricated Biocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Pillin, I.; Montrelay, N.; Bourmaud, A.; Grohens, Y. Effect of thermo-mechanical cycles on the physico-chemical properties of poly (lactic acid). Polym. Degrad. Stab. 2008, 93, 321–328. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Bledzki, A.; Reihmane, S.; Gassan, J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996, 59, 1329–1336. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Karaduman, Y.; Gokcan, D.; Onal, L. Effect of enzymatic pretreatment on the mechanical properties of jute fiber-reinforced polyester composites. J. Compos. Mater. 2013, 47, 1293–1302. [Google Scholar] [CrossRef]
- Su, Y.; Xian, H.; Shi, S.; Zhang, C.; Manik, S.M.N.; Mao, J.; Liu, H. Biodegradation of lignin and nicotine with white rot fungi for the delignification and detoxification of tobacco stalk. BMC Biotechnol. 2016, 16. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samir, M.A.S.A. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Laadila, M.A.; Brar, S.K.; Hernández-Galán, L.; Verma, M.; Surampalli, R.Y. Agro-industrial produced laccase for degradation of diclofenac and identification of transformation products. ACS Sustain. Chem. Eng. 2017, 5, 5772–5781. [Google Scholar] [CrossRef]
- Awafo, V.A.; Chahal, D.S.; Simpson, B.K.; Lê, G.B. Production of cellulase systems by selected mutants of Trichoderma reesei in solid-state fermentation and their hydrolytic potentials. Appl. Biochem. Biotechnol. 1996, 57, 461–470. [Google Scholar] [CrossRef]
- Gassara, F.; Brar, S.K.; Tyagi, R.D.; Verma, M.; Surampalli, R.Y. Screening of agro-industrial wastes to produce ligninolytic enzymes by Phanerochaete chrysosporium. Biochem. Eng. J. 2010, 49, 388–394. [Google Scholar] [CrossRef]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Jung, Y.R.; Park, J.M.; Heo, S.Y.; Hong, W.K.; Lee, S.M.; Oh, B.R.; Park, S.M.; Seo, J.W.; Kim, C.H. Cellulolytic enzymes produced by a newly isolated soil fungus Penicillium sp. TG2 with potential for use in cellulosic ethanol production. Renew. Energy 2015, 76, 66–71. [Google Scholar] [CrossRef]
- Tabka, M.; Herpoël-Gimbert, I.; Monod, F.; Asther, M.; Sigoillot, J. Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzym. Microb. Technol. 2006, 39, 897–902. [Google Scholar] [CrossRef]
- Shamsabadi, M.A.; Behzad, T.; Bagheri, R. Optimization of acid hydrolysis conditions to improve cellulose nanofibers extraction from wheat straw. Fibers Polym. 2015, 16, 579–584. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Chaali, M.; Lecka, J.; Suresh, G.; Salem, M.; Brar, S.K.; Hernandez-Galan, L.; Sevigny, J.; Avalos-Ramirez, A. Supplement comprising of laccase and citric acid as an alternative for antibiotics-in vitro triggers of melanin production. Eng. Life Sci. 2018, 18, 359–367. [Google Scholar] [CrossRef]
- Lee, S.-M.; Koo, Y.-M. Pilot-Scale Production of Cellulase Using Trichoderma reesei Rut C-30 Fed-Batch Mode. J. Microbiol. Biotechnol. 2001, 11, 229–233. [Google Scholar]
- Cao, Y.; Tan, H. Effects of cellulase on the modification of cellulose. Carbohydr. Res. 2002, 337, 1291–1296. [Google Scholar] [CrossRef]
- Yan, P.; Xu, Z.; Zhang, C.; Liu, X.; Xu, W.; Zhang, Z.C. Fractionation of lignin from eucalyptus bark using amine-sulfonate functionalized ionic liquids. Green Chem. 2015, 17, 4913–4920. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring Wood Degradation during Weathering by Cellulose Crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: A review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Sajna, V.P.; Mohanty, S.; Nayak, S.K. A study on thermal degradation kinetics and flammability properties of poly (lactic acid)/banana fiber/nanoclay hybrid bionanocomposites. Polym. Compos. 2015, 38, 2067–2079. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, L.; Liang, D.; Xiao, W.; Lin, J. Mechanical and Thermal Properties of PLA Biocomposites Reinforced by Coir Fibers. Int. J. Polym. Sci. 2017, 2017, 2178329. [Google Scholar] [CrossRef]
- Haafiz, M.M.; Hassan, A.; Khalil, H.A.; Khan, I.; Inuwa, I.M.; Islam, M.S.; Hossain, M.S.; Syakir, M.I.; Fazita, M.N. Bionanocomposite based on cellulose nanowhisker from oil palm biomass-filled poly (lactic acid). Polym. Test. 2015, 48, 133–139. [Google Scholar] [CrossRef]
- Laadila, M.A.; Hegde, K.; Rouissi, T.; Brar, S.K.; Galvez, R.; Sorelli, L.; Cheikh, R.B.; Paiva, M.; Abokitse, K. Green synthesis of novel biocomposites from treated cellulosic fibers and recycled bio-plastic polylactic acid. J. Clean. Prod. 2017, 164, 575–586. [Google Scholar] [CrossRef]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]
- Iwatake, A.; Nogi, M.; Yano, H. Cellulose nanofiber-reinforced polylactic acid. Compos. Sci. Technol. 2008, 68, 2103–2106. [Google Scholar] [CrossRef]
- Kasa, S.N.; Omar, M.F.; Abdullah, M.M.A.B.; Ismail, I.N.; Ting, S.S.; VAC, S.C.; VIZUREANU, P. Effect of Unmodified and Modified Nanocrystalline Cellulose Reinforced Polylactic Acid (PLA) Polymer Prepared by Solvent Casting Method. Mater. Plast. 2017, 54, 91. [Google Scholar]
- Robles, E.; Urruzola, I.; Labidi, J.; Serrano, L. Surface-modified nano-cellulose as reinforcement in poly (lactic acid) to conform new composites. Ind. Crop. Prod. 2015, 71, 44–53. [Google Scholar] [CrossRef]


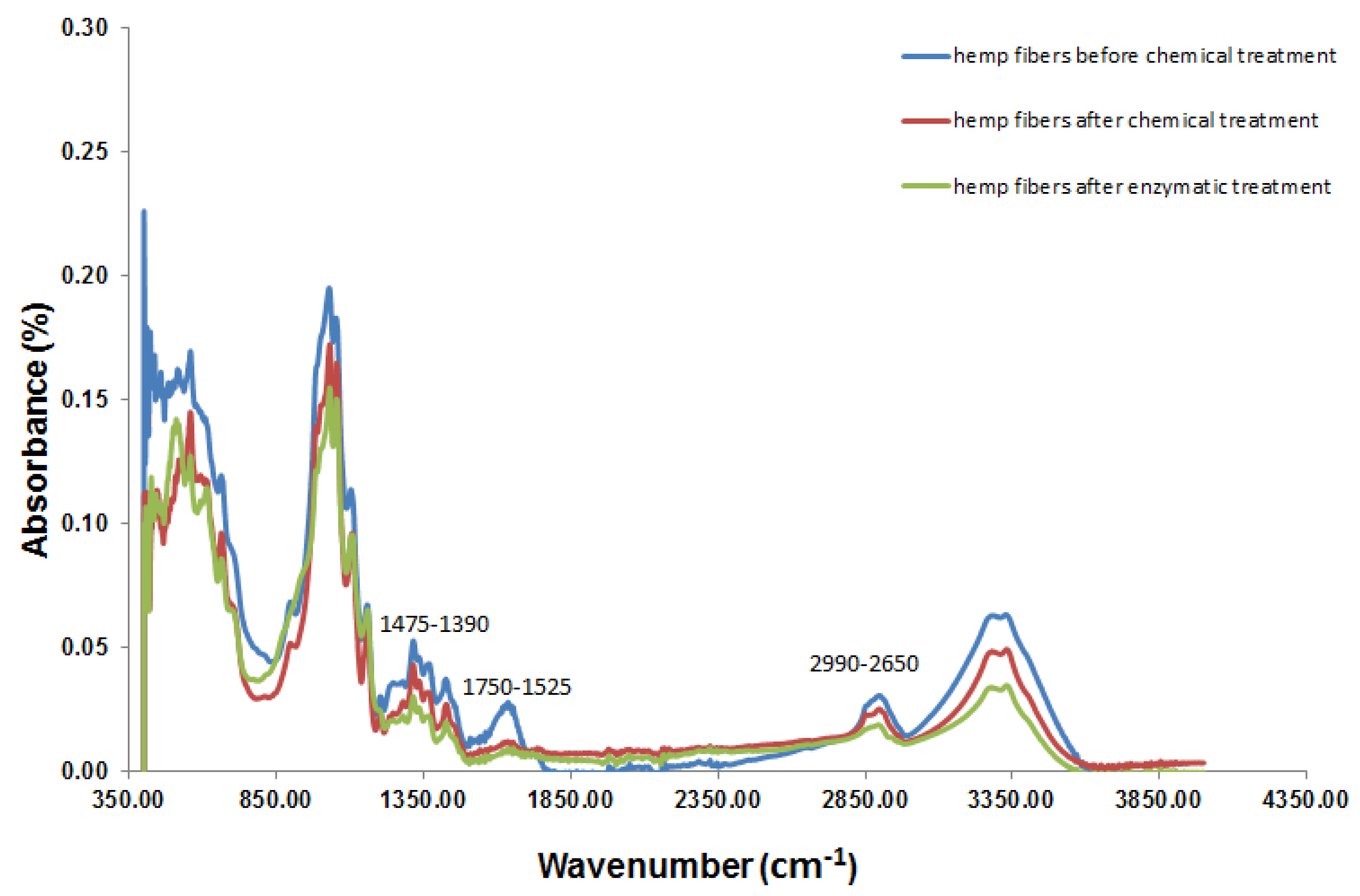

| Wave Number (cm−1) | Functional Groups | Percentage after Chemical Extraction | Percentage after Enzymatic Treatment |
|---|---|---|---|
| 3640–3000 | O–H, Cellulose [26] | 34.72 | 26.29 |
| 1750–1525 | C=O, Hemicellulose [26] | 65.75 | 18.23 |
| 1475–1390 | C=C, Lignin [26] | 71.74 | 54.35 |
| Formulation | Young’s Modulus (MPa) | Tensile Strength at Break (% Maximum Load: 90) (MPa) | Tensile Stress at Yield (Zero Slope) (MPa) |
|---|---|---|---|
| PLAr | 224.82 ± 21.60 | 512.07 ± 37.55 | 19.78 ± 0.06 |
| PLAr + 0.1% NCFs | 227.84 ± 28.62 | 413.11 ± 73.86 | 18.03 ± 1.88 |
| PLAr + 0.25% NCFs | 250.29 ± 5.48 | 323.76 ± 57.41 | 20.08 ± 0.81 |
| PLAr + 1% NCFs | 248.02 ± 8.18 | 229.58 ± 65.91 | 18.18 ± 0.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laadila, M.A.; Suresh, G.; Rouissi, T.; Kumar, P.; Brar, S.K.; Cheikh, R.B.; Abokitse, K.; Galvez, R.; Jacob, C. Biocomposite Fabrication from Enzymatically Treated Nanocellulosic Fibers and Recycled Polylactic Acid. Energies 2020, 13, 1003. https://doi.org/10.3390/en13041003
Laadila MA, Suresh G, Rouissi T, Kumar P, Brar SK, Cheikh RB, Abokitse K, Galvez R, Jacob C. Biocomposite Fabrication from Enzymatically Treated Nanocellulosic Fibers and Recycled Polylactic Acid. Energies. 2020; 13(4):1003. https://doi.org/10.3390/en13041003
Chicago/Turabian StyleLaadila, Mohamed Amine, Gayatri Suresh, Tarek Rouissi, Pratik Kumar, Satinder Kaur Brar, Ridha Ben Cheikh, Kofi Abokitse, Rosa Galvez, and Colin Jacob. 2020. "Biocomposite Fabrication from Enzymatically Treated Nanocellulosic Fibers and Recycled Polylactic Acid" Energies 13, no. 4: 1003. https://doi.org/10.3390/en13041003
APA StyleLaadila, M. A., Suresh, G., Rouissi, T., Kumar, P., Brar, S. K., Cheikh, R. B., Abokitse, K., Galvez, R., & Jacob, C. (2020). Biocomposite Fabrication from Enzymatically Treated Nanocellulosic Fibers and Recycled Polylactic Acid. Energies, 13(4), 1003. https://doi.org/10.3390/en13041003