Sulfur Conversion of Mixed Coal and Gangue during Combustion in a CFB Boiler
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal Quality Analysis of Design Coal
2.2. Thermodynamic Equilibrium Calculation of Sulfur-Containing Substances
2.2.1. Mechanism of Sulfur Evolution during Coal Combustion
2.2.2. Model Simplification and Simulation of Coal
2.3. Experimental Design on SO2 Emission and Fixation Characteristics
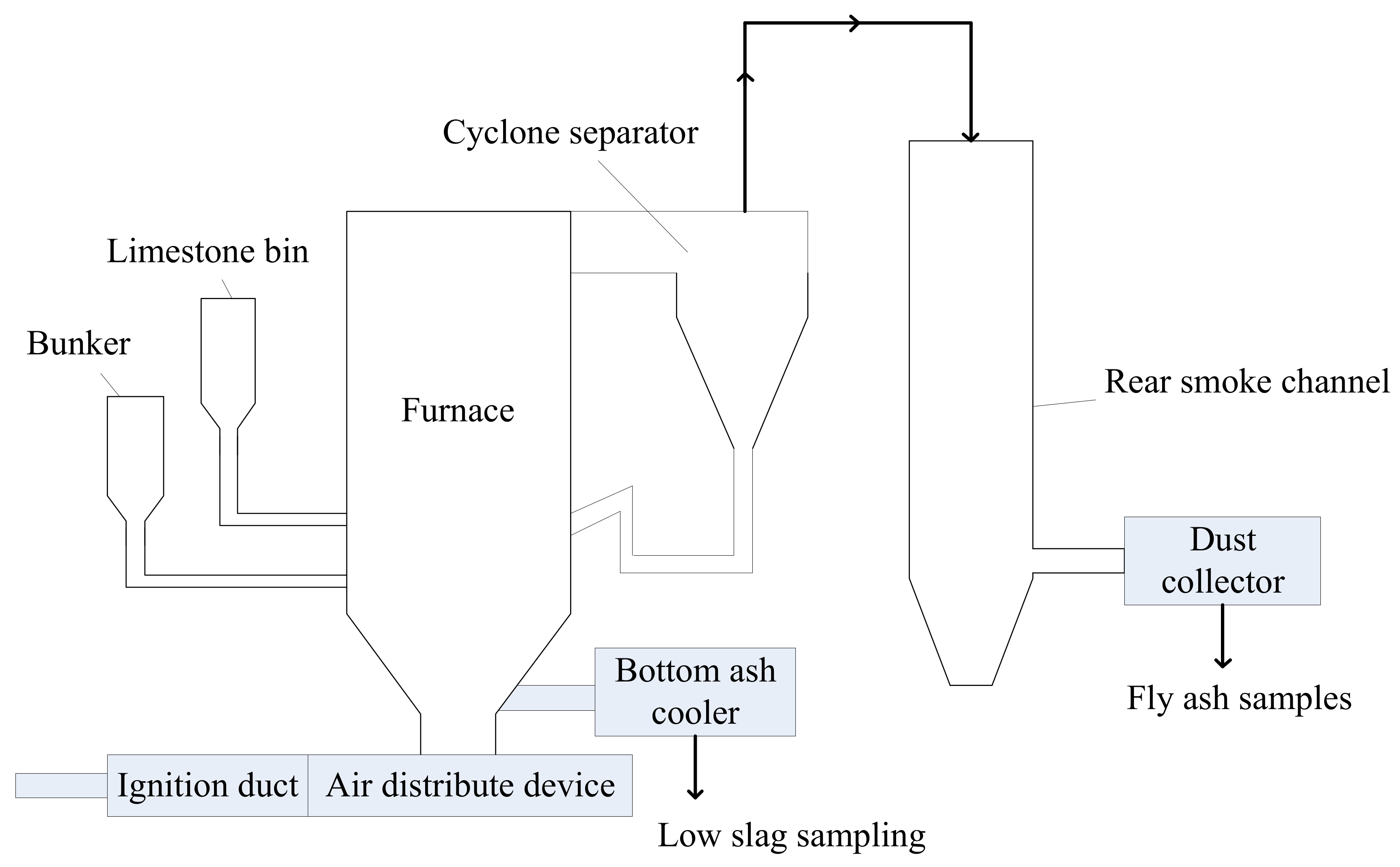
2.4. Experimental Study of Sulfur Conversion Process Based on 3 MWth Test Bench
3. Results & Discussion
3.1. Calculation of Oxidizing Atmosphere
3.1.1. Distribution and Transformation of Sulfur-Containing Phases
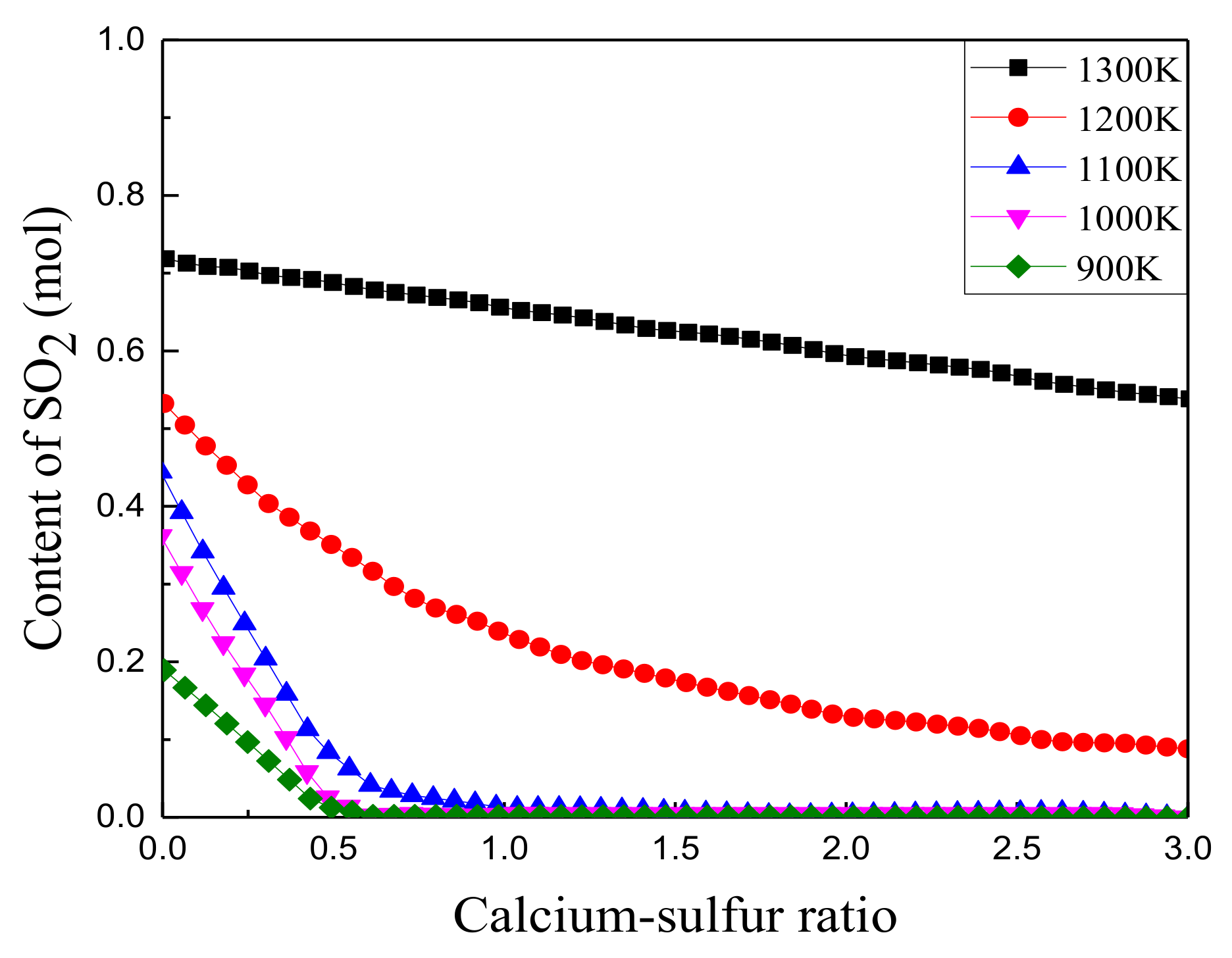
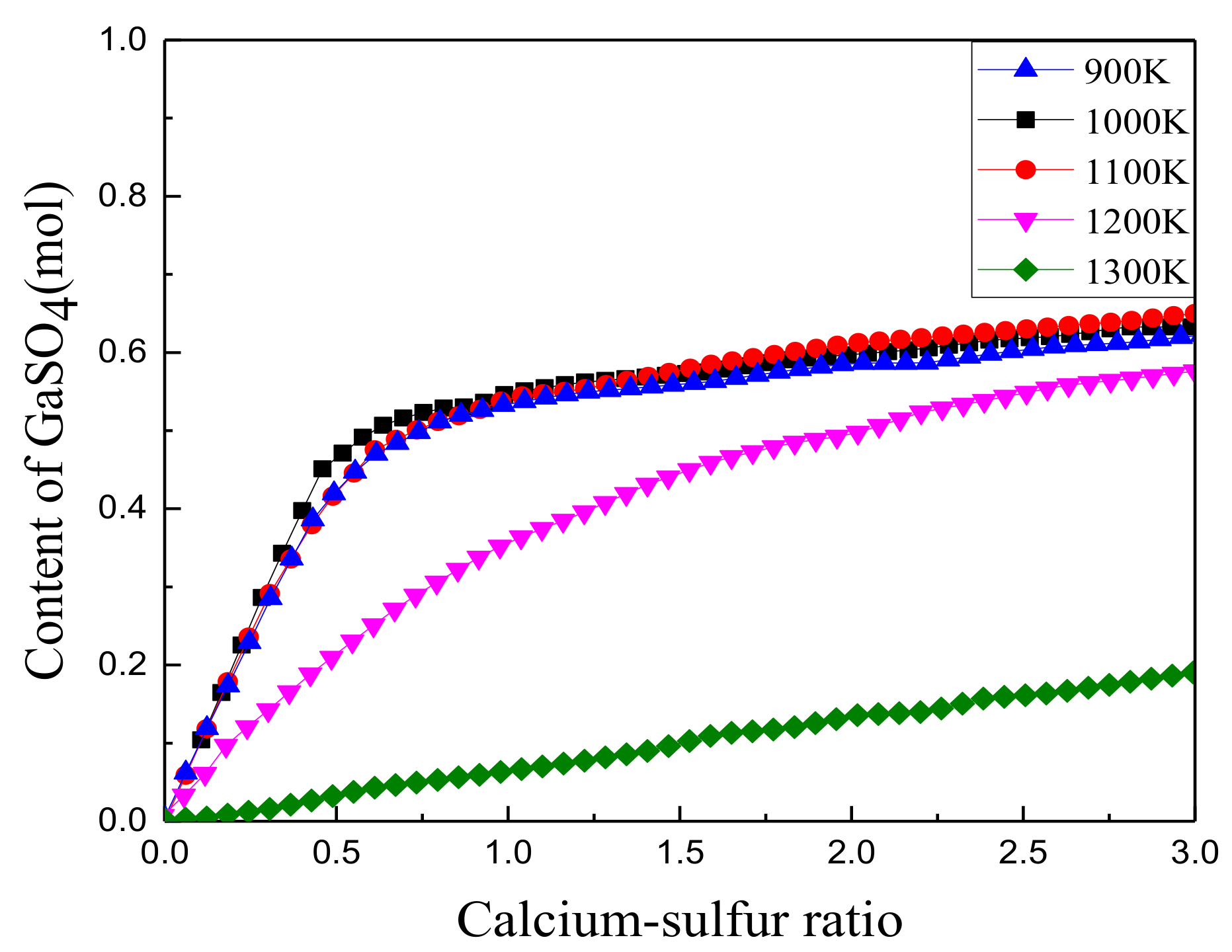
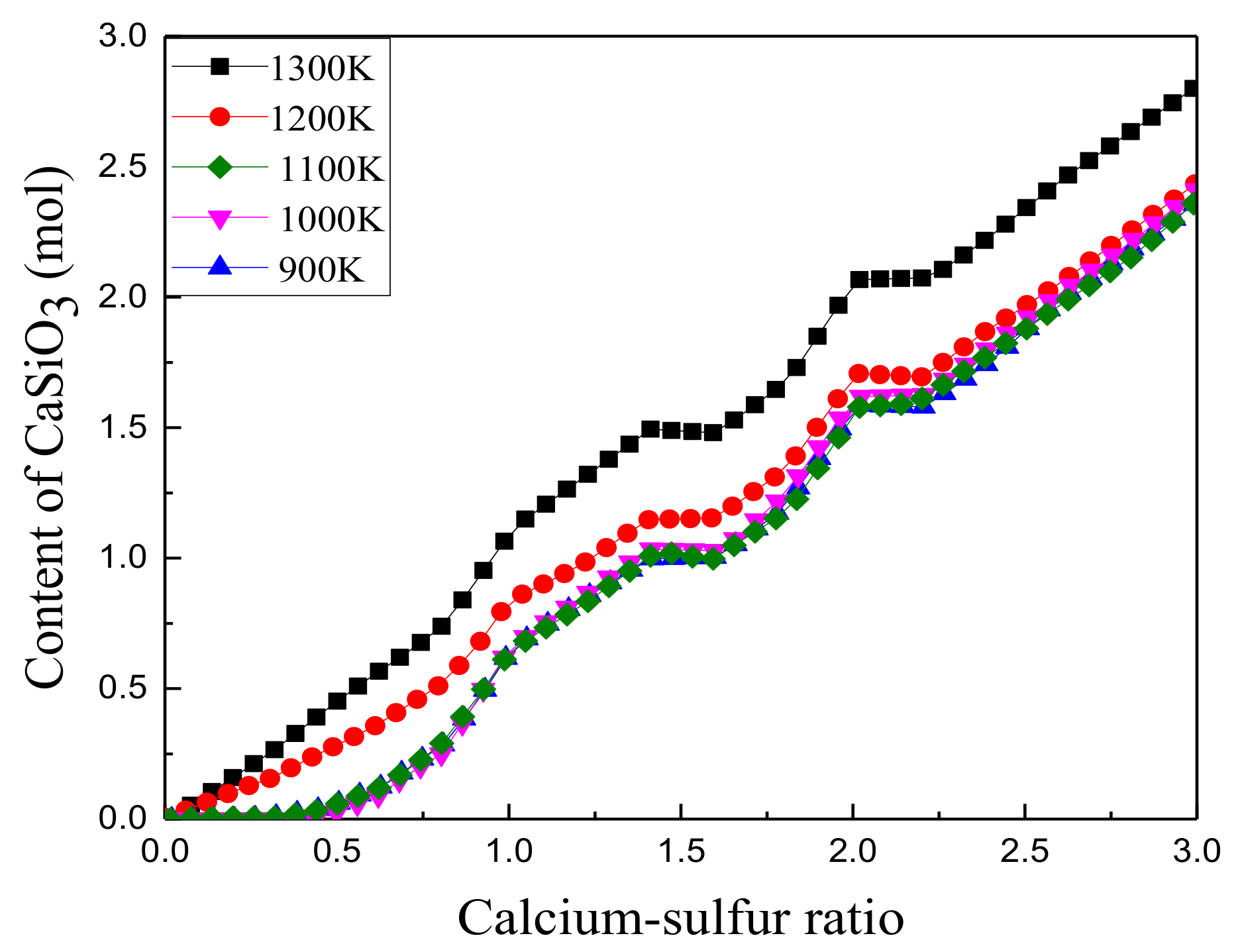
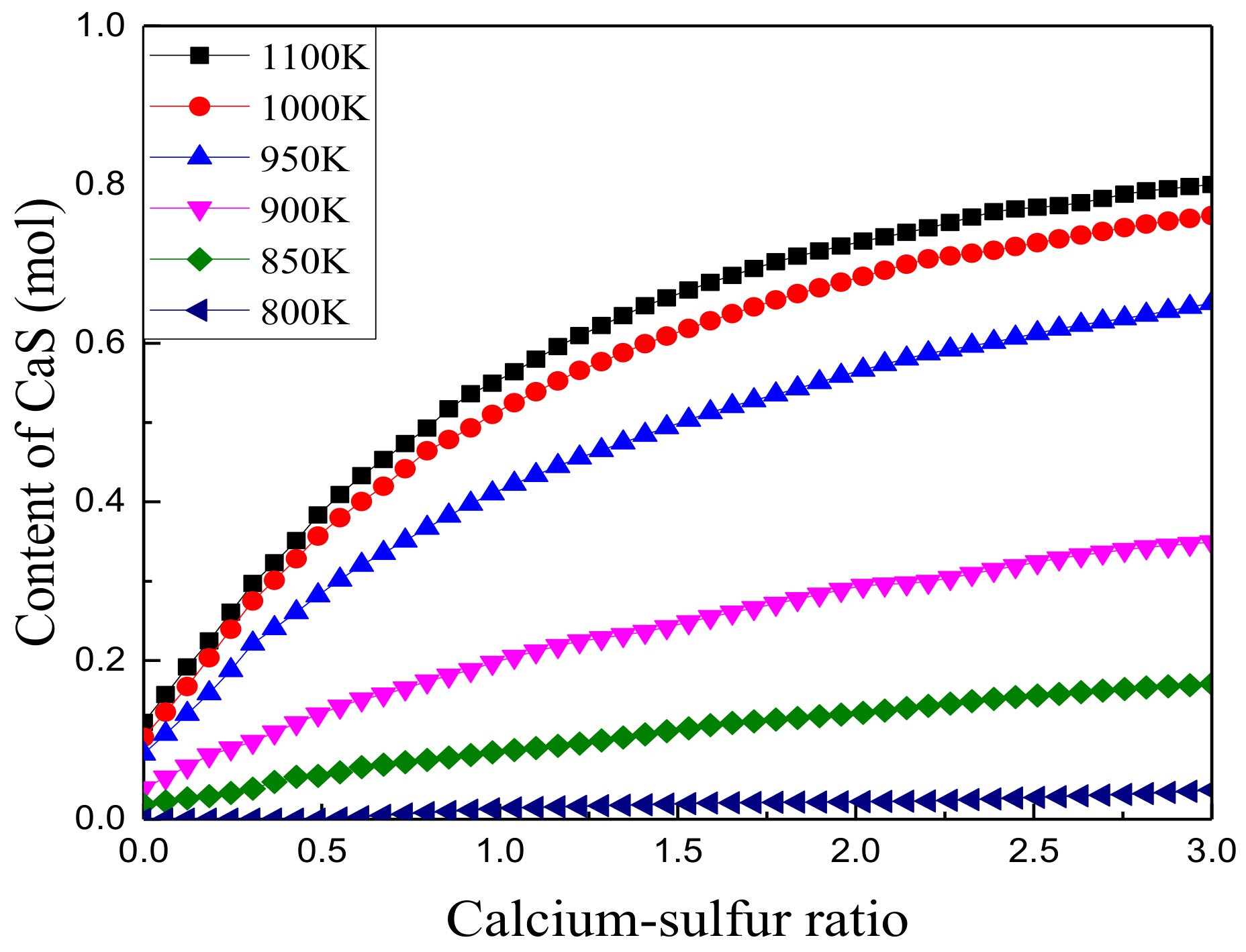
3.1.2. Effect of Calcium to Sulfur Ratio on Sulfur Fixation
3.2. Calculation of Reducing Atmosphere
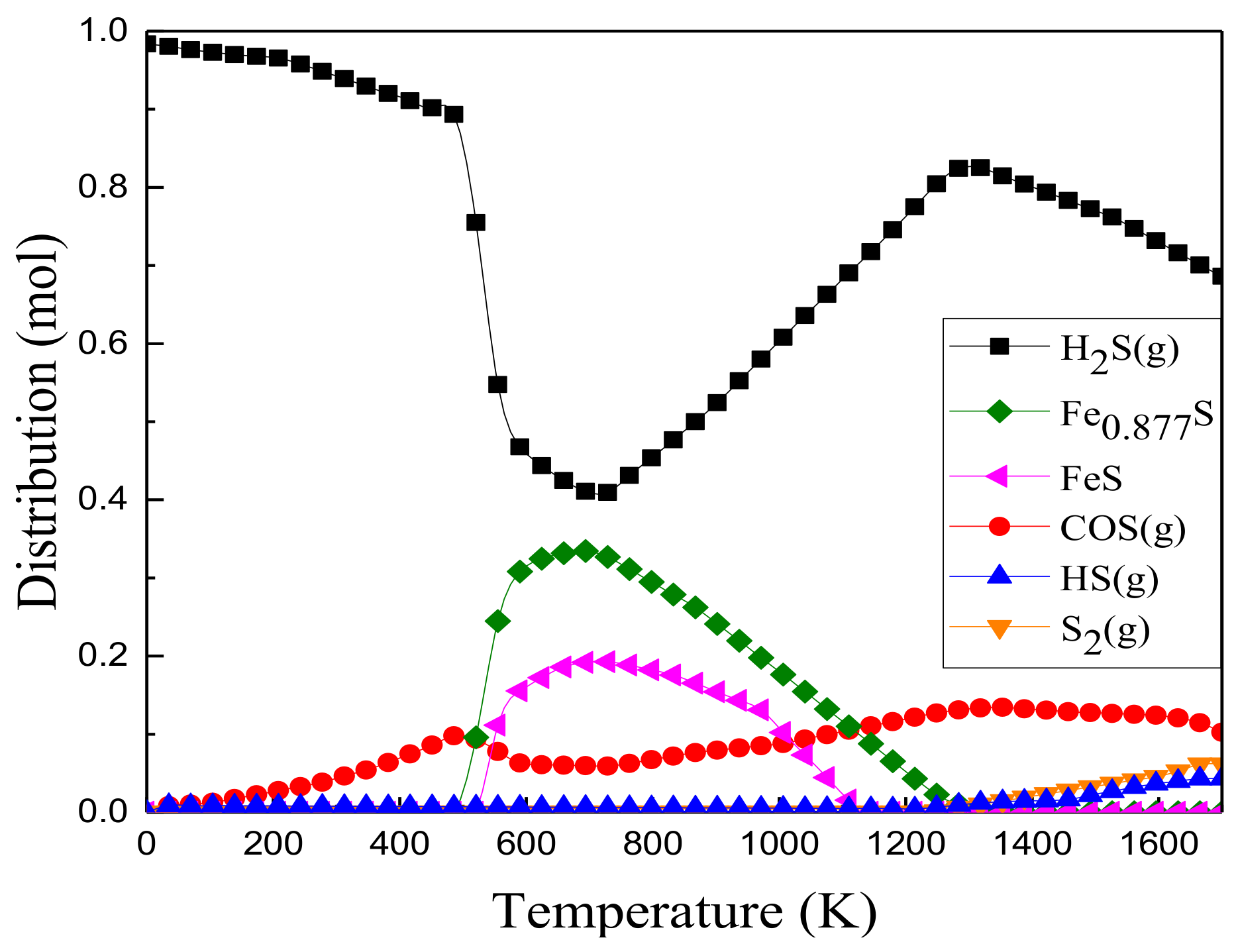
3.2.1. Distribution of Sulfur-Containing Phases
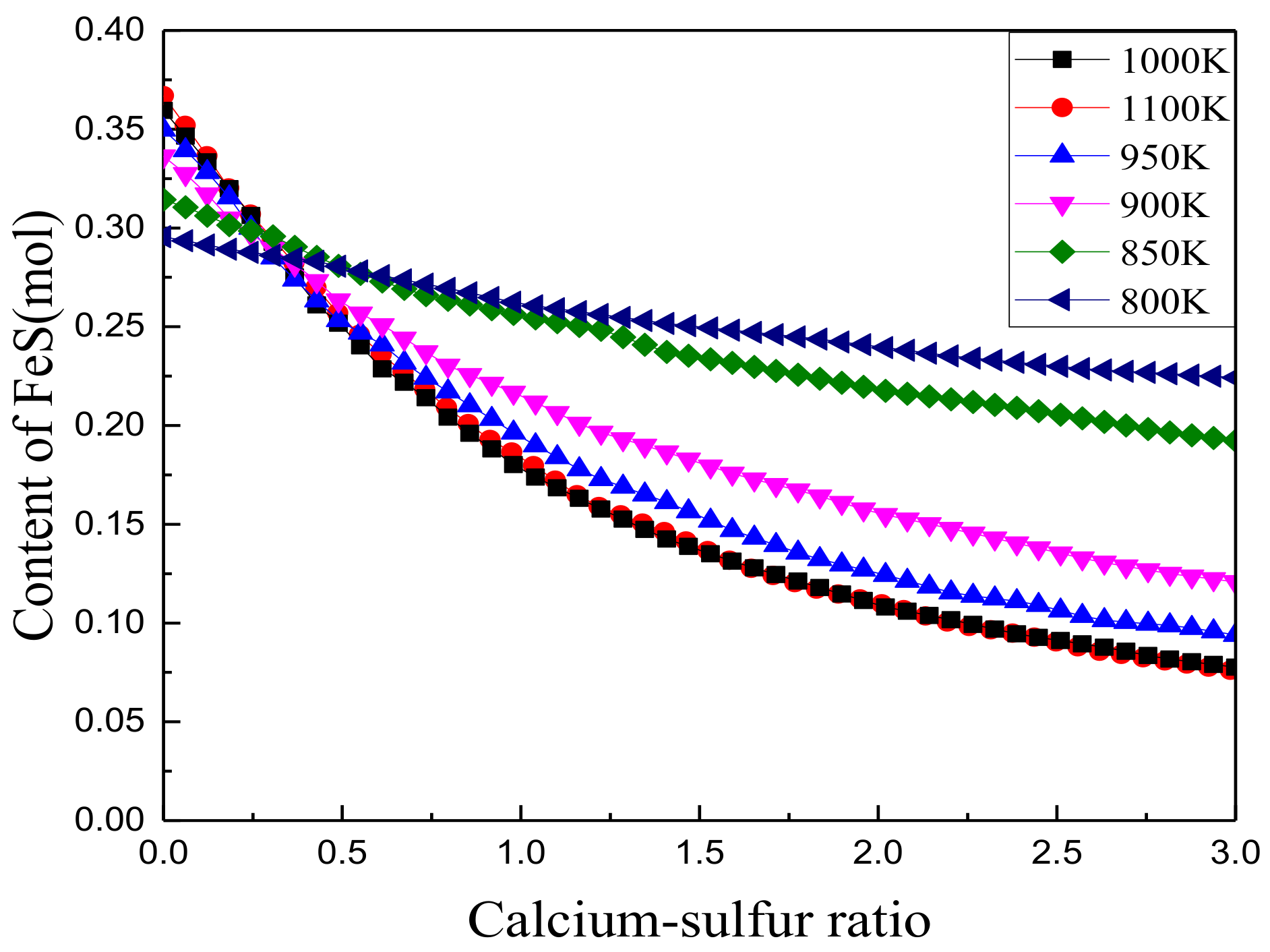
3.2.2. Effect of Iron Oxides
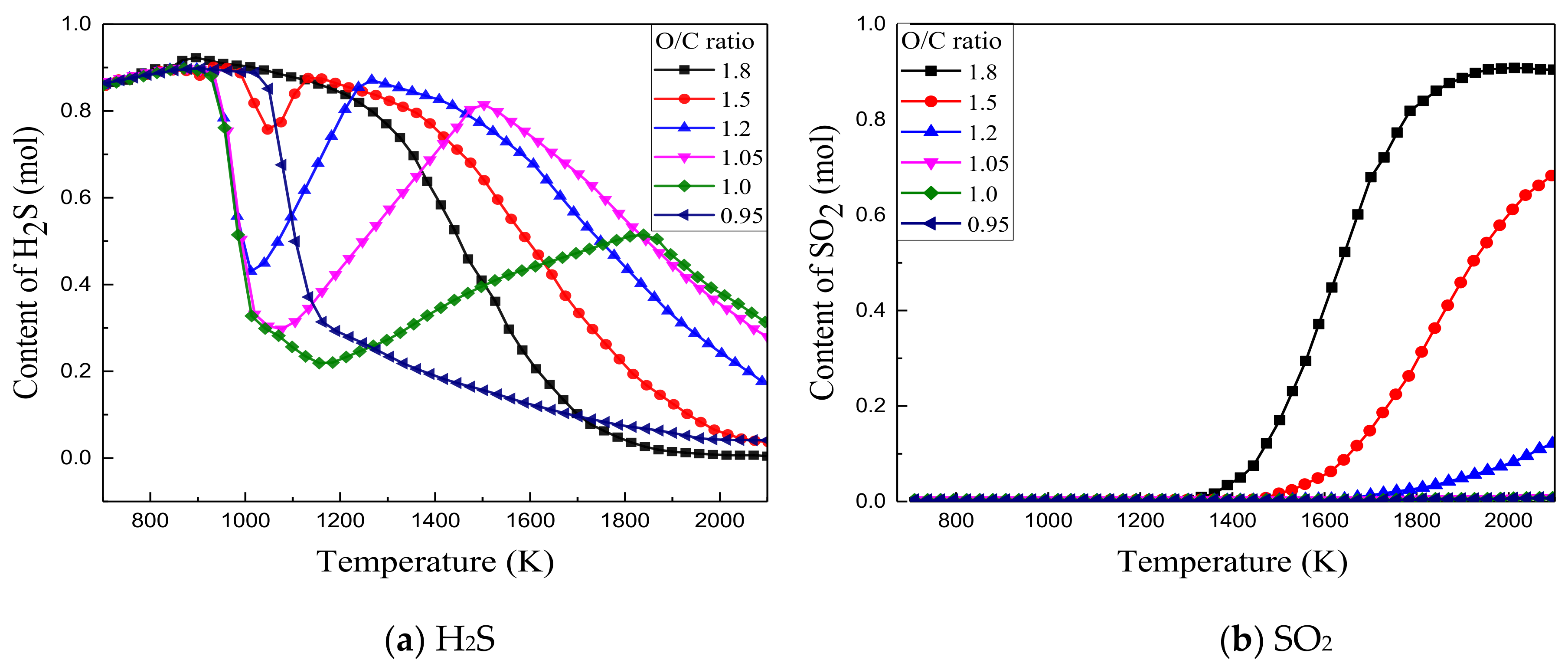
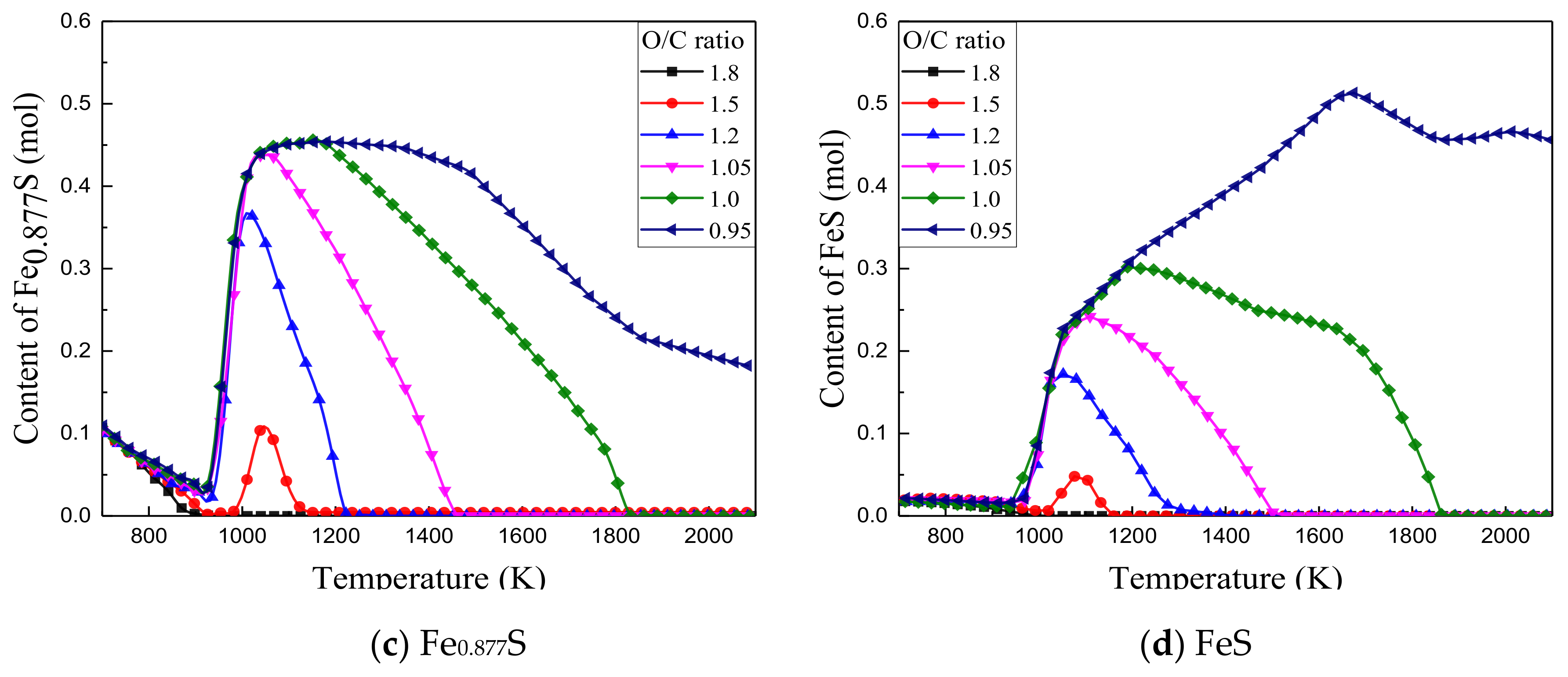
3.2.3. Effect of O/C
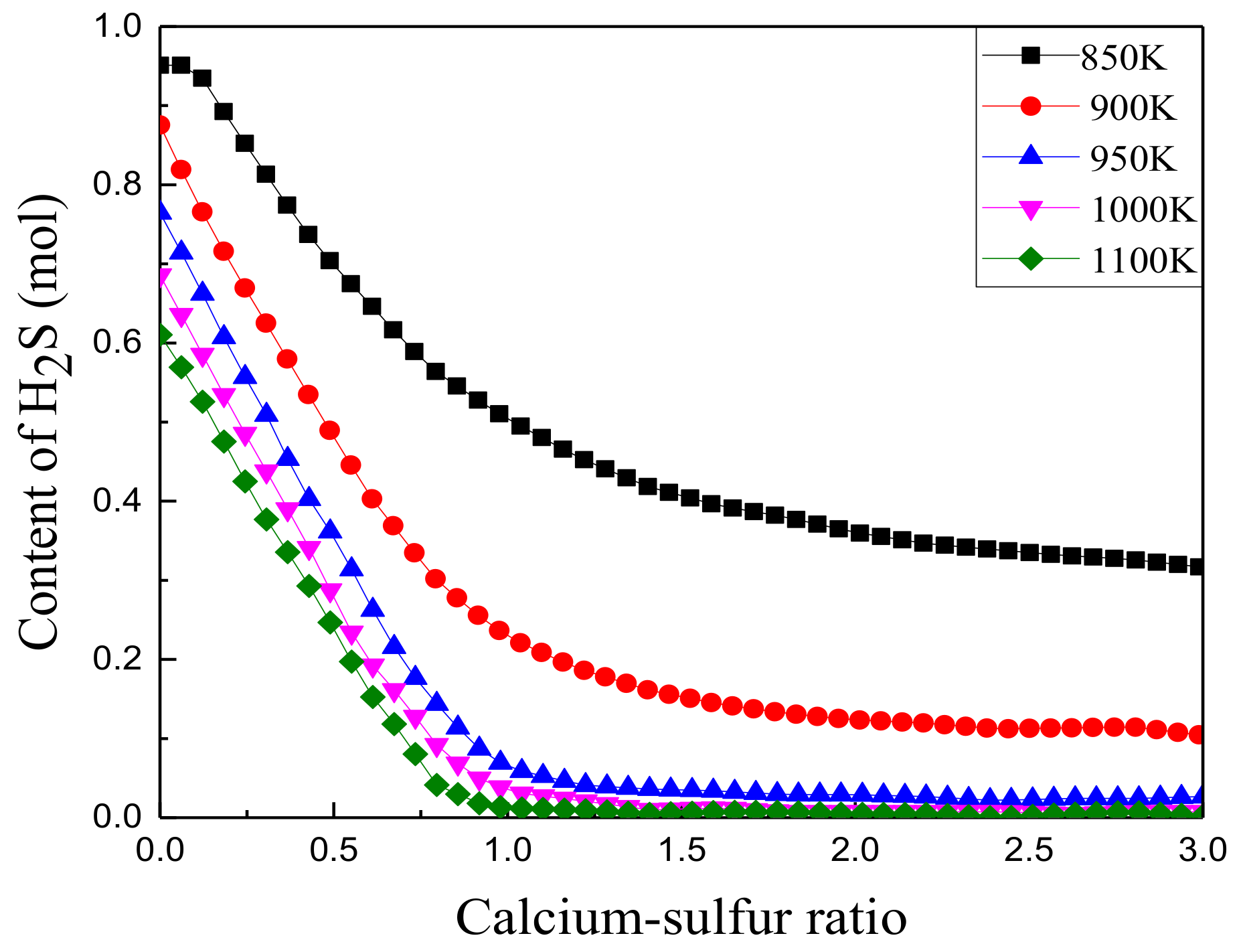
3.2.4. Effect of Calcium/Sulfur Ratio on Sulfur Fixation
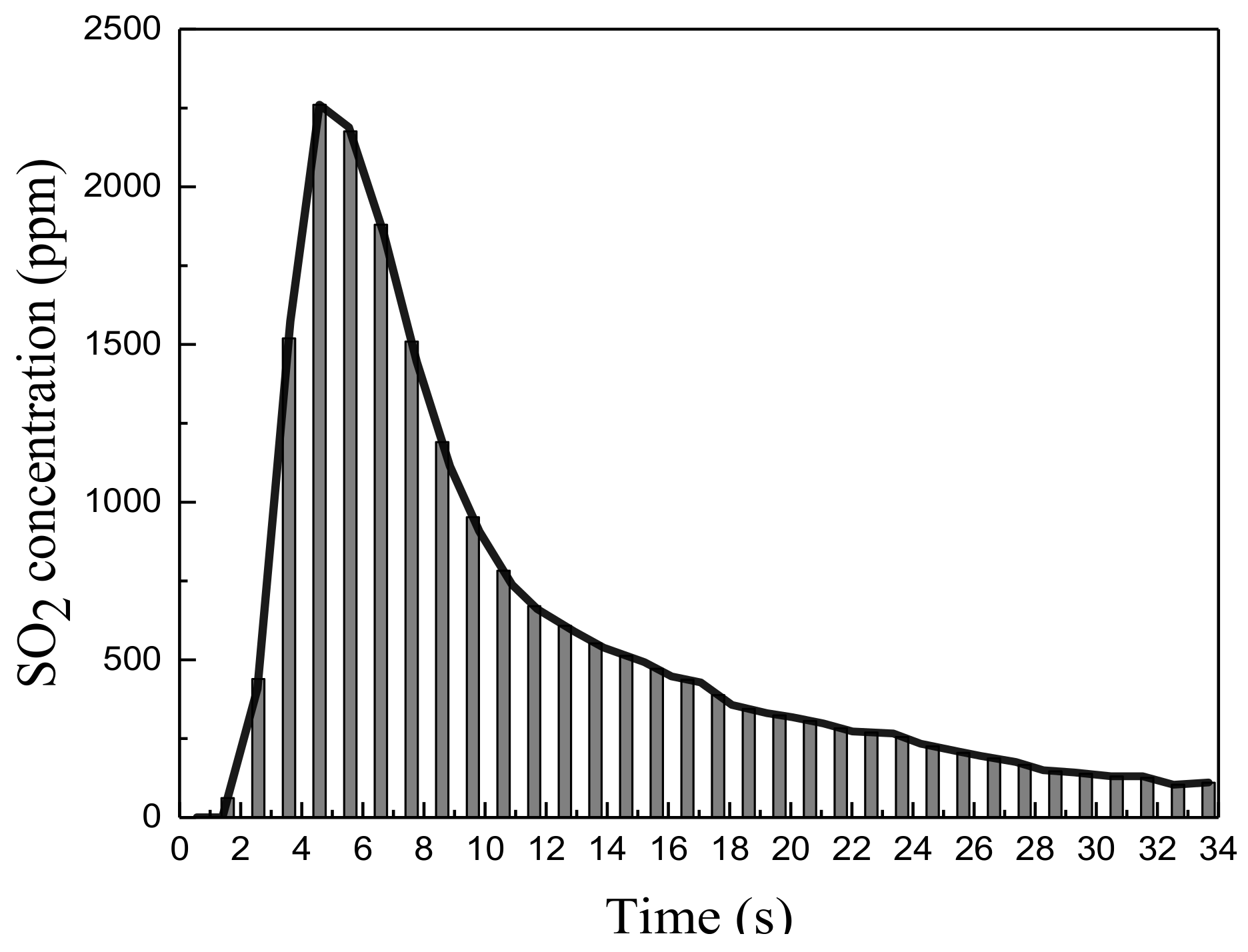
3.3. Analysis of Experimental Results
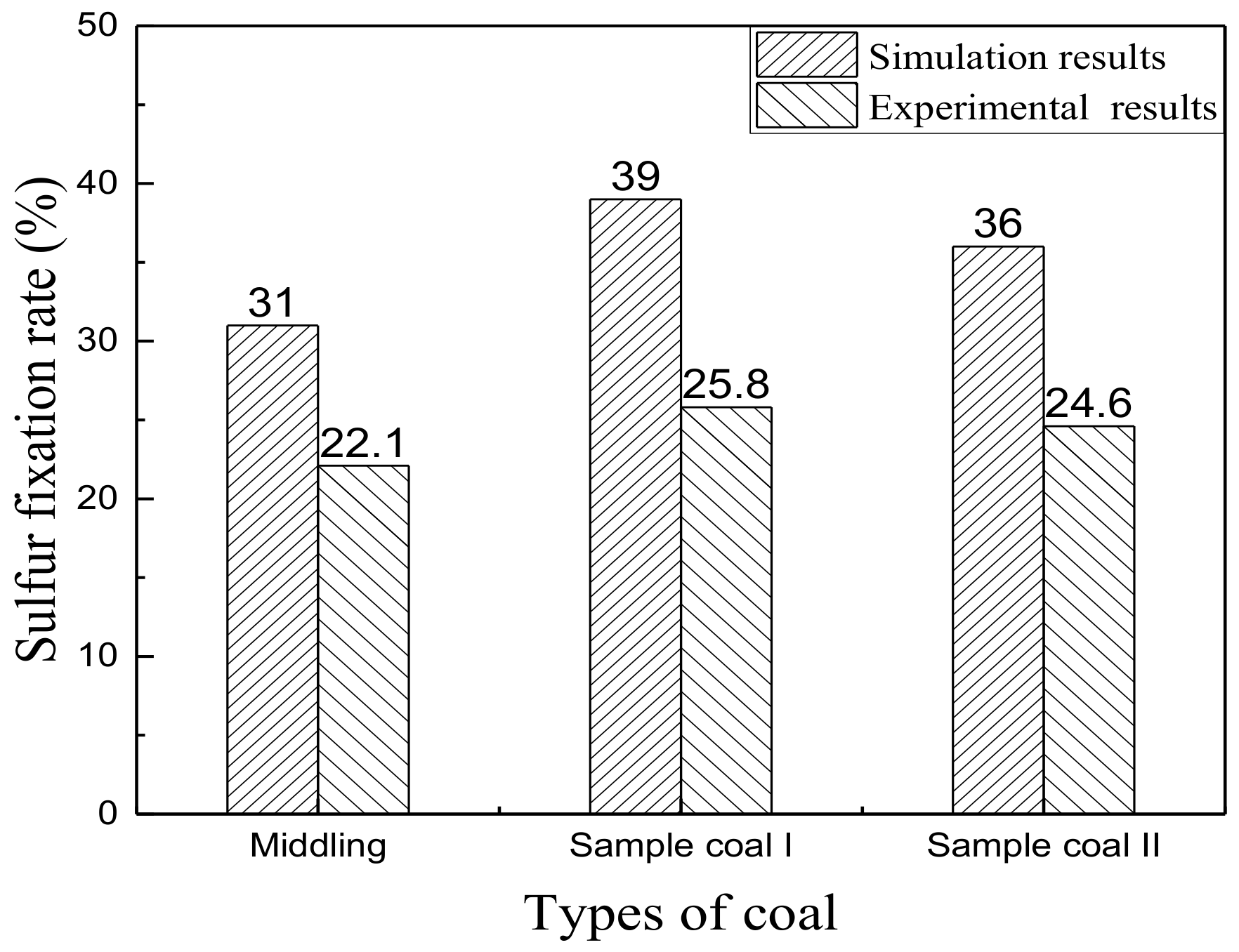
3.4. Literature Verification of Simulation Results
3.5. Experimental Analysis Based on 3 MWth Test Bench
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ren, A.P. The Planning of Low Calorific Value Coal Source in Shanxi Province and Its Influence on Shanxi Power Grid. Shanxi Electr. Power 2015, 47, 47–49. [Google Scholar]
- Wang, X.L. Research on comprehensive power generation utilization of energy-saving technology application for low calorific value coal based upon the low calorific value coal power generation project in Yonglong mining area. China Coal 2019, 45, 82–86. [Google Scholar]
- Li, C.; Zhao, L.; Ren, Q. Status analysis and development discussion of coal gangue comprehensive utilization. Environ. Dev. 2015, 27, 72–74. [Google Scholar]
- Liang, Y.S. Study on Present Situation and Progress of Coal Gangue Resource Utilization. Energy Energy Conserv. 2019, 72, 72–73. [Google Scholar]
- Tan, Q.Y.; Peng, F.; Peng, H.W.; Tang, X.S. Optimization Design of Two-Stage Desulfurization System for CFB Boilers. Electr. Power Constr. 2014, 35, 89–94. [Google Scholar]
- Jin, D.P.; Wang, M.Y.; Song, W.X. Research on Deep Desulfurization Process of CFB Boiler Tail Humidification and Activation. Power Syst. Eng. 2014, 30, 41–43. [Google Scholar]
- Weng, A.X.; Zhou, H.; Wang, Z.H.; Chi, Z.H.; Jiang, X.; Cen, K.F. Experimental Study on Formation of SO2 of Blending Coal Combustion in High Temperature. Boil. Technol. 2003, 34, 1–4. [Google Scholar]
- Yu, J.L.; Lucas, J.; Wall, T.; Liu, G.; Sheng, C.D. Modeling the development of char structure during the rapid heating of pulverized coal. Combust. Flame 2004, 136, 519–532. [Google Scholar] [CrossRef]
- Engin, B.; Atakul, H.; Unlu, A.; Olgun, Z. CFB combustion of low-grade lignites: Operating stability and emissions. J. Energy Inst. 2019, 92, 542–553. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, J.M. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Yrjas, P.; Iisa, K.; Hupa, M. Comparison of SO2 capture capacities of limestones and dolomites under pressure. Fuel 1995, 74, 359–400. [Google Scholar] [CrossRef]
- Dong, X.W.; Wang, H.; Liu, H.; Qin, J.R. Study on SO2 emission under various atmospheres during coal combustion. Acta Sci. Circumstantiae 2003, 23, 322–326. [Google Scholar]
- Shi, Y.; Zhong, W.Q.; Shao, Y.J.; Liu, X.J. Energy efficiency analysis of pressurized oxy-coal combustion system utilizing circulating fluidized bed. Appl. Therm. Eng. 2019, 150, 1104–1115. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, W.; Xu, M.X.; Wang, X.; Li, H.Y.; Lu, Q.G. The experimental study on nitrogen oxides and SO2 emission for oxy-fuel circulation fluidized bed combustion with high oxygen concentration. Fuel 2015, 146, 81–87. [Google Scholar] [CrossRef]
- Jia, L.; Tan, Y.; McCalden, D.; Wu, Y.; He, I.; Symonds, R.; Anthony, E.J. Commissioning of a 0.8 MWth CFBC for oxy-fuel combustion. Int. J. Greenh. Gas Control 2012, 7, 59–69. [Google Scholar] [CrossRef]
- Jia, L.; Tan, Y.; Anthony, E.J. Emissions of SO2 and NOx during Oxy-Fuel CFB Combustion Tests in a Mini-Circulating Fluidized Bed Combustion Reactor. Energy Fuels 2010, 24, 910–915. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B. Sulphur capture in fluidized bed boilers: The effect of reductive decomposition of CaSO4. Chem. Eng. Sci. 1989, 40, 1131–1141. [Google Scholar] [CrossRef]
- Belin, E. Babcock & Wilcox CFB boilers—Design and experience. Fuel Energy Abstr. 2002, 43, 272. [Google Scholar]
- Liu, D.B.; Li, W.; Li, S.Y.; Song, W.H.; Liu, D.F.; Kong, R.J. Transformation characteristics of sodium, chlorine and sulfur of Zhundong coal during O2/CO2 combustion in circulating fluidized bed. Energy 2019, 185, 254–261. [Google Scholar] [CrossRef]
- Chen, G.B.; Chatelier, S.; Lin, H.T.; Wu, F.H.; Lin, T.H. A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate. Energies 2018, 11, 3436. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.Z.; Qin, J.R.; Zheng, C.G. Experimental study on the desulfurization of calcium-based sorbent in CO2 enriched environment. J. Eng. Thermophys. 2001, 22, 374–377. [Google Scholar]
- Wang, H.; Zhang, L.Z.; Lu, X.H.; Zhen, C.G. Microstructure changes of calcium-based sorbent during desulfurization in O2/CO2 mixture. J. Eng. Thermophys. 2001, 22, 127–129. [Google Scholar]
- Wang, H.; Qiu, J.R.; Zheng, C.G. SO2 formation characteristics of coal in O2/CO2 mixture. J. Huazhong Univ. Sci. Technol. 2002, 30, 100–102. [Google Scholar]
- Zhang, Z.L.; Zeng, Q.D. Numerical simulation and experimental analysis on nitrogen and sulfur oxides emissions during the co-combustion of Longyan anthracite and sawmill sludge. Fuel 2019, 254, 115611. [Google Scholar] [CrossRef]
- Yang, N.N.; Guo, H.Q.; Liu, F.R.; Zhang, H.; Hu, Y.F.; Hu, R.S. Effects of atmospheres on sulfur release and its transformation behavior during coal thermolysis. Fuel 2018, 215, 446–453. [Google Scholar] [CrossRef]
- Hou, J.L.; Ma, Y.; Li, S.Y.; Shi, J.; He, L.; Li, J. Transformation of sulfur and nitrogen during Shenmu coal pyrolysis. Fuel 2018, 231, 134–144. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cheng, C.F.; Chen, C. Characteristics of polycyclic aromatic hydrocarbon release during spontaneous combustion of coal and gangue in the same coal seam. J. Loss Prev. Process Ind. 2018, 55, 392–399. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Q.; Luo, Z.; Eddings, E.; Ring, T.; Li, S.; Lin, J.; Xue, S.; Han, L.; Xie, G. Experimental and numerical investigation on sulfur transformation in pressurized oxy-fuel combustion of pulverized coal. Appl. Energy 2019, 253, 113542. [Google Scholar] [CrossRef]
- Xu, L.J.; Cheng, L.M.; Ji, J.Q.; Wang, Q.H.; Fang, M.X. A comprehensive CFD combustion model for supercritical CFB boilers. Particuology 2019, 43, 29–37. [Google Scholar] [CrossRef]
- Xiao, P.; Sun, X.B.; Xu, Z.Q. Test and study on selfdesulfuration behavior of coal sorts in 1 MWth CFB combustion test stand. Therm. Power Gener. 2004, 42, 30–33. [Google Scholar]
- Lu, Q.G.; Yong, Y.M.; Na, Y.J.; Bao, S.L.; Sun, Y.K.; He, J.; Jiao, W.H.; Gao, M. Influence of coal on SO2 and NOx emissions of circulating fluidized bed combustion. Proc. CSEE 2005, 25, 145–149. [Google Scholar]
- Zhao, P.B.; Wang, P.L.; Wang, H.T.; Liu, K.F.; Han, Y. Desulfuration efficiency Test and Study on Different Particle Size. Inn. Mong. Electr. Power 2010, 28, 24–26. [Google Scholar]
- Adanez, J.; Gayán, P.; Grasa, G.; Diego, L.F.D.; Armesto, L.; Cabanillas, A. Circulating fluidized bed combustion in the turbulent regime: Modelling of carbon combustion efficiency and sulphur retention. Fuel 2001, 80, 1405–1414. [Google Scholar] [CrossRef]
- Niu, S.L.; Han, K.H.; Lu, C.M. Release of sulfur dioxide and nitric oxide and characteristic of coal combustion under the effect of calcium based organic compounds. Chem. Eng. J. 2011, 168, 255–261. [Google Scholar] [CrossRef]
- Hlincik, T.; Buryan, P. Desulfurization of boiler flue gas by means of activated calcium oxide. Fuel Process. Technol. 2013, 111, 62–67. [Google Scholar] [CrossRef]
- Shao, L.M.; Fan, S.S.; Zhang, H.; Yao, Q.S.; He, P.J. SO2 and NOx emissions from sludge combustion in a CO2/O2 atmosphere. Fuel 2013, 109, 178–183. [Google Scholar] [CrossRef]
- Ma, L.; Guo, A.L.; Fang, Q.Y.; Wang, T.X.; Zhang, C.; Chen, G. Combustion interactions of blended coals in an O2/CO2 mixture in a drop tube furnace: Experimental investigation and numerical simulation. Appl. Therm. Eng. 2018, 145, 184–200. [Google Scholar] [CrossRef]
- Yan, J.; Lu, X.; Wang, Q.; Kang, Y.; Li, J.; Xu, Z.; Lei, X.; Zheng, X.; Fan, X.; Liu, Z. Study on the influence of secondary air on the distributions of flue gas composition at the lower part of a 600 MW supercritical CFB boiler. Fuel Process. Technol. 2019, 196, 106035. [Google Scholar] [CrossRef]
- Yang, T.H.; Zhou, J.H.; Li, R.D.; Cen, K.F. Kinetics for formation of CaS as sulphur intermediate retention. Proc. CSEE 2006, 26, 104–108. [Google Scholar]
- Duan, Y.Q.; Duan, L.B.; Anthony, E.J.; Zhao, C.S. Nitrogen and sulfur conversion during pressurized pyrolysis under CO2 atmosphere in fluidized bed. Fuel 2017, 189, 98–106. [Google Scholar] [CrossRef]






| Elemental Analysis (%) | |||||
| Gangue I | Gangue II | Middling | Sample Coal I | Sample Coal II | |
| Car | 14.32 | 9.38 | 46.22 | 33.46 | 31.48 |
| Har | 2.06 | 1.7 | 3.24 | 2.77 | 2.62 |
| Oar | 11.67 | 10.38 | 16.93 | 14.83 | 14.31 |
| Nar | 0.54 | 0.35 | 0.89 | 0.75 | 0.67 |
| Sar | 1.65 | 2.49 | 1.40 | 1.5 | 1.84 |
| Aar | 63.5 | 70.3 | 25.79 | 40.87 | 43.59 |
| Analysis of ash composition (%) | |||||
| Gangue I | Gangue II | Middling | Sample coal I | Sample coal II | |
| SiO2 | 49.25 | 50.28 | 48.35 | 48.71 | 49.12 |
| Al2O3 | 37.98 | 39.25 | 36.28 | 36.96 | 37.47 |
| Fe2O3 | 6.90 | 4.59 | 4.64 | 5.54 | 4.62 |
| CaO | 1.07 | 1.27 | 1.69 | 1.44 | 1.52 |
| MgO | 0.16 | 0.12 | 0.95 | 0.63 | 0.62 |
| TiO2 | 2.27 | 2.31 | 1.53 | 1.83 | 1.84 |
| K2O | 0.48 | 0.25 | 0.78 | 0.66 | 0.57 |
| Na2O | 0.32 | 0.38 | 0.42 | 0.38 | 0.40 |
| Middling | Sample Coal I | Sample Coal II | |
|---|---|---|---|
| Ca/S | 0.178 | 0.224 | 0.206 |
| Mg/S | 0.140 | 0.138 | 0.117 |
| K/S | 0.098 | 0.123 | 0.092 |
| Na/S | 0.080 | 0.107 | 0.099 |
| Alkaline-oxide/S | 0.496 | 0.592 | 0.515 |
| Component (mol) | Middling | Sample Coal I | Sample Coal II |
|---|---|---|---|
| C | 176.08 | 118.97 | 91.46 |
| H | 74.06 | 59.05 | 45.73 |
| O | 24.19 | 19.79 | 15.59 |
| N | 2.91 | 2.29 | 1.69 |
| S | 1 | 1 | 1 |
| SiO2 | 9.50 | 14.16 | 12.44 |
| Al2O3 | 4.19 | 6.32 | 5.58 |
| Fe2O3 | 0.34 | 0.60 | 0.44 |
| CaO | 0.36 | 0.45 | 0.41 |
| MgO | 0.28 | 0.28 | 0.23 |
| TiO2 | 0.23 | 0.40 | 0.35 |
| K2O | 0.10 | 0.12 | 0.09 |
| Na2O | 0.08 | 0.11 | 0.10 |
| Middling | Sample Coal I | Sample Coal II | |
|---|---|---|---|
| Total sulfur (mol) | 1 | 1 | 1 |
| SO2 released (mol) | 0.69 | 0.61 | 0.64 |
| Sulfur fixation rate (%) | 31 | 39 | 36 |
| Unit | Middling | Sample Coal I | Sample Coal II | Corrected Value of Middling | |
|---|---|---|---|---|---|
| Original C | mol | 176.08 | 118.97 | 91.46 | 176.08 |
| Original O | mol | 24.19 | 19.79 | 15.59 | 30.19 |
| Oxygen in air | mol | 76.16 | 52.14 | 40.17 | 76.16 |
| O/C ratio | 1 | 1.002 | 1.043 | 1.049 | 1.037 |
| Unit | Middling | Sample Coal I | Sample Coal II | |
|---|---|---|---|---|
| Actual release | mg | 2.18 | 2.73 | 2.61 |
| Total sulfur | % | 1.40 | 1.84 | 1.5 |
| Total S release | mg | 2.80 | 3.68 | 3.46 |
| Sulfur fixation rate | % | 22.1 | 25.8 | 24.6 |
| Unit | Sample Coal I | Sample Coal II | |
|---|---|---|---|
| Total sulfur | % | 1.85 | 1.72 |
| Test temperature range | K | 1180–1210 | 1110–1200 |
| Sulfur fixation rate | % | 35 | 20.4–31.6 |
| Serial Number | Item | Unit | Working Condition 1 | Working Condition 2 | Working Condition 3 | Working Condition 4 |
|---|---|---|---|---|---|---|
| 1 | Thermal power | MW | 2.27 | 2.84 | 2.99 | 1.01 |
| 2 | Amount of limestone | kg/h | 0 | 0 | 0 | 0 |
| 3 | Calcium-sulfur molar ratio | - | 0 | 0 | 0 | 0 |
| 4 | Bed temperature | K | 1140 | 1174 | 1197 | 1058 |
| 5 | Excess air factor (α) | - | 1.35 | 1.22 | 1.2 | 2.01 |
| 6 | Bottom ash share (αlz) | % | 71.8 | 71.8 | 71.8 | 71.8 |
| 7 | Bottom ash carbon content (Clz) | % | 1.94 | 4.22 | 3.26 | 1.39 |
| 8 | Fly ash share (αfh) | % | 28.2 | 28.2 | 28.2 | 28.2 |
| 9 | Carbon content of fly ash (Cfh) | % | 10.36 | 8.27 | 7.31 | 9.93 |
| 10 | Combustion efficiency (ηr) | % | 94.32 | 92.99 | 94.34 | 94.8 |
| 11 | Desulfurization efficiency (ηs) | % | 31.6 | 26.4 | 20.3 | 38 |
| Item | Unit | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|
| Thermal power | MW | 2.58 | 2.58 | 2.58 | 2.58 | 2.58 | 2.58 | 2.58 |
| Amount of limestone | kg/h | 177.5 | 177.5 | 177.5 | 177.5 | 177.5 | 256.9 | 174.3 |
| Ca/S | - | 3.7 | 3.7 | 3.7 | 3.7 | 3.7 | 5.36 | 3.64 |
| Bed temperature | K | 1166 | 1159 | 1119 | 1172 | 1176 | 1158 | 1152 |
| Excess air factor (α) | - | 1.22 | 1.21 | 1.22 | 1.22 | 1.24 | 1.2 | 1.21 |
| Bottom ash share (αlz) | % | 71 | 71 | 71 | 71 | 71 | 71 | 71 |
| Bottom ash carbon content (Clz) | % | 3.4 | 4.06 | 2.73 | 4.33 | 3.07 | 4.88 | 2.6 |
| Fly ash share (αfh) | % | 29 | 29 | 29 | 29 | 29 | 29 | 29 |
| Carbon content of fly ash (Cfh) | % | 5.68 | 7.14 | 7.54 | 6.09 | 4.74 | 5.32 | 9.93 |
| Combustion efficiency (ηr) | % | 93 | 91.39 | 92.9 | 91.63 | 93.94 | 90.46 | 91.83 |
| Desulfurization efficiency (ηs) | % | 94.3 | 94.19 | 88.55 | 88.24 | 88.88 | 96.51 | 88.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Du, Y.; Zeng, Y.; Kang, Z.; Sun, B. Sulfur Conversion of Mixed Coal and Gangue during Combustion in a CFB Boiler. Energies 2020, 13, 553. https://doi.org/10.3390/en13030553
Zhao L, Du Y, Zeng Y, Kang Z, Sun B. Sulfur Conversion of Mixed Coal and Gangue during Combustion in a CFB Boiler. Energies. 2020; 13(3):553. https://doi.org/10.3390/en13030553
Chicago/Turabian StyleZhao, Lizheng, Yanfei Du, Yusen Zeng, Zhizhong Kang, and Baomin Sun. 2020. "Sulfur Conversion of Mixed Coal and Gangue during Combustion in a CFB Boiler" Energies 13, no. 3: 553. https://doi.org/10.3390/en13030553
APA StyleZhao, L., Du, Y., Zeng, Y., Kang, Z., & Sun, B. (2020). Sulfur Conversion of Mixed Coal and Gangue during Combustion in a CFB Boiler. Energies, 13(3), 553. https://doi.org/10.3390/en13030553




