Impacts of Initial Sugar, Nitrogen and Calcium Carbonate on Butanol Fermentation from Sugarcane Molasses by Clostridium beijerinckii
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Nitrogen Supplementation
2.2. Culture Medium Preparation
2.3. Microorganism and Growth Conditions
2.4. Experiments
2.4.1. Effects of Various Nitrogen Sources on Batch Butanol Fermentation
2.4.2. Optimization, Experimental Design and Statistical Analysis
2.4.3. Verification Experiments
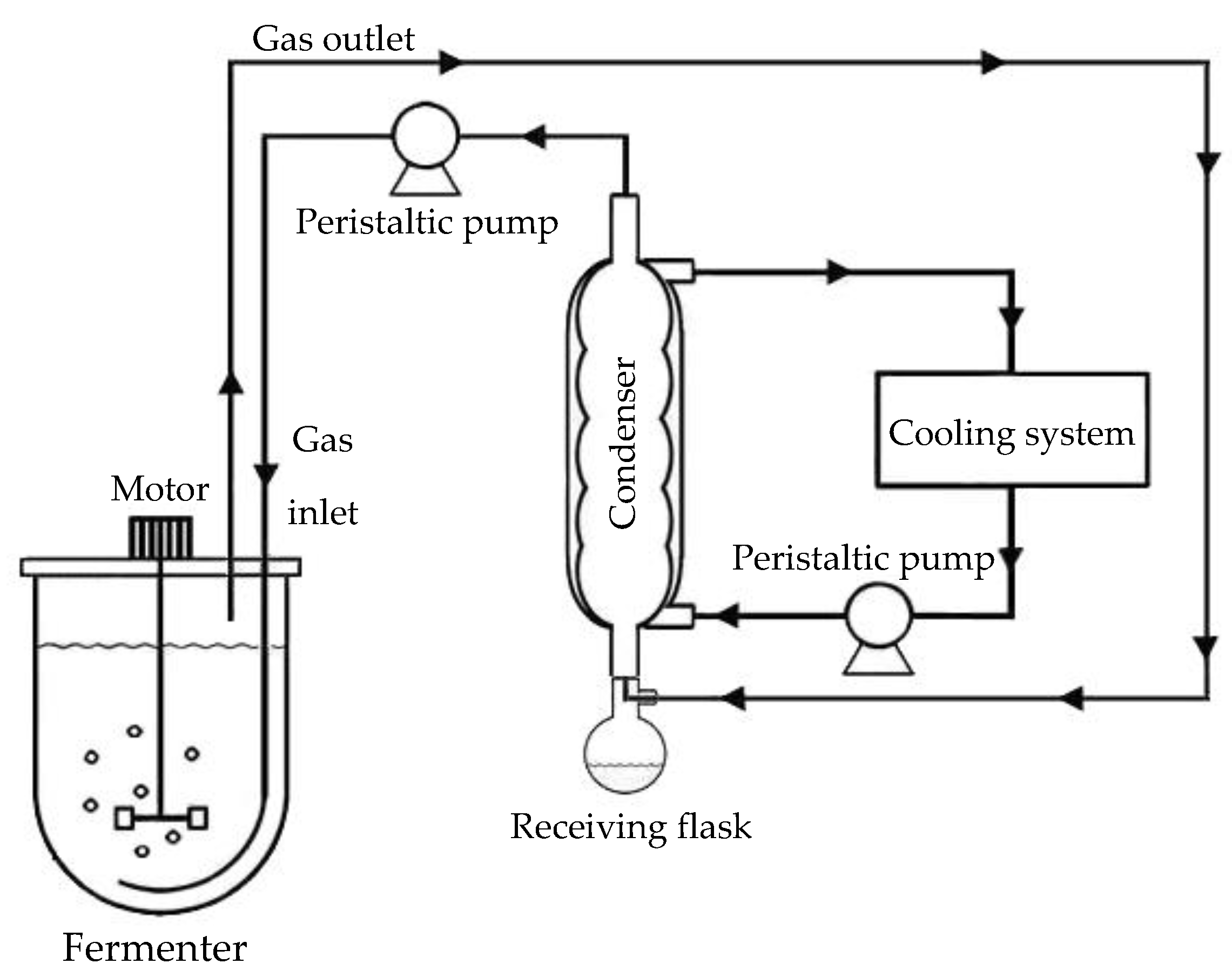
2.4.4. Batch Butanol Fermentation Coupled with a Gas Stripping System
2.5. Analytical Methods
3. Results and Discussion
3.1. Proximate Chemical Composition of Various Nitrogen Sources
3.2. Effects of Various Nitrogen Sources on Butanol Production
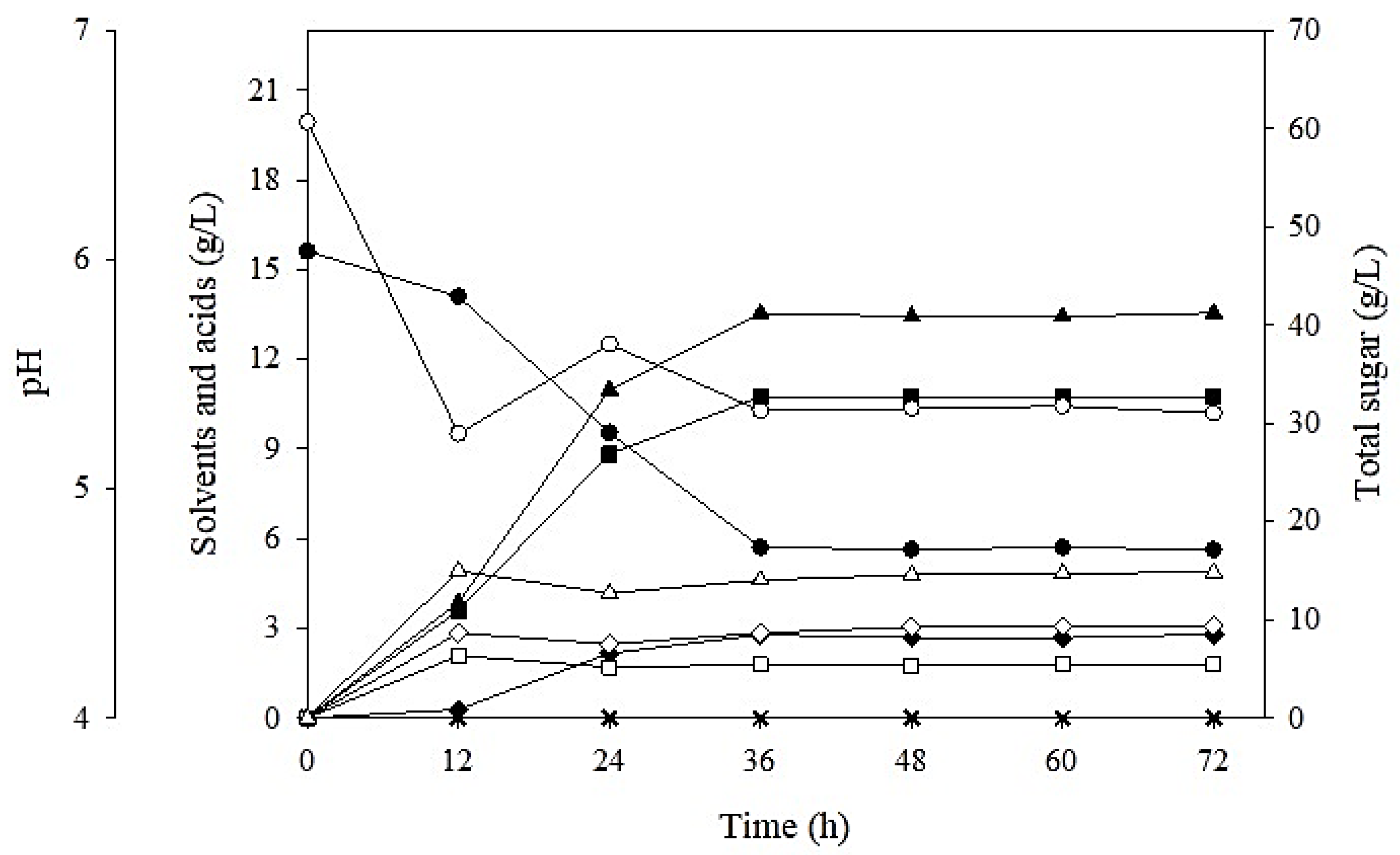
3.3. Batch Butanol Fermentation
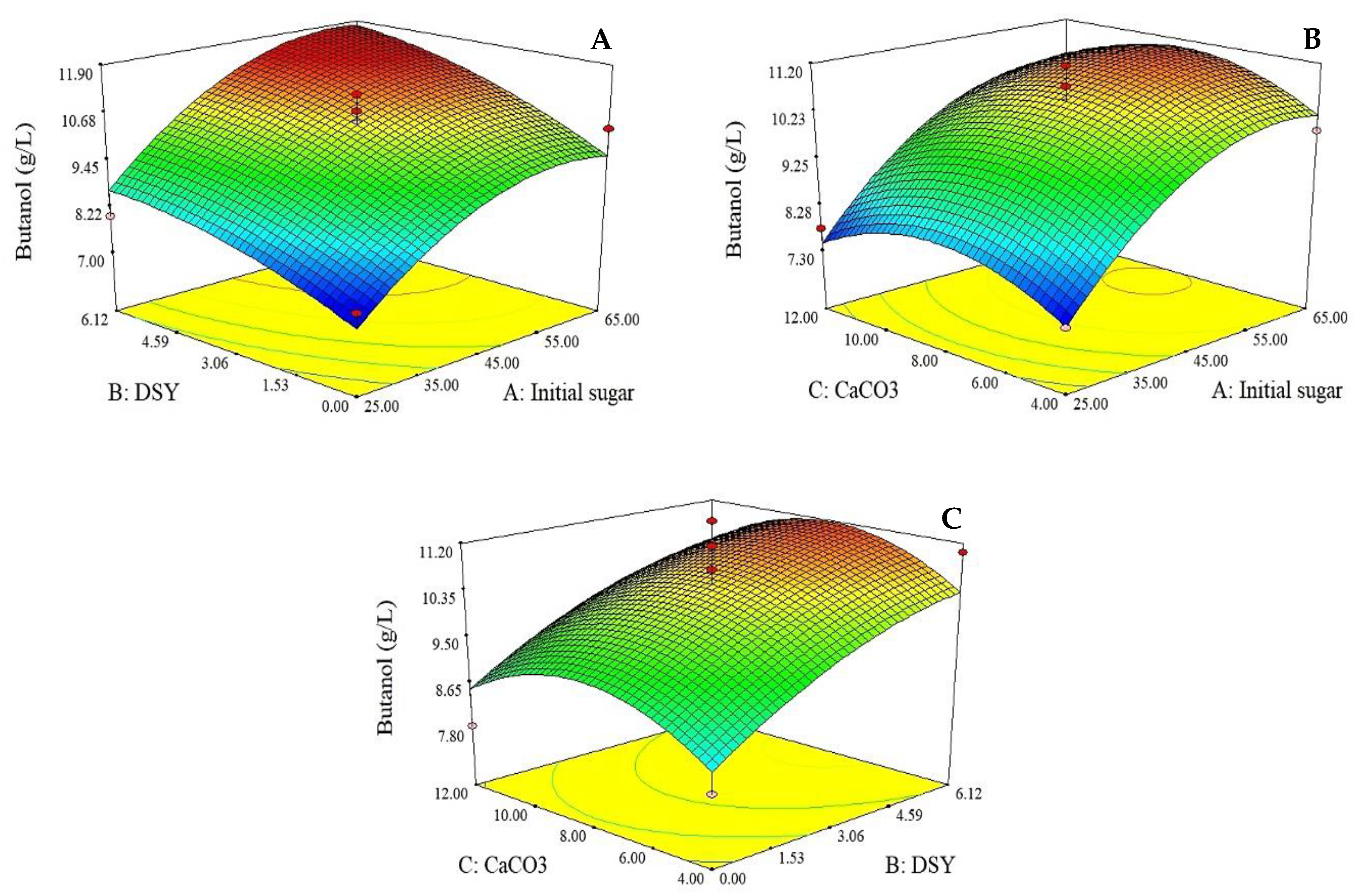
3.3.1. Impact of Initial Sugar, DSY and CaCO3 Concentrations on Butanol Production
1.634 × 10−3x2x3 − 2.358 × 10−3x12 − 0.037x22 − 0.051x32
3.3.2. Verification Experiment under the Optimized Medium Composition
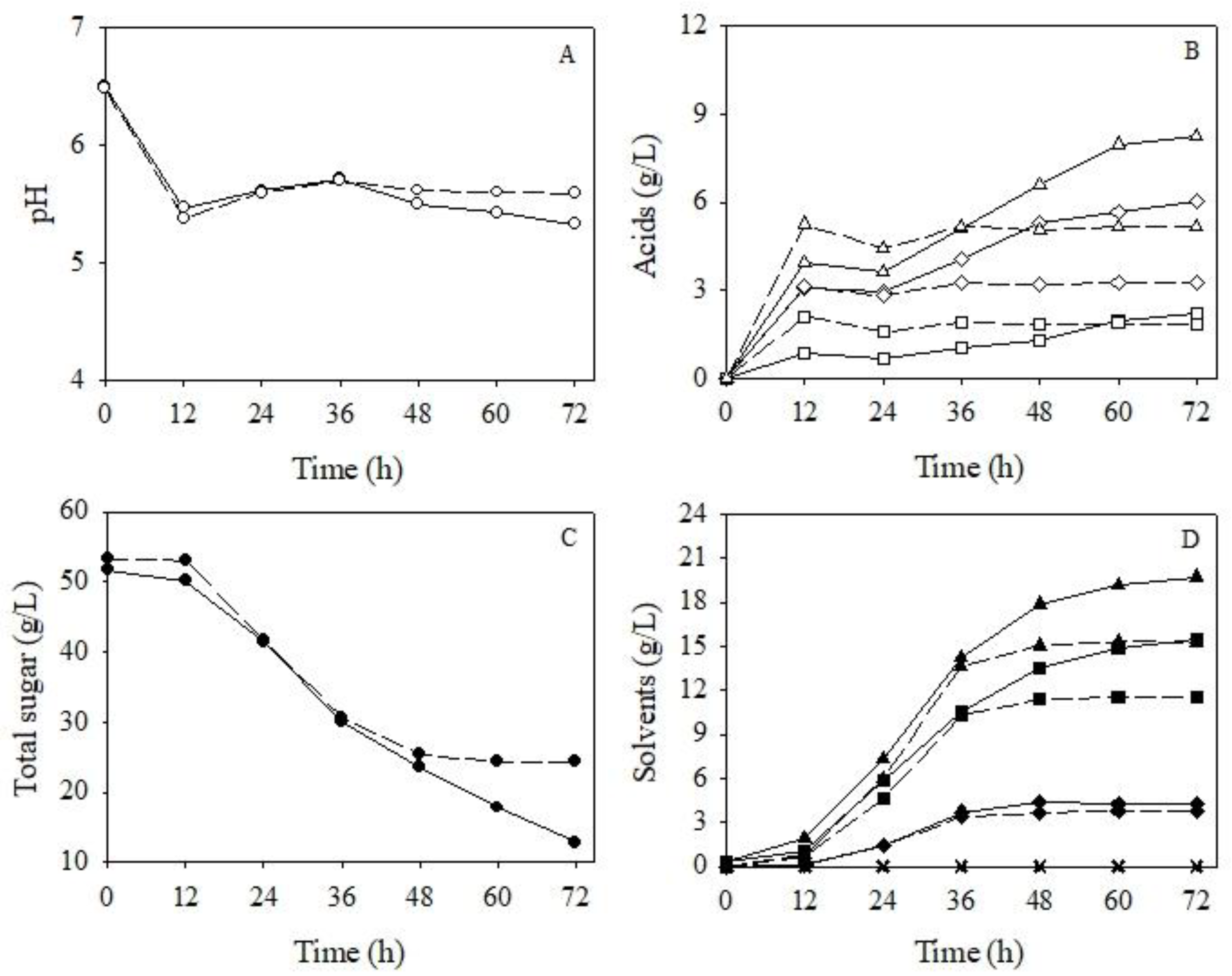
3.3.3. Improvement of Butanol Production by a Gas Stripping System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ezeji, T.C.; Milne, C.; Price, N.D.; Blaschek, H.P. Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol producing microorganisms. Appl. Microbiol. Biotechnol. 2010, 85, 1697–1712. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone butanol (AB) from liquefied corn starch, a commercial substrate, using Clostridium beijerinckii coupled with product recovery by gas stripping. J. Ind. Microbiol. Biotechnol. 2007, 34, 771–777. [Google Scholar] [CrossRef]
- Dürre, P. Fermentative Butanol Production. Ann. N. Y. Acad. Sci. 2008, 1125, 353–362. [Google Scholar] [CrossRef]
- Wechgama, K.; Laopaiboon, L.; Laopaiboon, P. Biobutanol production from agricultural raw materials by Clostridium spp. Chiang Mai J. Sci. 2017, 44, 394–405. [Google Scholar]
- Li, H.G.; Luo, W.; Gu, Q.Y.; Wang, Q.; Hu, W.J.; Yu, X.B. Acetone, butanol, and ethanol production from cane molasses using Clostridium beijerinckii mutant obtained low-energy ion beam implantation and N-methyl-N-nitro-N-nitrosoguanidine induction. Bioresour. Technol. 2013, 137, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Li, H.G.; Ma, X.X.; Zhang, Q.H.; Luo, W.; Wu, Y.Q.; Li, X.H. Enhanced butanol production by solvent tolerance Clostridium acetobutylicum SE25 from cassava flour in a fibrous bed bioreactor. Bioresour. Technol. 2016, 221, 412–418. [Google Scholar] [CrossRef]
- Thani, A.; Laopaiboon, P.; Laopaiboon, L. Improvement of a continuous ethanol fermentation from sweet sorghum stem juice using a cell recycling system. J. Biotechnol. 2017, 251, 21–29. [Google Scholar] [CrossRef]
- Pleissner, N.; Neu, A.K.; Mehlmann, K.; Schneider, R.; Quintero, G.P.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef]
- Richmond, C.; Ezeji, T.C. Stimulatory effects of calcium carbonate on butanol production by solventogenic Clostridium species. Continental J. Microbiol. 2011, 5, 18–28. [Google Scholar]
- YouSheng, L.; Jing, W.; XuMing, W.; XiaoHong, S. Optimization of butanol production from corn straw hydrolysate by Clostridium acetobutylicum using response surface method. Chinese Sci. Bull. 2011, 56, 1422–1428. [Google Scholar] [CrossRef]
- Han, B.; Ujor, V.; Lai, L.B.; Gopalan, V.; Ezeji, T.C. Use of proteomic analysis to elucidate the role of calcium in acetone-butanol-ethanol fermentation by Clostridium beijerinckii NCIMB 8052. Appl. Environ. Microbiol. 2013, 79, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Wechgama, K.; Laopaiboon, L.; Laopaiboon, P. Enhancement of batch butanol production from sugarcane molasses using nitrogen supplementation integrated with gas stripping for product recovery. Ind. Crops Prod. 2017, 95, 216–226. [Google Scholar] [CrossRef]
- Cai, D.; Chen, H.; Chen, C.; Hu, S.; Wang, Y.; Chang, Z.; Miao, Q.; Qin, P.; Wang, Z.; Wang, J.; et al. Gas stripping–pervaporation hybrid process for energy-saving product recovery from acetone-butanol-ethanol (ABE) fermentation broth. Chem. Eng. J. 2016, 287, 1–10. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Zhao, J.; Chen, L.; Ren, J.; Bai, F.; Yang, S.T. A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production. Biotechnol. Bioeng. 2016, 113, 120–129. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, J.; Yang, S.T.; Wei, D. Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour. Technol. 2012, 104, 380–387. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, J.; Lu, C.; Yang, S.T.; Bai, F.; Tang, I. High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol. Bioeng. 2012, 109, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhao, J.; Liu, F.; Lu, C.; Yang, S.T.; Bai, F.W. Two-stage in situ gas stripping for enhanced butanol fermentation and energy-saving product recovery. Bioresour. Technol. 2013, 135, 396–402. [Google Scholar] [CrossRef]
- Xue, C.; Du, G.Q.; Sun, J.X.; Chen, L.J.; Gao, S.S.; Yu, M.L.; Yang, S.T.; Bai, F.W. Characterization of gas stripping and its integration with acetone-butanol-ethanol fermentation for high-efficient butanol production and recovery. Biochem. Eng. J. 2014, 83, 55–61. [Google Scholar] [CrossRef]
- Rochon, E.; Ferrari, M.D.; Lareo, C. Integrated ABE fermentation-gas stripping process for enhanced butanol production from sugarcane-sweet sorghum juices. Biomass Bioenergy 2017, 98, 153–160. [Google Scholar] [CrossRef]
- Wiehn, M.; Staggs, K.; Wang, Y.C.; Nielsen, D.R. In situ butanol recovery from Clostridium acetobutylicum fermentations by expanded bed adsorption. Biotechnol. Prog. 2014, 30, 68–78. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Tang, I.; Zhao, J.; Bai, F.; Yang, S.T. Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Bioresour. Technol. 2016, 219, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Stoffers, M.; Górak, A. Continuous multi-stage extraction of n-butanol from aqueous solutions with 1-hexyl-3-methylimidazolium tetracyanoborate. Sep. Purif. Technol. 2013, 120, 415–422. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Production of acetone-butanol-ethanol (ABE) by a hyper-producing mutant stain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol. Prog. 1999, 15, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Sirisantimethakom, L.; Laopaiboon, L.; Sanchanda, P.; Chatleudmongkol, J.; Laopaiboon, P. Improvement of butanol production from sweet sorghum juice by Clostridium beijerinckii using an orthogonal array design. Ind. Crops Prod. 2016, 79, 287–294. [Google Scholar] [CrossRef]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol production by Clostridium beijerinckii. Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, A.; Goyal, A. Application of response surface methodology for glucan production from Leuconostoc dextranicum and its structural characterization. Carbohydr. Polym. 2009, 75, 150–156. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Acetone butanol ethanol (ABE) production from concentrated substrate: Reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl. Microbiol. Biotechnol. 2004, 63, 653–658. [Google Scholar] [CrossRef]
- William, H. Official Methods of Analysis of the AOAC; Association of Official Analytical Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Areesirisuk, A.; Laopaiboon, L.; Khongsay, N.; Laopaiboon, P. Improvement of gas chromatographic analysis for organic acids and solvents in acetone-butanol-ethanol fermentation from sweet sorghum juice. Afr. J. Biotechnol. 2010, 9, 6422–6429. [Google Scholar]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemometr. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Dong, H.; Mao, S.; Zhu, Y.; Wang, R.; Luan, G.; Li, Y. Formic acid triggers the “Acid Crash” of acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Appl. Environ. Microbiol. 2011, 77, 1674–1680. [Google Scholar] [CrossRef]
- Sridee, W.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. The use of dried spent yeast as a low-cost nitrogen supplement in ethanol fermentation from sweet sorghum juice under very high gravity conditions. Electron. J. Biotechnol. 2011, 14, 1–15. [Google Scholar] [CrossRef]
- Qureshi, N.; Lolas, A.; Blaschek, H.P. Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. J. Ind. Microbiol. Biotechnol. 2001, 26, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Al-Shorgani, N.K.N.; Kalil, M.S.; Yusoff, W.M.W. The effect of different nitrogen sources on biobutanol production using Clostridium saccharoperbutylacetonicum N1-4. Biotechnology 2011, 10, 280–285. [Google Scholar] [CrossRef]
- Costa, J.M. Solvent toxicity in the acetone-butanol fermentation. Proc. Ann. Biochem. Eng. 1981, 11, 83–90. [Google Scholar]
- Chen, F.; Peng, J.; Lei, D.; Liu, J.; Zhao, G. Optimization of genistein solubilization by κ-carrageenan hydrogel using response surface methodology. Food Sci. Hum. Wellness 2013, 2, 124–131. [Google Scholar] [CrossRef]
- Lai, M.C.; Traxler, R.W. A coupled two-stage continuous fermentation for solvent production by Clostridium acetobutylicum. Enzyme Microb. Technol. 1994, 16, 1021–1025. [Google Scholar] [CrossRef]
- Agarwal, L.; Isar, J.; Meghwanshi, G.K.; Saxena, R.K. A cost effective fermentative production of succinic acid from cane molasses and corn steep liquor by Escherichia coli. J. Appl. Microbiol. 2006, 100, 1348–1354. [Google Scholar] [CrossRef]
- Isar, J.; Rangaswamy, V. Improved n-butanol production by solvent tolerant Clostridium beijerinkii. Biomass Bioenergy 2012, 37, 9–15. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Y.; Sun, Z. Butanol production from cane molasses by Clostridium saccharobutylicum DSM 13864: Batch and semicontinuous fermentation. Appl. Biochem. Biotechnol. 2012, 166, 1896–1907. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Zheng, J.; Shi, Z.; Li, L. Yeast extract promotes phase shift of bio-butanol fermentation by Clostridium acetobutylicum ATCC824 using cassava as substrate. Bioresour. Technol. 2012, 125, 43–51. [Google Scholar] [CrossRef]
- Durán-Padilla, V.R.; Davila-Vazquez, G.; Chávez-Vela, N.A.; Tinoco-Valencia, J.R.; Jáuregui-Rincón, J. Iron effect on the fermentative metabolism of Clostridium acetobutylicum ATCC 824 using cheese whey as substrate. Biofuel Res. J. 2014, 4, 129–133. [Google Scholar] [CrossRef]
- Wu, Y.D.; Xue, C.; Chen, L.J.; Bai, F.W. Impact of zinc supplementation on the improved fructose/xylose utilization and butanol production during acetone-butanol-ethanol fermentation. J. Biosci. Bioeng. 2016, 121, 66–72. [Google Scholar] [CrossRef]
- Gomes, A.C.; Rodrigues, M.I.; Passos, D.F.; Castro, A.M.; Anna, L.M.S.; Pereira, N. Acetone-butanol-ethanol fermentation from sugarcane bagasse hydrolysates: Utilization of C5 and C6 sugars. Electron. J. Biotechnol. 2019, 42, 16–22. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Lu, C.; Dong, J.; Yang, S.T. Butanol production from wood pulping hydrolysate in an integrated fermentation-gas stripping process. Bioresour. Technol. 2013, 143, 467–475. [Google Scholar] [CrossRef]
- Qureshi, N.; Cotta, M.A.; Saha, B.C. Bioconversion of barley straw and corn stover to butanol (a biofuel) in integrated fermentation and simultaneous product recovery bioreactors. Food Bioprod. Process. 2014, 92, 298–308. [Google Scholar] [CrossRef]

| Variables | Variable Code | Level | ||
|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | ||
| Initial sugar (g/L) | a | 25 | 45 | 65 |
| Nitrogen source (g/L) a | b | 0 | 2 | 4 |
| CaCO3 (g/L) | c | 4 | 8 | 12 |
| Run Number | Coded Value | ||
|---|---|---|---|
| a | b | c | |
| 1 | 0 | 0 | 0 |
| 2 | +1 | −1 | 0 |
| 3 | −1 | +1 | 0 |
| 4 | +1 | +1 | 0 |
| 5 | 0 | 0 | 0 |
| 6 | +1 | 0 | −1 |
| 7 | −1 | 0 | +1 |
| 8 | +1 | 0 | +1 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | +1 | −1 |
| 11 | 0 | −1 | +1 |
| 12 | 0 | +1 | +1 |
| 13 | 0 | 0 | 0 |
| 14 | −1 | −1 | 0 |
| 15 | −1 | 0 | −1 |
| 16 | 0 | −1 | −1 |
| 17 | 0 | 0 | 0 |
| Composition (% Dry Weight) | Nitrogen Source a | |||||
|---|---|---|---|---|---|---|
| YE | DSY | RB | SM | Urea | (NH4)2SO4 | |
| Total carbohydrate | 8.98 | 36.67 | 51.95 | 39.71 | - b | - |
| Protein | 74.50 | 48.69 | 12.08 | 34.92 | - | - |
| Total fat | 0.07 | 2.94 | 10.44 | 13.08 | - | - |
| Moisture | 5.19 | 5.19 | 8.40 | 3.15 | - | - |
| Fibre | - | 0.35 | 8.21 | 6.60 | - | - |
| Ash | 11.26 | 6.16 | 8.92 | 2.54 | - | - |
| Nitrogen content | 11.92 | 7.79 | 1.93 | 5.59 | 46.62 | 21.19 |
| Nitrogen Source * | Acetone (g/L) | Butanol (g/L) | Ethanol (g/L) | ABE (g/L) | Acetic Acid (g/L) | Butyric Acid (g/L) | Total Acid (g/L) | SC ** (g/L) | t (h) | YB/S (g/g) | QB (g/L·h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| None | 4.42 ± 0.51 b | 8.53 ± 0.44 a | 0.77 ± 0.13 c | 13.72 ± 0.95 a | 3.67 ± 0.09 b | 1.58 ± 0.03 a | 5.25 ± 0.05 b | 27.25 ± 0.27 a | 60 | 0.31 ± 0.01 c | 0.14 ± 0.01 a |
| YE | 2.59 ± 0.23 a | 11.37 ± 0.55 d | 0.71 ± 0.08 c | 14.67 ± 0.23 ab | 2.43 ± 0.37 a | 2.00 ± 0.49 bc | 4.43 ± 0.12 a | 30.09 ± 0.76 b | 48 | 0.38 ± 0.01 d | 0.24 ± 0.01 d |
| DSY | 6.65 ± 0.11 e | 11.19 ± 0.01 d | 0.82 ± 0.24 c | 18.66 ± 0.36 e | 5.45 ± 0.05 d | 2.17 ± 0.01 c | 7.62 ± 0.05 e | 35.84 ± 0.55 f | 48 | 0.31 ± 0.01 c | 0.23 ± 0.00 d |
| RB | 6.08 ± 0.16 d | 10.34 ± 0.06 c | 0.48 ± 0.08 b | 16.89 ± 0.30 d | 5.21 ± 0.31 d | 1.76 ± 0.12 ab | 6.97 ± 0.43 d | 33.67 ± 0.20 de | 60 | 0.31 ± 0.00 c | 0.17 ± 0.00 bc |
| SM | 4.94 ± 0.08 bc | 9.81 ± 0.14 b | 0.00 ± 0.00 a | 14.75 ± 0.06 b | 4.28 ± 0.30 c | 2.19 ± 0.01 c | 6.47 ± 0.31 c | 33.28 ± 0.83 d | 60 | 0.29 ± 0.01 b | 0.16 ± 0.01 b |
| Urea | 5.21 ± 0.59 c | 10.61 ± 0.25 c | 0.02 ± 0.01 a | 15.83 ± 0.86 c | 4.51 ± 0.40 c | 2.66 ± 0.06 d | 7.17 ± 0.33 d | 34.56 ± 0.35 e | 60 | 0.31 ± 0.01 c | 0.18 ± 0.01 c |
| (NH4)2SO4 | 4.96 ± 0.02 bc | 8.54 ± 0.04 a | 0.85 ± 0.04 c | 14.35 ± 0.10 ab | 5.52 ± 0.02 d | 2.19 ± 0.01 c | 7.70 ± 0.03 e | 32.36 ± 0.24 c | 60 | 0.26 ± 0.00 a | 0.14 ± 0.00 a |
| Run No. | Real Value | Response (Butanol Concentration, g/L) | |||
|---|---|---|---|---|---|
| A | B | C | Experimental Value | Predicted Value | |
| 1 | 45 | 3.06 a | 8 | 11.00 ± 0.35 | 10.57 |
| 2 | 65 | 0 | 8 | 10.11 ± 0.17 | 9.60 |
| 3 | 25 | 6.12 b | 8 | 7.93 ± 0.06 | 8.67 |
| 4 | 65 | 6.12 | 8 | 11.50 ± 0.28 | 11.79 |
| 5 | 45 | 3.06 | 8 | 10.32 ± 0.33 | 10.57 |
| 6 | 65 | 3.06 | 4 | 9.79 ± 0.04 | 10.16 |
| 7 | 25 | 3.06 | 12 | 7.81 ± 0.05 | 7.45 |
| 8 | 65 | 3.06 | 12 | 10.06 ± 0.23 | 10.29 |
| 9 | 45 | 3.06 | 8 | 10.35 ± 0.30 | 10.57 |
| 10 | 45 | 6.12 | 4 | 11.01 ± 0.03 | 10.32 |
| 11 | 45 | 0 | 12 | 7.71 ± 0.11 | 8.54 |
| 12 | 45 | 6.12 | 12 | 10.75 ± 0.03 | 10.39 |
| 13 | 45 | 3.06 | 8 | 11.10 ± 0.45 | 10.57 |
| 14 | 25 | 0 | 8 | 7.35 ± 0.10 | 7.06 |
| 15 | 25 | 3.06 | 4 | 7.29 ± 0.04 | 7.34 |
| 16 | 45 | 0 | 4 | 7.89 ± 0.10 | 8.38 |
| 17 | 45 | 3.06 | 8 | 10.50 ± 0.15 | 10.57 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 31.04 | 9 | 3.45 | 7.24 | 0.0081 | significant |
| A: Initial sugar | 15.88 | 1 | 15.88 | 33.31 | 0.0007 | |
| B: DSY | 7.32 | 1 | 7.32 | 15.35 | 0.0058 | |
| C: CaCO3 | 0.029 | 1 | 0.029 | 0.060 | 0.8129 | |
| AB | 0.087 | 1 | 0.087 | 0.18 | 0.6820 | |
| AC | 1.00 × 10−4 | 1 | 1.00 × 10−4 | 2.10 × 10−4 | 0.9888 | |
| BC | 1.60 × 10−3 | 1 | 1.60 × 10−3 | 3.36 × 10−3 | 0.9554 | |
| A2 | 3.75 | 1 | 3.75 | 7.86 | 0.0264 | |
| B2 | 0.50 | 1 | 0.50 | 1.04 | 0.3416 | |
| C2 | 2.80 | 1 | 2.80 | 5.88 | 0.0458 | |
| Residual | 3.34 | 7 | 0.48 | |||
| Lack of Fit | 2.77 | 3 | 0.92 | 6.55 | 0.0505 | not significant |
| Pure Error | 0.56 | 4 | 0.14 | |||
| Cor Total | 34.38 | 16 |
| Container | Medium | PB (g/L) | QB (g/L·h) | YB/S (g/g) | t (h) |
|---|---|---|---|---|---|
| 1-L screw-capped bottle | P2 medium | 10.47 ± 0.12 a | 0.22 ± 0.00 a | 0.34 ± 0.00 a | 48 |
| Molasses with no supplement * | 8.53 ± 0.44 b | 0.14 ± 0.01 b | 0.31 ± 0.01 a | 60 | |
| Molasses under optimum condition ** | 11.38 ± 0.02 c | 0.32 ± 0.00 c | 0.40 ± 0.01 b | 36 | |
| 2-L stirred-tank reactor (STR) | Molasses under optimum condition ** | 11.42 ± 0.03 c | 0.24 ± 0.00 d | 0.40 ± 0.01 b | 48 |
| Substrate | Microorganism | Nutrient Supplement | Butanol | Reference | |
|---|---|---|---|---|---|
| PB (g/L) | QB (g/L·h) | ||||
| Pretreated molasses (60 g/L of sugar) | C. beijerinckii L175 | Urea, 3 g/L (NH4)2SO4, 3 g/L | 6.60 7.70 | - - | [5] |
| Sugarcane molasses (60 g/L of sugar) | C. saccharobutylicum DSM 13864 | CaCO3, 3.2 g/L; (NH4)2SO4, 2 g/L; K2HPO4, 0.5 g/L; corn steep powder, 10 g/L and MnSO4·H2O, 0.01 g/L | 11.86 | 0.33 | [40] |
| Sugarcane molasses (50 g/L of sugar) | C. beijerinckii TISTR 1461 | Dried spent yeast, 6 g/L and CaCO3, 6.6 g/L | 11.38 | 0.32 | This study |
| Corn straw hydrolysate (42–44 g/L of sugar) | C. acetobutylicum CICC 8008 | CaCO3, 5 g/L | 6.20 | - | [10] |
| (a) Cassava (65%–70% w/w) (b) Corn (50% w/w) | C. acetobutylicum ATCC 824 | Yeast extract, 2.5 g/L | 10.56 9.57 | 0.24 0.20 | [41] |
| Cheese whey (55 g/L) | C. acetobutylicum ATCC 824 | Yeast extract, 5 g/L; CaCO3, 18 g/L and FeSO4·7H2O, 20 mg/L | 7.13 | - | [42] |
| Xylose (70 g/L) | C. acetobutylicum L7 | P2 and ZnSO4·7H2O, 0.001 g/L | 8.30 | 0.10 | [43] |
| Sugarcane bagasse hydrolysate (50 g/L) | C. acetobutylicum DSM 6228 | Yeast extract, 1 g/L; MnSO4, 0.01 g/L; MgSO4, 0.2 g/L; KH2PO4, 0.5 g/L; K2HPO4, 0.5 g/L; sodium acetate, 0.01 g/L and FeSO4·7H2O, 0.01 g/L | 9.10 | 0.25 | [44] |
| System | Initial Sugar (g/L) | Sugar Utilized (g/L) | SC (%) | Acetone (g/L) | Butanol (g/L) | Ethanol (g/L) | ABE (g/L) | YB/S (g/g) | QB (g/L·h) |
|---|---|---|---|---|---|---|---|---|---|
| Without gas stripping | 53.25 ± 0.11 | 28.88 ± 0.47 | 54.23 ± 0.47 | 3.63 ± 0.33 | 11.42 ± 0.04 | 0 | 15.05 ± 0.37 | 0.40 | 0.24 |
| With gas stripping | |||||||||
| …: Condensate | - | - | - | 0.63 ± 0.06 | 107 ± 0.10 | 0 | 107.63 ± 0.08 | - | - |
| …: Fermenter | 52.05 ± 0.57 | 38.98 ± 0.30 | 74.89 ± 0.30 | 3.57 ± 0.21 | 9.39 ± 0.14 | 0 | 12.96 ± 0.18 | 0.39 | 0.21 |
| …: Total | 52.05 ± 0.57 | 38.98 ± 0.30 | 74.89 ± 0.30 | 4.08 ± 0.11 | 15.33 ± 0.25 | 0 | 19.46 ± 0.37 | 0.39 | 0.21 |
| Substrate | Strain | Initial Sugar (g/L) | PB (g/L) | QB (g/L·h) | YB/S (g/g) | Reference |
|---|---|---|---|---|---|---|
| Glucose | C. beijerinckii BA101 | 60 | 16.4 | 0.42 | 0.27 | [45] |
| Saccharified liquefied cornstarch | C. beijerinckii BA101 | 64.3 | 17.6 | 0.26 | 0.27 | [2] |
| Wood pulping hydrolysate | C. beijerinckii CC101 | 42 | 9.38 | 0.13 | 0.29 | [46] |
| Glucose | C. acetobutylicum JB200 | 80 | 19.8 | 0.41 | 0.25 | [17] |
| Glucose | C. beijerinckii P260 | 62 | 13.89 | 0.19 | 0.25 | [47] |
| Sugarcane juice | C. acetobutylicum ABE1201 | 60 | 13.98 | 0.18 | 0.24 | [14] |
| Sugarcane molasses | C. beijerinckii TISTR 1461 | 52 | 15.33 | 0.21 | 0.39 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narueworanon, P.; Laopaiboon, L.; Phukoetphim, N.; Laopaiboon, P. Impacts of Initial Sugar, Nitrogen and Calcium Carbonate on Butanol Fermentation from Sugarcane Molasses by Clostridium beijerinckii. Energies 2020, 13, 694. https://doi.org/10.3390/en13030694
Narueworanon P, Laopaiboon L, Phukoetphim N, Laopaiboon P. Impacts of Initial Sugar, Nitrogen and Calcium Carbonate on Butanol Fermentation from Sugarcane Molasses by Clostridium beijerinckii. Energies. 2020; 13(3):694. https://doi.org/10.3390/en13030694
Chicago/Turabian StyleNarueworanon, Patthranit, Lakkana Laopaiboon, Niphaphat Phukoetphim, and Pattana Laopaiboon. 2020. "Impacts of Initial Sugar, Nitrogen and Calcium Carbonate on Butanol Fermentation from Sugarcane Molasses by Clostridium beijerinckii" Energies 13, no. 3: 694. https://doi.org/10.3390/en13030694
APA StyleNarueworanon, P., Laopaiboon, L., Phukoetphim, N., & Laopaiboon, P. (2020). Impacts of Initial Sugar, Nitrogen and Calcium Carbonate on Butanol Fermentation from Sugarcane Molasses by Clostridium beijerinckii. Energies, 13(3), 694. https://doi.org/10.3390/en13030694




