High Channel Density Ceramic Microchannel Reactor for Syngas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Coating
2.2. Catalyst Characterisation
2.3. Catalytic Activity
3. Results and Discussion
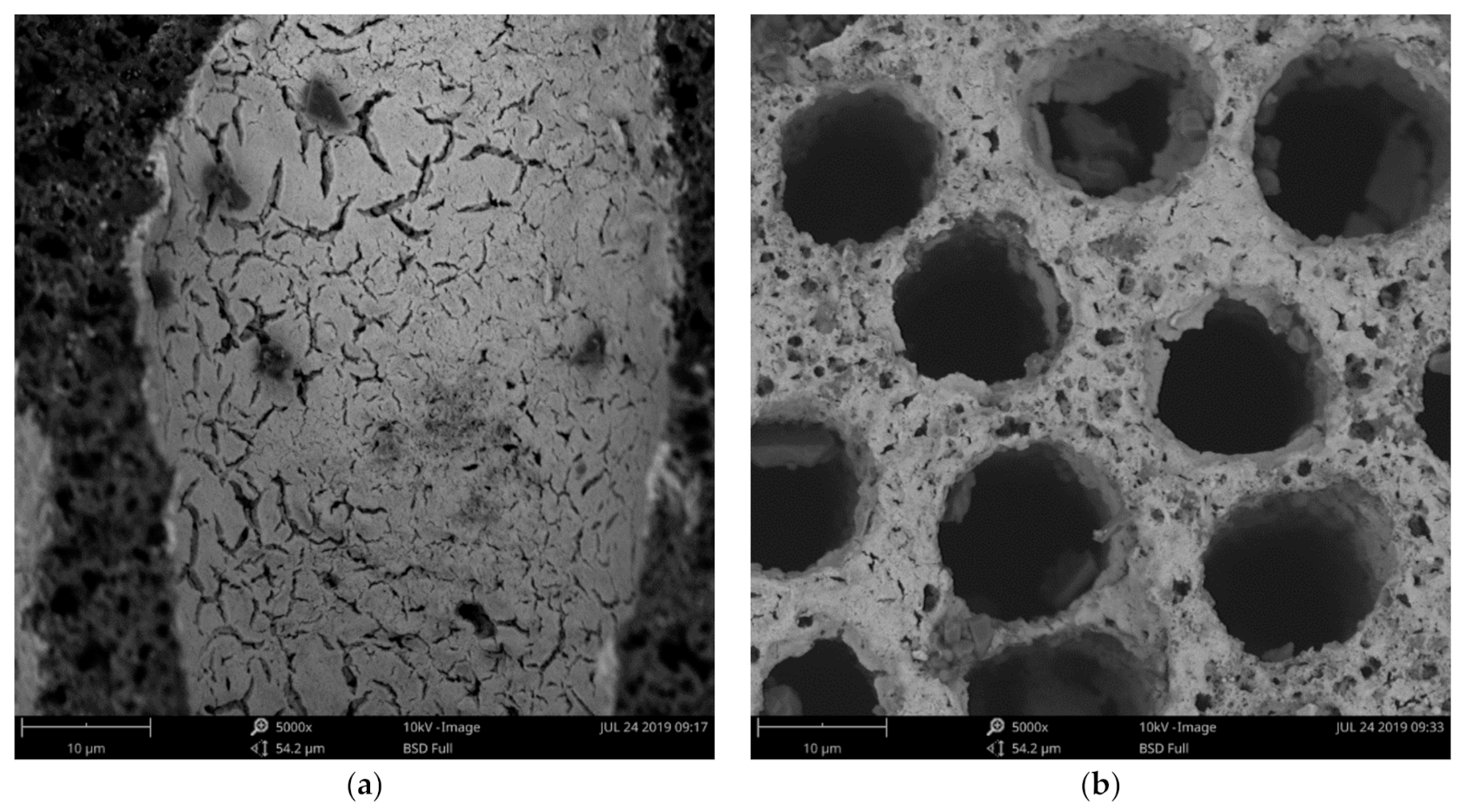
3.1. Physicochemical Properties
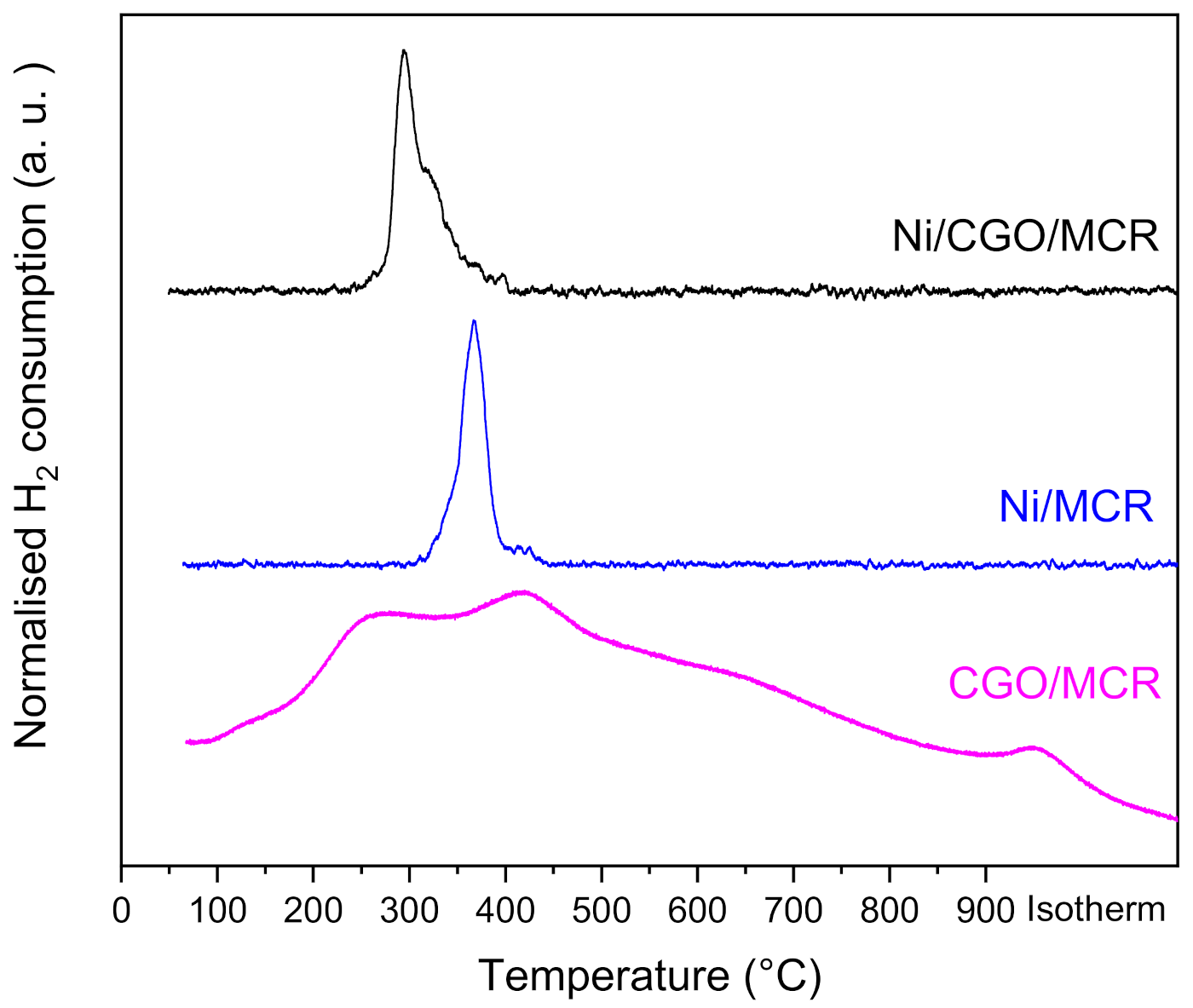
3.2. Reducibility
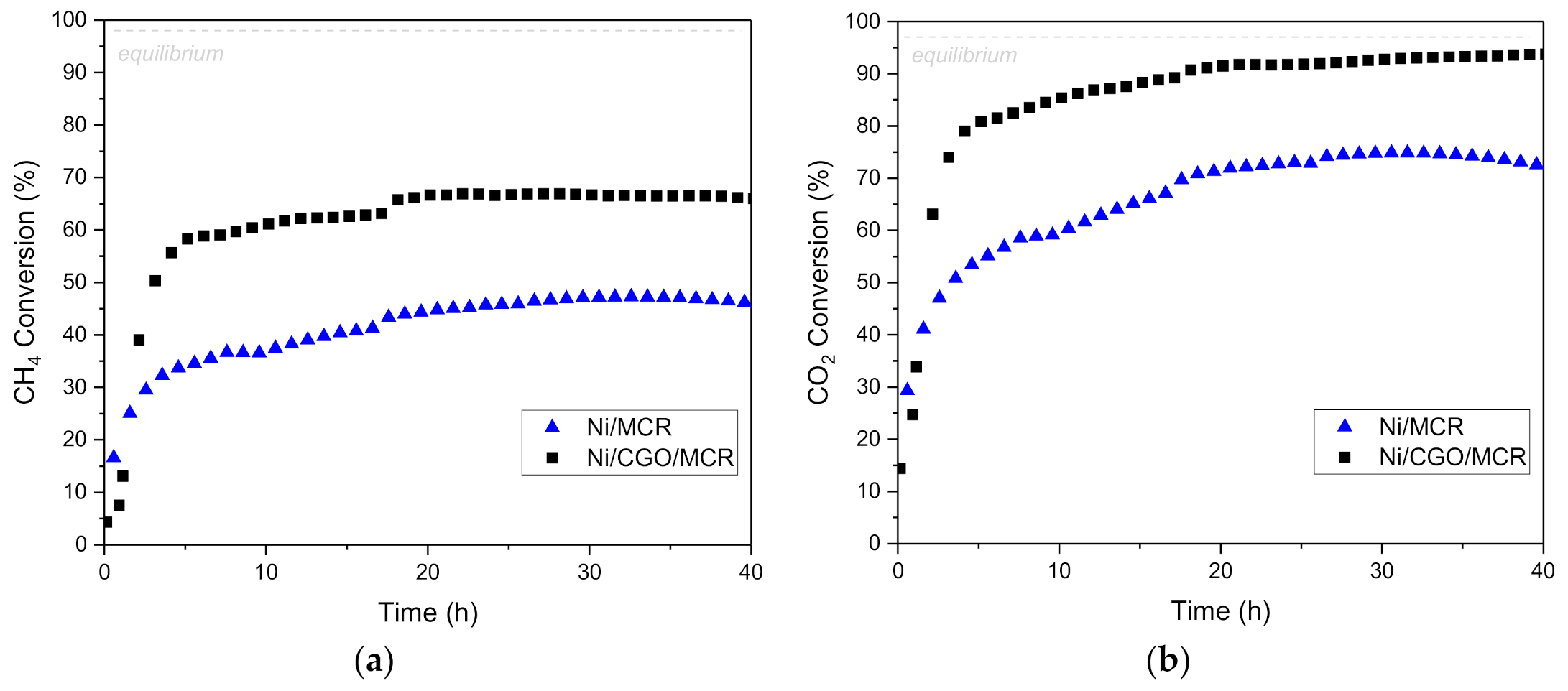
3.3. Catalytic Performance: DRM
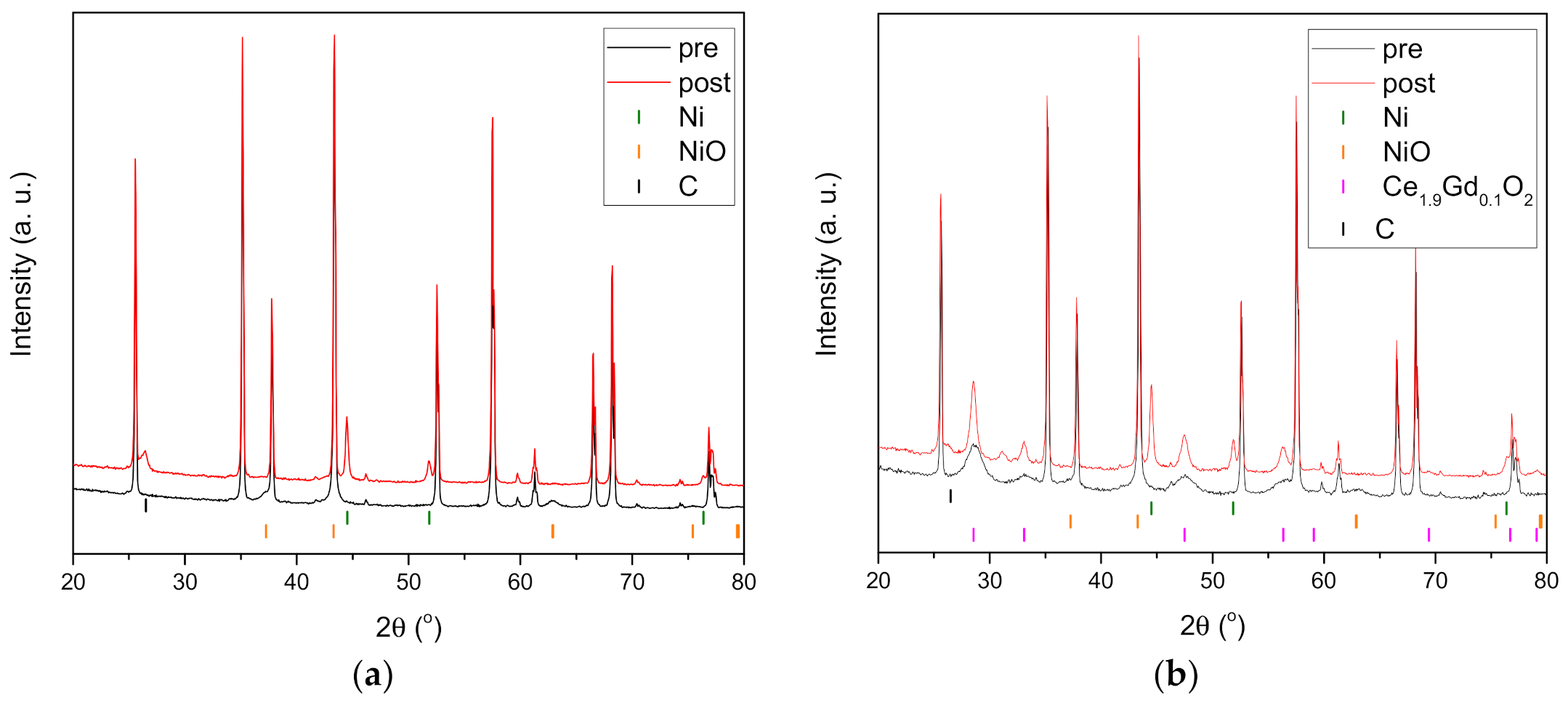
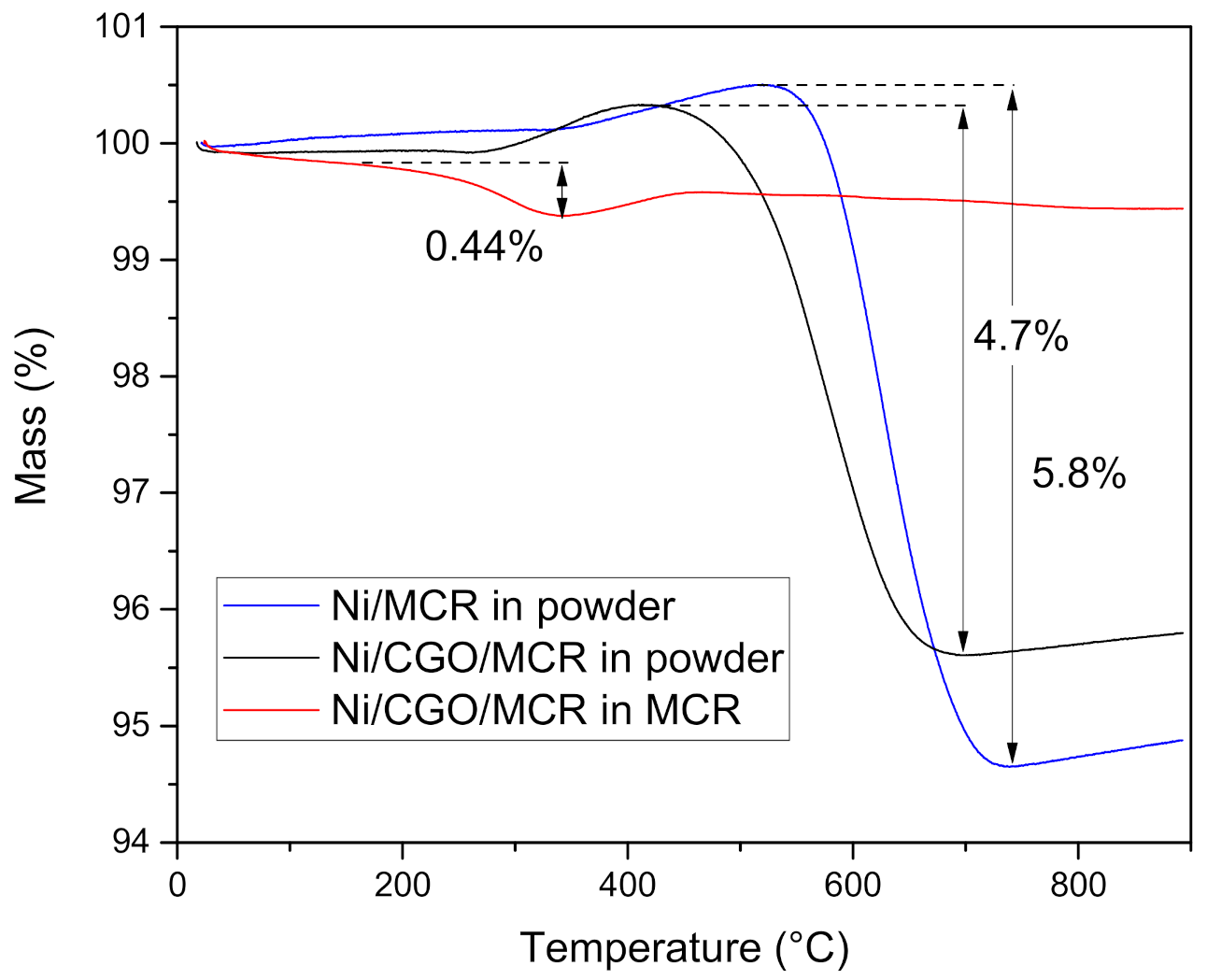
3.4. Characterisation Post Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Le Saché, E.; Johnson, S.; Pastor-Pérez, L.; Amini Horri, B.; Reina, T.R. Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source. Energies 2019, 12, 1007. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Pérez, L.; Garcilaso, V.; Watson, D.J.; Centeno, M.A.; Odriozola, J.A.; Reina, T.R. Flexible syngas production using a La2Zr2-xNixO7-δ pyrochlore-double perovskite catalyst: Towards a direct route for gas phase CO2 recycling. Catal. Today 2019, 357, 583–589. [Google Scholar] [CrossRef]
- Le Saché, E.; Santos, J.L.; Smith, T.J.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Multicomponent Ni-CeO2 nanocatalysts for syngas production from CO2/CH4 mixtures. J. CO2 Util. 2018, 25, 68–78. [Google Scholar] [CrossRef]
- Stroud, T.; Smith, T.J.; le Saché, E.; Santos, J.L.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B 2018, 224, 125–135. [Google Scholar] [CrossRef]
- Kohn, M.P.; Castaldi, M.J.; Farrauto, R.J. Auto-thermal and dry reforming of landfill gas over a Rh/γAl2O3 monolith catalyst. Appl. Catal. B 2010, 94, 125–133. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C.; De Felicis, D.; Basoli, F.; Battocchio, C.; Tuti, S.; Licoccia, S.; Di Bartolomeo, E. Ni supported on γ-Al2O3 promoted by Ru for the dry reforming of methane in packed and monolithic reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Kim, H.; You, Y.-W.; Heo, I.; Chang, T.-S.; Hong, J.S.; Lee, K.B.; Suh, J.K. Development of Monolithic Catalyst System with Co-Ru-Zr for CO2 (dry) Reforming of Methane: Enhanced Coke Tolerance. Clean Technol. 2017, 23, 314–324. [Google Scholar]
- Leba, A.; Yıldırım, R. Determining most effective structural form of nickel-cobalt catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 4268–4283. [Google Scholar] [CrossRef]
- Soloviev, S.O.; Kapran, A.Y.; Orlyk, S.N.; Gubareni, E.V. Carbon dioxide reforming of methane on monolithic Ni/Al2O3-based catalysts. J. Nat. Gas Chem. 2011, 20, 184–190. [Google Scholar] [CrossRef]
- Gao, X.; Liu, G.; Wei, Q.; Yang, G.; Masaki, M.; Peng, X.; Yang, R.; Tsubaki, N. Carbon nanofibers decorated SiC foam monoliths as the support of anti-sintering Ni catalyst for methane dry reforming. Int. J. Hydrogen Energy 2017, 42, 16547–16556. [Google Scholar] [CrossRef]
- Fukuhara, C.; Hyodo, R.; Yamamoto, K.; Masuda, K.; Watanabe, R. A novel nickel-based catalyst for methane dry reforming: A metal honeycomb-type catalyst prepared by sol–gel method and electroless plating. Appl. Catal. A 2013, 468, 18–25. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Ji, S.; Huang, B.; Li, C. Preparation of Ni-Based Metal Monolithic Catalysts and a Study of Their Performance in Methane Reforming with CO2. ChemSusChem 2008, 1, 527–533. [Google Scholar] [CrossRef]
- Zhai, X.; Cheng, Y.; Zhang, Z.; Jin, Y.; Cheng, Y. Steam reforming of methane over Ni catalyst in micro-channel reactor. Int. J. Hydrogen Energy 2011, 36, 7105–7113. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Álvarez, A.; Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Montes, M.; Odriozola, J.A. Influence of the shape of Ni catalysts in the glycerol steam reforming. Appl. Catal. B 2012, 123–124, 379–390. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Blay, V.; Álvarez, A.; Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Intensifying glycerol steam reforming on a monolith catalyst: A reaction kinetic model. Chem. Eng. J. 2016, 306, 933–941. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Sanz, O.; Italiano, C.; Vita, A.; Montes, M.; Specchia, S. Analysis of Ru/La-Al2O3 catalyst loading on alumina monoliths and controlling regimes in methane steam reforming. Chem. Eng. J. 2018, 334, 1792–1807. [Google Scholar] [CrossRef]
- Katheria, S.; Deo, G.; Kunzru, D. Washcoating of Ni/MgAl2O4 Catalyst on FeCralloy Monoliths for Steam Reforming of Methane. Energy Fuels 2017, 31, 3143–3153. [Google Scholar] [CrossRef]
- Kawamura, Y.; Ogura, N.; Yamamoto, T.; Igarashi, A. A miniaturized methanol reformer with Si-based microreactor for a small PEMFC. Chem. Eng. Sci. 2006, 61, 1092–1101. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Luqmanulhakim, B.; Matthew James, W. Monolithic substrate support catalyst design considerations for steam methane reforming operation. Rev. Chem. Eng. 2018, 34, 481–501. [Google Scholar]
- Papadopoulou, C.; Matralis, H.; Verykios, X. Utilization of Biogas as a Renewable Carbon Source: Dry Reforming of Methane. In Catalysis for Alternative Energy Generation; Guczi, L., Erdôhelyi, A., Eds.; Springer: New York, NY, USA, 2012; pp. 57–127. [Google Scholar]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Aspects of CO2-reforming of Methane. In Studies in Surface Science and Catalysis; Curry-Hyde, H.E., Howe, R.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 81, pp. 25–41. [Google Scholar]
- Rodrigues, M.T.; Zonetti, P.C.; Alves, O.C.; Sousa-Aguiar, E.F.; Borges, L.E.P.; Appel, L.G. RWGS reaction employing Ni/Mg(Al,Ni)O—The role of the O vacancies. Appl. Catal. A 2017, 543, 98–103. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Ramirez Reina, T.; Alvarez Moreno, A.; Ivanova, S.; Odriozola Gordón, J.A.; Centeno Gallego, M.Á. Influence of Vanadium or Cobalt Oxides on the CO Oxidation Behavior of Au/MOx/CeO2-Al2O3 Systems. ChemCatChem 2012, 4, 512–520. [Google Scholar] [CrossRef]
- Reina, T.; Papadopoulou, E.; Palma, S.; Ivanova, S.; Centeno, M.; Ioannides, T.; Odriozola, J.A. Could an efficient WGS catalyst be useful in the CO-PrOx reaction? Appl. Catal. B 2014, 150, 554–563. [Google Scholar] [CrossRef]
- Gurav, H.R.; Dama, S.; Samuel, V.; Chilukuri, S. Influence of preparation method on activity and stability of Ni catalysts supported on Gd doped ceria in dry reforming of methane. J. CO2 Util. 2017, 20, 357–367. [Google Scholar] [CrossRef]
- Ruiz-Trejo, E.; Sirman, J.; Baikov, Y.M.; Kilner, J. Oxygen ion diffusivity, surface exchange and ionic conductivity in single crystal Gadolinia doped Ceria. Solid State Ion. 1998, 113, 565–569. [Google Scholar] [CrossRef]
- Llorca, J.; Casanovas, A.; Trifonov, T.; Rodríguez, A.; Alcubilla, R. First use of macroporous silicon loaded with catalyst film for a chemical reaction: A microreformer for producing hydrogen from ethanol steam reforming. J. Catal. 2008, 255, 228–233. [Google Scholar] [CrossRef]
- Divins, N.J.; Trifonov, T.; Rodríguez, Á.; Llorca, J. A Million-Microchannel Multifuel Steam Reformer for hydrogen production. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Pla, D.; Salleras, M.; Morata, A.; Garbayo, I.; Gerbolés, M.; Sabaté, N.; Divins, N.J.; Casanovas, A.; Llorca, J.; Tarancón, A. Standalone ethanol micro-reformer integrated on silicon technology for onboard production of hydrogen-rich gas. Lab Chip 2016, 16, 2900–2910. [Google Scholar] [CrossRef] [PubMed]
- Florea, M.; Postole, G.; Matei-Rutkovska, F.; Urda, A.; Neaţu, F.; Massin, L.; Gelin, P. Influence of Gd and Pr doping on the properties of ceria: Texture, structure, redox behaviour and reactivity in CH4/H2O reactions in the presence of H2S. Catal. Sci. Technol. 2018, 8, 1333–1348. [Google Scholar] [CrossRef]
- Zhao, P.; Qin, F.; Huang, Z.; Sun, C.; Shen, W.; Xu, H. Morphology-dependent oxygen vacancies and synergistic effects of Ni/CeO2 catalysts for N2O decomposition. Catal. Sci. Technol. 2018, 8, 276–288. [Google Scholar] [CrossRef]
- Dow, W.-P.; Wang, Y.-P.; Huang, T.-J. Yttria-Stabilized Zirconia Supported Copper Oxide Catalyst: I. Effect of Oxygen Vacancy of Support on Copper Oxide Reduction. J. Catal. 1996, 160, 155–170. [Google Scholar] [CrossRef]
- le Saché, E.; Pastor-Pérez, L.; Watson, D.; Sepúlveda-Escribano, A.; Reina, T.R. Ni stabilised on inorganic complex structures: Superior catalysts for chemical CO2 recycling via dry reforming of methane. Appl. Catal. B 2018, 236, 458–465. [Google Scholar] [CrossRef]
- Guharoy, U.; Le Saché, E.; Cai, Q.; Reina, T.R.; Gu, S. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane. J. CO2 Util. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, W.; Wang, J.; Li, Z.; Ma, J. Characterization and Analysis of Carbon Deposited during the Dry Reforming of Methane over Ni/La2O3/Al2O3 Catalysts. Chin. J. Catal. 2009, 30, 1076–1084. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zheng, X. The deposition of coke from methane on a Ni/MgAl2O4 catalyst. Carbon 2007, 45, 1314–1321. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saché, E.l.; Tsaousis, P.; Reina, T.R.; Ruiz-Trejo, E. High Channel Density Ceramic Microchannel Reactor for Syngas Production. Energies 2020, 13, 6472. https://doi.org/10.3390/en13236472
Saché El, Tsaousis P, Reina TR, Ruiz-Trejo E. High Channel Density Ceramic Microchannel Reactor for Syngas Production. Energies. 2020; 13(23):6472. https://doi.org/10.3390/en13236472
Chicago/Turabian StyleSaché, Estelle le, Panayiotis Tsaousis, Tomas Ramirez Reina, and Enrique Ruiz-Trejo. 2020. "High Channel Density Ceramic Microchannel Reactor for Syngas Production" Energies 13, no. 23: 6472. https://doi.org/10.3390/en13236472
APA StyleSaché, E. l., Tsaousis, P., Reina, T. R., & Ruiz-Trejo, E. (2020). High Channel Density Ceramic Microchannel Reactor for Syngas Production. Energies, 13(23), 6472. https://doi.org/10.3390/en13236472





