Reversible Molten Catalytic Methane Cracking Applied to Commercial Solar-Thermal Receivers
Abstract
1. Introduction
2. Materials and Methods
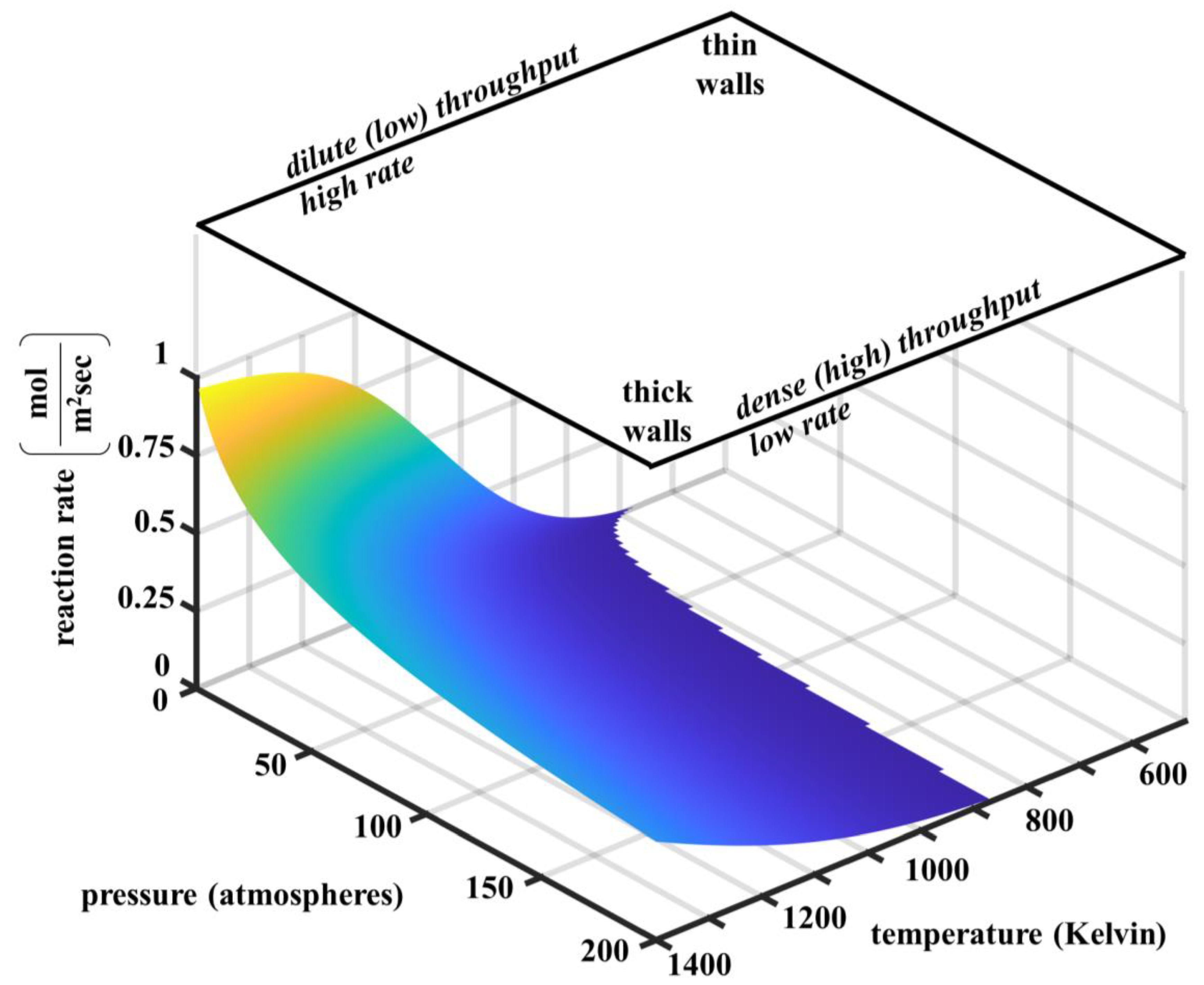
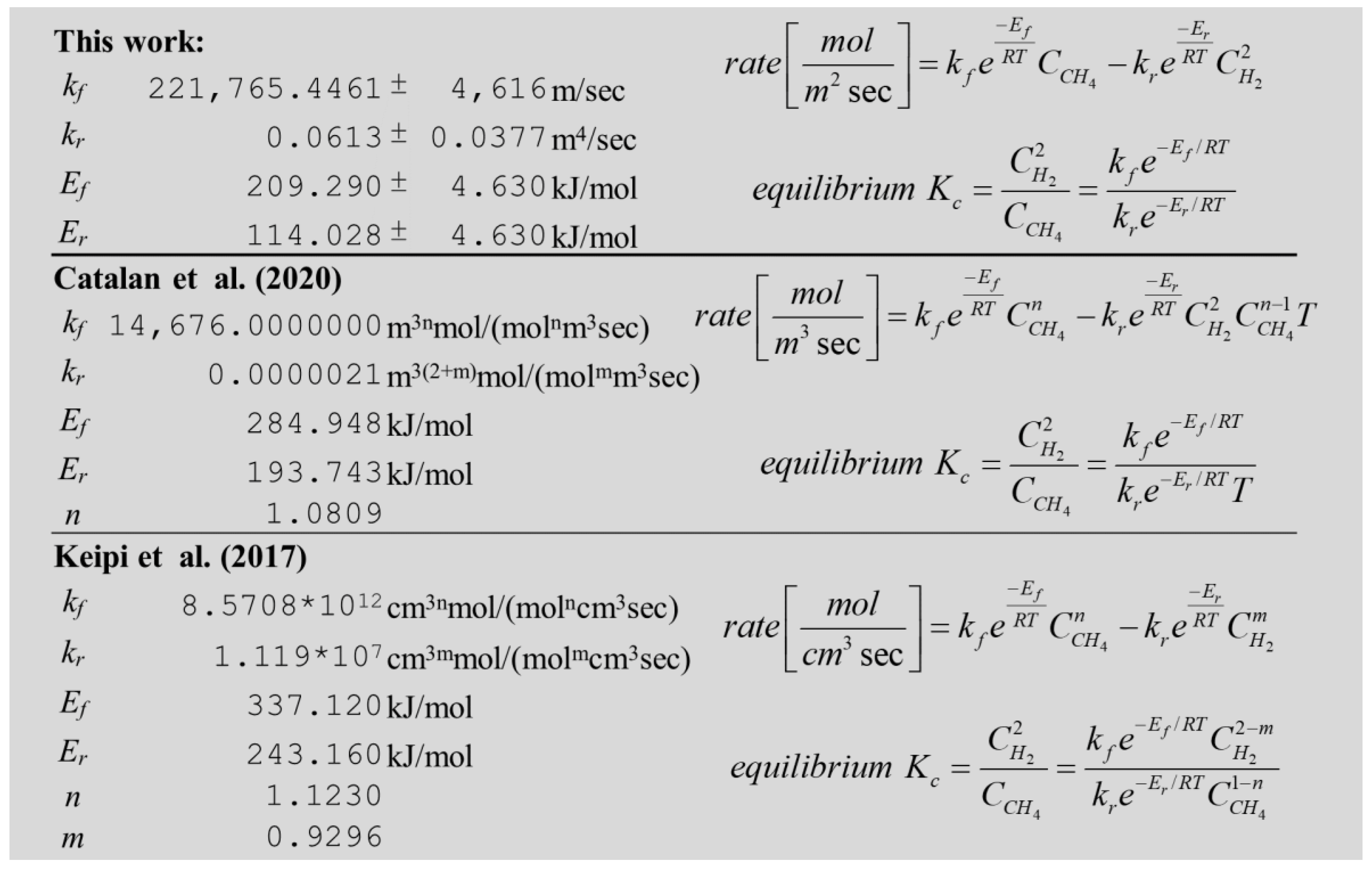
2.1. Reversible Catalytic Molten Methane Cracking Kinetics
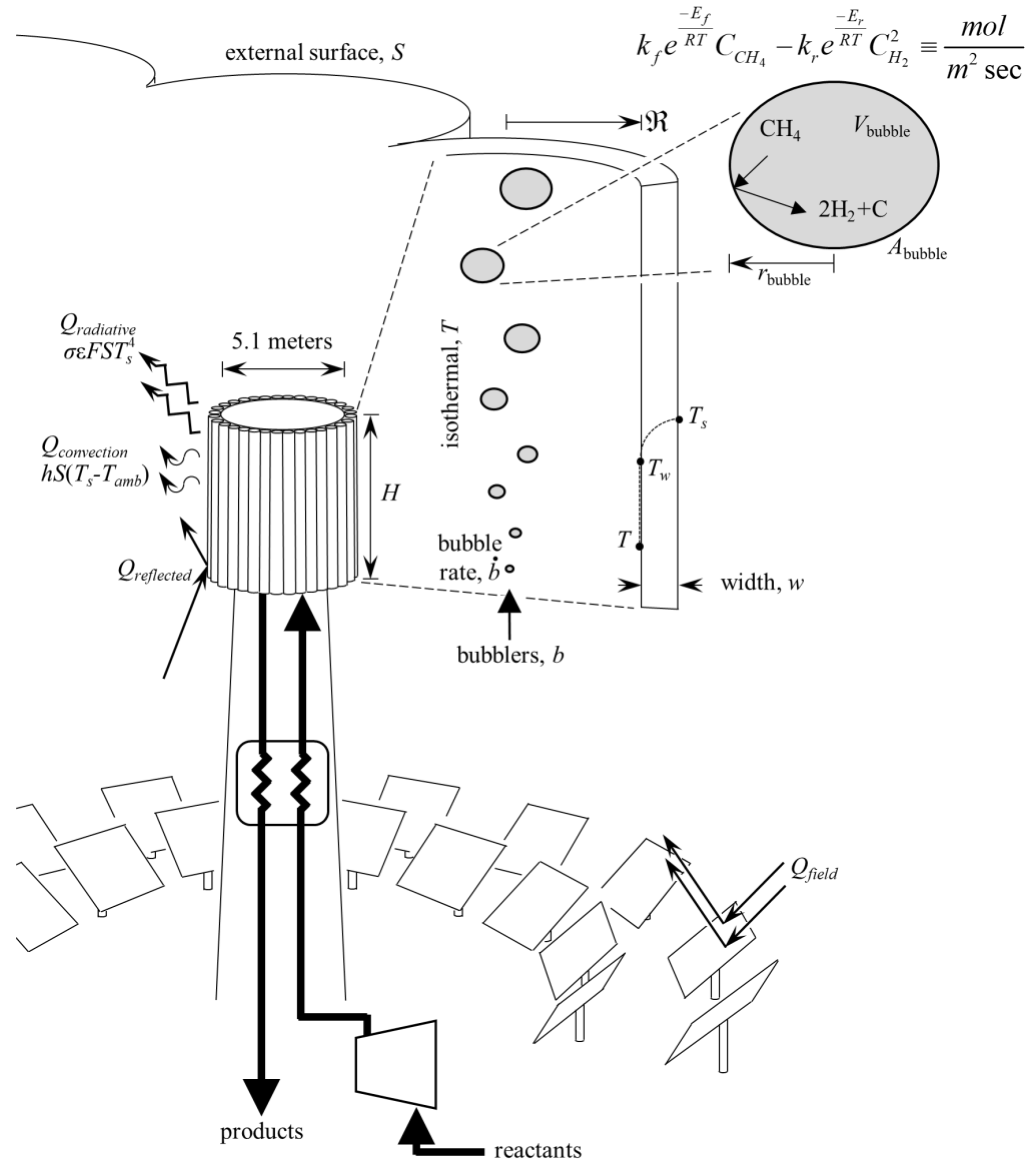
2.2. Thermal Reactor Model
3. Results
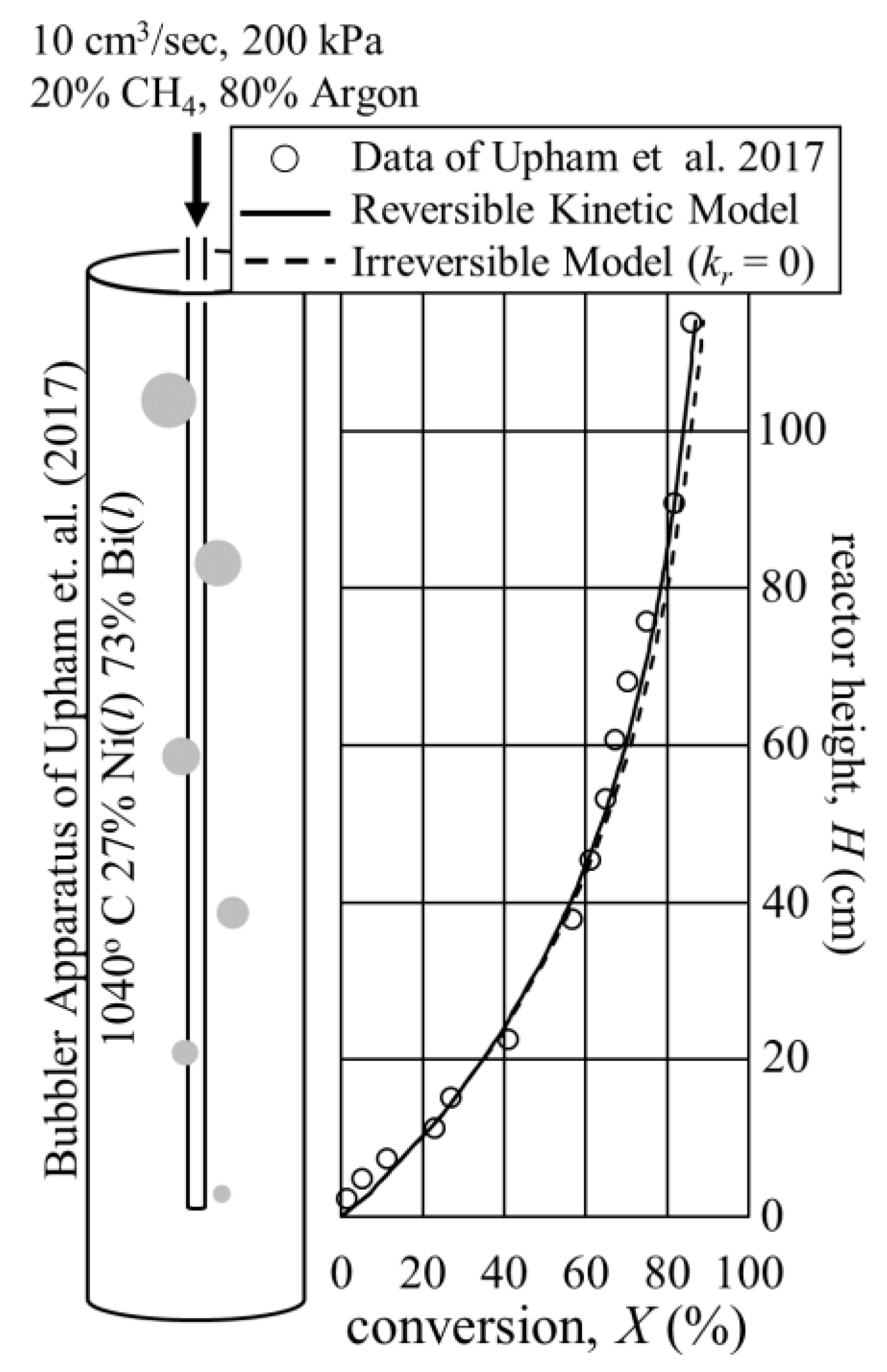
3.1. Validation of Reversible Catalytic Molten Methane Cracking Kinetics
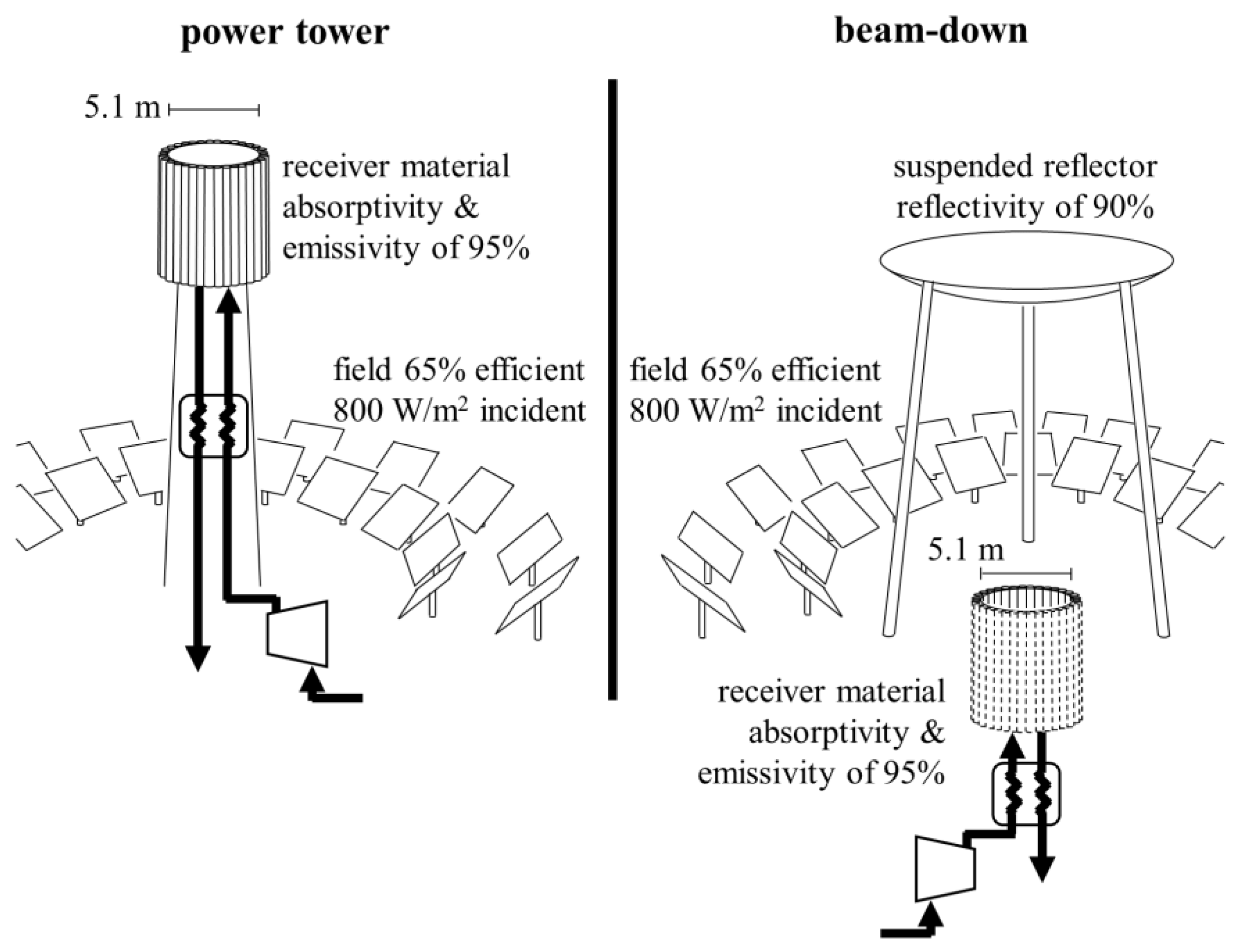
3.2. Solar Reactor Screening and Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Greek Variable Definitions
| α | fractional | effective absorptivity of a receiver surface from ray tracing |
| ε | fractional | effective emissivity of a receiver surface from ray tracing |
| η | % | energy efficiency of a solar receiver in hydrogen production |
| ø | meters | diameter of a vessel. |
| ρ | kg/m3 | density of molten 27% nickel, 73% bismuth [33,34] |
| ρgas | kg/m4 | gas density |
| ρinlet | kg/m5 | gas density at a reactor tube inlet temperature and pressure |
| σ | W/(m2K4) | Stefan–Boltzmann constant [5.67…×10–8 W/(m2K4)] |
| σYS(T) | Pa | Inconel temperature-dependent yield strength [46] |
Appendix A.2. English Variable Definitions
| Abubble | m2 | area of bubble |
| Abubblers | m2 | area of gas emanation on a gas distributor |
| Atube | m2 | cross-sectional area of a bubbler (reactor) tube |
| b | bubbles | number of bubbles initiated by a gas distributor |
| ḃ | bubbles/sec | rate of bubble emanation from a gas distributor |
| Cp,i | J/(mol·Kelvin) | heat capacity of chemical species i |
| Ef | J/mol | forward reaction activation energy (Table 1) |
| Er | J/mol | reverse reaction activation energy (Table 1) |
| F | fractional | view factor [16] |
| g | m/s2 | acceleration of gravity (9.81 m/s2) |
| Gr | unitless | Grashoff number |
| h | W/(m2Kelvin) | convective heat transfer coefficient to air [43] |
| hwall | W/(m2Kelvin) | convective heat transfer coefficient to molten metal [44] |
| H | meters | reactor tube manifold height |
| ΔHrxn | J/mol | enthalpy of reaction |
| kf | m/sec | forward Arrhenius preexponential (Table 1) |
| kr | m4/sec | reverse Arrhenius preexponential (Table 1) |
| k(T) | W/(m·Kelvin) | Inconel thermal conductivity at temperature T [46] |
| Kc | mol/m3 | concentration equilibrium constant |
| n | integer | number of tubes in a receiver manifold |
| ṅinlet | mol/sec | mole flow of reactor tube gaseous feed |
| nC | moles | moles of solid carbon |
| nCH4 | moles | moles of gaseous methane |
| nH2 | moles | moles of gaseous hydrogen |
| ṅC | mol/sec | mole flow of solid carbon |
| ṅCH4 | mol/sec | mole flow of gaseous methane |
| ṅH2 | mol/sed | mole flow of gaseous hydrogen |
| Nu | unitless | Nusselt number |
| N(r) | W/m2 | flux at radial coordinate r in an Inconel tube wall |
| Pe | unitless | Peclet number |
| Pinlet | Pascals | reactor tube feed pressure |
| P(z) | Pascals | axial pressure in a reactor tube at elevation z |
| Qconvection | Watts | receiver losses to ambient air by natural convection |
| Qfield | Watts | solar energy incident on a heliostat field reflective area |
| Qradiative | Watts | receiver emissive losses by radiation |
| Qwall | Watts | energy that trespasses a reactor tube interior wall |
| Q(z) | m3/sec | volumetric flow at elevation z in a reactor tube |
| r | meters | radial coordinate |
| rbubble | meters | radius of a bubble |
| rbubble,inlet | meters | initial radius of a bubble entering a reactor tube |
| R | J/(mol·Kelvin) | gas constant |
| ℜ | meters | inner radius of a reactor tube |
| S | m2 | surface area of a solar receiver |
| t | seconds | time coordinate |
| T | Kelvin | isothermal reaction temperature |
| Ts | Kelvin | outer Inconel solar receiver surface temperature |
| Tw | Kelvin | inner Inconel reactor tube wall temperature |
| vbubbler | m/sec | initial velocity of a bubble entering a reactor tube |
| V | m3/sec | feed volumetric flow into a reactor tube |
| Vbubble | m3 | volume of a bubble |
| Vbubble,inlet | m3 | initial volume of a bubble entering a reactor tube |
| w | meters | reactor tube Inconel wall thickness |
| X | % | methane conversion to hydrogen gas |
| yCH4 | fractional | mole fraction of gaseous methane |
| yH2 | fractional | mole fraction of gaseous hydrogen |
| z | meters | axial coordinate (elevation in a reactor tube) |
| Z | unitless | compressibility factor from the SRK equation of state [35] |
Appendix B
Physical Property Correlations
| Species | a1 | a2 | a3 | a4 |
|---|---|---|---|---|
| CH4 | 18.386286 | 5.470402(10−2) | 1.034479(10−5) | −9.833387(10−9) |
| C | −5.394667 | 5.812952(10−2) | −4.177213(10−5) | −1.071678(10−8) |
| H2 | 28.653517 | 7.762754(10−4) | 1.324842(10−7) | 6.664873(10−10) |
References
- Loutzenhiser, P.G.; Muroyama, A.P. A review of the state-of-the-art in solar-driven gasification processes with carbonaceous materials. Sol. Energy 2017, 156, 93–100. [Google Scholar] [CrossRef]
- Palmer, C.; Upham, D.C.; Smart, S.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 2020, 3, 83–89. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Prasher, R.; Majumdar, A. Five thermal energy grand challenges for decarbonization. Nat. Energy 2020, 5, 635–637. [Google Scholar] [CrossRef]
- Ehrhart, B.D.; Muhich, C.L.; Al-Shankiti, I.; Weimer, A.W. System efficiency for two-step metal oxide solar thermochemical hydrogen production—Part 3: Various methods for achieving low oxygen partial pressures in the reduction reaction. Int. J. Hydrogen Energy 2016, 41, 19904–19914. [Google Scholar] [CrossRef]
- Ehrhart, B.D.; Muhich, C.L.; Al-Shankiti, I.; Weimer, A.W. System efficiency for two-step metal oxide solar thermochemical hydrogen production—Part 1: Thermodynamic model and impact of oxidation kinetics. Int. J. Hydrogen Energy 2016, 41, 19881–19893. [Google Scholar] [CrossRef]
- Ehrhart, B.D.; Muhich, C.L.; Al-Shankiti, I.; Weimer, A.W. System efficiency for two-step metal oxide solar thermochemical hydrogen production—Part 2: Impact of gas heat recuperation and separation temperatures. Int. J. Hydrogen Energy 2016, 41, 19894–19903. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: A review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Scheffe, J.R. Advances and trends in redox materials for solar thermochemical fuel production. Sol. Energy 2017, 156, 3–20. [Google Scholar] [CrossRef]
- Bader, R.; Lipiński, W. Solar thermochemical processes. In Solar Energy; World Scientific: Hackensack, NJ, USA, 2016; pp. 345–394. [Google Scholar]
- Binotti, M.; Di Marcoberardino, G.; Biassoni, M.; Manzolini, G. Solar hydrogen production with cerium oxides thermochemical cycle. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville NY, USA, 2017; Volume 1850, p. 100002. [Google Scholar]
- Giostri, A.; Binotti, M.; Sterpos, C.; Lozza, G. Small scale solar tower coupled with micro gas turbine. Renew. Energy 2020, 147, 570–583. [Google Scholar] [CrossRef]
- Weger, L.; Abánades, A.; Butler, T. Methane cracking as a bridge technology to the hydrogen economy. Int. J. Hydrogen Energy 2017, 42, 720–731. [Google Scholar] [CrossRef]
- Pacheco, J.E.; Bradshaw, R.W.; Dawson, D.B.; De la Rosa, W.; Rockwell, G.; Goods, H.S.; Hale, M.J.; Jacobs, P. Final Tests and Evaluation Results from the Solar Two Project; Sandia National Labs: Albuqerque, NM, USA, 2002; pp. 1–21. [Google Scholar]
- Ho, C.K.; Iverson, B.D. Review of high-temperature central receiver designs for concentrating solar power. Renew. Sustain. Energy Rev. 2014, 29, 835–846. [Google Scholar] [CrossRef]
- Howell, J.R.; Menguc, M.P.; Siegel, R. Thermal Radiation Heat Transfer; CRC Press: Boca Raton, FL, USA, 2010; ISBN 1-4398-9455-8. [Google Scholar]
- Diago, M.; Calvet, N.; Armstrong, P.R. Net power maximization from a faceted beam-down solar concentrator. Sol. Energy 2020, 204, 476–488. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes; Pearson Education: London UK, 2008; ISBN 0-13-245918-3. [Google Scholar]
- Parkinson, B.; Tabatabaei, M.; Upham, D.C.; Ballinger, B.J.; Greig, C.; Smart, S.; McFarland, E. Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. Int. J. Hydrogen Energy 2018, 43, 2540–2555. [Google Scholar] [CrossRef]
- Trommer, D.; Hirsch, D.; Steinfeld, A. Kinetic investigation of the thermal decomposition of CH4 by direct irradiation of a vortex-flow laden with carbon particles. Int. J. Hydrogen Energy 2004, 29, 627–633. [Google Scholar] [CrossRef]
- Paxman, D.; Trottier, S.; Flynn, M.R.; Kostiuk, L.; Secanell, M. Experimental and numerical analysis of a methane thermal decomposition reactor. Int. J. Hydrogen Energy 2017, 42, 25166–25184. [Google Scholar] [CrossRef]
- Steinberg, M. Production of hydrogen and methanol from natural gas with reduced CO2 emission. Int. J. Hydrogen Energy 1998, 23, 419–425. [Google Scholar] [CrossRef]
- Blackwood, J.D. The kinetics of the system carbon-hydrogen-methane. Aust. J. Chem. 1962, 15, 397–408. [Google Scholar] [CrossRef]
- Blackwood, J.D. The reaction of Carbon with Hydrogen at High Pressure. Aust. J. Chem. 1959, 12, 14–28. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Andrew, K.F.; Brassart, F.A. The reaction of hydrogen with graphite at 1200° to 1650 °C. J. Electrochem. Soc. 1965, 112, 49–52. [Google Scholar] [CrossRef]
- Hedden, K. The formation of methane from hydrogen and carbon at high temperatures and pressures. Z. Elektrochem. Angew. Phys. Chem. 1962, 60, 125–131. [Google Scholar]
- Parolin, G.; Borgognga, A.; Iaquaniello, G.; Salladini, A.; Cerbelli, S. Deactivation-induced dynamics of the reaction front in a fixed-bed catalytic membrane reactor: Methane cracking as a case study. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Pring, J.N.; Fairlie, D.M. X.—The methane equilibrium. J. Chem. Soc. Trans. 1912, 101, 91–103. [Google Scholar] [CrossRef]
- Catalan, L.J.; Rezaei, E. Coupled hydrodynamic and kinetic model of liquid metal bubble reactor for hydrogen production by noncatalytic thermal decomposition of methane. Int. J. Hydrogen Energy 2020, 45, 2486–2503. [Google Scholar] [CrossRef]
- Keipi, T.; Li, T.; Løvås, T.; Tolvanen, H.; Konttinen, J. Methane thermal decomposition in regenerative heat exchanger reactor: Experimental and modeling study. Energy 2017, 135, 823–832. [Google Scholar] [CrossRef]
- Luyben, W.L. Principles and Case Studies of Simultaneous Design; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-92708-3. [Google Scholar]
- Rawlings, J.B.; Ekerdt, J.G. Chemical Reactor Analysis and Design Fundamentals; Nob Hill Pub, LLC.: Madison WS, USA, 2002; ISBN 0-615-11884-4. [Google Scholar]
- Cahill, J.A.; Kirshenbaum, A.D. The density of liquid bismuth from its melting point to its normal boiling point and an estimate of its critical constants. J. Inorg. Nucl. Chem. 1963, 25, 501–506. [Google Scholar] [CrossRef]
- Grosse, A.V.; Kirshenbaum, A. The densities of liquid iron and nickel and an estimate of their critical temperature. J. Inorg. Nucl. Chem. 1963, 25, 331–334. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles; Wiley: New York, NY, USA, 1998; Volume 25. [Google Scholar]
- Eldridge, R.B.; Fair, J.R. Sieve-tray extractor continuous-phase mixing. Ind. Eng. Chem. Res. 1999, 38, 218–222. [Google Scholar] [CrossRef]
- Eldridge, R.B.; Humphrey, J.L.; Fair, J.R. Continuous Phase Mixing on Crossflow Extraction Sieve Trays. Sep. Sci. Technol. 1987, 22, 1121–1134. [Google Scholar] [CrossRef]
- Davies, R.M.; Taylor, G.I. The mechanics of large bubbles rising through extended liquids and through liquids in tubes. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1950, 200, 375–390. [Google Scholar]
- Bohn, M.S. Experimental investigation of the direct absorption receiver concept. Energy 1987, 12, 227–233. [Google Scholar] [CrossRef]
- Martinek, J.; Weimer, A.W. Design considerations for a multiple tube solar reactor. Sol. Energy 2013, 90, 68–83. [Google Scholar] [CrossRef]
- Klingensmith, L. Development of Extruded Heavy-Wall Large Diameter Nickel-Base Alloy Piping for AUSC Power Plants. In Proceedings of the Asian Coalition for Climate and Energy, Shanghai, China, 23–26 August 2011; pp. 272–298. [Google Scholar]
- Boehm, R.F. A Review of Convective Loss Data from Solar Central Receivers. J. Sol. Energy Eng. 1987, 109, 101–107. [Google Scholar] [CrossRef]
- Metals, U.S.O. of N.R.C. on the B.P. of L. Liquid-Metals Handbook; US Government Printing Office: Washington DC, USA, 1954; Volume 2.
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N.; Meredith, R.E. Transport phenomena. J. Electrochem. Soc. 1961, 108, 78C. [Google Scholar] [CrossRef]
- Incoloy Alloy 800H & 800HT; Special Metals Corporation: Huntington, WV, USA, 2004.
- Behar, O.; Khellaf, A.; Mohammedi, K. A review of studies on central receiver solar thermal power plants. Renew. Sustain. Energy Rev. 2013, 23, 12–39. [Google Scholar] [CrossRef]
- Bhargav, K.R.; Gross, F.; Schramek, P. Life Cycle Cost Optimized Heliostat Size for Power Towers. Energy Procedia 2014, 49, 40–49. [Google Scholar] [CrossRef]
- Mancini, T.R.; Gary, J.A.; Kolb, G.J.; Ho, C.K. Power Tower Technology Roadmap and Cost Reduction Plan; SAND2011-2419; Sandia National Laboratories: Albuquerque, NM, USA, 2011; p. 7.
- Rowe, S.C.; Groehn, A.J.; Palumbo, A.W.; Chubukov, B.A.; Clough, D.E.; Weimer, A.W.; Hischier, I. Worst-case losses from a cylindrical calorimeter for solar simulator calibration. Opt. Express 2015, 23, A1309–A1323. [Google Scholar] [CrossRef]
- Rowe, S.C.; Hischier, I.; Palumbo, A.W.; Chubukov, B.A.; Wallace, M.A.; Viger, R.; Lewandowski, A.; Clough, D.E.; Weimer, A.W. Nowcasting, predictive control, and feedback control for temperature regulation in a novel hybrid solar-electric reactor for continuous solar-thermal chemical processing. Sol. Energy 2018, 174, 474–488. [Google Scholar] [CrossRef]
- Skinrood, A.C.; Brumleve, T.D.; Schafer, C.T.; Yokomizo, C.T.; Leonard, C.M., Jr. Status Report on a High Temperature Solar Energy System; Sandia National Lab. (Albuquerque-NM): Albuquerque, NM, USA, 1974. [Google Scholar]
- Slocum, A.H.; Codd, D.S.; Buongiorno, J.; Forsberg, C.W.; McKrell, T.J.; Nave, J.-C.; Papanicolas, C.N.; Ghobeity, A.; Noone, C.J.; Passerini, S.; et al. Concentrated solar power on demand. Sol. Energy 2011, 85, 1519–1529. [Google Scholar] [CrossRef]
- Smith, J.M. Introduction to Chemical Engineering Thermodynamics; ACS Publications: Washington, DC, USA, 2004; ISBN 0073104450. [Google Scholar]
- Yu, X. Thermodynamics and Kinetics of Gasification Reactions of Metallurgical Cokes. Ph.D. Thesis, University of London, London, UK, 1988. [Google Scholar]
- De Bokx, P.K.; Kock, A.H.; Boellaard, E.; Klop, W.; Geus, J.W. The formation of filamentous carbon on iron and nickel catalysts: I. Thermodynamics. J. Catal. 1985, 96, 454–467. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical reaction engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
- Wetzel, T.; Pacio, J.; Marocco, L.; Weisenburger, A.; Heinzel, A.; Hering, W.; Schroer, C.; Muller, G.; Konys, J.; Stieglitz, R.; et al. Liquid metal technology for concentrated solar power systems: Contributions by the German research program. AIMS Energy 2014, 2, 89–98. [Google Scholar] [CrossRef]
- Sefkow, E.A.B. The Design of a Calorimeter to Measure Concentrated Solar Flux. Master’s Thesis, The University of Minnesota, Minnesota, MN, USA, 2013. [Google Scholar]
- Sparrow, E.M. Radiation Heat Transfer; Routledge and CRC Press: Boca Raton, FL, USA, 2018; ISBN 1-351-42010-0. [Google Scholar]
- Sarwar, J.; Georgakis, G.; Kouloulias, K.; Kakosimos, K.E. Experimental and numerical investigation of the aperture size effect on the efficient solar energy harvesting for solar thermochemical applications. Energy Convers. Manag. 2015, 92, 331–341. [Google Scholar] [CrossRef]
- Berenguel, M.; Rubio, F.R.; Valverde, A.; Lara, P.J.; Arahal, M.R.; Camacho, E.F.; López, M. An artificial vision-based control system for automatic heliostat positioning offset correction in a central receiver solar power plant. Sol. Energy 2004, 76, 563–575. [Google Scholar] [CrossRef]
- Relloso, S.; García, E.A. Tower Technology Cost Reduction Approach after Gemasolar Experience. Energy Procedia 2015, 69, 1660–1666. [Google Scholar] [CrossRef]
- Binotti, M.; De Giorgi, P.; Sánchez, D.; Manzolini, G. Comparison of Different Strategies for Heliostats Aiming Point in Cavity and External Tower Receivers. J. Sol. Energy Eng. 2016, 138, 021008. [Google Scholar] [CrossRef]
- Kang, D.; Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic methane pyrolysis in molten MnCl2-KCl. Appl. Catal. B Environ. 2019, 254, 659–666. [Google Scholar] [CrossRef]
- Kuipers, J.A.M.; Van Swaaij, W.P.M. Application of computational fluid dynamics to chemical reaction engineering. Rev. Chem. Eng. 1997, 13, 1–118. [Google Scholar] [CrossRef]
- Roeb, M.; Neises, M.; Monnerie, N.; Sattler, C.; Pitz-Paal, R. Technologies and trends in solar power and fuels. Energy Environ. Sci. 2011, 4, 2503–2511. [Google Scholar] [CrossRef]
- Vant-Hull, L. Issues with Beam-down Concepts. Energy Procedia 2014, 49, 257–264. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]

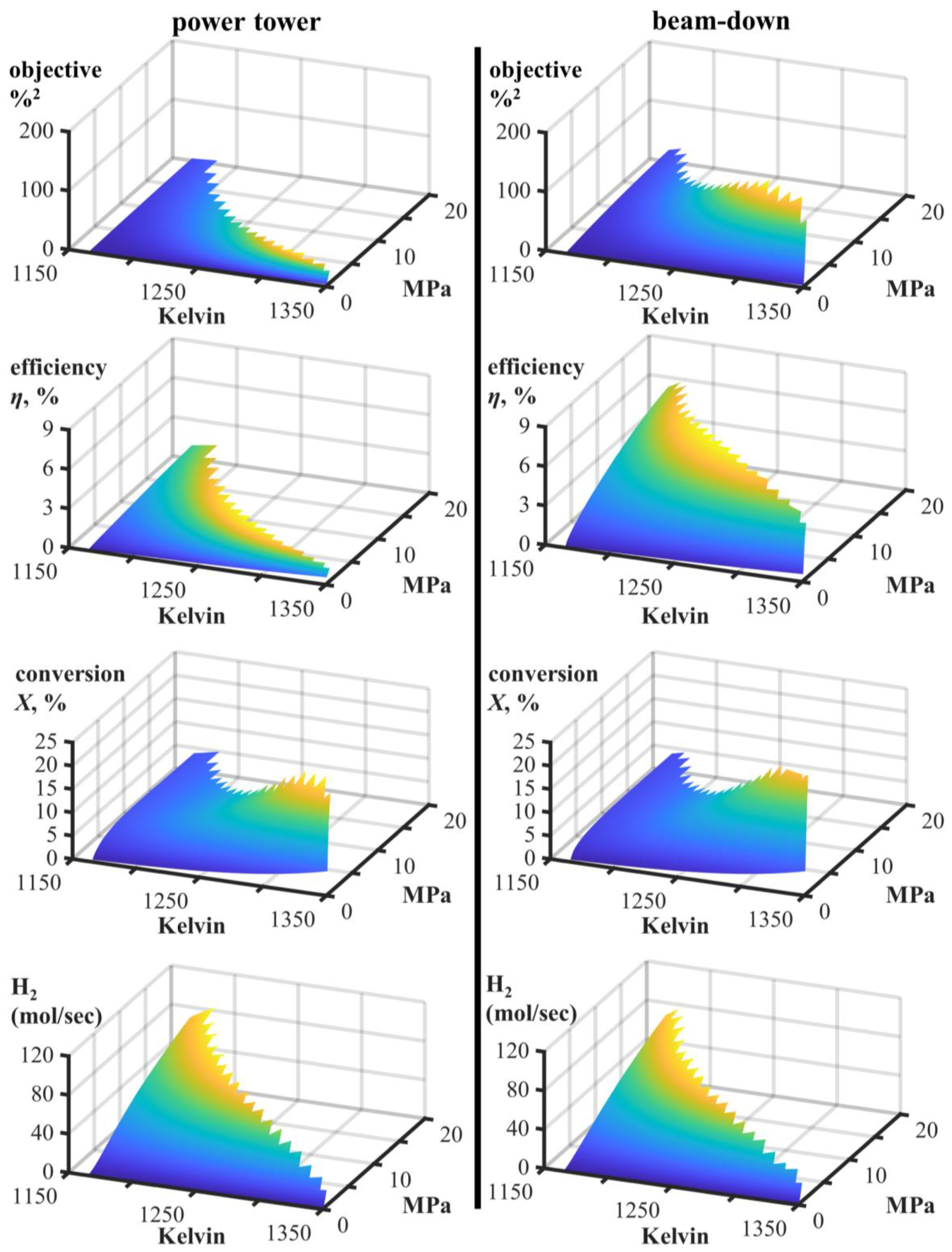
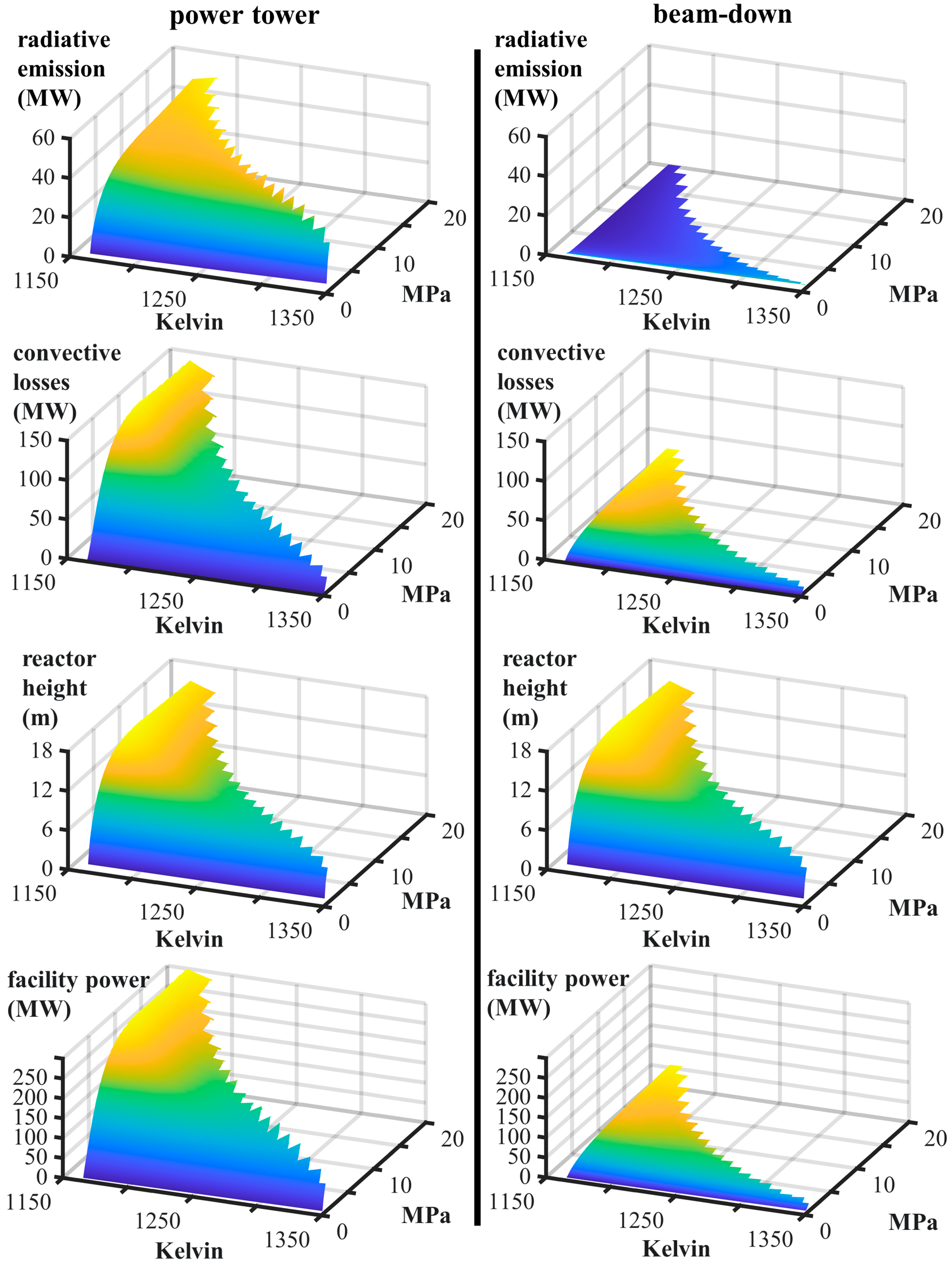
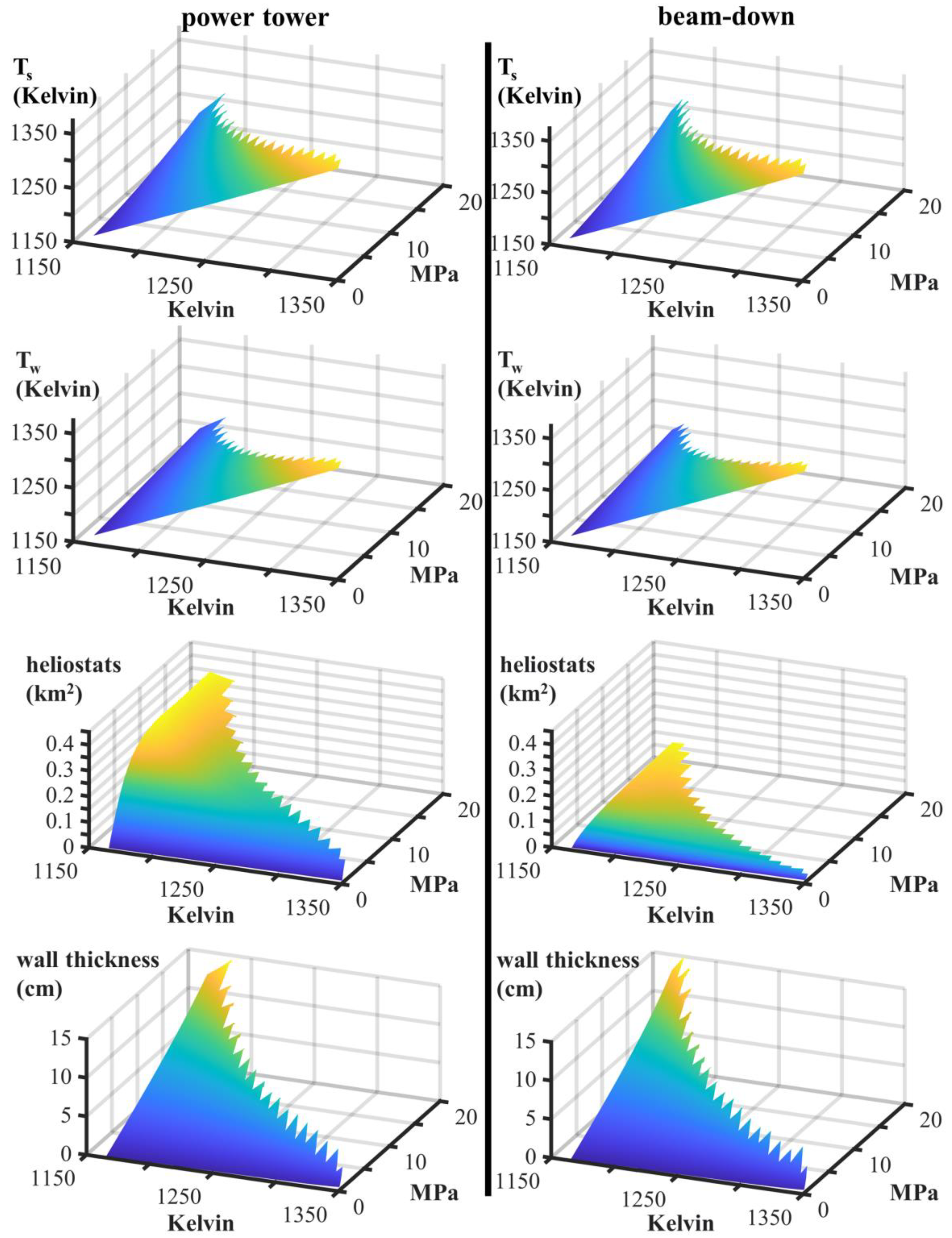
| Power Tower | Beam-Down | ||||
|---|---|---|---|---|---|
| Best Objective | Best Efficiency | Best Objective | Best Efficiency | ||
| objective, ηX | %2 | 33 | 22 | 152 | 102 |
| efficiency, η | % | 1.4 | 1.7 | 7.22 | 9 |
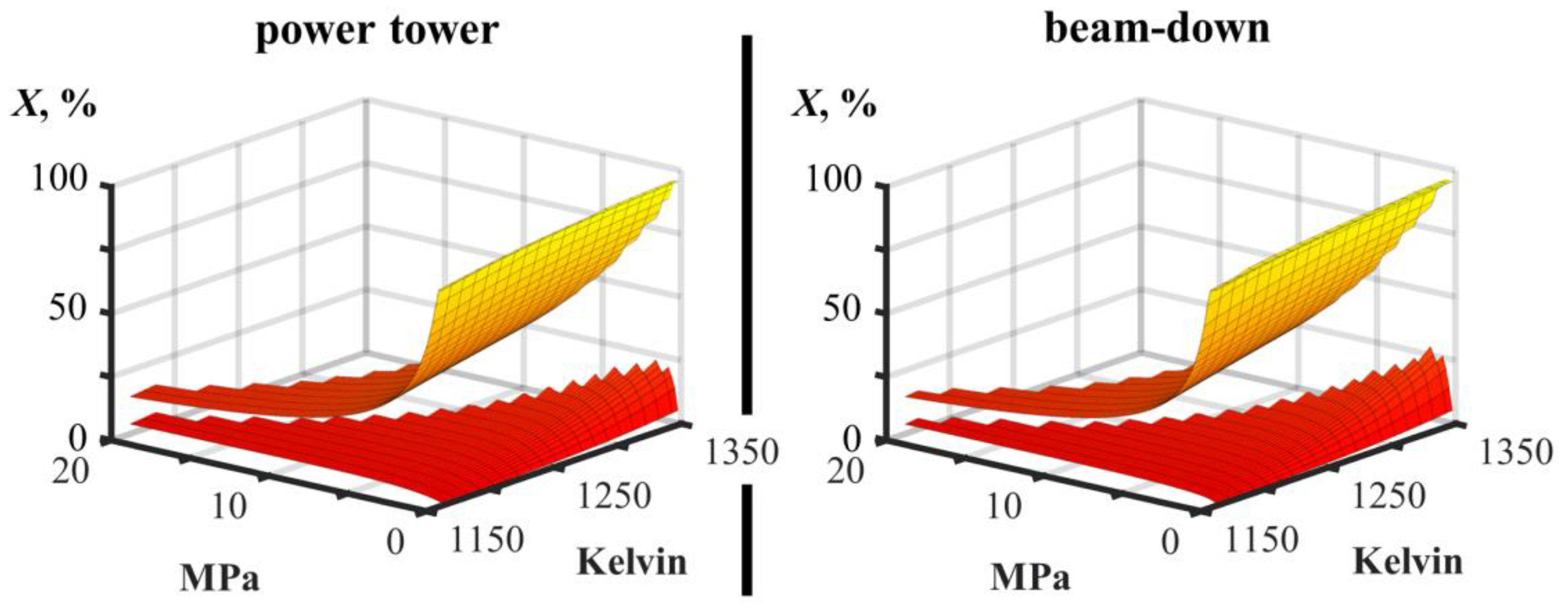
| conversion X | % | 23.6 | 12.9 | 21.1 | 11.3 |
| isothermal reaction, T | Kelvin | 1325 | 1265 | 1315 | 1255 |
| inlet pressure, Pinlet | atm | 21.71 | 65.13 | 24.67 | 69.10 |
| facility power, Qfield | MW | 144.3 | 190.0 | 24.7 | 34.1 |
| convective losses, Qconvective | MW | 39.6 | 72.5 | 10.5 | 15.22 |
| radiative losses, Qradiative | MW | 38.1 | 42.4 | 1.4 | 1.00 |
| H2 produced | mol/sec | 44.88 | 73.73 | 39.23 | 64.31 |
| reactor height, H | meters | 8.5 | 10.4 | 8.5 | 10.4 |
| tube radius, ℜ | meters | 0.48 | 0.48 | 0.41 | 0.45 |
| surface temperature, Ts | Kelvin | 1365 | 1330 | 1348 | 1318 |
| wall temperature, Tw | Kelvin | 1350 | 1300 | 1337 | 1289 |
| wall thickness, w | cm | 6.10 | 8.40 | 3.85 | 7.47 |
| heliostat area, Amirrors | km2 | 0.18 | 0.24 | 0.03 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowe, S.C.; Ariko, T.A.; Weiler, K.M.; Spana, J.T.E.; Weimer, A.W. Reversible Molten Catalytic Methane Cracking Applied to Commercial Solar-Thermal Receivers. Energies 2020, 13, 6229. https://doi.org/10.3390/en13236229
Rowe SC, Ariko TA, Weiler KM, Spana JTE, Weimer AW. Reversible Molten Catalytic Methane Cracking Applied to Commercial Solar-Thermal Receivers. Energies. 2020; 13(23):6229. https://doi.org/10.3390/en13236229
Chicago/Turabian StyleRowe, Scott C., Taylor A. Ariko, Kaylin M. Weiler, Jacob T. E. Spana, and Alan W. Weimer. 2020. "Reversible Molten Catalytic Methane Cracking Applied to Commercial Solar-Thermal Receivers" Energies 13, no. 23: 6229. https://doi.org/10.3390/en13236229
APA StyleRowe, S. C., Ariko, T. A., Weiler, K. M., Spana, J. T. E., & Weimer, A. W. (2020). Reversible Molten Catalytic Methane Cracking Applied to Commercial Solar-Thermal Receivers. Energies, 13(23), 6229. https://doi.org/10.3390/en13236229






