Effect of Iron Oxide Nanoparticles on the Properties of Water-Based Drilling Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide NPs
2.2. Coating of Xanthan Gum on Iron Oxide NPs (Fe-XG)
2.3. Formulation of Drilling Fluid
2.4. Drilling Fluid and NPs Characterization
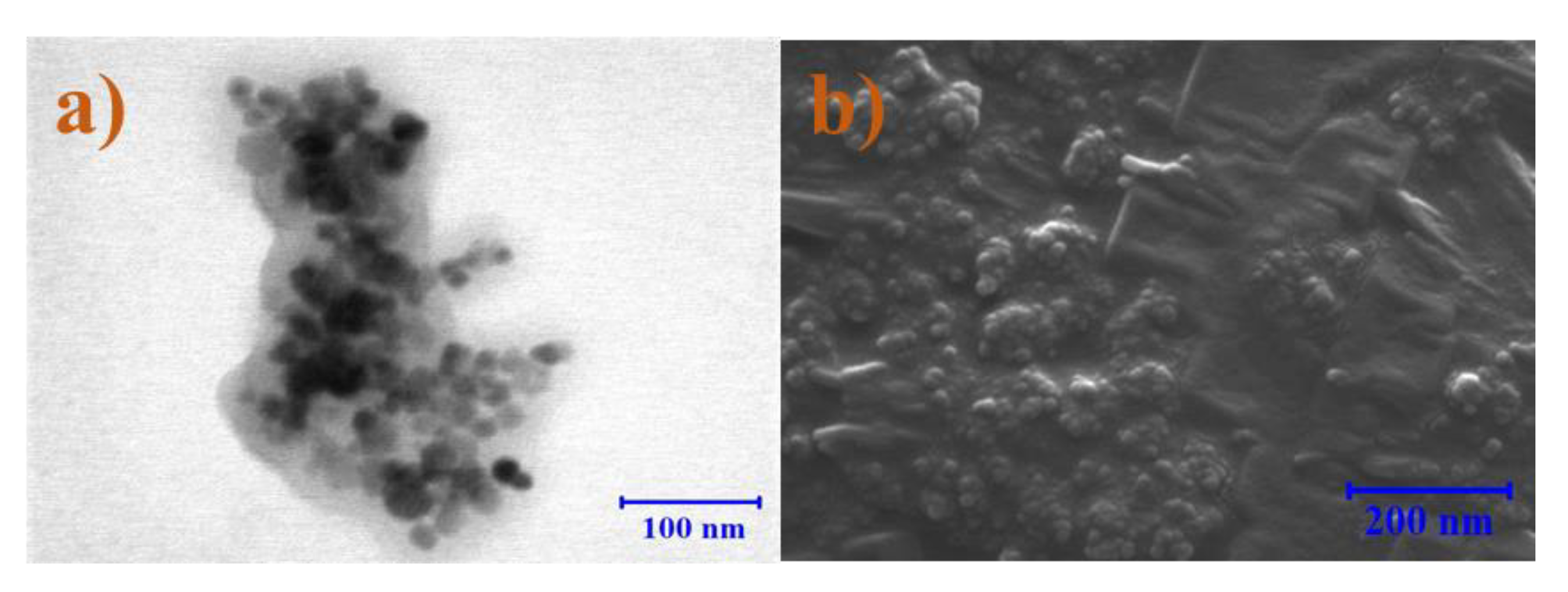
2.4.1. Scanning Transmission Electron Microscopy (STEM)
2.4.2. Microscale and Elemental Analysis
2.4.3. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
2.4.4. Viscosity
2.4.5. Mechanical Friction Measurement
2.4.6. Fluid Loss Measurement
2.4.7. Inductively Coupled Optical Atomic Emission Spectrometry (ICP-OES)
2.4.8. Viscoelastic Measurement
3. Results and Discussion
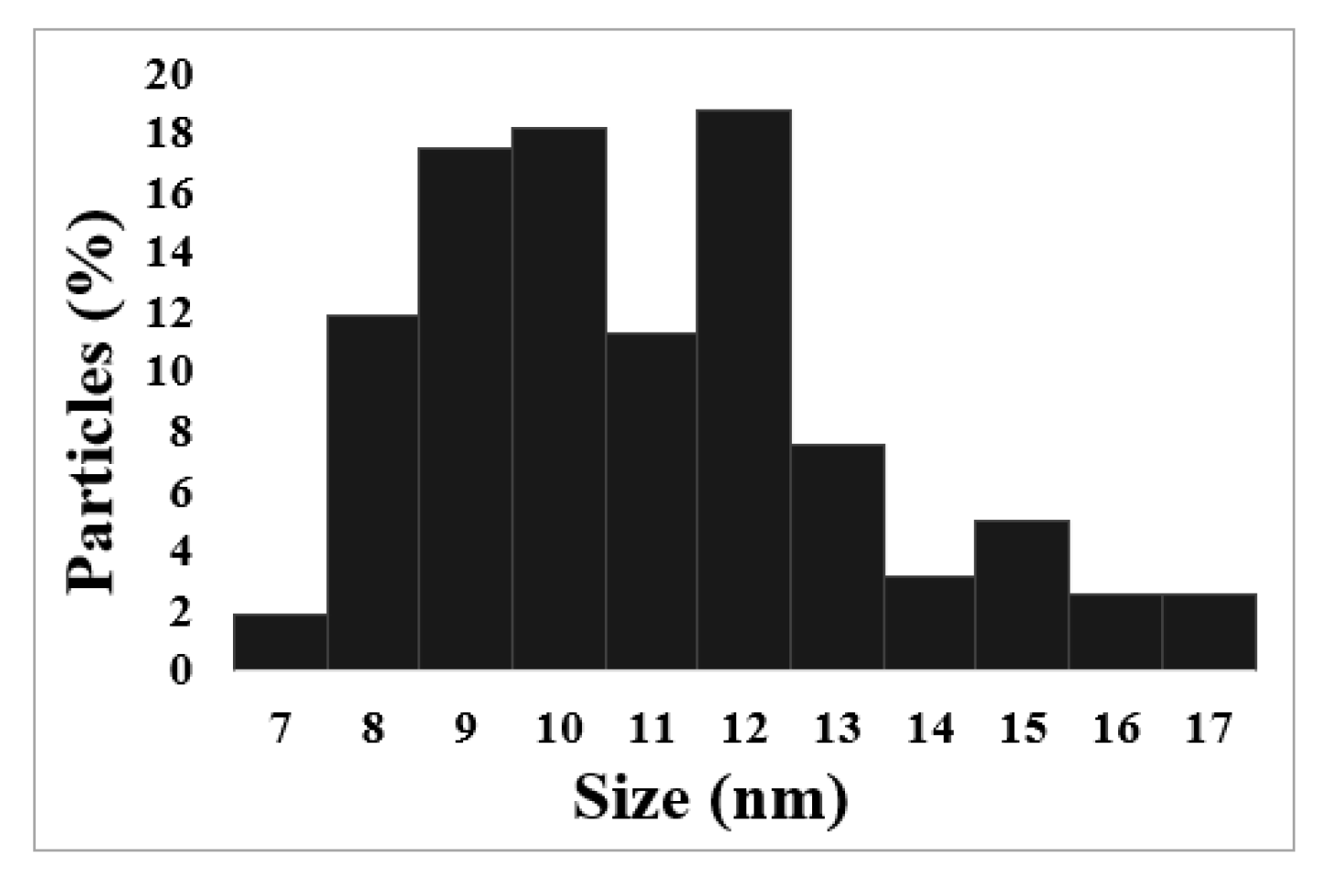
3.1. Size Distribution of Iron Oxide and Fe-XG NPs
3.2. Hydrodynamic Size and Surface Charge of NPs
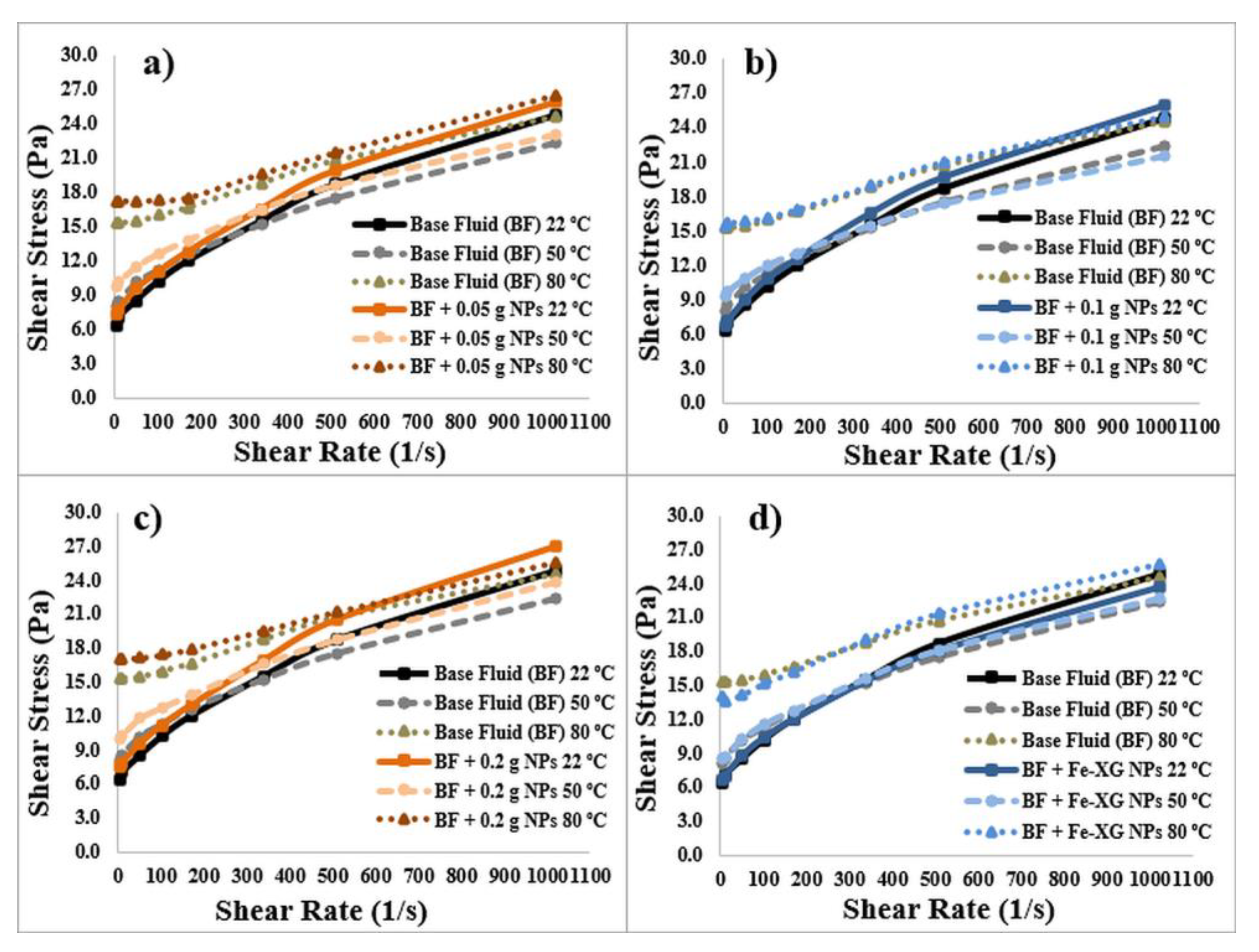
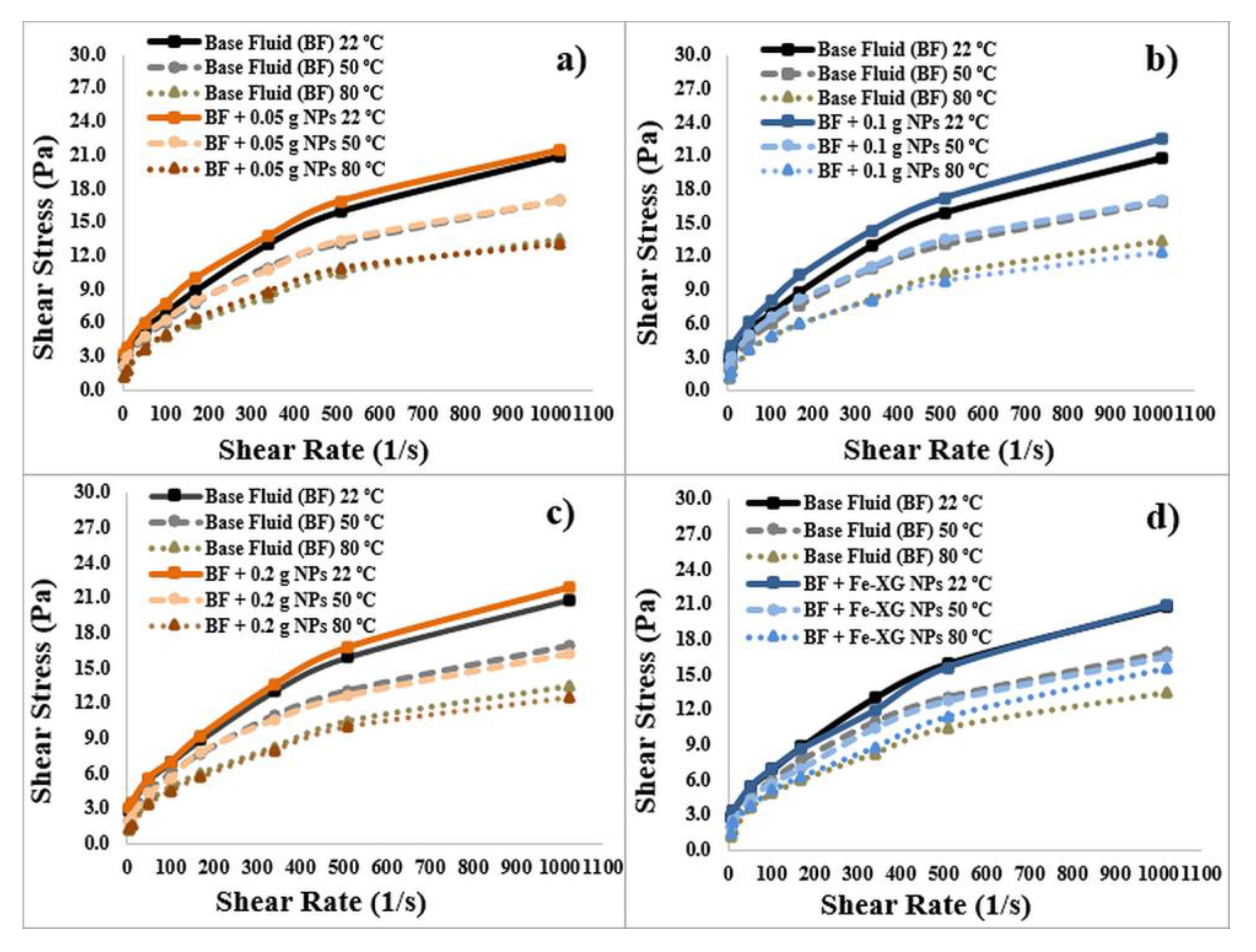
3.3. Rheological Parameters of the Drilling Fluids
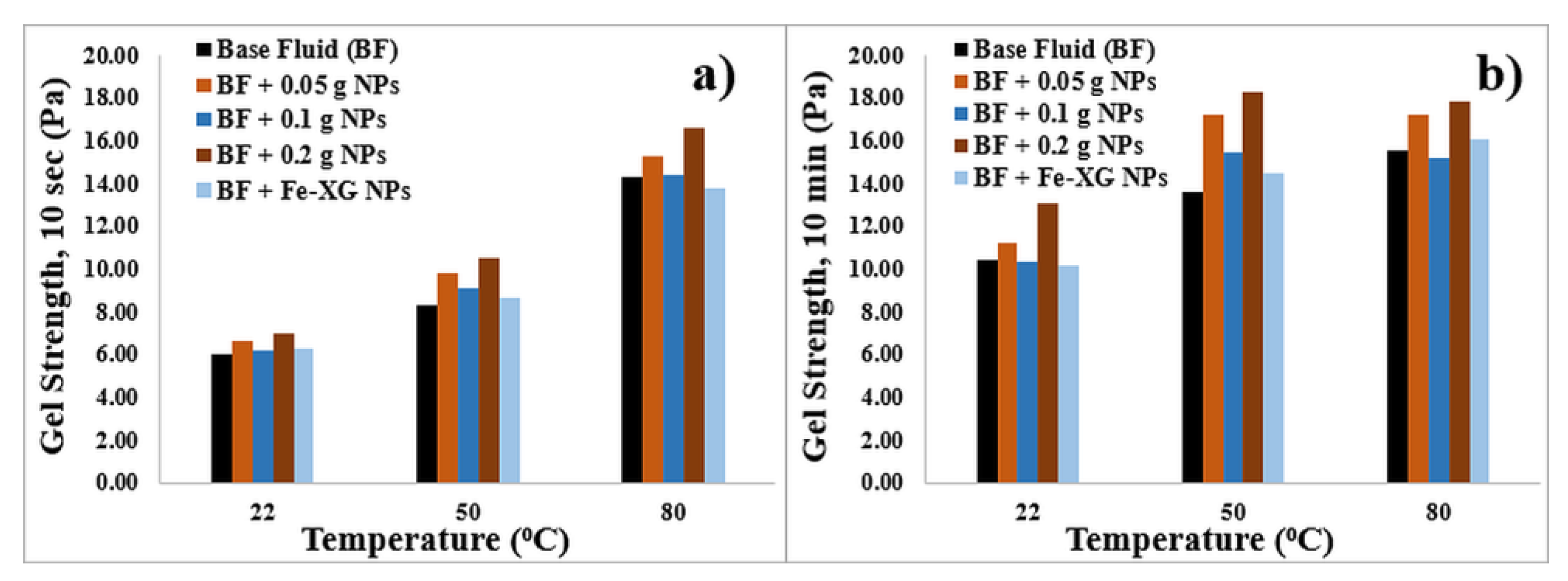
3.3.1. Bentonite-Based Fluids
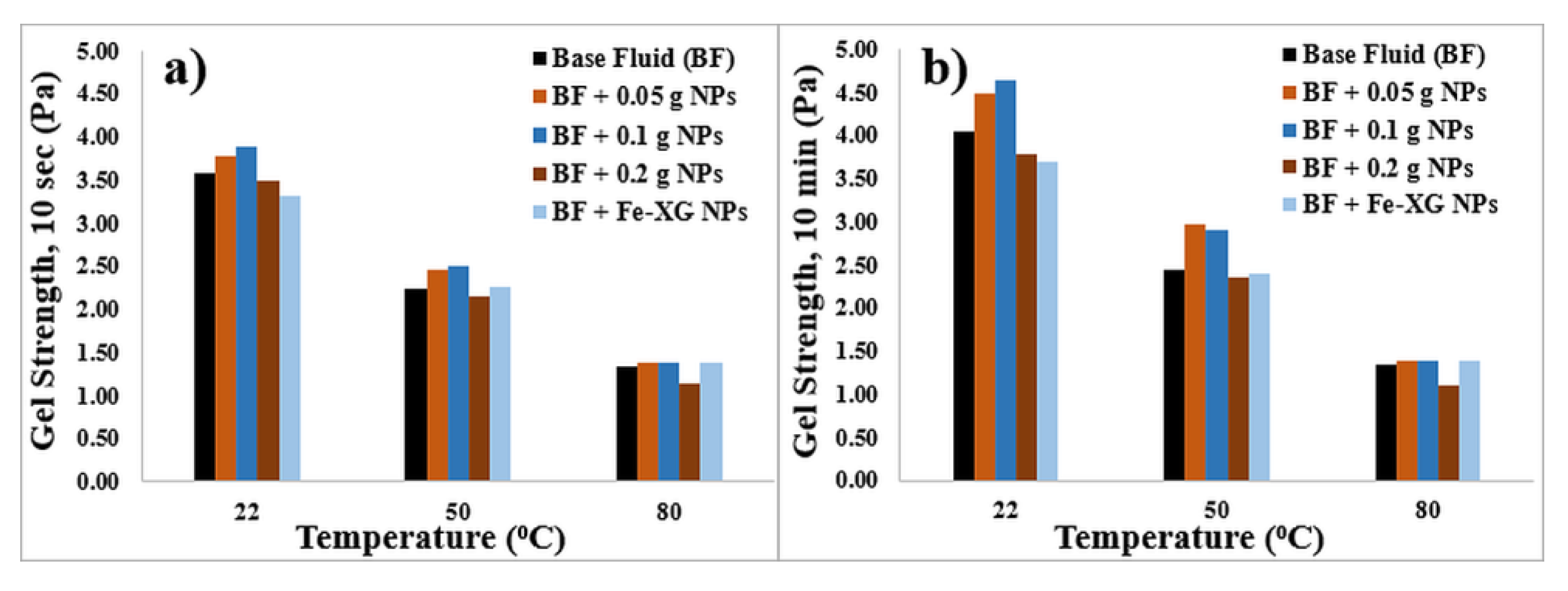
3.3.2. KCL-Based Fluids
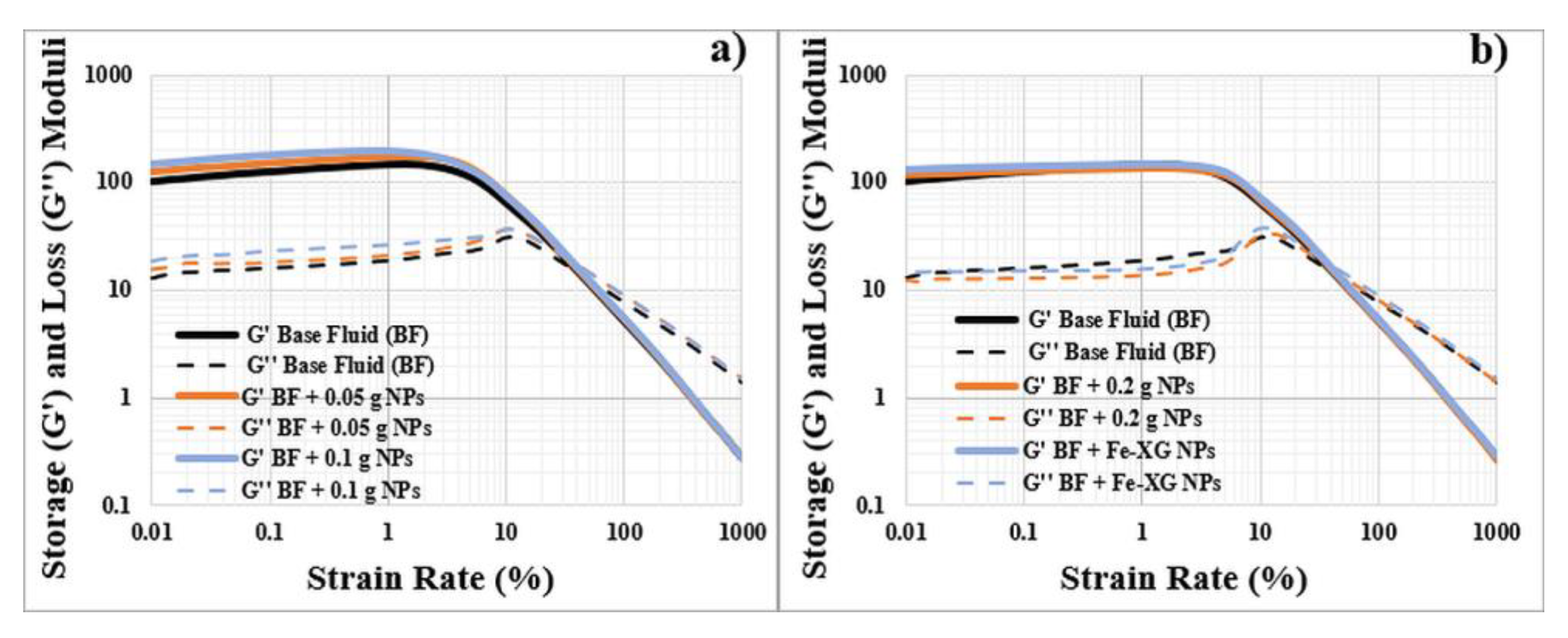
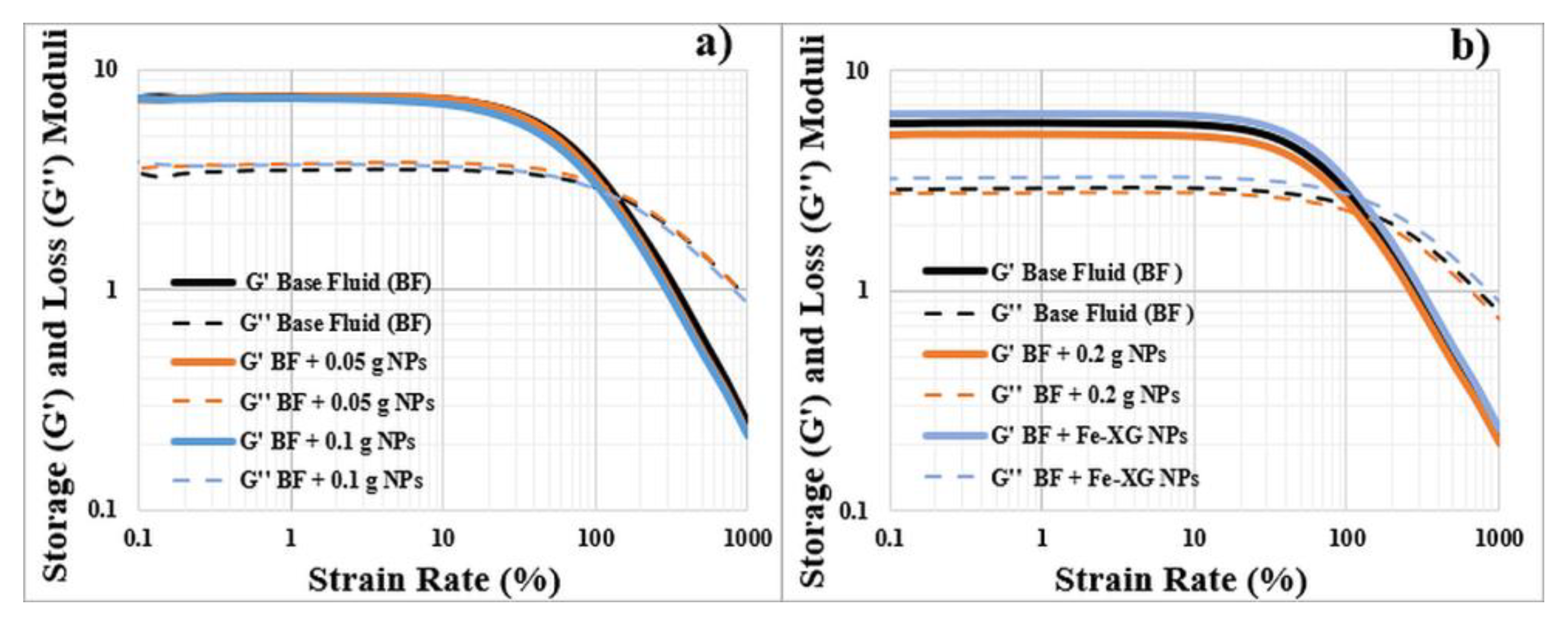
3.4. Viscoelasticity
3.4.1. Bentonite-Based Fluids
3.4.2. KCL-Based Fluids
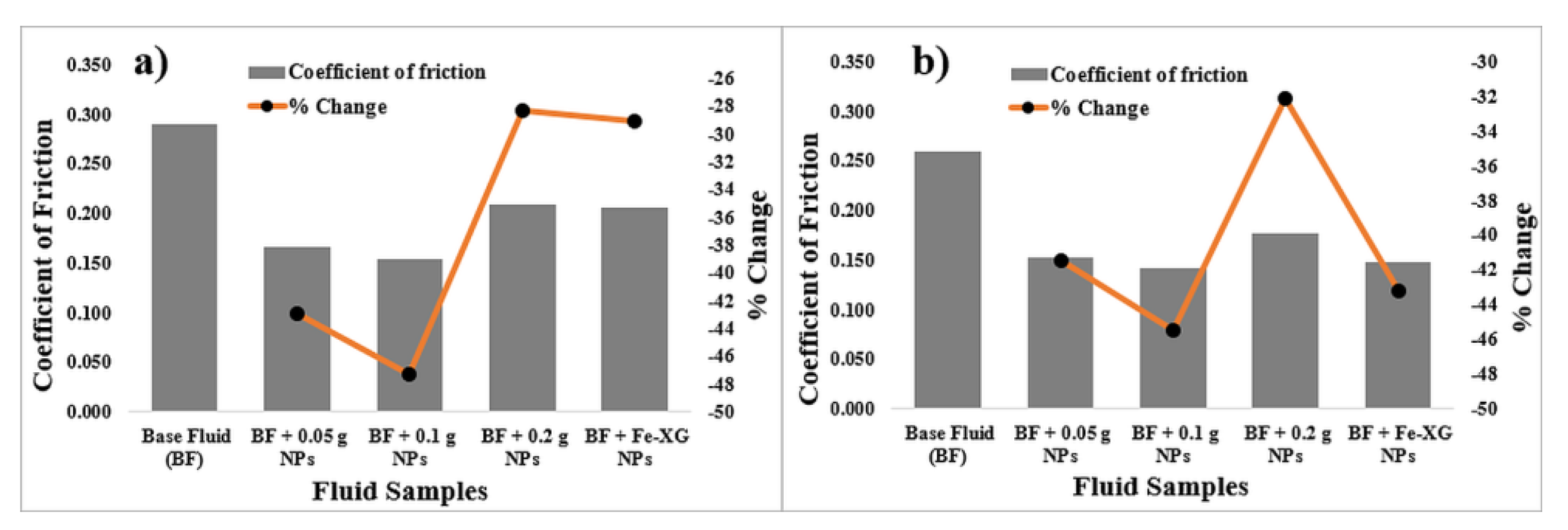
3.5. Mechanical Friction
3.5.1. Bentonite-Based Fluids
3.5.2. KCl-Based Fluids
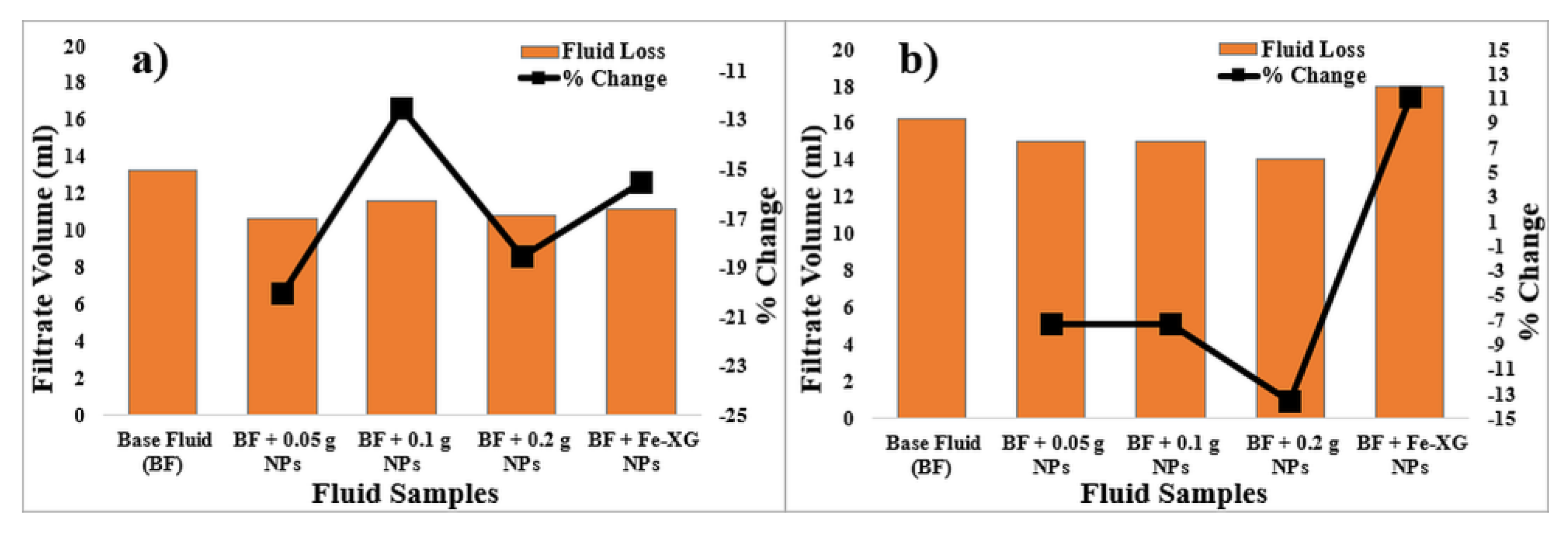
3.6. Effect of NPs on the Fluid Loss
3.6.1. Bentonite-Based Fluids
3.6.2. KCL-Based Fluids
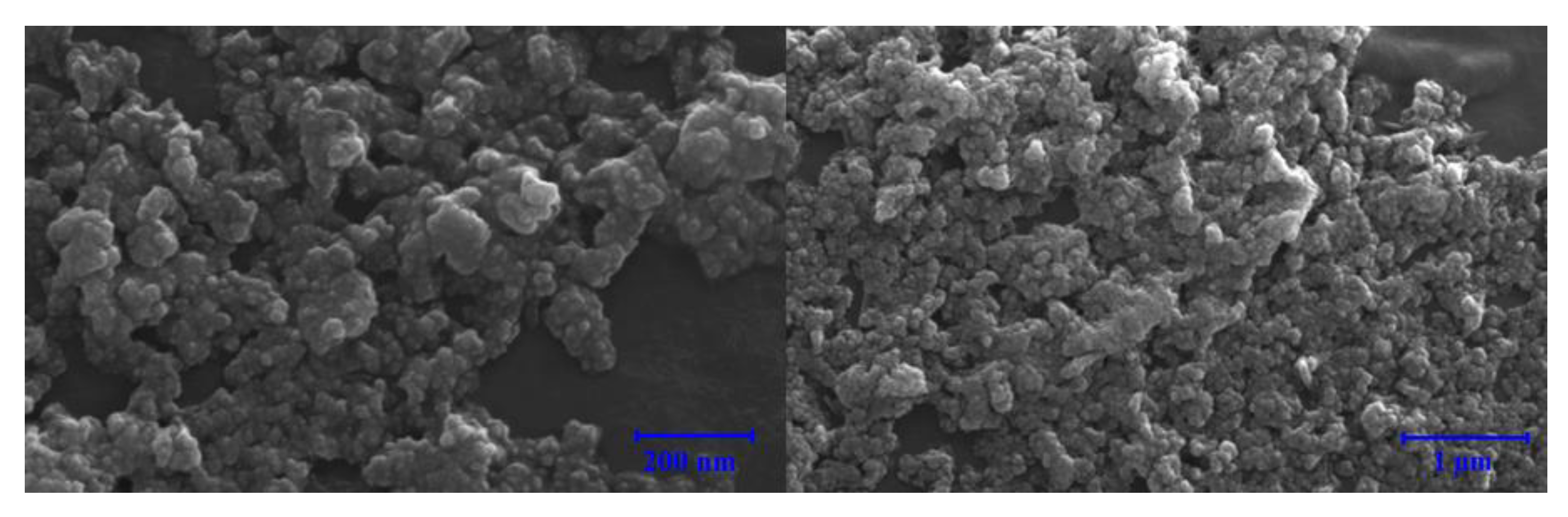
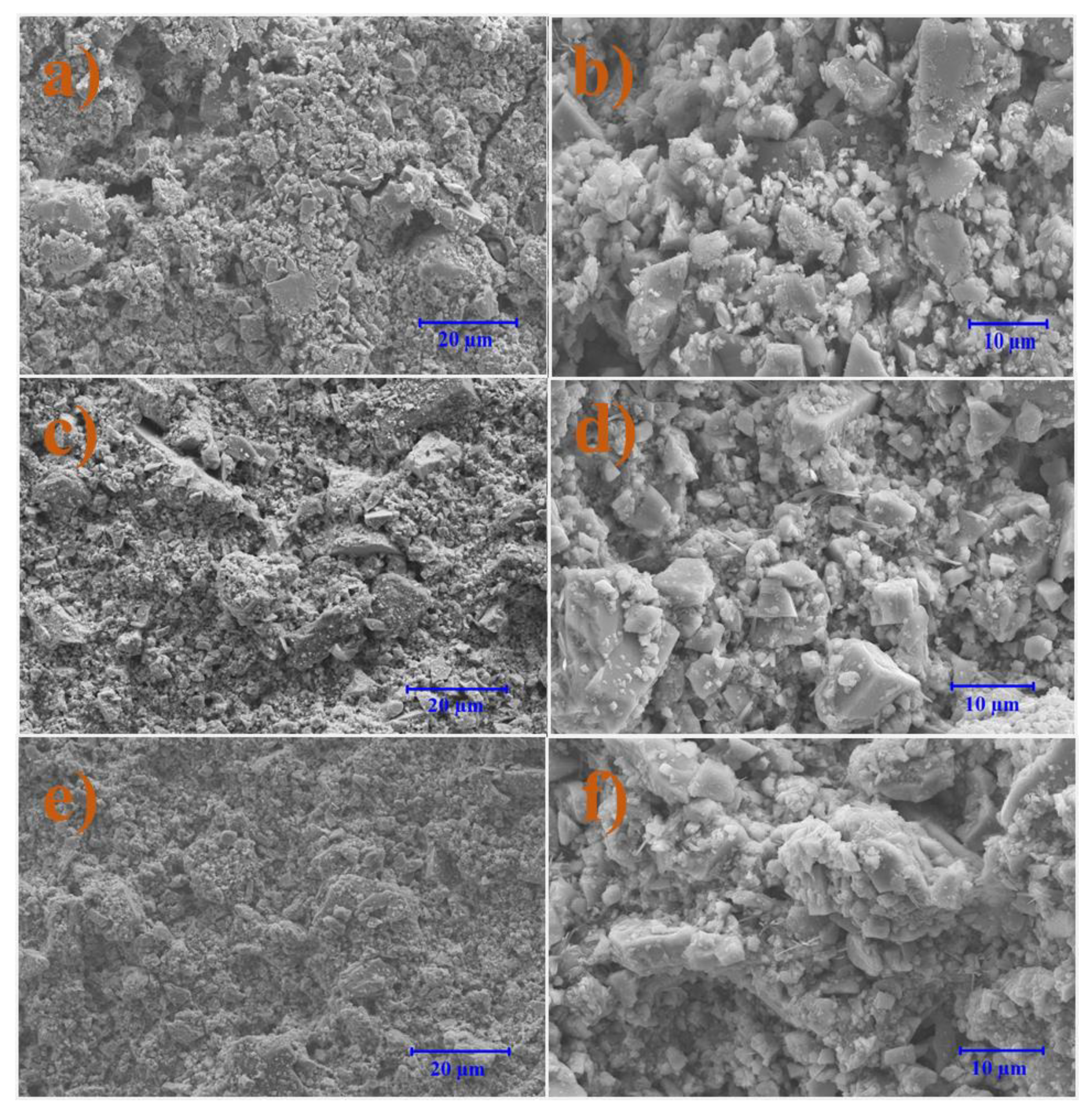
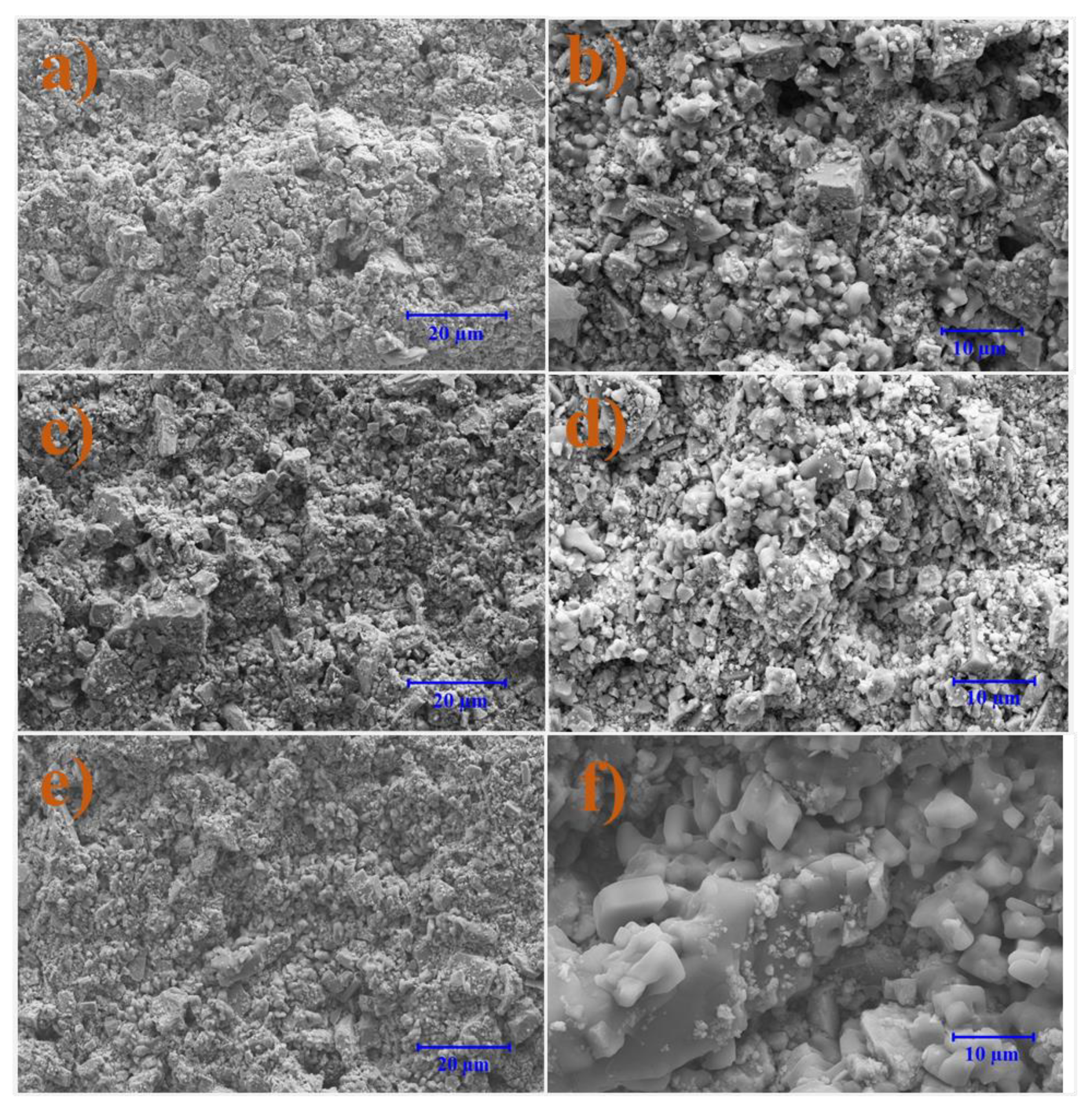
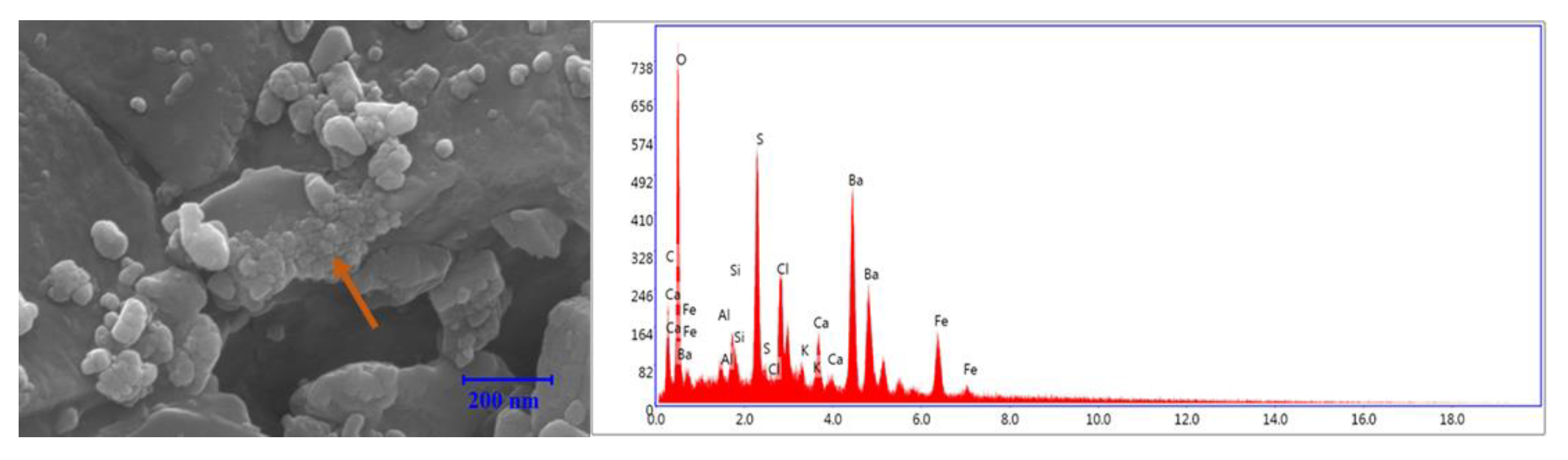
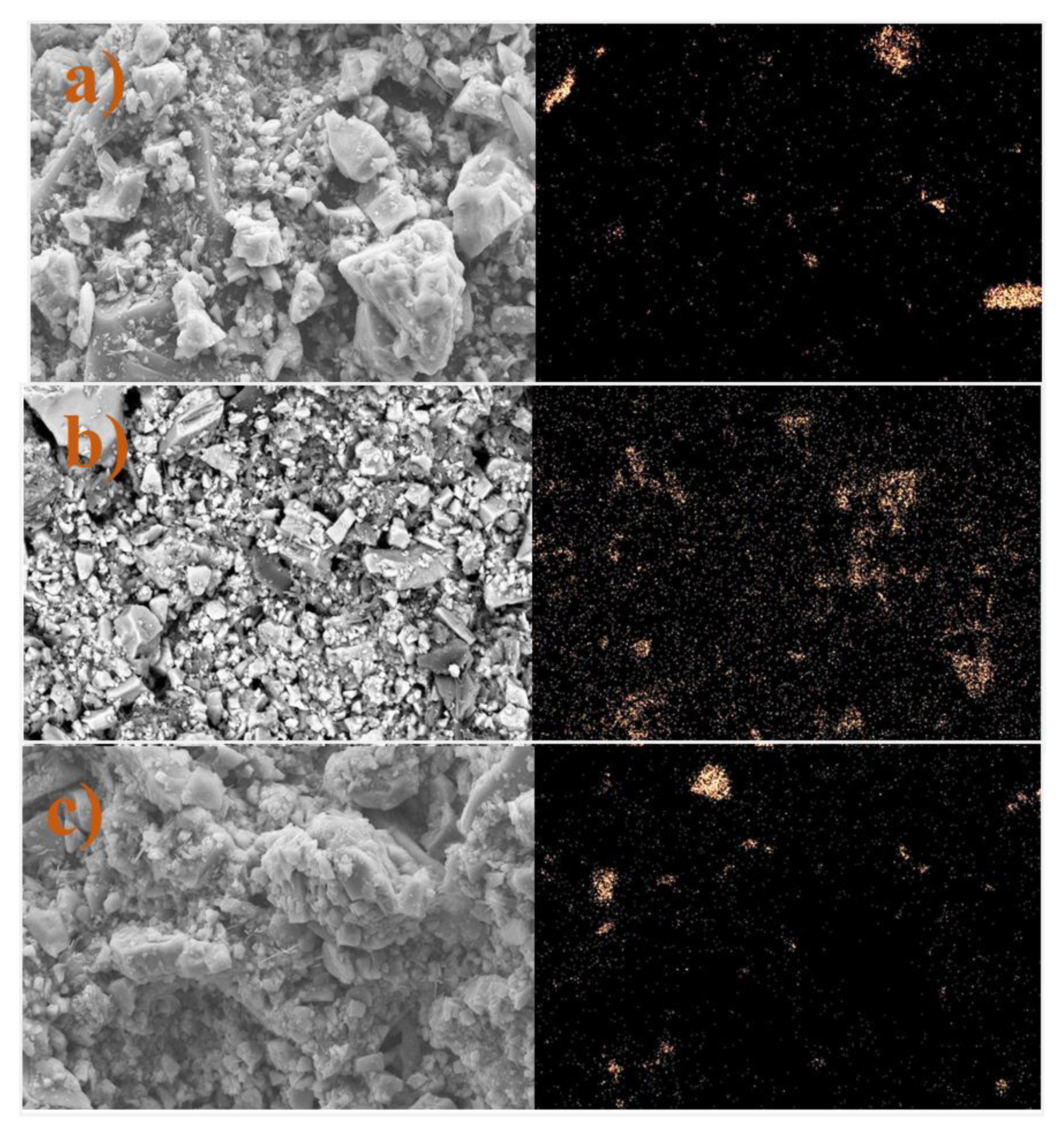
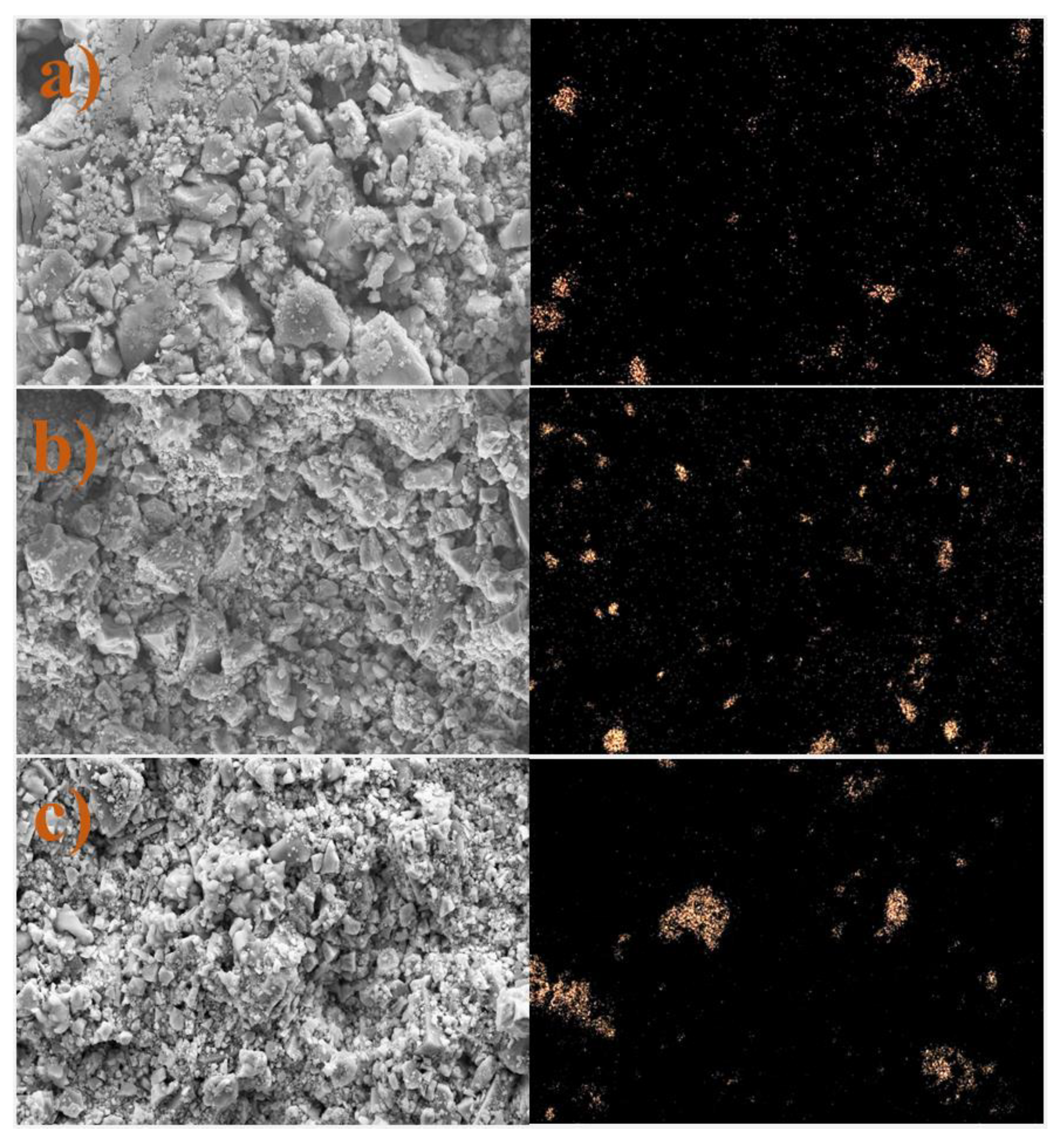
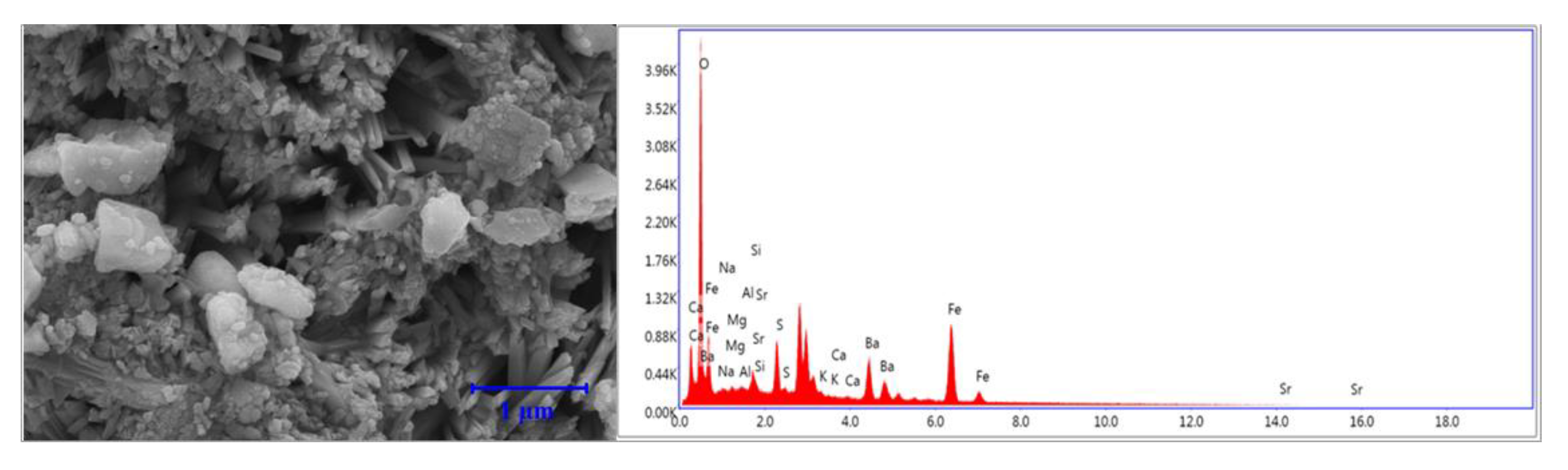
3.7. Microscale Analysis of the Filter Cake
3.7.1. Bentonite-Based Fluids
3.7.2. KCl-Based Fluids
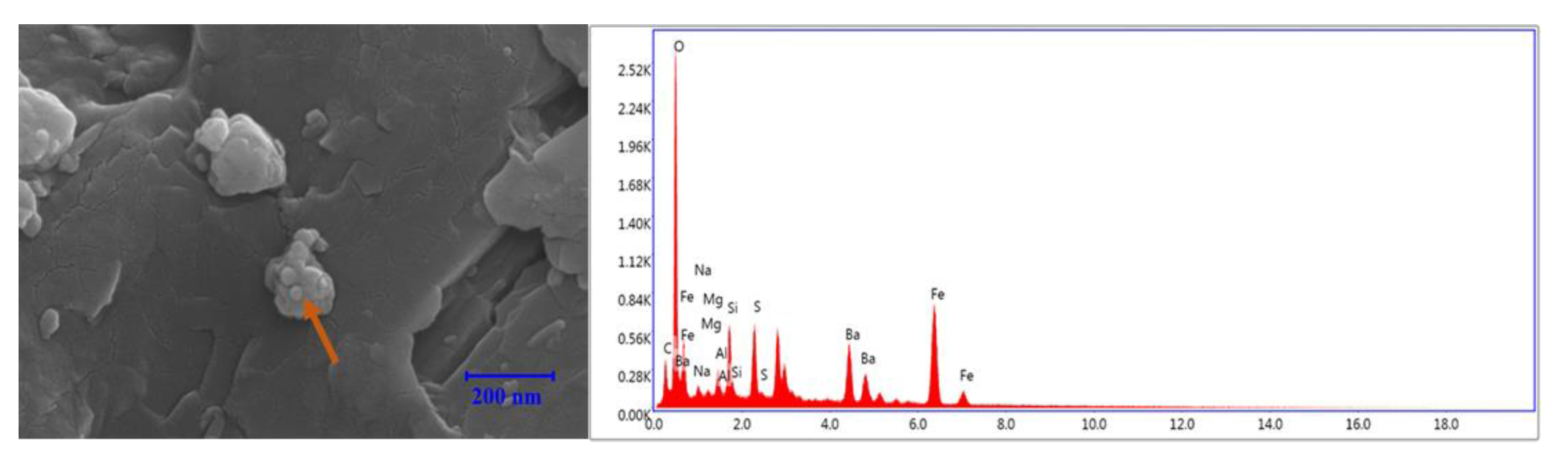
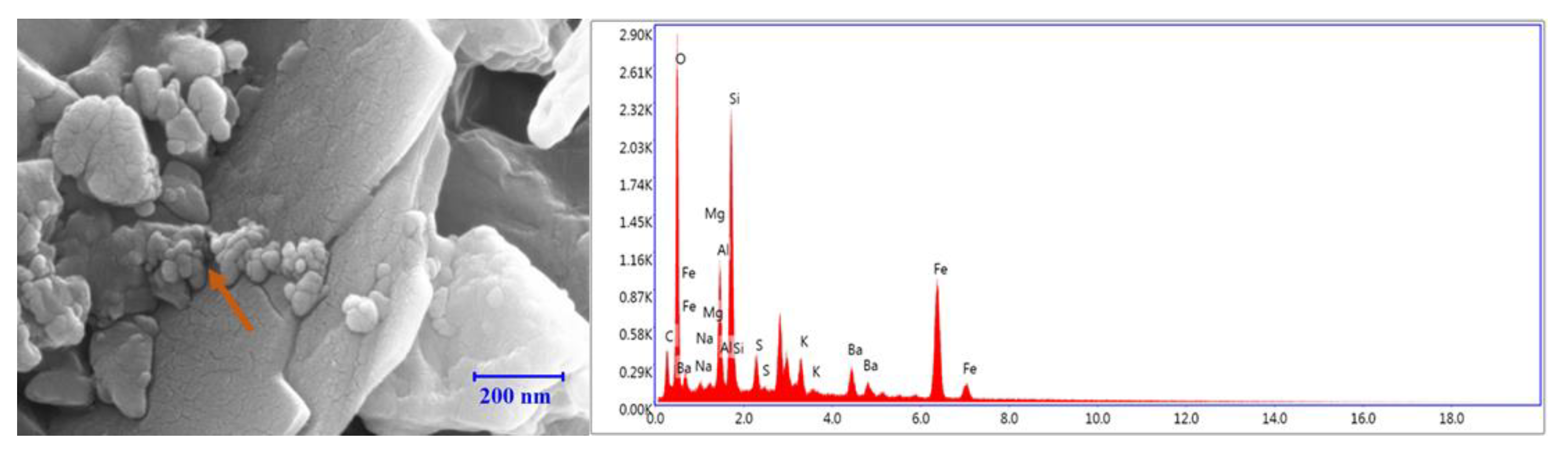
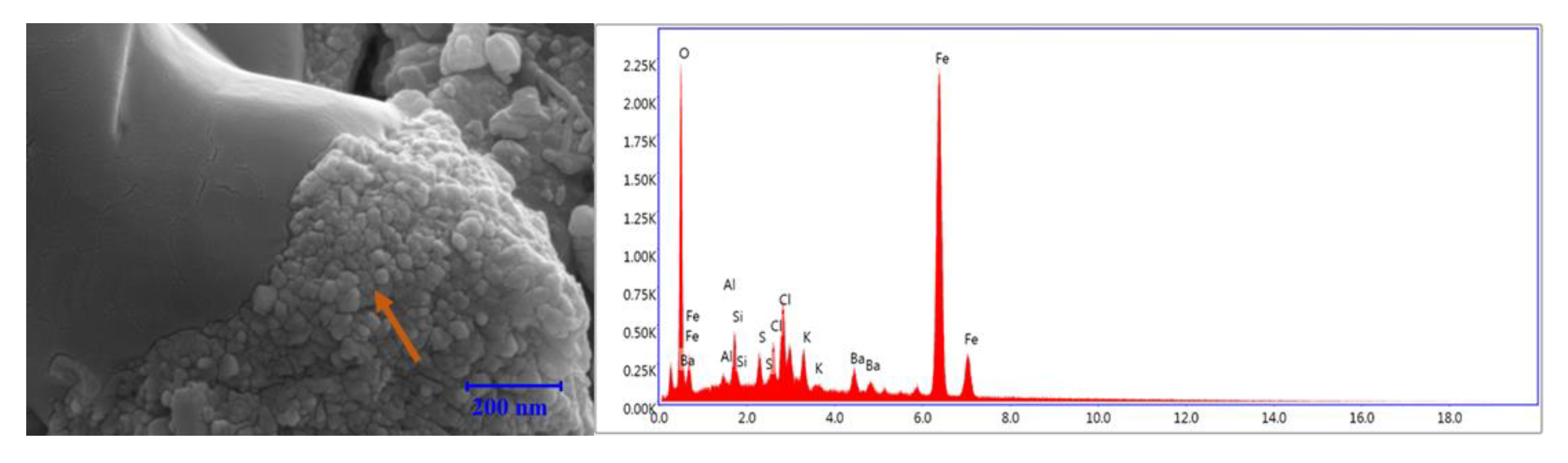
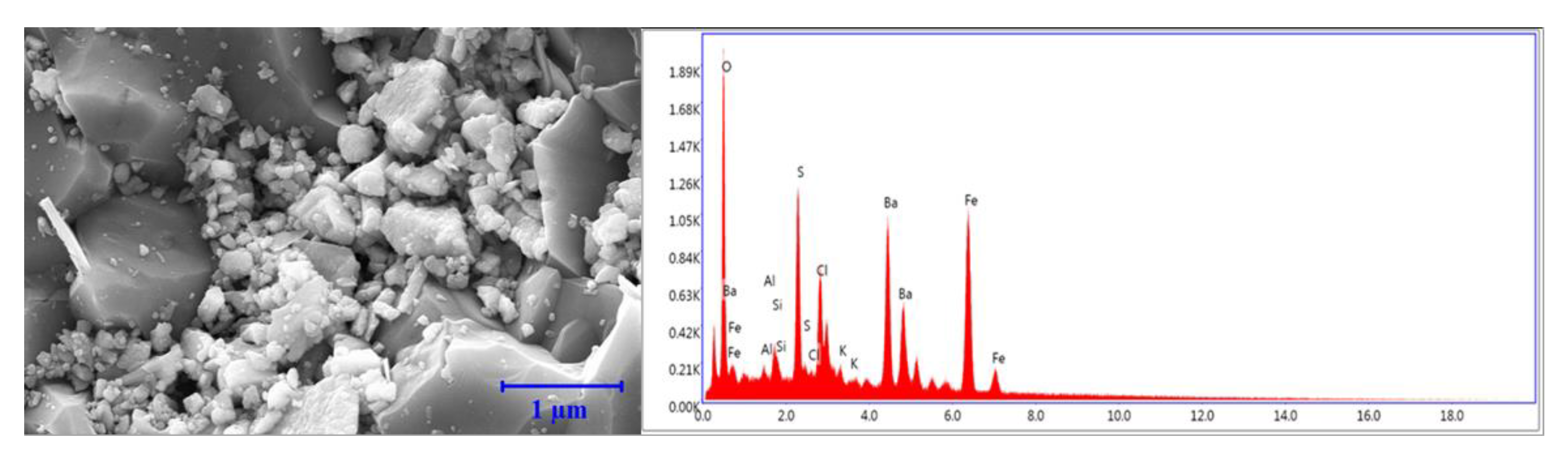
3.8. EDS Analysis
3.8.1. Bentonite-Based Fluids
3.8.2. KCl-Based Fluids
3.9. Elemental Analysis of Filtrate
3.10. Magnetic Recovery
4. Conclusions
- ➢
- Results indicate that NPs can modify the rheological parameters of bentonite-based fluids. NPs increases the viscosity and gel strength of the bentonite-based fluids owing to the agglomeration of magnetic NPs.
- ➢
- A polymer coating on the NPs slightly reduces the gel strength values at high temperature for bentonite-based fluids.
- ➢
- Small concentration of NPs was sufficient to reduce the coefficient of friction and fluid loss values of the bentonite-based fluids. NPs with 0.019 wt% (0.1 g) and 0.0095 wt% (0.05 g) reduces the coefficient of friction and fluid loss by 47% and 20%, respectively.
- ➢
- Fe-XG NPs also reduces the coefficient of friction by 29%, while fluid loss values were reduced by 16% for bentonite-based fluids.
- ➢
- Filter cake analysis shows that NPs filled the spaces in the structure of the cake and uniform distribution of particles kept the fluid loss values to a minimum compared to the base fluid.
- ➢
- In addition, elemental analysis shows the even distribution of the NPs throughout the structure of the filter cake.
- ➢
- In the case of KCl-based fluids, the addition of NPs has a slight impact on rheological parameters owing to the lesser stability of the fluid system due to presence of high salt concentration.
- ➢
- In case of coefficient of friction and fluid loss, NPs do provide improvements. Addition of 0.038 (0.2 g) wt%, and 0.019 wt% (0.1 g) of NPs reduces fluid loss and friction by 14% and 45%, respectively. However, Fe-XG NPs increase the fluid loss value by 11%.
- ➢
- Microscale analysis of the cake shows that NPs do interact with the salt and reduce the cake’s permeability by forming compact structures.
- ➢
- Fe-XG NPs increases the fluid loss values due to an increase in size and adsorption of polymer on the salt surface, creating voids in the cake structure.
- ➢
- This work also shows that magnetic recovery of NPs also provides further information regarding the interaction of NPs with other additives in the drilling fluid system.
- ➢
- This study shows that low concentration of NPs is sufficient to improve the properties of drilling fluids. Also, NPs can reduce the fluid loss values and friction values of KCl-based fluids without affecting the rheological parameters.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bourgoyne, A.T.; Millhelm, K.K.; Chenevert, M.E.; Young, F.S., Jr. Applied Drilling Engineering; Textbook Series Vol. 2; Society of Petroleum Engineering: Richardson, TX, USA, 1986. [Google Scholar]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Introduction to Drilling Fluids. In Composition and Properties of Drilling and Completion Fluids; Gulf Professional Publishing: Houston, TX, USA, 2017. [Google Scholar]
- He, S.; Liang, L.; Zeng, Y.; Ding, Y.; Lin, Y.; Liu, X. The influence of water-based drilling fluid on mechanical property of shale and the wellbore stability. Petroleum 2016, 2, 61–66. [Google Scholar] [CrossRef]
- Ewy, R.T.; Morton, E.K. Wellbore-Stability Performance of Water-Based Mud Additives. SPE Drill. Complet. 2009, 24, 390–397. [Google Scholar] [CrossRef]
- Jones, T.G.J.; Hughes, T.L. Drilling Fluid Suspensions. Adv. Chem. Ser. 1996, 251. [Google Scholar] [CrossRef]
- Al-Yasiri, M.; Awad, A.; Pervaiz, S.; Wen, D. Influence of silica nanoparticles on the functionality of water-based drilling fluids. J. Pet. Sci. Eng. 2019, 179, 504–512. [Google Scholar] [CrossRef]
- Agarwal, S.; Phuoc, T.X.; Soong, Y.; Martello, D.; Gupta, R.K. Nanoparticle-stabilised invert emulsion drilling fluids for deep-hole drilling of oil and gas. Can. J. Chem. Eng. 2013, 91, 1641–1649. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids; Gulf Professional Publishing: Houston, TX, USA, 2012. [Google Scholar] [CrossRef]
- Abdo, J.; Haneef, M.D. Nano-Enhanced Drilling Fluids: Pioneering Approach to Overcome Uncompromising Drilling Problems. J. Energy Resour. Technol. 2012, 134, 014501. [Google Scholar] [CrossRef]
- Abdo, J.; AL-Sharji, H.; Hassan, E. Effects of nano-sepiolite on rheological properties and filtration loss of water-based drilling fluids. Surf. Interface Anal. 2016, 48, 522–526. [Google Scholar] [CrossRef]
- Ghanbari, S.; Kazemzadeh, E.; Soleymani, M.; Naderifar, A. A facile method for synthesis and dispersion of silica nanoparticles in water-based drilling fluid. Colloid Polym. Sci. 2016, 294, 381–388. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V.; Sharma, V.P. Evaluation of polyacrylamide-grafted-polyethylene glycol/silica nanocomposite as potential additive in water based drilling mud for reactive shale formation. J. Nat. Gas Sci. Eng. 2015, 26, 526–537. [Google Scholar] [CrossRef]
- Katende, A.; Boyou, N.V.; Ismail, I.; Chung, D.Z.; Sagala, F.; Hussein, N.; Ismail, M.S. Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 645–673. [Google Scholar] [CrossRef]
- Sadeghalvaad, M.; Sabbaghi, S. The effect of the TiO2/polyacrylamide nanocomposite on water-based drilling fluid properties. Powder Technol. 2015, 272, 113–119. [Google Scholar] [CrossRef]
- Alvi, M.A.A.; Belayneh, M.; Saasen, A.; Fjelde, K.K.; Aadnøy, B.S. Effect of MWCNT and MWCNT functionalized -OH and -COOH nanoparticles in laboratory water based drilling fluid. In Proceedings of the ASME 37th International Conference on Ocean, Offshore & Arctic Engineering (OMAE), Madrid, Spain, 17–22 June 2018. [Google Scholar] [CrossRef]
- Aftab, A.; Ismail, A.R.; Khokhar, S.; Ibupoto, Z.H. Novel zinc oxide nanoparticles deposited acrylamide composite used for enhancing the performance of water-based drilling fluids at elevated temperature conditions. J. Pet. Sci. Eng. 2016, 146, 1142–1157. [Google Scholar] [CrossRef]
- Halali, M.A.; Ghotbi, C.; Tahmasbi, K.; Ghazanfari, M.H. The Role of Carbon Nanotubes in Improving Thermal Stability of Polymeric Fluids: Experimental and Modeling. Ind. Eng. Chem. Res. 2016, 55, 7514–7534. [Google Scholar] [CrossRef]
- Song, K.; Wu, Q.; Li, M.C.; Wojtanowicz, A.K.; Dong, L.; Zhang, X.; Ren, S.; Lei, T. Performance of low solid bentonite drilling fluids modified by cellulose nanoparticles. J. Nat. Gas Sci. Eng. 2016, 34, 1403–1411. [Google Scholar] [CrossRef]
- Song, K.; Wu, Q.; Li, M.; Ren, S.; Dong, L.; Zhang, X.; Lei, T.; Kojima, Y. Water-based bentonite drilling fluids modified by novel biopolymer for minimizing fluid loss and formation damage. Colloids Surf. A Physicochem. Eng. Asp. 2016, 507, 58–66. [Google Scholar] [CrossRef]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Mao, H.; Qiu, Z.; Shen, Z.; Huang, W. Hydrophobic associated polymer based silica nanoparticles composite with core-shell structure as a filtrate reducer for drilling fluid at utra-high temperature. J. Pet. Sci. Eng. 2015, 129, 1–14. [Google Scholar] [CrossRef]
- Taha, N.M.; Lee, S. Nano Graphene Application Improving Drilling Fluids Performance. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. [Google Scholar] [CrossRef]
- Fazelabdolabadi, B.; Khodadadi, A.A.; Sedaghatzadeh, M. Thermal and rheological properties improvement of drilling fluids using functionalized carbon nanotubes. Appl. Nanosci. 2015, 5, 651–659. [Google Scholar] [CrossRef]
- Sadegh Hassani, S.; Amrollahi, A.; Rashidi, A.; Soleymani, M.; Rayatdoost, S. The effect of nanoparticles on the heat transfer properties of drilling fluids. J. Pet. Sci. Eng. 2016, 146, 183–190. [Google Scholar] [CrossRef]
- Kang, Y.; She, J.; Zhang, H.; You, L.; Song, M. Strengthening shale wellbore with silica nanoparticles drilling fluid. Petroleum 2016, 2, 189–195. [Google Scholar] [CrossRef]
- Nwaoji, C.O.; Hareland, G.; Husein, M.; Nygaard, R.; Zakaria, M.F. Wellbore strengthening- nano-particle drilling fluid experimental design using hydraulic fracture apparatus. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 5–7 March 2013. [Google Scholar] [CrossRef]
- Sharma, M.M.; Zhang, R.; Chenevert, M.E.; Ji, L.; Guo, Q.; Friedheim, J. A new family of nanoparticle based drilling fluids. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar] [CrossRef]
- Aramendiz, J.; Imqam, A. Water-based drilling fluid formulation using silica and graphene nanoparticles for unconventional shale applications. J. Pet. Sci. Eng. 2019, 179, 742–749. [Google Scholar] [CrossRef]
- Jung, Y.; Barry, M.; Lee, J.-K.; Tran, P.; Soong, Y.; Martello, D.; Chyu, M. Effect of nanoparticle-additives on the rheological properties of clay-based fluids at high temperature and high pressure. In Proceedings of the AADE National Technical Conference and Exhibition, Houston, TX, USA, 12–14 April 2011; pp. 1–4. [Google Scholar]
- Barry, M.M.; Jung, Y.; Lee, J.K.; Phuoc, T.X.; Chyu, M.K. Fluid filtration and rheological properties of nanoparticle additive and intercalated clay hybrid bentonite drilling fluids. J. Pet. Sci. Eng. 2015, 127, 338–346. [Google Scholar] [CrossRef]
- Mahmoud, O.; Nasr-El-Din, H.A.; Vryzas, Z.; Kelessidis, V.C. Characterization of filter cake generated by nanoparticle-based drilling fluid for HP/HT applications. In Proceedings of the SPE International Conference on Oilfield Chemistry, Montgomery, TX, USA, 3–5 April 2017. [Google Scholar] [CrossRef]
- Vryzas, Z.; Mahmoud, O.; Nasr-El-Din, H.; Zaspalis, V.; Kelessidis, V.C. Incorporation of Fe3O4 nanoparticles as drilling fluid additives for improved drilling operations. In Proceedings of the ASME 35th International Conference on Ocean, Offshore & Arctic Engineering (OMAE), Busan, Korea, 19–24 June 2016. [Google Scholar] [CrossRef]
- Vryzas, Z.; Zaspalis, V.; Nalbantian, L.; Mahmoud, O.; Nasr-El-Din, H.A.; Kelessidis, V.C. A comprehensive approach for the development of new iron oxide nanoparticles giving smart drilling fluids with superior properties for HP/HT applications. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016. [Google Scholar] [CrossRef]
- Mahmoud, O.; Nasr-El-Din, H.A.; Vryzas, Z.; Kelessidis, V.C. Effect of ferric oxide nanoparticles on the properties of filter cake formed by calcium bentonite-based drilling muds. SPE Drill. Complet. 2018, 33. [Google Scholar] [CrossRef]
- Mahmoud, O.; Nasr-El-din, H.A.; Vryzas, Z.; Kelessidis, V.C. Using ferric oxide and silica nanoparticles to develop modified calcium bentonite drilling fluids. SPE Drill. Complet. 2018, 33. [Google Scholar] [CrossRef]
- Alvi, M.A.A.; Belayneh, M.; Saasen, A.; AadnØy, B.S. The effect of micro-sized boron nitride BN and iron trioxide Fe2O3 nanoparticles on the properties of laboratory bentonite drilling fluid. In Proceedings of the SPE Norway One Day Seminar, Bergen, Norway, 18 April 2018. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Guo, J.; Su, Q.; Zhuo, X. Effects of inhibitor KCl on shale expansibility and mechanical properties. Petroleum 2019, 5, 407–412. [Google Scholar] [CrossRef]
- Puddu, M.; Paunescu, D.; Stark, W.J.; Grass, R.N. Magnetically recoverable, thermostable, hydrophobic DNA/silica encapsulates and their application as invisible oil tags. ACS Nano 2014, 8, 2677–2685. [Google Scholar] [CrossRef]
- Torsvik, A.; Myrseth, V.; Opedal, N.; Lund, B.; Saasen, A.; Ytrehus, J.D. Rheological comparison of bentonite based and KCl/polymer based drilling fluids. Annu. Trans. Nord. Rheol. Soc. 2014, 22, 219–224. [Google Scholar]
- API. Recommended Practice for Field Testing Water-Based Drilling Fluids; 13B-1 APIRP; API: Washington, DC, USA, 2009. [Google Scholar]
- Sharma, A.; Foppen, J.W.; Banerjee, A.; Slimani, S.; Bachhar, N.; Peddis, D.; Bandyopadhyay, S. Magnetic Nanoparticles to Unique DNA Tracers—Effect of Functionalization on Physico-Chemical Properties. Nanoscale Res. Lett. 2020. [Google Scholar] [CrossRef]
- Darley, H.C.H.; Gray, G.R. Composition and Properties of Drilling and Completion Fluids; Gulf Professional Publishing: Houston, TX, USA, 1988. [Google Scholar]
- Vryzas, Z.; Kelessidis, V.C.; Nalbantian, L.; Zaspalis, V.; Gerogiorgis, D.I.; Wubulikasimu, Y. Effect of temperature on the rheological properties of neat aqueous Wyoming sodium bentonite dispersions. Appl. Clay Sci. 2017, 136, 26–36. [Google Scholar] [CrossRef]
- Mouzon, J.; Bhuiyan, I.U.; Hedlund, J. The structure of montmorillonite gels revealed by sequential cryo-XHR-SEM imaging. J. Colloid Interface Sci. 2016, 465, 58–66. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Kamal, M.S.; Al-Harthi, M.A. Rheological and filtration properties of clay-polymer systems: Impact of polymer structure. Appl. Clay Sci. 2018, 160, 226–237. [Google Scholar] [CrossRef]
- Vryzas, Z.; Nalbandian, L.; Zaspalis, V.T.; Kelessidis, V.C. How different nanoparticles affect the rheological properties of aqueous Wyoming sodium bentonite suspensions. J. Pet. Sci. Eng. 2019, 173, 941–954. [Google Scholar] [CrossRef]
- Chang, W.Z.; Leong, Y.K. Ageing and collapse of bentonite gels—Effects of Li, Na, K and Cs ions. Rheologica Acta 2014, 53, 109–122. [Google Scholar] [CrossRef]
- Bui, B.; Saasen, A.; Maxey, J.; Ozbayoglu, M. Viscoelastic Properties of Oil-Based Drilling Fluids. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 33–47. [Google Scholar]
- Saasen, A.; Liu, D.; Marken, C.D. Prediction of barite sag potential of drilling fluids from rheological measurements. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 28 February–2 March 1995. [Google Scholar] [CrossRef]
- Barnes, H.A.; Nguyen, Q.D. Rotating vane rheometry—A review. J. Nonnewtonian Fluid Mech. 2001, 98, 1–14. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Andrade, D.E.V.; Franco, A.T.; Negrão, C.O.R. Correlation between the gel-liquid transition stress and the storage modulus of an oil-based drilling fluid. J. Nonnewton Fluid Mech. 2016, 231, 6–10. [Google Scholar] [CrossRef]
- Vryzas, Z.; Mahmoud, O.; Nasr-El-din, H.A.; Kelessidis, V.C. Development and testing of novel drilling fluids using Fe2O3 and SiO2 nanoparticles for enhanced drilling operations. In Proceedings of the International Petroleum Technology Conference (IPTC), Doha, Qatar, 6–9 December 2015. [Google Scholar] [CrossRef]
| Material | Base Fluid | Base Fluid + NPs |
|---|---|---|
| Water | 350 mL | 350 mL |
| Soda ash | 4.8 g | 4.8 g |
| Xanthan gum | 0.71 g | 0.71 g |
| Bentonite | 10.04 g | 10.04 g |
| Barite | 183 g | 183 g |
| Iron oxide NPs in water | - | 0.05, 0.1, 0.2 g |
| Material | Base Fluid | Base Fluid + NPs |
|---|---|---|
| Water | 350 mL | 350 mL |
| Soda ash | 0.75 g | 0.75 g |
| Xanthan gum | 1.5 g | 1.5 g |
| KCl | 24.80 g | 24.80 g |
| Barite | 175 g | 175 g |
| Iron oxide NPs in water | - | 0.05, 0.1, 0.2 g |
| Material | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Iron oxide NPs at 25 °C | 273.5 ± 5.95 | −31.3 ± 0.27 |
| Fe-XG NPs at 25 °C | 487.60 ± 8.55 | −39.23 ± 0.59 |
| Iron oxide NPs at 50 °C | 381.17 ± 19.58 | −20.17 ± 0.23 |
| Fe-XG NPs at 50 °C | 673.57 ± 65.45 | −34.37 ± 0.42 |
| Samples | Yield Stress (Pa) | Flow Point (Pa) |
|---|---|---|
| Base fluid (BF) | 4.6 | 8.5 |
| BF + 0.05 g NPs | 5.6 | 9.3 |
| BF + 0.1 g NPs | 4.9 | 9.5 |
| BF + 0.2 g NPs | 5.2 | 8.9 |
| BF + Fe-XG NPs | 5.1 | 9.4 |
| Samples | Yield Stress (Pa) | Flow Point (Pa) |
|---|---|---|
| Base fluid (BF-1) | 2.0 | 4.9 |
| BF-1 + 0.05 g NPs | 2.0 | 4.6 |
| BF-1 + 0.1 g NPs | 1.9 | 4.4 |
| Base fluid (BF-2) | 1.6 | 4.3 |
| BF-2 + 0.2 g NPs | 1.4 | 3.7 |
| BF-2 + Fe-XG NPs | 2.0 | 4.6 |
| Elements | Base Fluid | Base Fluid + 0.05 g NPs | Base Fluid + 0.2 g NPs | Base Fluid + Fe-XG |
|---|---|---|---|---|
| Barium, Ba (mg/L) | 0.31 | 0.27 | 0.27 | 0.30 |
| Calcium Ca (mg/L) | 0.7 | 1.0 | 0.5 | 0.6 |
| Copper Cu (mg/L) | 0.7 | 0.91 | 0.6 | 0.6 |
| Iron Fe (mg/L) | n.d. | n.d. | 0.09 | <0.06 |
| Potassium K (mg/L) | 237 | 108 | 123 | 35 |
| Magnesium Mg (mg/L) | 2.2 | 2.1 | 2.3 | 2.4 |
| Sodium Na (mg/L) | 5906 | 5579 | 5460 | 5370 |
| Silicon Si (mg/L) | 5.6 | 5.6 | 4.5 | 5.3 |
| Strontium Sr (mg/L) | 0.9 | 1.3 | 0.5 | 0.3 |
| Aluminium Al (mg/L) | 1.4 | <0.5 | 2.0 | 2.3 |
| Elements | Base Fluid | Base Fluid + 0.05 g NPs | Base Fluid + 0.2 g NPs | Base Fluid + Fe-XG |
|---|---|---|---|---|
| Barium, Ba (mg/L) | 1.8 | 1.2 | 1.4 | 1.6 |
| Calcium Ca (mg/L) | <3 | <3 | 2.1 | 3.7 |
| Copper Cu (mg/L) | 1.9 | 1.5 | 1.5 | 1.4 |
| Iron Fe (mg/L) | n.d. | n.d. | <0.3 | <0.3 |
| Potassium K (mg/L) | 36,144 | 35,564 | 33,400 | 32,700 |
| Magnesium Mg (mg/L) | 4 | 4 | 4 | 3.2 |
| Sodium Na (mg/L) | 894 | 826 | 820 | 850 |
| Silicon Si (mg/L) | <3 | <3 | 2.4 | 2.6 |
| Strontium Sr (mg/L) | 9.3 | 8.6 | 8.3 | 8.3 |
| Aluminium Al (mg/L) | <5 | <5 | <5 | <5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvi, M.A.A.; Belayneh, M.; Bandyopadhyay, S.; Minde, M.W. Effect of Iron Oxide Nanoparticles on the Properties of Water-Based Drilling Fluids. Energies 2020, 13, 6718. https://doi.org/10.3390/en13246718
Alvi MAA, Belayneh M, Bandyopadhyay S, Minde MW. Effect of Iron Oxide Nanoparticles on the Properties of Water-Based Drilling Fluids. Energies. 2020; 13(24):6718. https://doi.org/10.3390/en13246718
Chicago/Turabian StyleAlvi, Muhammad Awais Ashfaq, Mesfin Belayneh, Sulalit Bandyopadhyay, and Mona Wetrhus Minde. 2020. "Effect of Iron Oxide Nanoparticles on the Properties of Water-Based Drilling Fluids" Energies 13, no. 24: 6718. https://doi.org/10.3390/en13246718
APA StyleAlvi, M. A. A., Belayneh, M., Bandyopadhyay, S., & Minde, M. W. (2020). Effect of Iron Oxide Nanoparticles on the Properties of Water-Based Drilling Fluids. Energies, 13(24), 6718. https://doi.org/10.3390/en13246718






