Investigation of the Fuel Utilization Factor in PEM Fuel Cell Considering the Effect of Relative Humidity at the Cathode
Abstract
1. Introduction
2. Theory Analysis of the Fuel Utilization
2.1. Definition of the Fuel Utilization Factor
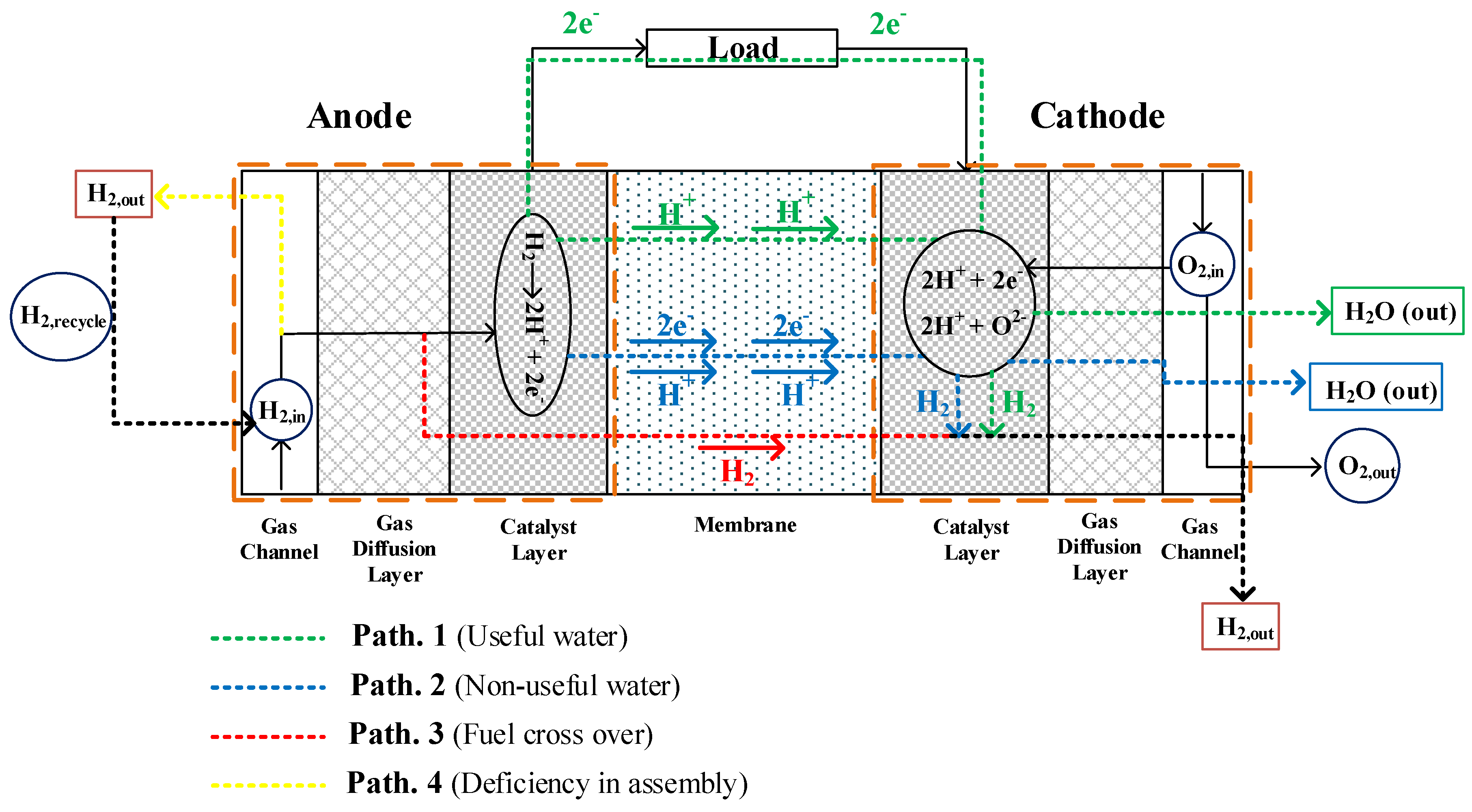
2.2. A Novel Scenario for H₂ Consumption
- Path No. 1: The input hydrogen in the catalyst layer is converted into H+ and electron. Hydrogen ions pass through the membrane and the electrons move through the external circuit to the cathode, generating current. In this path, the hydrogen ion also passes through the membrane on the other side (“Cathode”) and then can react either with the free electrons and re-generate hydrogen, or react with the oxygen ion due to the shown reduction reaction in the cathode side and generate water. The water generated in this state is called “useful water”.
- Path No. 2: In this path, the input hydrogen in the catalytic layer is converted into H+ ions and electrons, but the electrons produced along with the hydrogen ions pass through the membrane. In this state, electrons pass through the membrane using the momentum drag. That means the movement of hydrogen ions causes dragging of electrons. Hydrogen ions and electrons react in the cathode side after passing through the membrane and re-generate hydrogen. Although it may be possible that the hydrogen ion passing through the membrane reacts with the oxygen ion due to a reduction reaction in the cathode side and generates liquid water, this will be “non-useful water” since the generation of this water is not accompanied by the production of an electric current. In this state, part of the hydrogen is consumed and generates water, but it is not useful. It is obvious that most of the produced electrons in the catalytic layer move through the external circuit to the cathode, which results in the production of external current. However, a small number of electrons produced in the catalytic layer travel through the membrane to the cathode due to the polarity created. To further clarify “Path No. 2”, it can be said that the faradic efficiency of a PEMFC is not completely 100% for two reasons:
- (i)
- The short circuit current, which can arise because of the electrons transfer across the membrane. Such a current is negligible in normal operating conditions despite the membrane can be assumed as a dielectric. This last contribution can be very important as a consequence either of the membrane ageing or the same manufacturing flaws. In the worst case, such a conduction can also arise from the local contact between the two electrodes;
- (ii)
- The gas crossover across the membrane, which in this case can be associated with “Path No. 3”.
- Path No. 3: Hydrogen passes through the membrane without participating in the oxidation reaction, which is called fuel crossover. Hydrogen passing through the membrane leaves the cathode side of the fuel cell. The amount of hydrogen in this path is negligible, even at high temperatures and high currents.
- Path No. 4: In this path, the reason for not having an effective hydrogen passage is due to either the lack of proper design of the flow channels or the failure to assemble the components of the fuel cell correctly. This will cause the hydrogen to move out of the output channel in the anode side without having a chance to participate in the oxidation reaction.
3. Experimental Measurement
4. Results and Discussion
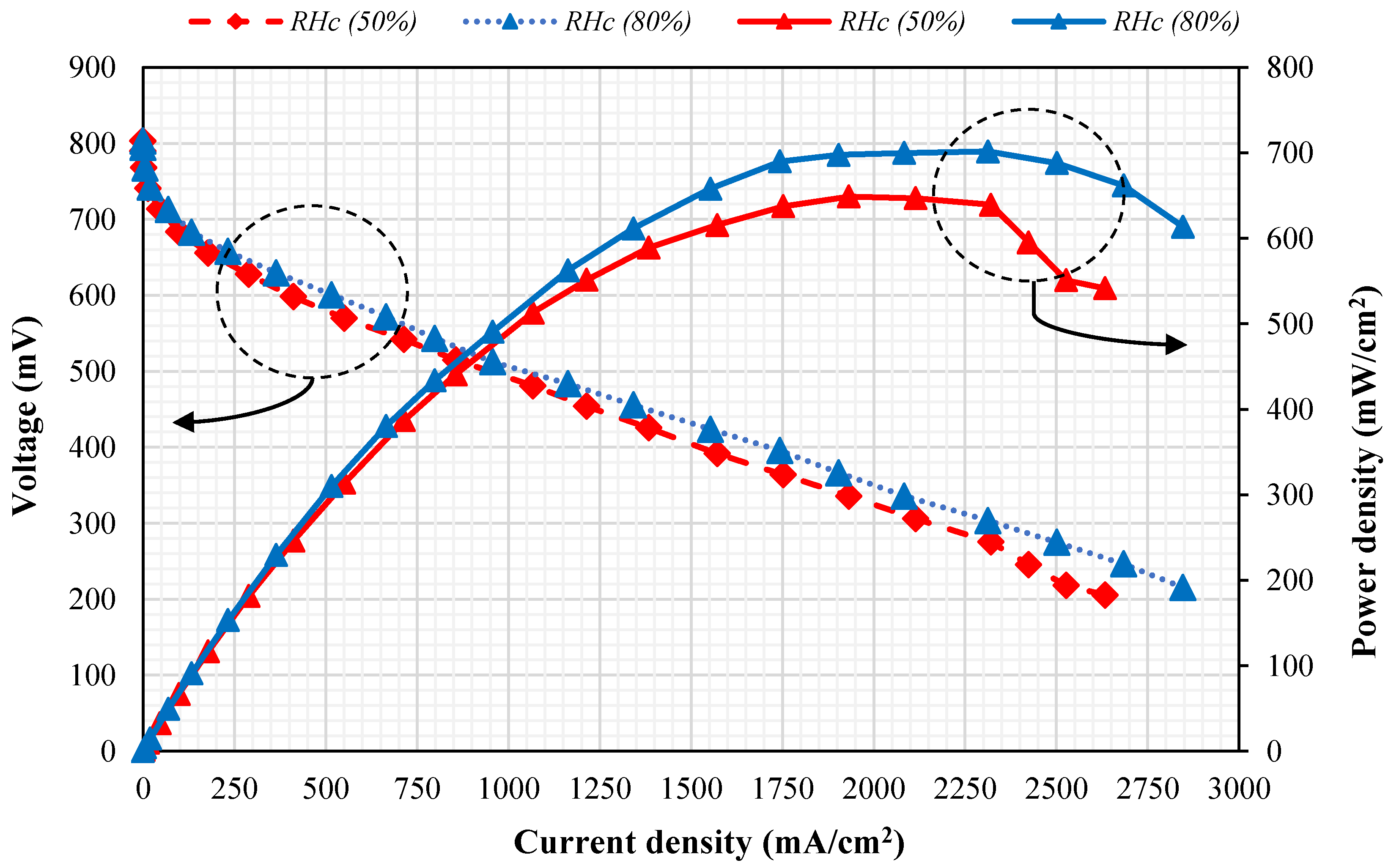
4.1. The Effect of Cathode Relative Humidity on PEMFC Power Density
- At a current density lower than 500 mA/cm2, increasing the relative humidity of the cathode side does not affect the polarization curve. In fact, in this current density range, the effective factor showing the catalytic effectiveness is the activation losses. The relative humidity change has no effect on the activation losses.
- At current density values between 500 and 1750 mA/cm2, an increase in relative humidity leads to a gradual increase in the fuel-cell performance curve. Considering the range of medium current densities, the most important losses are the ohmic losses; therefore, increasing the relative humidity of the cathode side causes the membrane to hydrate and the ohmic losses to decrease.
- At current densities larger than 1750 mA/cm2, the difference between the two performance curves is relatively constant up to a current density of about 2200 mA/cm2 for 50% and 80% relative humidity. On the other hand, at current densities larger than 2200 mA/cm2, the performance curve with a relative humidity of 50% shows a sudden decrease not seen for 80% relative humidity. As the current density depends on the concentration of the reactants at the catalyst layer, it can be concluded that in high current density, the most important voltage loss is due to concentration losses; the main cause of it is the high reaction rate inside the fuel cell and the exothermic nature of the half-reaction of the cathode side. Therefore, increasing the relative humidity can lead to enhancement of the cell’s performance through the further transfer of water into the cell and pushing the membrane to the full hydrated state, despite the increase in the reaction rate inside the fuel cell. It should be noted that excessive moisture might result in a flooding condition in the cathode.
4.2. Calculation of the Modified Fuel Utilization Factor by Using the Parameter δ
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| e− | Electron |
| F | Faraday constant (96,487 C·mol−1) |
| GDL | Gas Diffusion Layer |
| H2 | Hydrogen |
| H2O | Water |
| H+ | Hydrogen ion |
| I | Current (A) |
| n | Number of electrons in the electrochemical reaction |
| Amount of consumable hydrogen (mol·s−1) | |
| O2 | Oxygen |
| OCV | Open circuit voltage (V) |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| RH | Relative humidity (%) |
| UF | Fuel utilization |
| Modified fuel utilization | |
| Greek Letters | |
| Correction factor for fuel utilization | |
| Superscripts | |
| a | anode |
| c | cathode |
| con | consumed |
| in | inlet |
| out | outlet |
References
- Ghadamian, H.; Saboohi, Y. Quantitative analysis of irreversibilities causes voltage drop in fuel cell (simulation & modeling). Electrochim. Acta 2004, 50, 699–704. [Google Scholar]
- Ghadamian, H.; Bakhtary, K.; Namini, S.S. An algorithm for optimum design and macro-model development in PEMFC with exergy and cost considerations. J. Power Sources 2006, 163, 87–92. [Google Scholar] [CrossRef]
- Kazeminasab, B.; Rowshanzamir, S.; Ghadamian, H. Multi-objective multivariable optimization of agglomerated cathode catalyst layer of a proton exchange membrane fuel cell. Bulg. Chem. Commun. 2015, 47, 38–48. [Google Scholar]
- Kim, H.Y.; Kim, K. Numerical study on the effects of gas humidity on proton-exchange membrane fuel cell performance. Int. J. Hydrogen Energy 2016, 41, 11776–11783. [Google Scholar] [CrossRef]
- Ozen, D.N.; Timurkutluk, B.; Altinisik, K. Effects of operation temperature and reactant gas humidity levels on performance of PEM fuel cells. Renew. Sustain. Energy Rev. 2016, 59, 1298–1306. [Google Scholar] [CrossRef]
- Kazeminasab, B.; Rowshanzamir, S.; Ghadamian, H. Nitrogen doped graphene/cobalt-based catalyst layers of a PEM fuel cell: Performance evaluation and multi-objective optimization. Korean J. Chem. Eng. 2017, 34, 2978–2983. [Google Scholar] [CrossRef]
- Ou, K.; Yuan, W.W.; Choi, M.; Yang, S.; Kim, Y.B. Performance increase for an open-cathode PEM fuel cell with humidity and temperature control. Int. J. Hydrog. Energy 2017, 42, 29852–29862. [Google Scholar] [CrossRef]
- Muirhead, D.; Banerjee, R.; George, M.G.; Ge, N.; Shrestha, P.; Liu, H.; Lee, J.; Bazylak, A. Liquid water saturation and oxygen transport resistance in polymer electrolyte membrane fuel cell gas diffusion layers. Electrochim. Acta 2018, 274, 250–265. [Google Scholar] [CrossRef]
- Hasheminasab, M.; Kermani, M.J.; Nourazar, S.S.; Khodsiani, M.H. A novel experimental based statistical study for water management in proton exchange membrane fuel cells. Appl. Energy 2020, 264, 114713. [Google Scholar] [CrossRef]
- Wang, B.; Wu, K.; Xi, F.; Xuan, J.; Xie, X.; Wang, X.; Jiao, K. Numerical analysis of operating conditions effects on PEMFC with anode recirculation. Energy 2019, 173, 844–856. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mojica, F.; Sarker, M. Abel Chuang, P.Y. Development of 1-D multiphysics PEMFC model with dry limiting current experimental validation. Electrochim. Acta 2019, 320, 134601. [Google Scholar] [CrossRef]
- Jeon, D.H.; Kim, K.N.; Baek, S.M.; Nam, J.H. The effect of relative humidity of the cathode on the performance and the uniformity of PEM fuel cells. Int. J. Hydrog. Energy 2011, 36, 12499–12511. [Google Scholar] [CrossRef]
- Abdollahzadeha, M.; Pascoa, J.C.; Ranjbar, A.A.; Esmaili, Q. Analysis of PEM (Polymer Electrolyte Membrane) fuel cell cathode two-dimensional modeling. Energy 2014, 68, 478–494. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sasou, H.; Kurihara, R.; Nakamura, S.; Kano, A.; Tanaka, K.; Aoki, T.; Ogami, Y. High fuel utilization operation of pure hydrogen fuel cells. Int. J. Hydrog. Energy 2008, 33, 6262–6269. [Google Scholar] [CrossRef]
- Iranzo, A.; Boillat, P.; Biesdorf, J.; Salva, A. Investigation of the liquid water distributions in a 50 cm2 PEM fuel cell: Effects of reactants relative humidity, current density, and cathode stoichiometry. Energy 2015, 82, 914–921. [Google Scholar] [CrossRef]
- Muirhead, D.; Banerjee, R.; Lee, J.; George, M.G.; Ge, N.; Liu, H.; Chevalier, S.; Hinebaugh, J.; Han, K.; Bazylak, A. Simultaneous characterization of oxygen transport resistance and spatially resolved liquid water saturation at high-current density of polymer electrolyte membrane fuel cells with varied cathode relative humidity. Int. J. Hydrog. Energy 2017, 42, 29472–29483. [Google Scholar] [CrossRef]
- Yousefkhani, M.B.; Ghadamian, H.; Massoudi, A.; Aminy, M. Quantitative and qualitative investigation of the fuel utilization and introducing a novel calculation idea based on transfer phenomena in a polymer electrolyte membrane (PEM) fuel cell. Energy Convers. Manag. 2017, 131, 90–98. [Google Scholar] [CrossRef]
- Farhat, Z.N. Modeling of catalyst layer microstructural refinement and catalyst utilization in a PEM fuel cell. J. Power Sources 2004, 138, 68–78. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, J.Y.; Jo, D.H.; Jung, H.W.; Kim, S.H. Polarization characteristics and fuel utilization in anode-supported solid oxide fuel cell using three-dimensional simulation. Korean J. Chem. Eng. 2011, 28, 143–148. [Google Scholar] [CrossRef]
- Han, I.; Jeong, J.; Shin, H.K. PEM fuel-cell stack design for improved fuel utilization. Int. J. Hydrog. Energy 2013, 38, 11996–12006. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; University of Connecticut: Storrs, CT, USA; Elsevier: San Diego, CA, USA, 2005; pp. 33–34. [Google Scholar]
- Arif, M.; Cheung, S.C.P.; Andrews, J. A systematic approach for matching simulated and experimental polarization curves for a PEM fuel cell. Int. J. Hydrogen Energy 2019, 45, 2206–2223. [Google Scholar] [CrossRef]

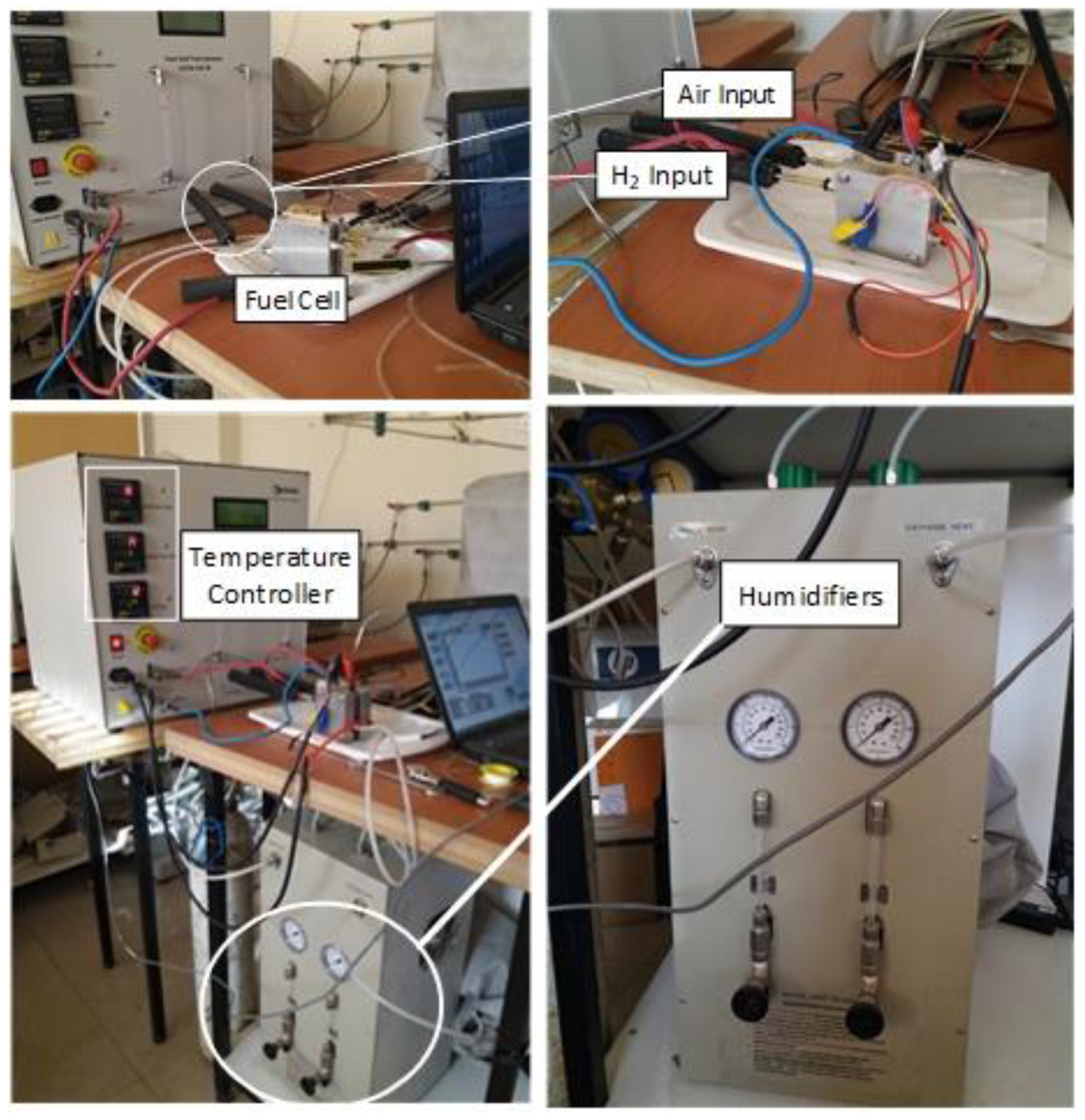

| Characteristic | Description/Value |
|---|---|
| Cell parameters | |
| Flow channel type | Single serpentine |
| Flow collectors material | Stainless steel |
| End plate material | Aluminum alloy |
| Maximum operating temperature (°C) | 90 |
| Maximum operating pressure (bar) | 2 |
| Active surface area (cm2) | 5 |
| Operating conditions | |
| Anode pressure (bar) | 1 |
| Cathode pressure (bar) | 1 |
| Inlet flow rate of H2 (lit/min) | 0.3 |
| Inlet flow rate of O2 (lit/min) | 0.3 |
| Cell Temperature (°C) | 60 |
| Anode Temperature (°C) | 60 |
| Cathode Temperature (°C) | 56 |
| Anode relative humidity (%) | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghban Yousefkhani, M.; Ghadamian, H.; Daneshvar, K.; Alizadeh, N.; Rincon Troconis, B.C. Investigation of the Fuel Utilization Factor in PEM Fuel Cell Considering the Effect of Relative Humidity at the Cathode. Energies 2020, 13, 6117. https://doi.org/10.3390/en13226117
Baghban Yousefkhani M, Ghadamian H, Daneshvar K, Alizadeh N, Rincon Troconis BC. Investigation of the Fuel Utilization Factor in PEM Fuel Cell Considering the Effect of Relative Humidity at the Cathode. Energies. 2020; 13(22):6117. https://doi.org/10.3390/en13226117
Chicago/Turabian StyleBaghban Yousefkhani, Mojtaba, Hossein Ghadamian, Keyvan Daneshvar, Nima Alizadeh, and Brendy C. Rincon Troconis. 2020. "Investigation of the Fuel Utilization Factor in PEM Fuel Cell Considering the Effect of Relative Humidity at the Cathode" Energies 13, no. 22: 6117. https://doi.org/10.3390/en13226117
APA StyleBaghban Yousefkhani, M., Ghadamian, H., Daneshvar, K., Alizadeh, N., & Rincon Troconis, B. C. (2020). Investigation of the Fuel Utilization Factor in PEM Fuel Cell Considering the Effect of Relative Humidity at the Cathode. Energies, 13(22), 6117. https://doi.org/10.3390/en13226117





