The Influence of Porous Structure on the Electrochemical Properties of LiFe0.5Mn0.5PO4 Cathode Material Prepared by Mechanochemically Assisted Solid-State Synthesis
Abstract
1. Introduction
2. Materials and Methods
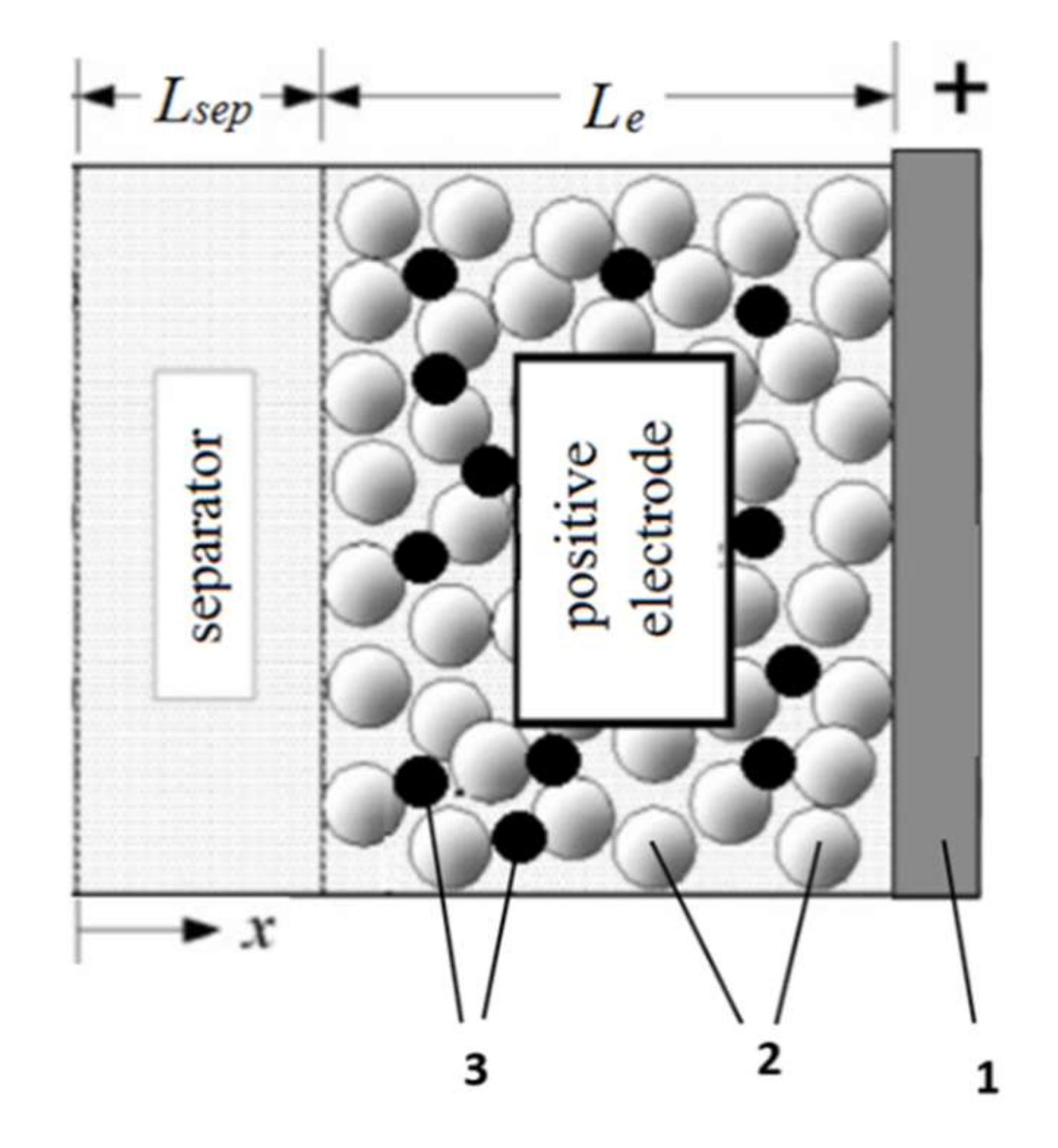
3. Mathematic Modeling
4. Results
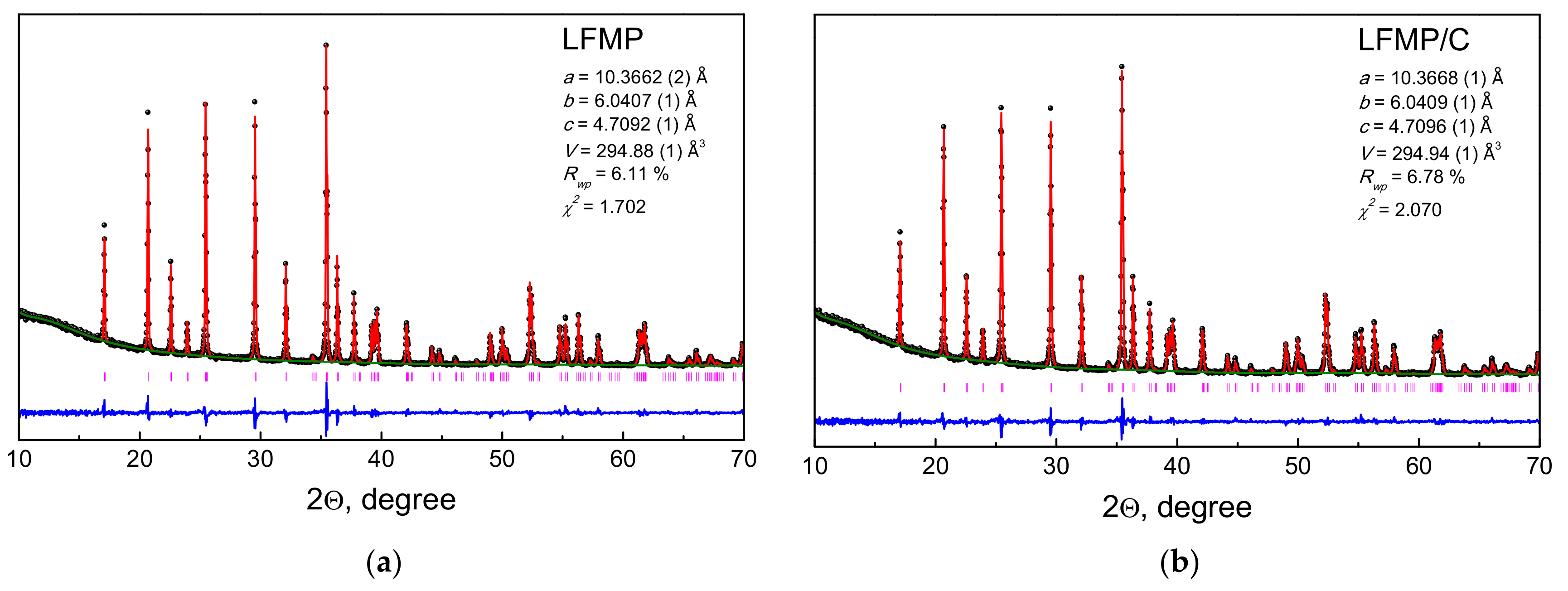
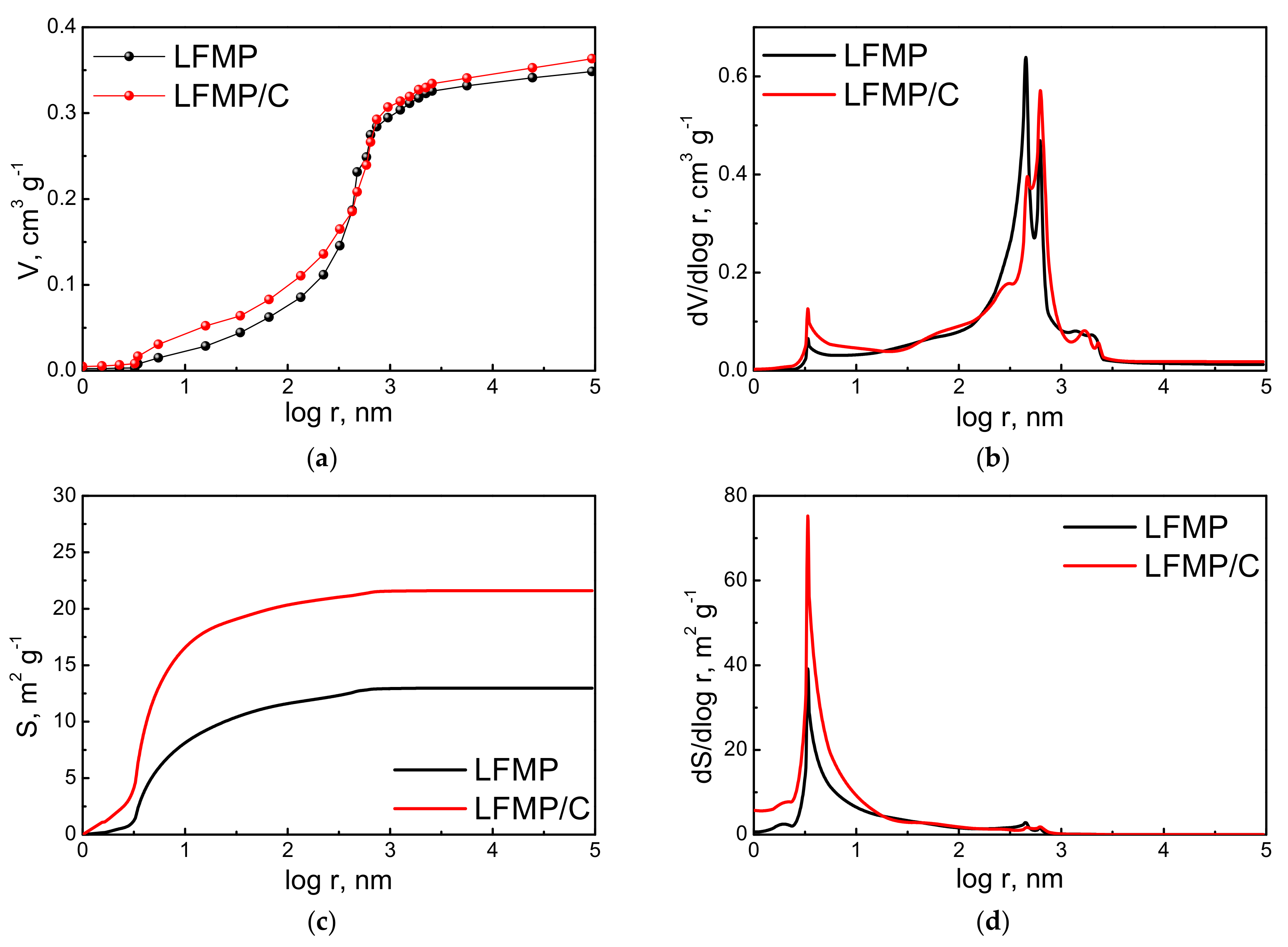
4.1. Structure, Morphology, and Porosity of the LiFe0.5Mn0.5PO4 (LFMP) Samples
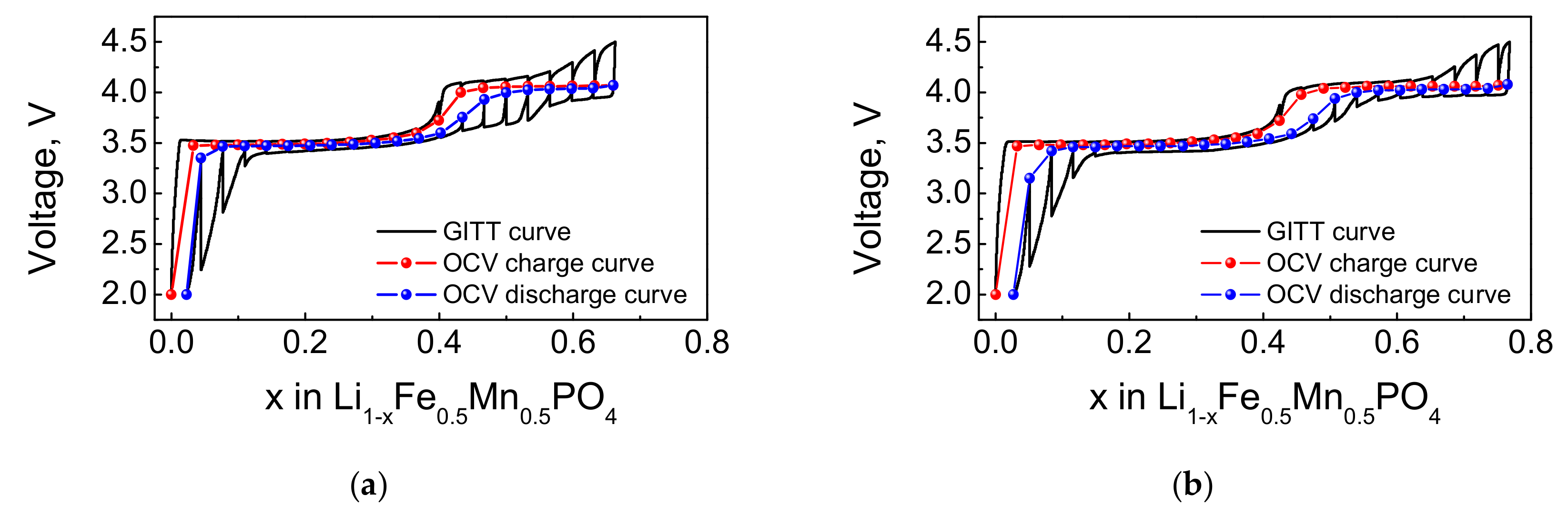
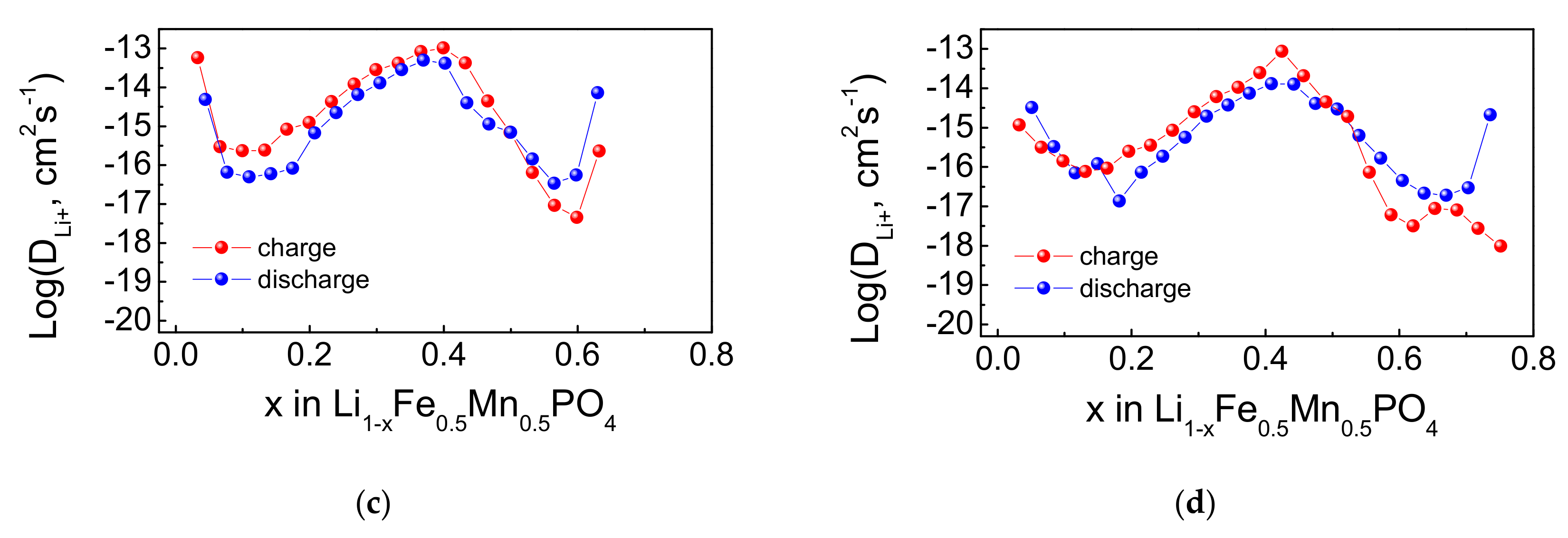
4.2. Electrochemical Properties
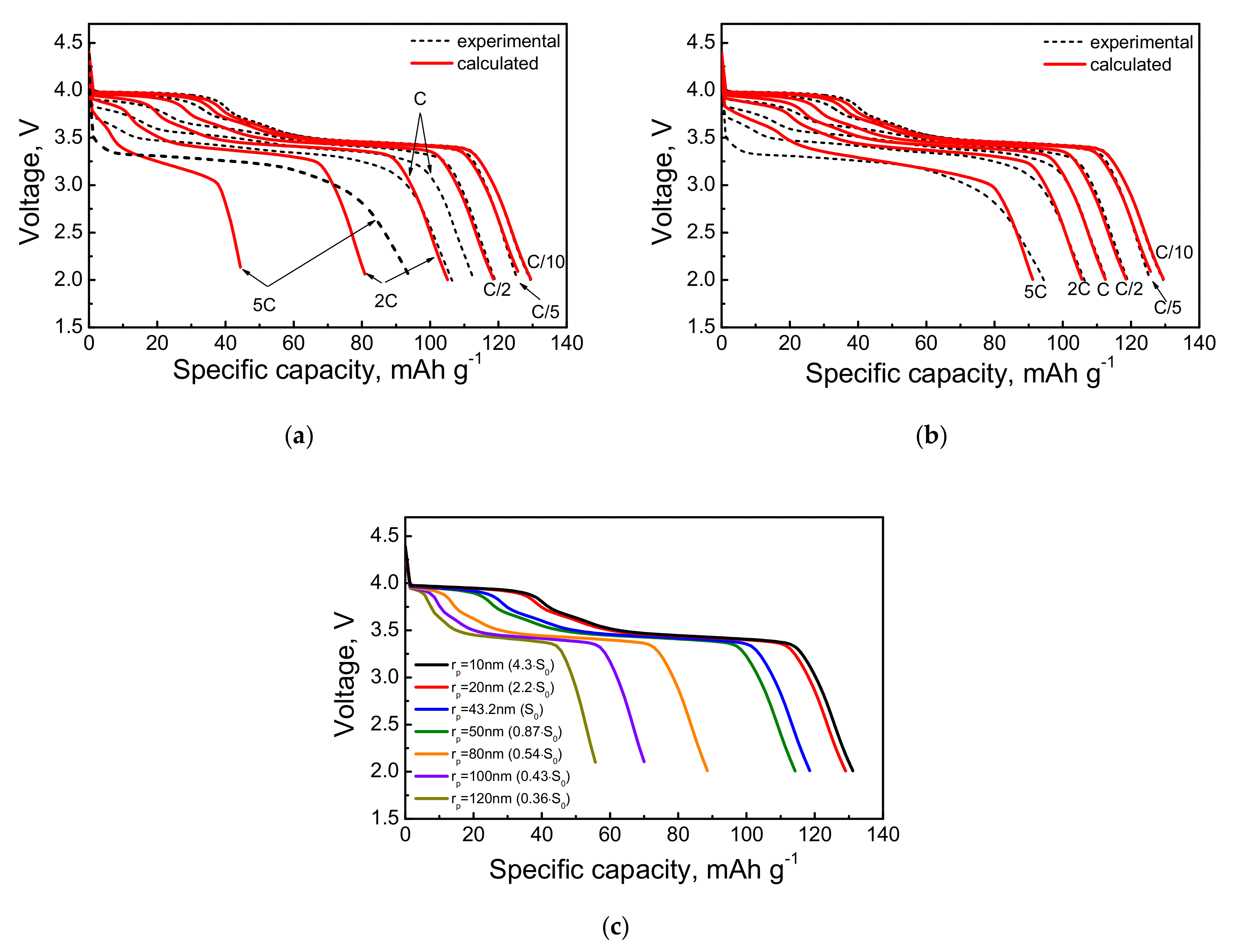
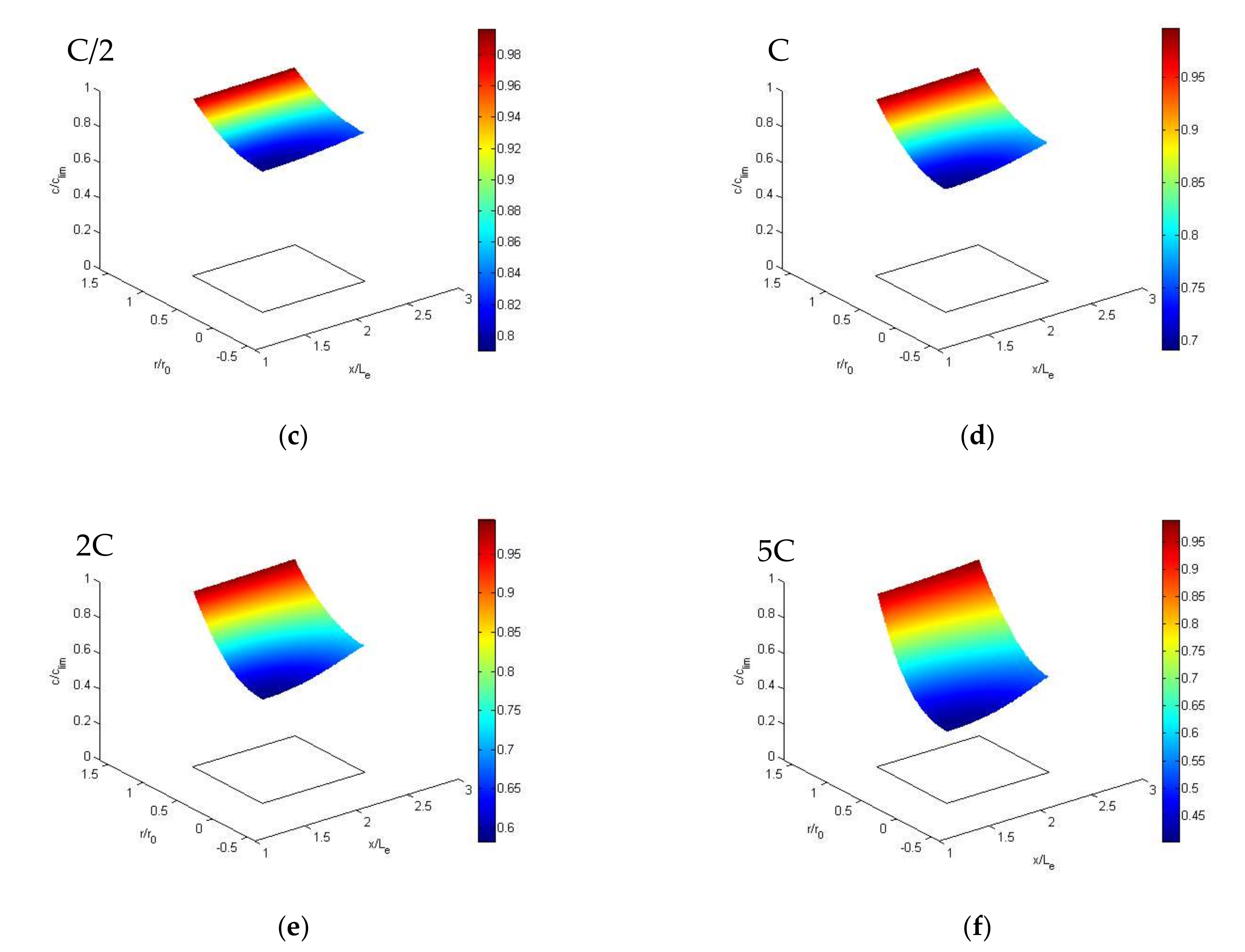
4.3. Calculation Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, P.S.; Chen, G.F. General introduction to porous materials. In Porous Materials. Processing and Applications, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar] [CrossRef]
- Pei, Z.; Li, Z.; Zheng, X. Porous materials for lithium-ion batteries. J. Nanosci. Nanotechnol. 2016, 16, 9028–9049. [Google Scholar] [CrossRef]
- Vu, A.; Qian, Y.; Stein, A. Porous electrode materials for lithium-ion batteries-how to prepare them and what makes them special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Song, J.; Kim, J.; Kang, T.; Kim, D. Design of a porous cathode for ultrahigh performance of a Li ion battery: An overlooked pore distribution. Sci. Rep. 2017, 7, 42521. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wang, C.-W.; Zhang, X.; Sastry, A.M. Porous cathode optimization for lithium cells: Ionic and electronic conductivity, capacity, and selection of materials. J. Power Sources 2010, 195, 2851–2862. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Filippov, A.N.; Bagotsky, V.S. Structural Properties of Porous Materials and Powders Used in Different Fields of Science and Technology; Springer: London, UK, 2014. [Google Scholar]
- Deng, Y.; Yang, C.; Zou, K.; Zhao, X.; Chen, G. Recent advances of Mn-rich LiFe1−yMnyPO4 (0.5 <y <1.0) cathode materials for high energy density lithium ion batteries. Adv. Energy Mater. 2017, 7, 1601958. [Google Scholar] [CrossRef]
- Chizmadzhev, Y.; Markin, V.; Tarasevich, M.; Chirkov, Y. Macrokinetics of Processes in Porous Media; Nauka: Moscow, Russia, 1971. [Google Scholar]
- Newman, J.; Tiedemann, W. Porous electrode theory with battery applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Atlung, S.; West, K.; Jacobsen, T. Dynamic aspects of solid solution cathodes for electrochemical power sources. J. Electrochem. Soc. 1979, 126, 1311–1321. [Google Scholar] [CrossRef]
- Polymer, L.; Cell, I.; Doyle, M.; Fuller, T.F.; Newman, J.; Soc, J.E. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Chadha, T.S.; Suthar, B.; Rife, D.; Subramanian, V.R.; Biswas, P. Model based analysis of one-dimensional oriented lithium-ion battery electrodes. J. Electrochem. Soc. 2017, 164, E3114–E3121. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and optimization of the dual lithium ion insertion cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef]
- Xue, N.; Du, W.; Gupta, A.; Shyy, W.; Sastry, A.M.; Martins, J.R.R.A. Optimization of a single lithium-ion battery cell with a gradient-based algorithm. J. Electrochem. Soc. 2013, 160, A1071–A1078. [Google Scholar] [CrossRef]
- Bernardi, D.M.; Go, J.-Y. Analysis of pulse and relaxation behavior in lithium-ion batteries. J. Power Sources 2011, 196, 412–427. [Google Scholar] [CrossRef]
- Kumaresan, K.; Mikhaylik, Y.; White, R.E. A mathematical model for a lithium–sulfur cell. J. Electrochem. Soc. 2008, 155, A576–A582. [Google Scholar] [CrossRef]
- Bograchev, D.A.; Volfkovich, Y.M.; Dubasova, V.S.; Nikolenko, A.F.; Ponomareva, T.A.; Sosenkin, V.E. Development and experimental verification of a mathematical model of lithium ion battery. Russ. J. Electrochem. 2013, 49, 115–123. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Koch, B.J. Modeling lithium intercalation of single-fiber carbon microelectrodes. J. Electrochem. Soc. 1996, 143, 600–608. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Koch, B.J. Electrochemistry of intercalation materials. Charge-transfer reaction and intercalate diffusion in porous electrodes. J. Electrochem. Soc. 1999, 146, 833–839. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Bagotzky, V.S. The method of standard porosimetry. 2. Investigation of the formation of porous structures. J. Power Sources 1994, 48, 339–348. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Sakars, A.V.; Volinsky, A.A. Application of the standard porosimetry method for nanomaterials. Int. J. Nanotechnol. 2005, 2, 292–302. [Google Scholar] [CrossRef]
- Valøen, L.O.; Reimers, J.N. Transport properties of LiPF6-based Li-Ion battery electrolytes. J. Electrochem. Soc. 2005, 152, 882–891. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Slobodyuk, A.B.; Petrov, S.A. Submicron LiFe1-yMnyPO4 solid solutions prepared by mechanochemically assisted carbothermal reduction: The structure and properties. Electrochim. Acta 2012, 59, 404–411. [Google Scholar] [CrossRef]
- Kosova, N.V.; Kulova, T.L.; Nikolskaya, N.F.; Podgornova, O.A.; Rychagov, A.Y.; Sosenkin, V.E.; Volfkovich, Y.M. Effect of porous structure of LiCoPO4 on its performance in hybrid supercapacitor. J. Solid State Electrochem. 2019, 23, 1981–1990. [Google Scholar] [CrossRef]
- Krachkovskiy, S.A.; Bazak, J.D.; Fraser, S.; Halalay, I.C.; Goward, G.R. Determination of mass transfer parameters and ionic association of LiPF6: Organic carbonates solutions. J. Electrochem. Soc. 2017, 164, A912–A916. [Google Scholar] [CrossRef]
- Fang, W.; Kwon, O.J.; Wang, C. Electrochemical–thermal modeling of automotive Li-ion batteries and experimental validation using a three-electrode cell. Int. J. Energy Res. 2010, 34, 107–115. [Google Scholar] [CrossRef]
- Cai, L.; White, R.E. Mathematical modeling of a lithium ion battery with thermal effects in COMSOL Inc. Multiphysics (MP) software. J. Power Sources 2011, 196, 5985–5989. [Google Scholar] [CrossRef]
- Prada, E.; Di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Simplified electrochemical and thermal model of LiFePO4-graphite Li-ion batteries for fast charge applications. J. Electrochem. Soc. 2012, 159, A1508–A1519. [Google Scholar] [CrossRef]
- Troxler, Y.; Wu, B.; Marinescu, M.; Yufit, V.; Patel, Y.; Marquis, A.J.; Brandon, N.P.; Offer, G.J. The effect of thermal gradients on the performance of lithium-ion batteries. J. Power Sources 2014, 247, 1018–1025. [Google Scholar] [CrossRef]
- West, W.C.; Soler, J.; Smart, M.C.; Ratnakumar, B.V.; Firdosy, S.; Ravi, V. Electrochemical behavior of layered solid solution Li2MnO3−LiMO2 (M = Ni, Mn, Co) Li-ion cathodes with and without alumina coatings. J. Electrochem. Soc. 2011, 158, A883–A889. [Google Scholar] [CrossRef]



| Parameter | LFMP | LFMP/C |
|---|---|---|
| Average logarithmic pore radius, nm | 2.4 | 2.3 |
| Porosity per weight, cm3·g−1 | 0.35 | 0.36 |
| Porosity per volume, cm3·cm−3 | 0.54 | 0.54 |
| Meso- and macro-pore surface per weight (Sme), m2·g−1 | 12.97 | 21.60 |
| Meso- and macro-pore surface per volume, m2·cm−3 | 20.03 | 31.97 |
| Total pore surface per weight (St), m2·g−1 | 17.97 | 31.29 |
| Calculated total specific surface area (St,cal.), m2·g−1 | 42.42 |
| Parameter | Value | Source |
|---|---|---|
| Archie’s exponent coefficient of cathode | 1.5 | fitting |
| Archie’s exponent coefficient of separator | 1.5 | fitting |
| Conductivity of cathode, S·m−1 | 0.001 | fitting |
| Cationic transport number | 0.32 | [25] |
| Electrolyte diffusion coefficient, m2·s−1 | 3.5 × 10−10 | [25] |
| Reaction rate coefficient | 6 × 10−8 | fitting |
| Transfer coefficient | 0.5 | assumed |
| Initial solid-state concentration, mol·m−3 | 2.14 × 104 | fitting |
| Solid-state diffusion coefficient, m2·s−1 | 1.8 × 10−19 (see Table 3) | fitting |
| Cycling Rate | 0.1C | 0.2C | 0.5C | 1C | 2C | 5C |
|---|---|---|---|---|---|---|
| Diffusion coefficient, m2·s−1 | 1.8 × 10−19 | 1.8 × 10−19 | 1.8 × 10−19 | 2.5 × 10−19 | 3.8 × 10−19 | 6.2 × 10−19 |
| Acceleration factor of the diffusion | 1.0 | 1.0 | 1.0 | 1.4 | 2.1 | 3.4 |
| Return temperature 1000/T | 3.38 | 3.38 | 3.38 | 3.32 | 3.29 | 3.24 |
| Temperature estimated from the slope of the curve (acceleration factor of the diffusion), °C [11] | 23 | 23 | 23 | 28 | 31 | 36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bograchev, D.A.; Volfkovich, Y.M.; Sosenkin, V.E.; Podgornova, O.A.; Kosova, N.V. The Influence of Porous Structure on the Electrochemical Properties of LiFe0.5Mn0.5PO4 Cathode Material Prepared by Mechanochemically Assisted Solid-State Synthesis. Energies 2020, 13, 542. https://doi.org/10.3390/en13030542
Bograchev DA, Volfkovich YM, Sosenkin VE, Podgornova OA, Kosova NV. The Influence of Porous Structure on the Electrochemical Properties of LiFe0.5Mn0.5PO4 Cathode Material Prepared by Mechanochemically Assisted Solid-State Synthesis. Energies. 2020; 13(3):542. https://doi.org/10.3390/en13030542
Chicago/Turabian StyleBograchev, Daniil A., Yury M. Volfkovich, Valentin E. Sosenkin, Olga A. Podgornova, and Nina V. Kosova. 2020. "The Influence of Porous Structure on the Electrochemical Properties of LiFe0.5Mn0.5PO4 Cathode Material Prepared by Mechanochemically Assisted Solid-State Synthesis" Energies 13, no. 3: 542. https://doi.org/10.3390/en13030542
APA StyleBograchev, D. A., Volfkovich, Y. M., Sosenkin, V. E., Podgornova, O. A., & Kosova, N. V. (2020). The Influence of Porous Structure on the Electrochemical Properties of LiFe0.5Mn0.5PO4 Cathode Material Prepared by Mechanochemically Assisted Solid-State Synthesis. Energies, 13(3), 542. https://doi.org/10.3390/en13030542





