Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders
Abstract
1. Introduction
2. Experimental
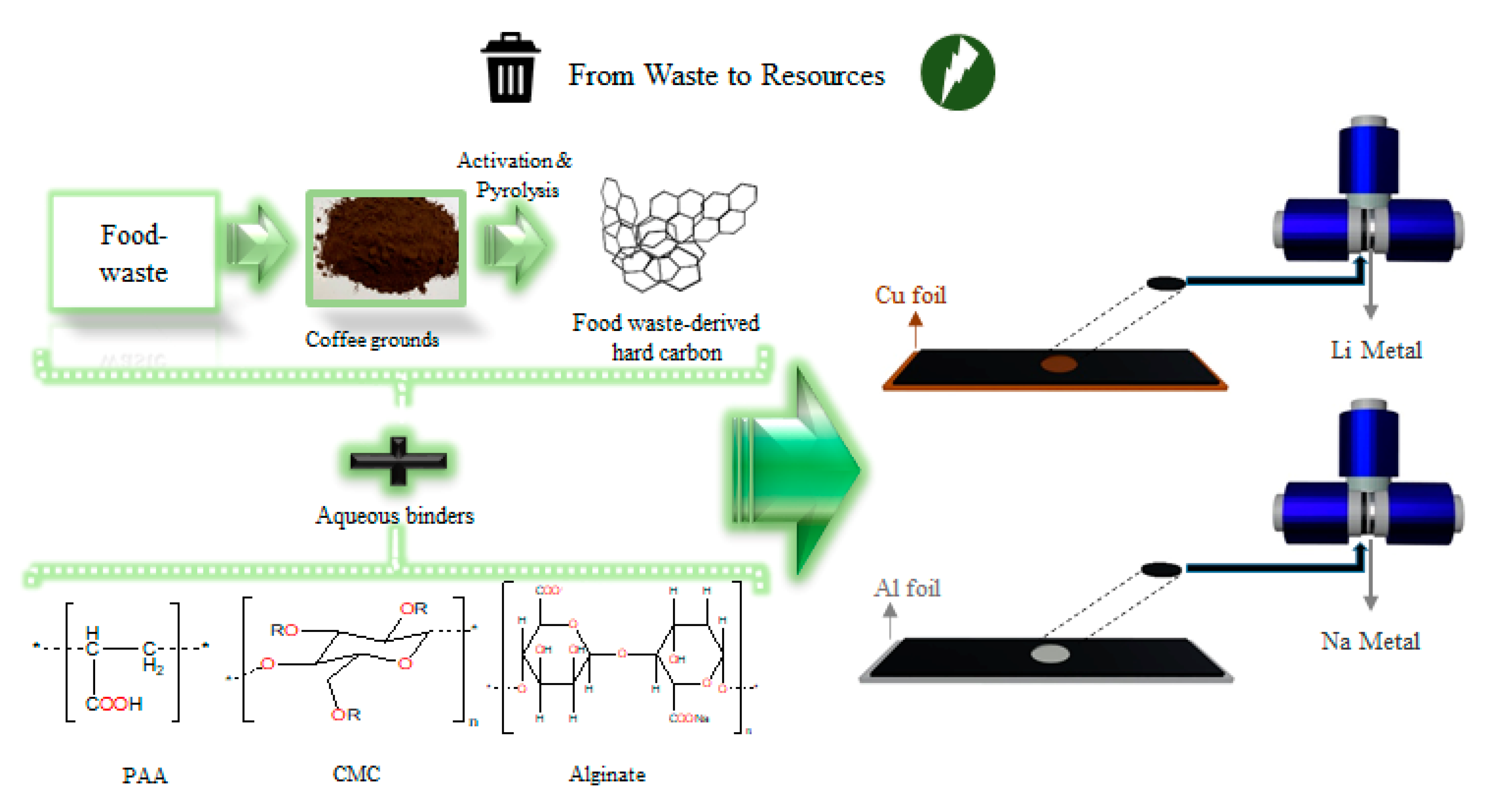
2.1. Synthesis of Coffee Ground-Derived Hard Carbon
2.2. Material Characterization
2.3. Electrodes Preparation and Cell Assembling
2.4. Electrochemical Characterization
3. Result and Discussion
3.1. Chemical, Structural, and Morphological Characterization of the Material
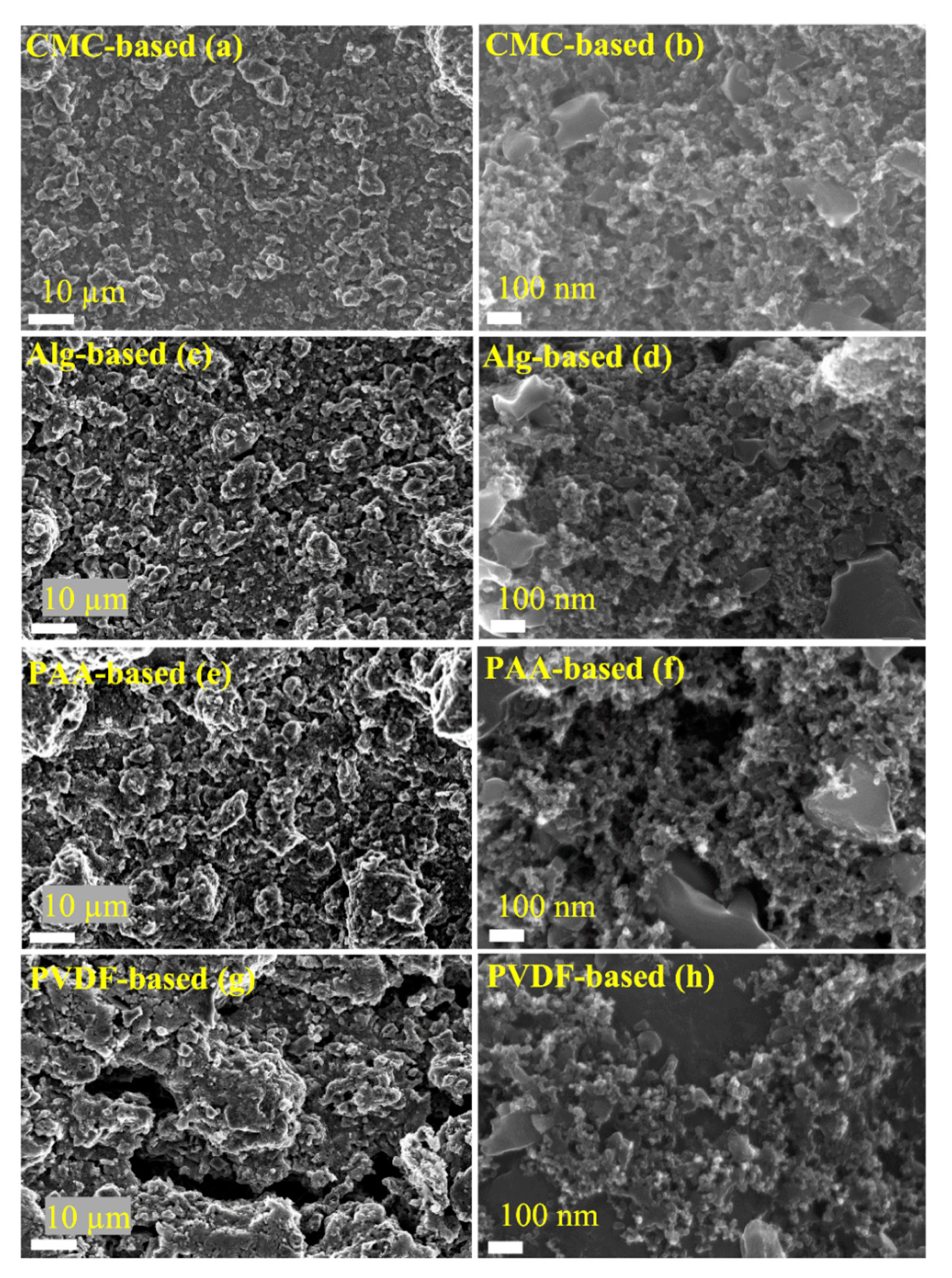
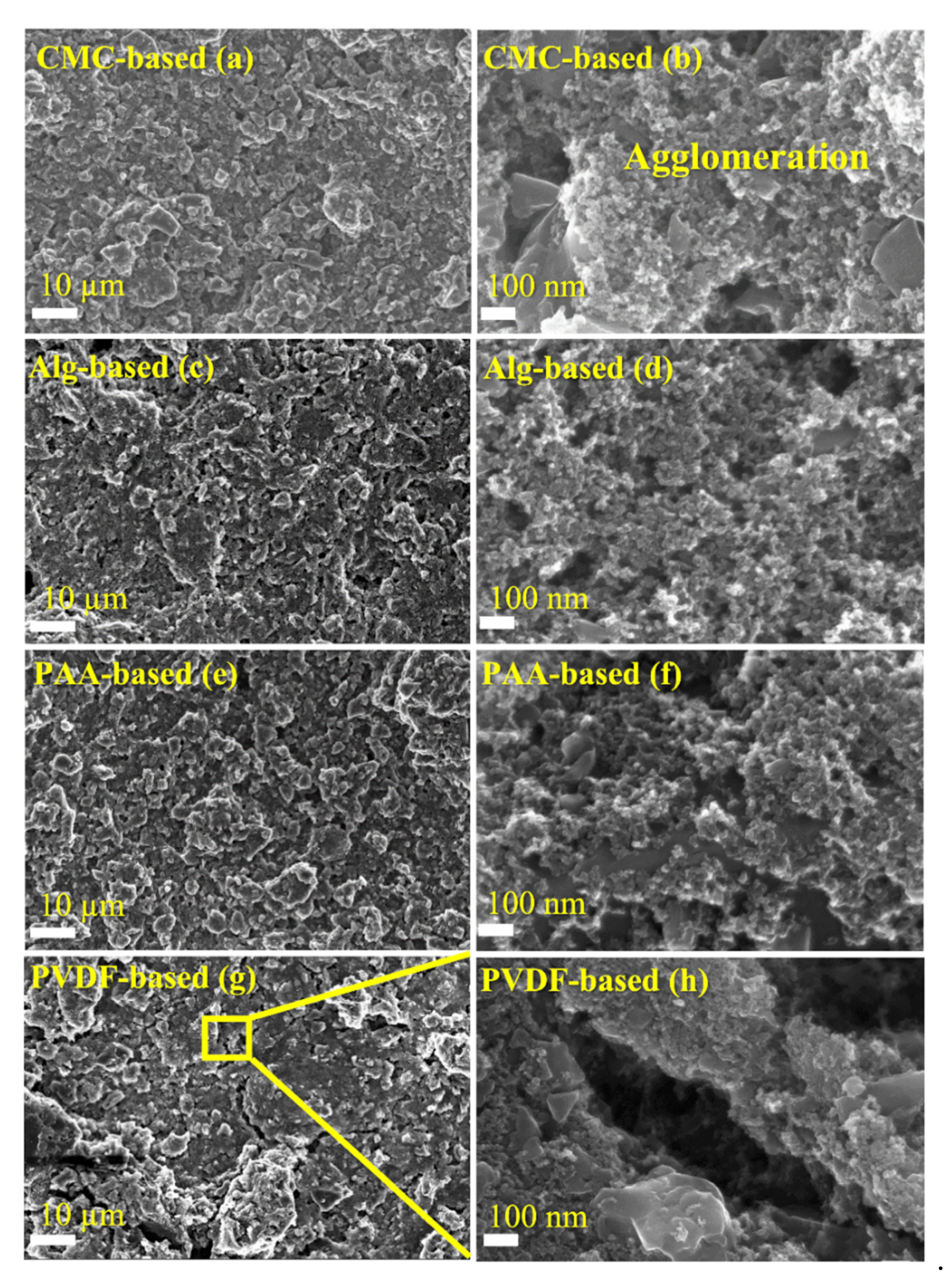
3.2. Structural and Morphological Characterization of the Electrodes
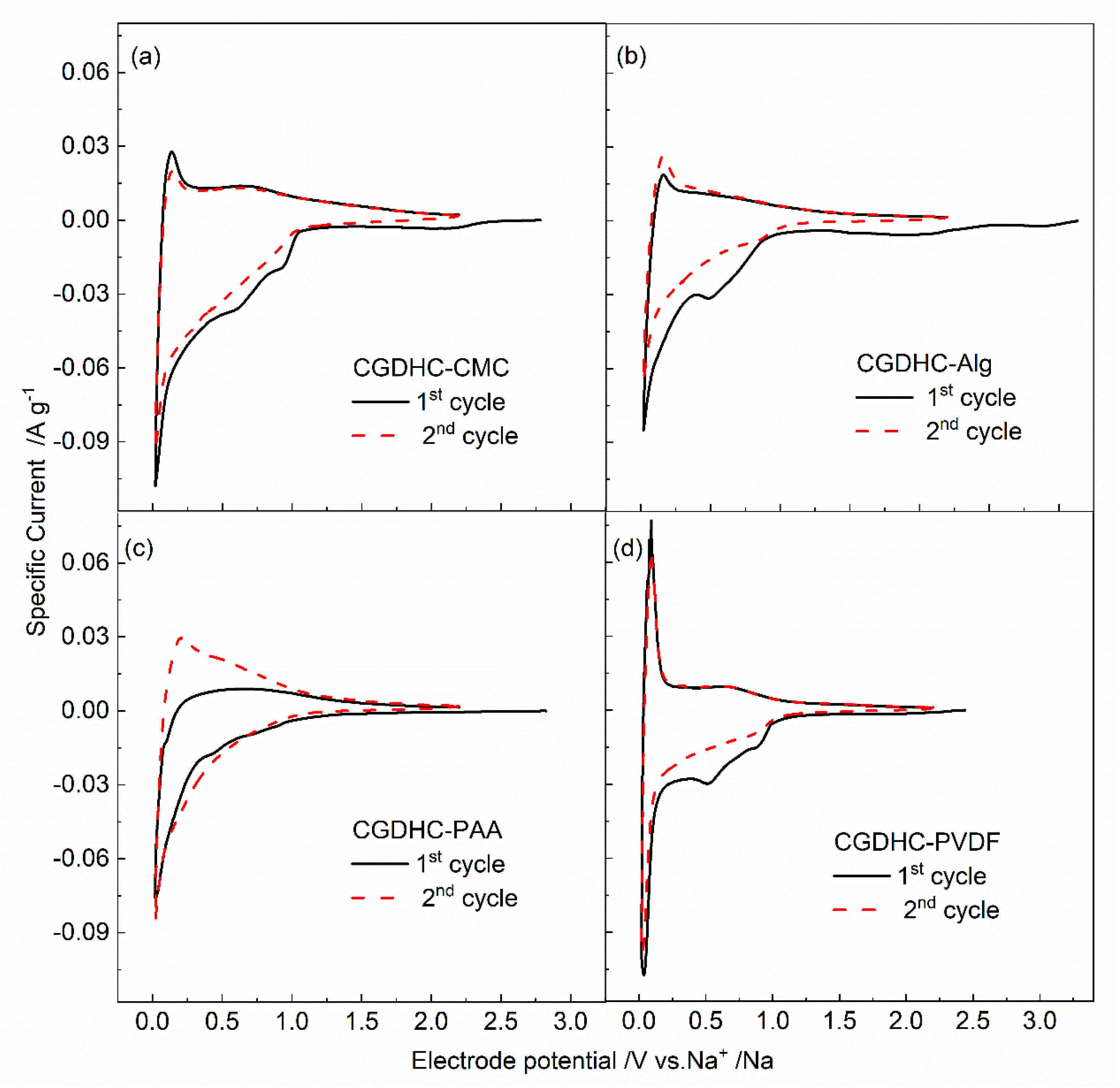
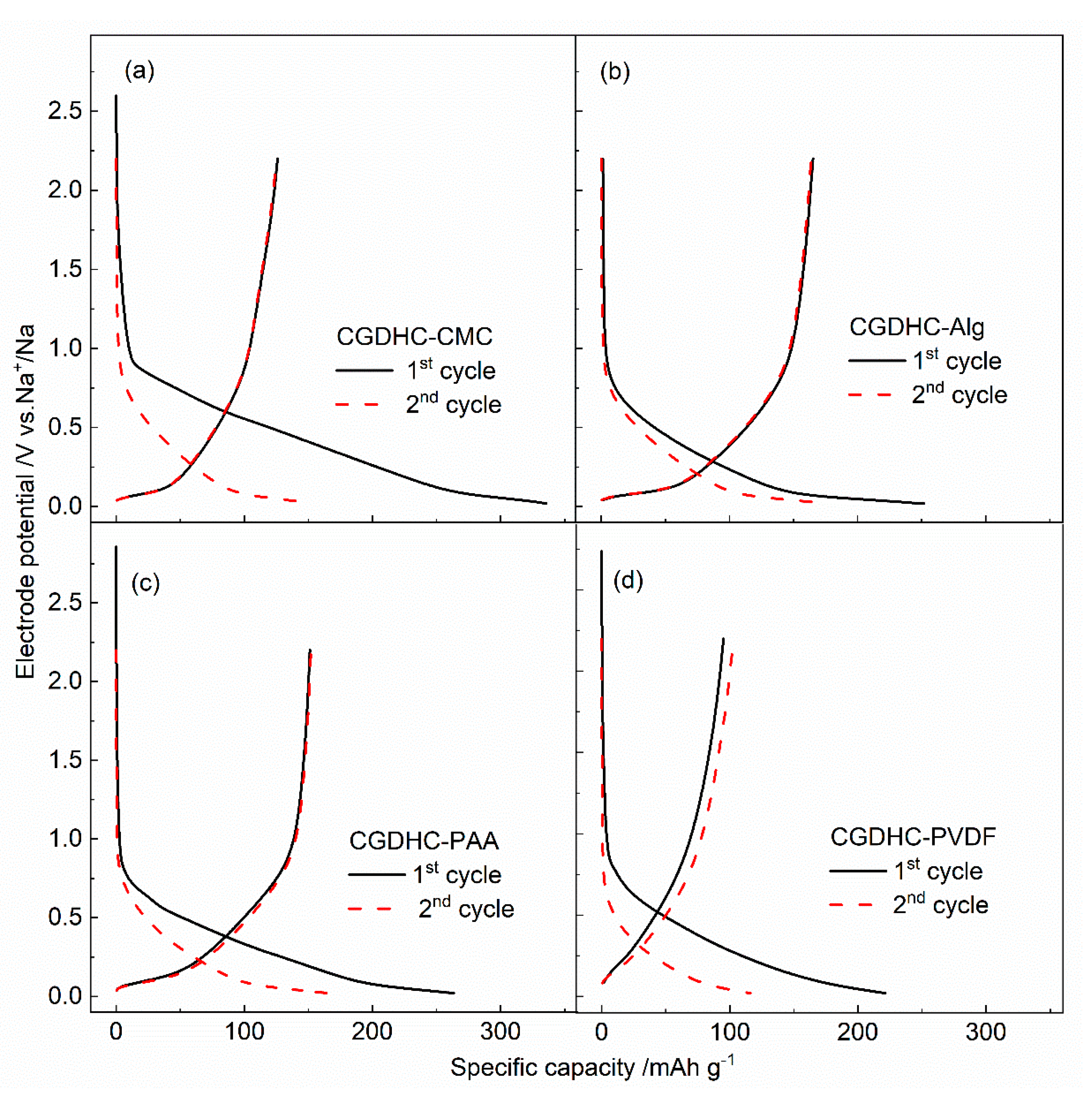
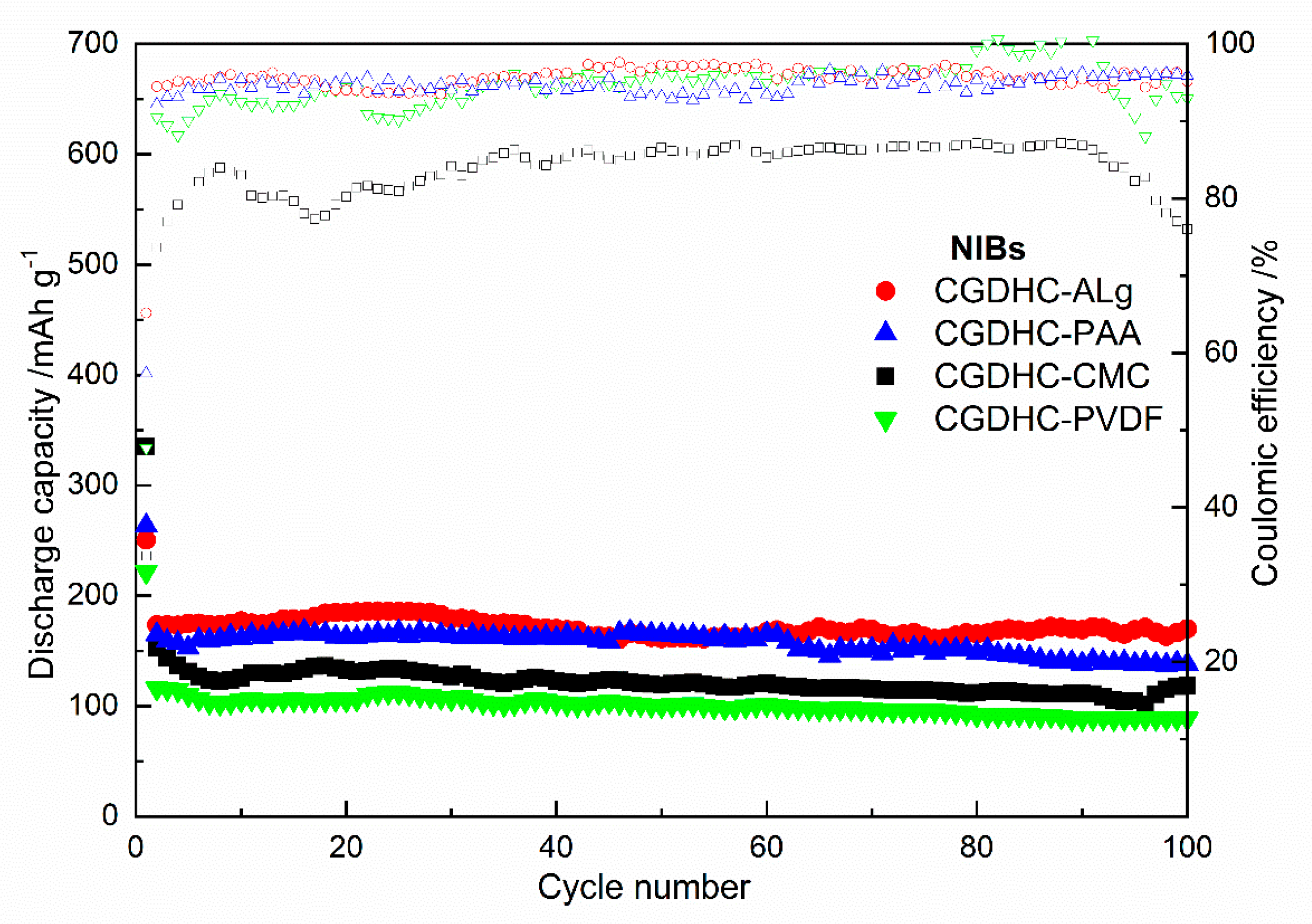
3.3. Electrochemical Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Huang, Y.; Zhao, C.; Shao, X.; Zhu, Z. Rose-derived 3D carbon nanosheets for high cyclability and extended voltage supercapacitors. Electrochim. Acta 2018, 291, 287–296. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Darjazi, H.; Hosseiny Davarani, S.S.; Moazami, H.R.; Yousefi, T.; Tabatabaei, F. Evaluation of charge storage ability of chrome doped Mn2O3 nanostructures derived by cathodic electrodeposition. Prog. Nat. Sci. Mater. Int. 2016, 26, 523–527. [Google Scholar] [CrossRef]
- Notohara, H.; Urita, K.; Moriguchi, I. Tin Oxide Electrodes in Li and Na-Ion Batteries; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128159248. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Eilers-Rethwisch, M.; Hildebrand, S.; Evertz, M.; Ibing, L.; Dagger, T.; Winter, M.; Schappacher, F.M. Comparative study of Sn-doped Li[Ni0.6Mn0.2Co0.2-xSnx]O2 cathode active materials (x = 0–0.5) for lithium ion batteries regarding electrochemical performance and structural stability. J. Power Sources 2018, 397, 68–78. [Google Scholar] [CrossRef]
- Hou, H.; Shao, L.; Zhang, Y.; Zou, G.; Chen, J.; Ji, X. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Q.; Sun, B.; Gu, J.; Yu, B.; Zhang, W.; Zhang, D. N-doped catalytic graphitized hard carbon for high-performance lithium/sodium-ion batteries. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Y.; Chen, J. Advanced Organic Electrode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Balogun, M.S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon N. Y. 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Jache, B.; Binder, J.O.; Abe, T.; Adelhelm, P. A comparative study on the impact of different glymes and their derivatives as electrolyte solvents for graphite co-intercalation electrodes in lithium-ion and sodium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 14299–14316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.S.; Li, H.; Chen, L.; Huang, X. A superior low-cost amorphous carbon anode made from pitch and lignin for sodium-ion batteries. J. Mater. Chem. A 2015, 4, 96–104. [Google Scholar] [CrossRef]
- Li, H.; Shen, F.; Luo, W.; Dai, J.; Han, X.; Chen, Y.; Yao, Y.; Zhu, H.; Fu, K.; Hitz, E.; et al. Carbonized-leaf Membrane with Anisotropic Surfaces for Sodium-ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple-Biowaste-Derived Hard Carbon as a Powerful Anode Material for Na-Ion Batteries. ChemElectroChem 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef] [PubMed]
- Väli, R.; Jänes, A.; Thomberg, T.; Lust, E. Synthesis and characterization of D-glucose derived nanospheric hard carbon negative electrodes for lithium- and sodium-ion batteries. Electrochim. Acta 2017, 253, 536–544. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon nanosheet frameworks derived from peat moss as high performance sodium ion battery anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef]
- Lv, W.; Wen, F.; Xiang, J.; Zhao, J.; Li, L.; Wang, L.; Liu, Z.; Tian, Y. Peanut shell derived hard carbon as ultralong cycling anodes for lithium and sodium batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.S.; Titirici, M.M.; Chen, L.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From Charge Storage Mechanism to Performance: A Roadmap toward High Specific Energy Sodium-Ion Batteries through Carbon Anode Optimization. Adv. Energy Mater. 2018, 8, 1–33. [Google Scholar] [CrossRef]
- Velez, V.; Ramos-Sánchez, G.; Lopez, B.; Lartundo-Rojas, L.; González, I.; Sierra, L. Synthesis of novel hard mesoporous carbons and their application as anodes for Li and Na ion batteries. Carbon N. Y. 2019, 147, 214–226. [Google Scholar] [CrossRef]
- Dachowski, R.; Kostrzewa, P. The Use of Waste Materials in the Construction Industry. Procedia Eng. 2016, 161, 754–758. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Yoo, M.; Frank, C.W.; Mori, S.; Yamaguchi, S. Effect of poly(vinylidene fluoride) binder crystallinity and graphite structure on the mechanical strength of the composite anode in a lithium ion battery. Polymer 2003, 44, 4197–4204. [Google Scholar] [CrossRef]
- Xu, J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Dou, S.X. The effect of different binders on electrochemical properties of LiNi 1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Chou, S.L.; Pan, Y.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Duan, J.; Hong, K.; Xue, L.; Huang, Y. Effects of binders on electrochemical performance of nitrogen-doped carbon nanotube anode in sodium-ion battery. Electrochim. Acta 2015, 174, 970–977. [Google Scholar] [CrossRef]
- Beda, A.; Taberna, P.; Simon, P.; Ghimbeu, C.M.; Beda, A.; Taberna, P.; Simon, P.; Matei, C.; Hard, G. Hard carbons derived from green phenolic resins for Na-ion batteries To cite this version: HAL Id: Hal-02022742. Carbon 2018, 139, 248–257. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Liu, X.; Xiao, L.; Cao, Y. A green route to synthesize low-cost and high-performance hard carbon as promising sodium-ion battery anodes from sorghum stalk waste. Green Energy Environ. 2017, 2, 310–315. [Google Scholar] [CrossRef]
- Li, Y.; Ni, B.; Li, X.; Wang, X.; Zhang, D.; Zhao, Q.; Li, J.; Lu, T.; Mai, W.; Pan, L. High-Performance Na-Ion Storage of S-Doped Porous Carbon Derived from Conjugated Microporous Polymers. Nano-Micro Lett. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Luo, C.; Fan, X.; Ma, Z.; Gao, T.; Wang, C. Self-Healing Chemistry between Organic Material and Binder for Stable Sodium-Ion Batteries. Chem 2017, 3, 1050–1062. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Zhong, Y.; Shen, T.; Wang, X.; Xia, X.; Tu, J. Natural biomass-derived carbons for electrochemical energy storage. Mater. Res. Bull. 2017, 88, 234–241. [Google Scholar] [CrossRef]
- Ajuria, J.; Redondo, E.; Arnaiz, M.; Mysyk, R.; Rojo, T.; Goikolea, E. Lithium and sodium ion capacitors with high energy and power densities based on carbons from recycled olive pits. J. Power Sources 2017, 359, 17–26. [Google Scholar] [CrossRef]
- Ni, J.; Huang, Y.; Gao, L. A high-performance hard carbon for Li-ion batteries and supercapacitors application. J. Power Sources 2013, 223, 306–311. [Google Scholar] [CrossRef]
- Dell’Era, A.; Pasquali, M.; Tarquini, G.; Scaramuzzo, F.A.; De Gasperis, P.; Prosini, P.P.; Mezzi, A.; Tuffi, R.; Cafiero, L. Carbon powder material obtained from an innovative high pressure water jet recycling process of tires used as anode in alkali ion (Li, Na) batteries. Solid State Ion. 2018, 324, 20–27. [Google Scholar] [CrossRef]
- Wang, H.; Umeno, T.; Mizuma, K.; Yoshio, M. Highly conductive bridges between graphite spheres to improve the cycle performance of a graphite anode in lithium-ion batteries. J. Power Sources 2008, 175, 886–890. [Google Scholar] [CrossRef]
- García, A.; Culebras, M.; Collins, M.N.; Leahy, J.J. Stability and rheological study of sodium carboxymethyl cellulose and alginate suspensions as binders for lithium ion batteries. J. Appl. Polym. Sci. 2018, 135, 11–13. [Google Scholar] [CrossRef]
- Guerfi, A.; Kaneko, M.; Petitclerc, M.; Mori, M.; Zaghib, K. LiFePO4 water-soluble binder electrode for Li-ion batteries. J. Power Sources 2007, 163, 1047–1052. [Google Scholar] [CrossRef]
- Komaba, S.; Okushi, K.; Ozeki, T.; Yui, H.; Katayama, Y.; Miura, T.; Saito, T.; Groult, H. Polyacrylate modifier for graphite anode of lithium-ion batteries. Electrochem. Solid-State Lett. 2009, 12, 107–110. [Google Scholar] [CrossRef]
- Nobili, F.; Dsoke, S.; Mancini, M.; Tossici, R.; Marassi, R. Electrochemical investigation of polarization phenomena and intercalation kinetics of oxidized graphite electrodes coated with evaporated metal layers. J. Power Sources 2008, 180, 845–851. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Li, D.; Lu, P.; Hou, F.; Liang, J. Enhanced electrochemical stability of carbon-coated antimony nanoparticles with sodium alginate binder for sodium-ion batteries. Prog. Nat. Sci. Mater. Int. 2018, 28, 205–211. [Google Scholar] [CrossRef]
- Zarrabeitia, M.; Nobili, F.; Muñoz-Márquez, M.Á.; Rojo, T.; Casas-Cabanas, M. Direct observation of electronic conductivity transitions and solid electrolyte interphase stability of Na2Ti3O7 electrodes for Na-ion batteries. J. Power Sources 2016, 330, 78–83. [Google Scholar] [CrossRef]
- Zarrabeitia, M.; Muñoz-Márquez, M.Á.; Nobili, F.; Rojo, T.; Casas-Cabanas, M. Influence of using metallic na on the interfacial and transport properties of Na-Ion batteries. Batteries 2017, 3, 16. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
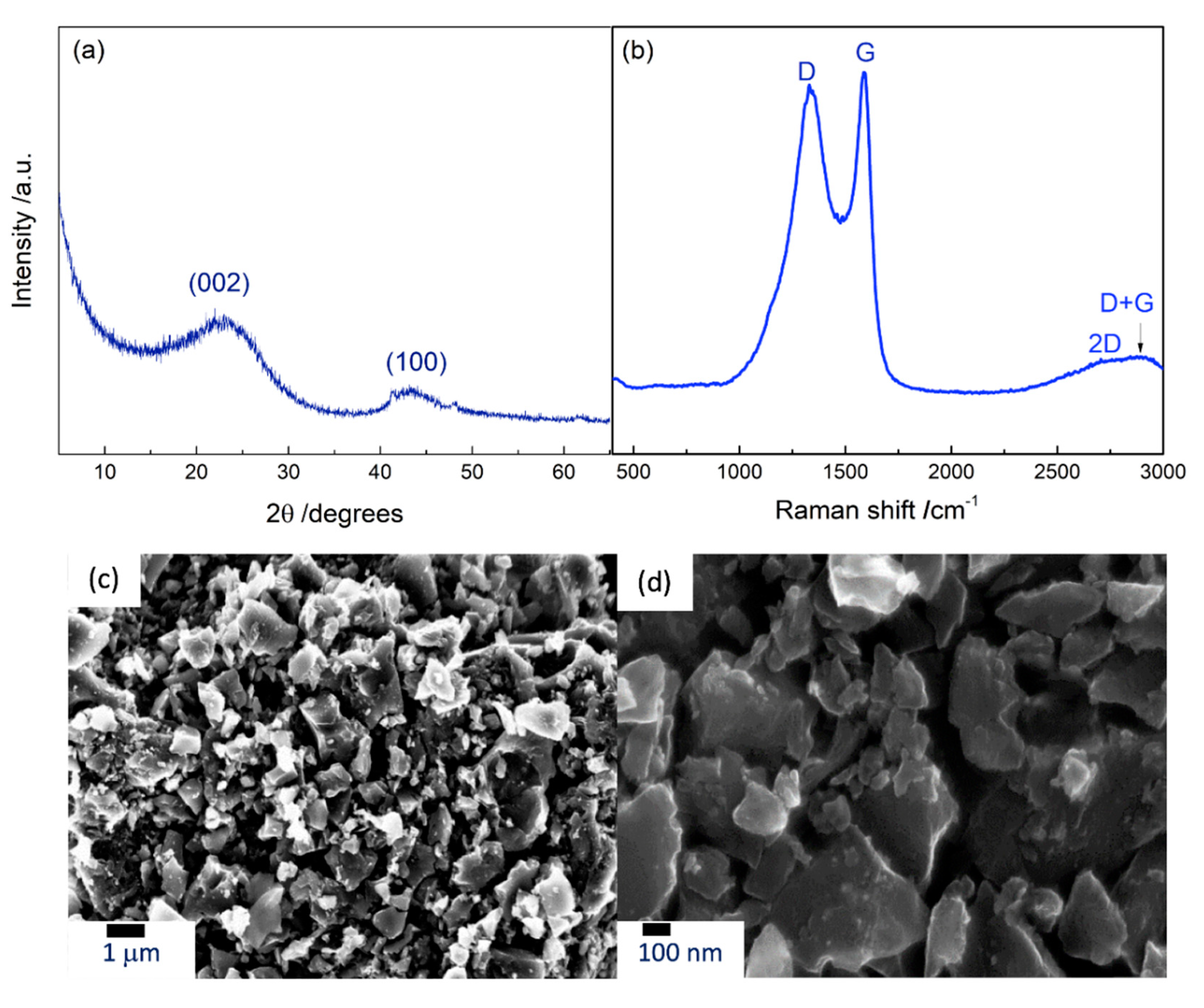
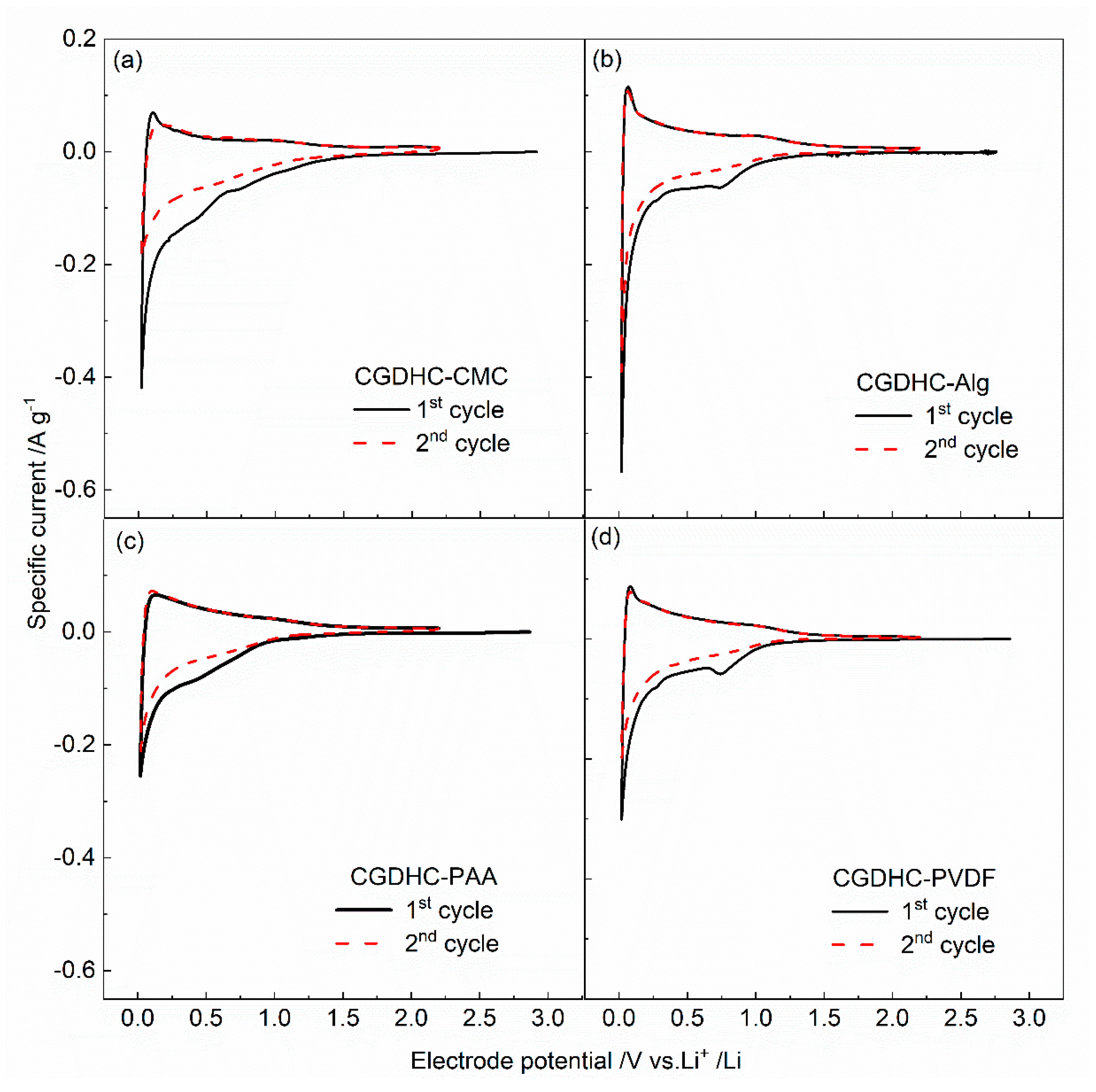
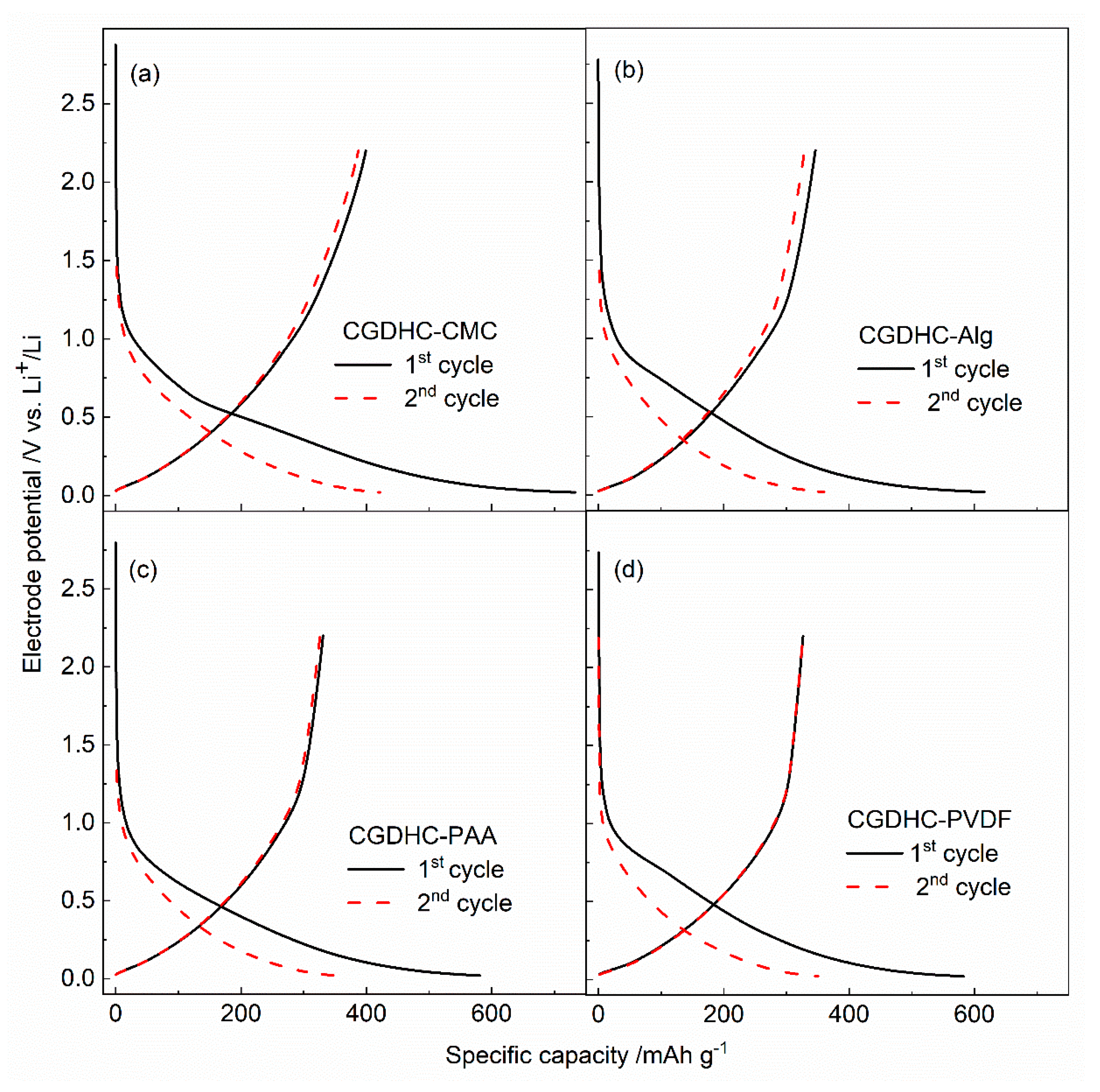
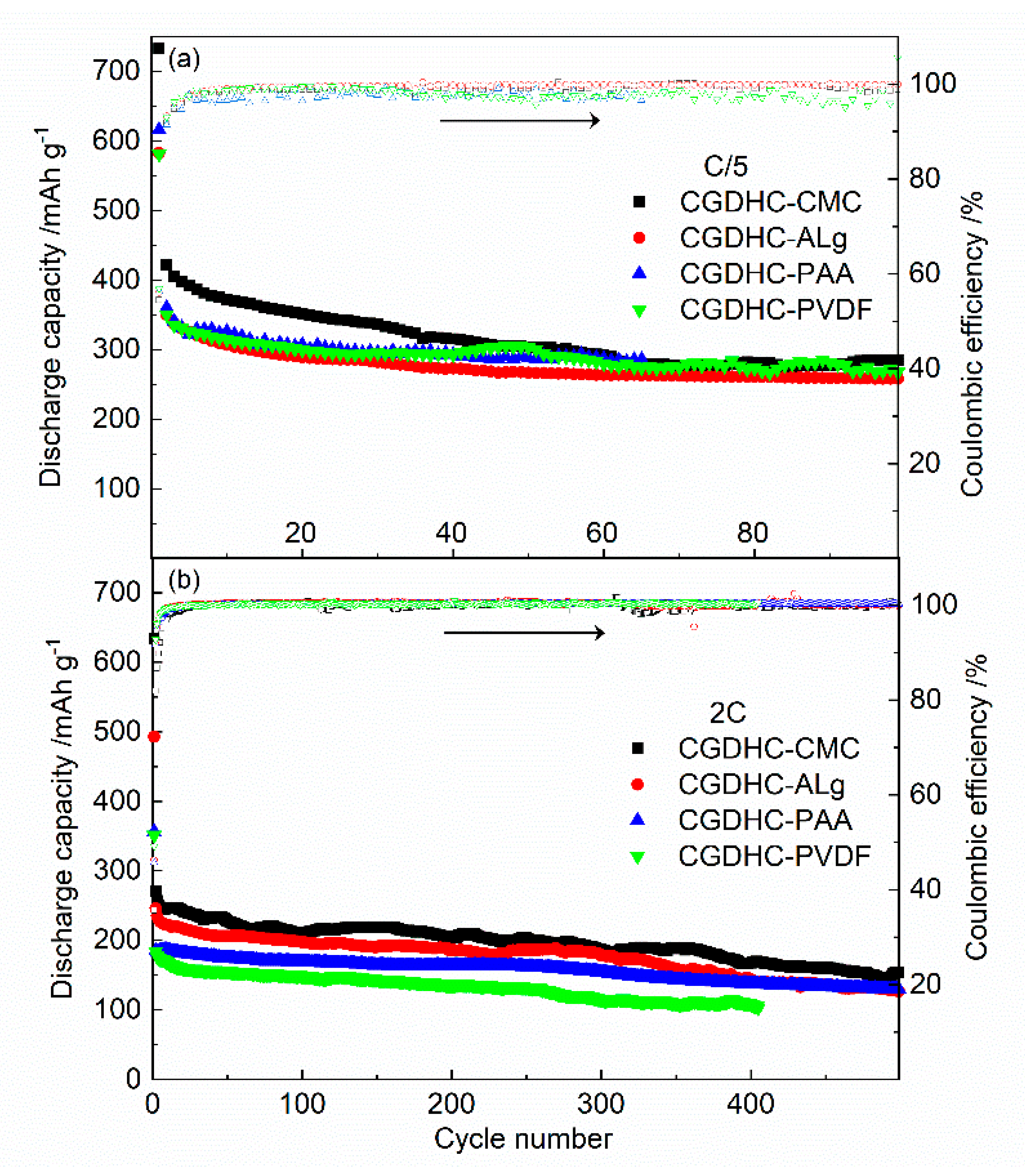
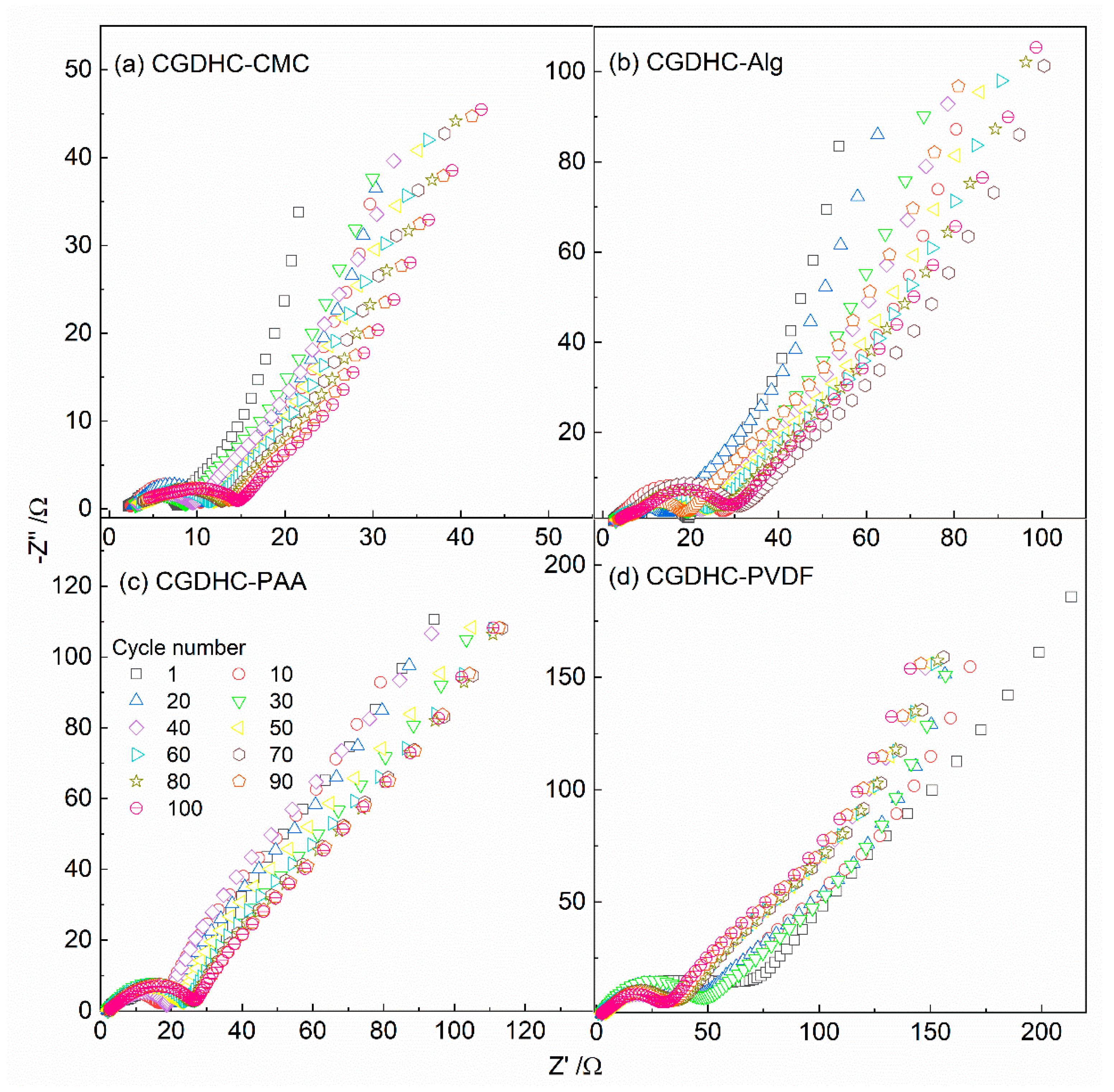

| Sample | d002 | Lc (nm) | n | ID/IG | La (nm) |
|---|---|---|---|---|---|
| CGDHC-Powder | 0.386 | 0.732 | 1.89 | 0.965 | 19.92 |
| Samples | First Discharge Capacity (mAh g−1) | First Charge Capacity (mAh g−1) | Coulombic Efficiency | Second Discharge Capacity (mAh g−1) | Capacity Retention after 100 Cycles |
|---|---|---|---|---|---|
| CGDHC-CMC | 732.4 | 399.1 | 54% | 421.8 | 70.0% |
| CGDHC-Alg | 616.0 | 346.3 | 56% | 360.2 | 72.8% |
| CGDHC-PAA | 581.3 | 330.7 | 57% | 350.3 | 76.3% |
| CGDHC-PVDF | 582.6 | 326.5 | 56% | 350.1 | 73.7% |
| Samples | First Discharge Capacity (mAh g−1) | First Charge Capacity (mAh g−1) | Coulombic Efficiency | Second Discharge Capacity (mAh g−1) | Capacity Retention after 100 Cycles |
|---|---|---|---|---|---|
| CGDHC-CMC | 335.5 | 113.0 | 34% | 152.6 | 77.7% |
| CGDHC-Alg | 250.4 | 163.2 | 65% | 173.5 | 98.0% |
| CGDHC-PAA | 263.3 | 151.0 | 57% | 164.8 | 83.7% |
| CGDHC-PVDF | 221.3 | 105.5 | 47% | 115.9 | 76.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darjazi, H.; Staffolani, A.; Sbrascini, L.; Bottoni, L.; Tossici, R.; Nobili, F. Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders. Energies 2020, 13, 6216. https://doi.org/10.3390/en13236216
Darjazi H, Staffolani A, Sbrascini L, Bottoni L, Tossici R, Nobili F. Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders. Energies. 2020; 13(23):6216. https://doi.org/10.3390/en13236216
Chicago/Turabian StyleDarjazi, Hamideh, Antunes Staffolani, Leonardo Sbrascini, Luca Bottoni, Roberto Tossici, and Francesco Nobili. 2020. "Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders" Energies 13, no. 23: 6216. https://doi.org/10.3390/en13236216
APA StyleDarjazi, H., Staffolani, A., Sbrascini, L., Bottoni, L., Tossici, R., & Nobili, F. (2020). Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders. Energies, 13(23), 6216. https://doi.org/10.3390/en13236216





