Maximising Yield and Engine Efficiency Using Optimised Waste Cooking Oil Biodiesel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biodiesel Production Using WCO
2.3. Biodiesel Property Analysis
2.4. Method for Biodiesel Yield Optimisation
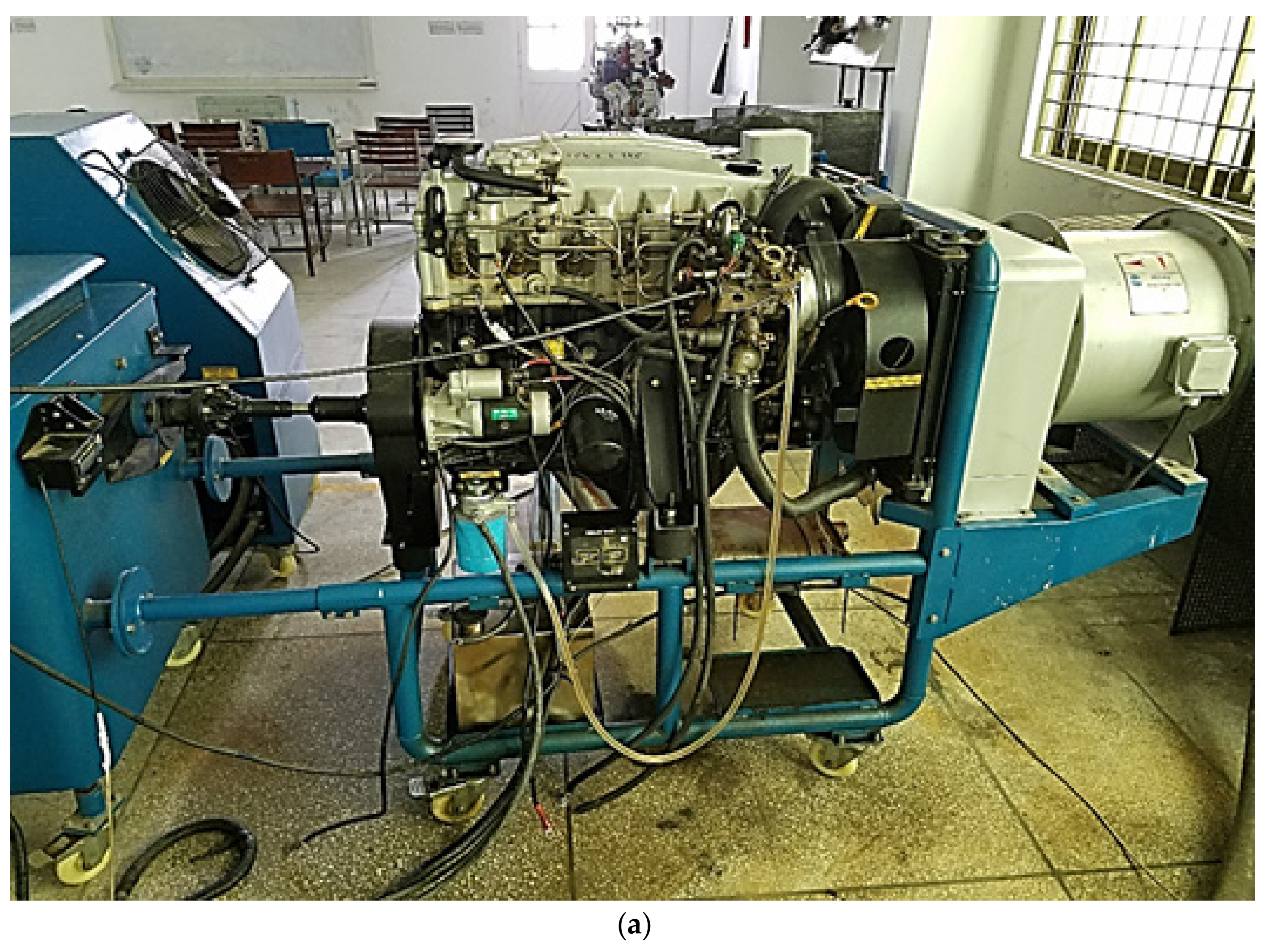
2.5. Engine Setup
3. Results and Analysis
3.1. Physicochemical Properties of Biodiesel
3.2. Model Fitting and ANOVA
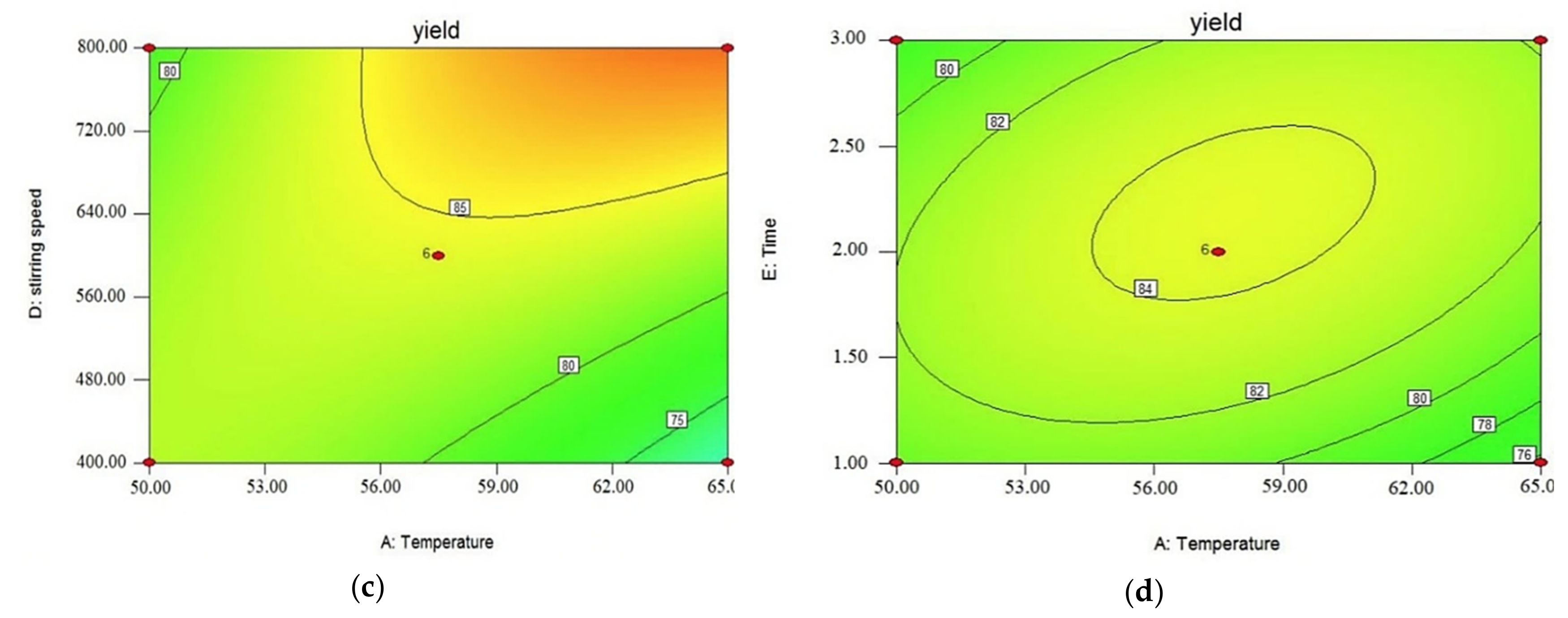
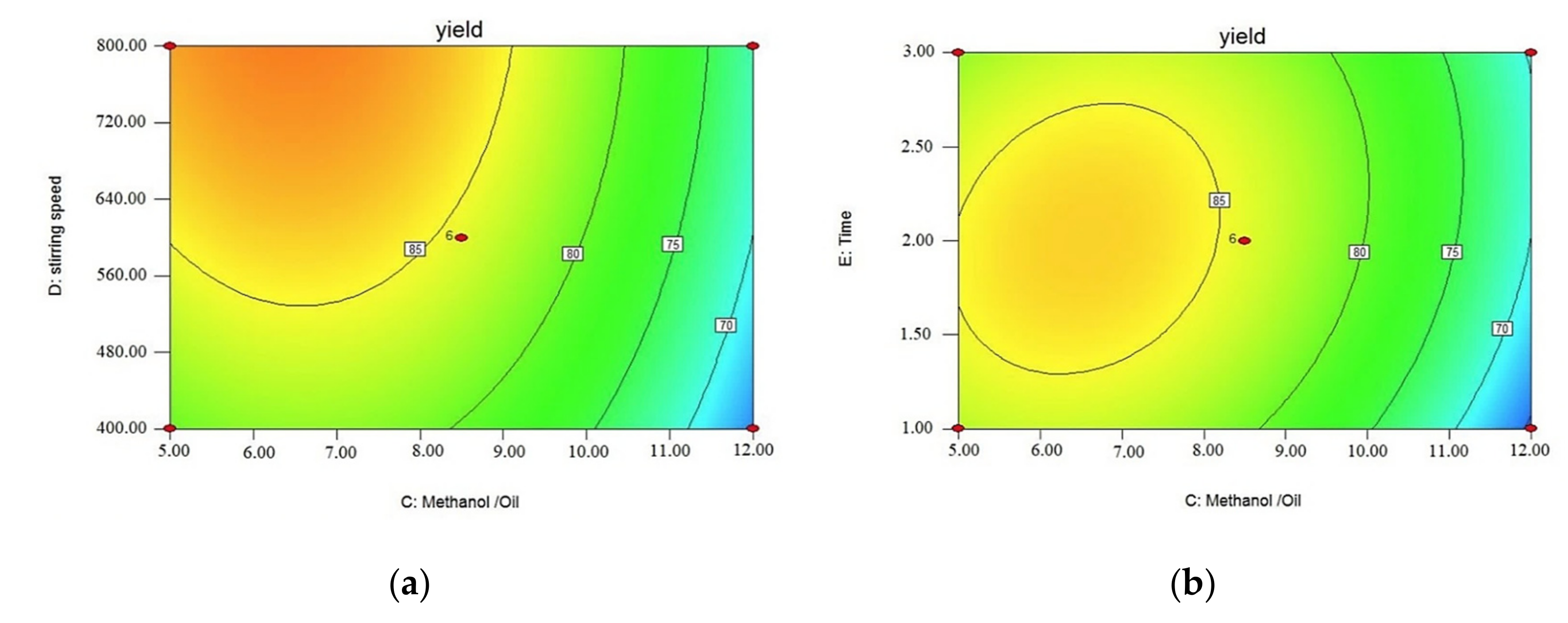
3.3. Effect of Operating Parameters
3.4. Performance Characteristics
3.4.1. Brake Torque and Brake Power
3.4.2. Brake Specific Fuel Consumption and Brake Thermal Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ANOVA | Analysis of Variance |
| ANFIS | Adaptive neuro-fuzzy inference system |
| AV | Acid value |
| BSFC | Brake specific fuel consumption |
| BP | Brake power |
| BTE | Brake thermal efficiency |
| BT | Brake torque |
| CCD | Central Composite Design |
| CI | Compression ignition |
| DI | Direct injection |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid |
| GCMS | Gas chromatography-mass spectroscopy |
| HSD | High-speed diesel |
| HPLC | High-Performance Liquid Chromatography |
| LHV | Lower heating value |
| NMR | Nuclear magnetic resonance |
| RSM | Response surface methodology |
| WCO | Waste cooking oil |
References
- Soudagar, M.E.M.; Banapurmath, N.R.; Afzal, A.; Hossain, N.; Abbas, M.M.; Haniffa, M.A.C.M.; Naik, B.; Ahmed, W.; Nizamuddin, S.; Mubarak, N.M. Study of diesel engine characteristics by adding nanosized zinc oxide and diethyl ether additives in Mahua biodiesel–diesel fuel blend. Sci. Rep. 2020, 10, 15326. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Soudagar, M.E.M.; Afzal, A.; Mujtaba, M.; Fattah, I.M.R.; Naik, B.; Mulla, M.H.; Badruddin, I.A.; Khan, T.M.Y.; Raju, V.D.; et al. Enhancement in Combustion, Performance, and Emission Characteristics of a Diesel Engine Fueled with Ce-ZnO Nanoparticle Additive Added to Soybean Biodiesel Blends. Energies 2020, 13, 4578. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Masjuki, H.H.; Kalam, M.A.; Noor, F.; Farooq, M.; Ong, H.C.; Gul, M.; Soudagar, M.E.M.; Bashir, S.; Fattah, I.M.R.; et al. Effect of Additivized Biodiesel Blends on Diesel Engine Performance, Emission, Tribological Characteristics, and Lubricant Tribology. Energies 2020, 13, 3375. [Google Scholar] [CrossRef]
- Baudry, G.; Macharis, C.; Vallée, T. Can microalgae biodiesel contribute to achieve the sustainability objectives in the transport sector in France by 2030? A comparison between first, second and third generation biofuels though a range-based Multi-Actor Multi-Criteria Analysis. Energy 2018, 155, 1032–1046. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Naqvi, M.; Saleem, M.W.; Hussain, J.; Naqvi, S.R.; Jahangir, S.; Jazim Usama, H.M.; Idrees, S.; Anukam, A. Use of gasoline, LPG and LPG-HHO blend in SI engine: A comparative performance for emission control and sustainable environment. Processes 2020, 8, 74. [Google Scholar] [CrossRef]
- Masjuki, H.H.; Ruhul, A.M.; Mustafi, N.N.; Kalam, M.A.; Arbab, M.I.; Rizwanul Fattah, I.M. Study of production optimization and effect of hydroxyl gas on a CI engine performance and emission fueled with biodiesel blends. Int. J. Hydrog. Energy 2016, 41, 14519–14528. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Rizwanul Fattah, I.M.; Sanjid, A. Feasibility of diesel–biodiesel–ethanol/bioethanol blend as existing CI engine fuel: An assessment of properties, material compatibility, safety and combustion. Renew. Sustain. Energy Rev. 2014, 32, 379–395. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118. [Google Scholar] [CrossRef]
- Serin, H.; Yıldızhan, Ş. Hydrogen addition to tea seed oil biodiesel: Performance and emission characteristics. Int. J. Hydrog. Energy 2018, 43, 18020–18027. [Google Scholar] [CrossRef]
- Imran, S.; Korakianitis, T.; Shaukat, R.; Farooq, M.; Condoor, S.; Jayaram, S. Experimentally tested performance and emissions advantages of using natural-gas and hydrogen fuel mixture with diesel and rapeseed methyl ester as pilot fuels. Appl. Energy 2018, 229, 1260–1268. [Google Scholar] [CrossRef]
- Imran, S.; Emberson, D.R.; Hussain, A.; Ali, H.; Ihracska, B.; Korakianitis, T. Performance and specific emissions contours throughout the operating range of hydrogen-fueled compression ignition engine with diesel and RME pilot fuels. Alex. Eng. J. 2015, 54, 303–314. [Google Scholar] [CrossRef]
- Alagu, K.; Venu, H.; Jayaraman, J.; Raju, V.D.; Subramani, L.; Appavu, P.; Dhanasekar, S. Novel water hyacinth biodiesel as a potential alternative fuel for existing unmodified diesel engine: Performance, combustion and emission characteristics. Energy 2019, 179, 295–305. [Google Scholar] [CrossRef]
- Vigneswaran, R.; Annamalai, K.; Dhinesh, B.; Krishnamoorthy, R. Experimental investigation of unmodified diesel engine performance, combustion and emission with multipurpose additive along with water-in-diesel emulsion fuel. Energy Convers. Manag. 2018, 172, 370–380. [Google Scholar] [CrossRef]
- Yunus Khan, T.M.; Badruddin, I.A.; Ankalgi, R.F.; Badarudin, A.; Hungund, B.S.; Ankalgi, F.R. Biodiesel Production by Direct Transesterification Process via Sequential Use of Acid–Base Catalysis. Arab. J. Sci. Eng. 2018, 43, 5929–5936. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the art of catalysts for biodiesel production. Front. Energy Res. 2020. [Google Scholar] [CrossRef]
- Vicente, G.; Martı́nez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Mekala, M.; Goli, V.R. Kinetics of esterification of methanol and acetic acid with mineral homogeneous acid catalyst. Chin. J. Chem. Eng. 2015, 23, 100–105. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Likozar, B.; Levec, J. Effect of process conditions on equilibrium, reaction kinetics and mass transfer for triglyceride transesterification to biodiesel: Experimental and modeling based on fatty acid composition. Fuel Process. Technol. 2014, 122, 30–41. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Kale, P.; Geremew, B.; Adeloju, S.B.; Nizami, M.; Ayoub, M. Optimization of process variables for biodiesel production by transesterification of flaxseed oil and produced biodiesel characterizations. Renew. Energy 2019, 139, 1272–1280. [Google Scholar] [CrossRef]
- Klofutar, B.; Golob, J.; Likozar, B.; Klofutar, C.; Žagar, E.; Poljanšek, I. The transesterification of rapeseed and waste sunflower oils: Mass-transfer and kinetics in a laboratory batch reactor and in an industrial-scale reactor/separator setup. Bioresour. Technol. 2010, 101, 3333–3344. [Google Scholar] [CrossRef] [PubMed]
- Fattah, I.M.R.; Yip, H.L.; Jiang, Z.; Yuen, A.C.Y.; Yang, W.; Medwell, P.R.; Kook, S.; Yeoh, G.H.; Chan, Q.N. Effects of flame-plane wall impingement on diesel combustion and soot processes. Fuel 2019, 255, 115726. [Google Scholar] [CrossRef]
- Yip, H.L.; Rizwanul Fattah, I.M.; Yuen, A.C.Y.; Yang, W.; Medwell, P.R.; Kook, S.; Yeoh, G.H.; Chan, Q.N. Flame–Wall Interaction Effects on Diesel Post-injection Combustion and Soot Formation Processes. Energy Fuels 2019, 33, 7759–7769. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Rashedul, H.K.; Masjuki, H.H.; Kalam, M.A.; Ashraful, A.M.; Ashrafur Rahman, S.M.; Shahir, S.A. The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers. Manag. 2014, 88, 348–364. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Nirmala, N.; Dawn, S.S.; Harindra, C. Analysis of performance and emission characteristics of Waste cooking oil and Chlorella variabilis MK039712.1 biodiesel blends in a single cylinder, four strokes diesel engine. Renew. Energy 2020, 147, 284–292. [Google Scholar] [CrossRef]
- Akcay, M.; Yilmaz, I.T.; Feyzioglu, A. Effect of hydrogen addition on performance and emission characteristics of a common-rail CI engine fueled with diesel/waste cooking oil biodiesel blends. Energy 2020, 212, 118538. [Google Scholar] [CrossRef]
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a waste cooking oil biodiesel mixture. Energy Convers. Manag. 2014, 87, 676–686. [Google Scholar] [CrossRef]
- Nayak, M.G.; Vyas, A.P. Optimization of microwave-assisted biodiesel production from Papaya oil using response surface methodology. Renew. Energy 2019, 138, 18–28. [Google Scholar] [CrossRef]
- Mostafaei, M.; Javadikia, H.; Naderloo, L. Modeling the effects of ultrasound power and reactor dimension on the biodiesel production yield: Comparison of prediction abilities between response surface methodology (RSM) and adaptive neuro-fuzzy inference system (ANFIS). Energy 2016, 115, 626–636. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. Optimization of lipid extraction from Salvinia molesta for biodiesel production using RSM and its FAME analysis. Environ. Sci. Pollut. Res. 2016, 23, 14047–14055. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Divya, N.; Jha, M.K. Application of RSM for Optimizing the Biodiesel Production Catalyzed by Calcium Methoxide. In Sustainable Engineering; Springer: Singapore, 2019; pp. 75–83. [Google Scholar]
- Latchubugata, C.S.; Kondapaneni, R.V.; Patluri, K.K.; Virendra, U.; Vedantam, S. Kinetics and optimization studies using Response Surface Methodology in biodiesel production using heterogeneous catalyst. Chem. Eng. Res. Des. 2018, 135, 129–139. [Google Scholar] [CrossRef]
- Jamshaid, M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Arslan, A.; Alwi, A.; Khuong, L.S.; Alabdulkarem, A.; Syahir, A.Z. Production optimization and tribological characteristics of cottonseed oil methyl ester. J. Clean. Prod. 2019, 209, 62–73. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N.; Rahman, M.M. Optimisation of Second-Generation Biodiesel Production from Australian Native Stone Fruit Oil Using Response Surface Method. Energies 2018, 11, 2566. [Google Scholar] [CrossRef]
- Veljković, V.B.; Veličković, A.V.; Avramović, J.M.; Stamenković, O.S. Modeling of biodiesel production: Performance comparison of Box–Behnken, face central composite and full factorial design. Chin. J. Chem. Eng. 2019, 27, 1690–1698. [Google Scholar] [CrossRef]
- Chai, M.; Tu, Q.; Lu, M.; Yang, Y.J. Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel Process. Technol. 2014, 125, 106–113. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Masjuki, H.H.; Kalam, M.A.; Wakil, M.A.; Ashraful, A.M.; Shahir, S.A. Experimental investigation of performance and regulated emissions of a diesel engine with Calophyllum inophyllum biodiesel blends accompanied by oxidation inhibitors. Energy Convers. Manag. 2014, 83, 232–240. [Google Scholar] [CrossRef]
- Santos, T.; Gomes, J.F.; Puna, J. Liquid-liquid equilibrium for ternary system containing biodiesel, methanol and water. J. Environ. Chem. Eng. 2018, 6, 984–990. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Masjuki, H.H.; Kalam, M.A.; Mofijur, M.; Abedin, M.J. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers. Manag. 2014, 79, 265–272. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Sudalaiyadum Perumal, A.; Jayaraman, J.; Mani, J.; Ramanujam, P. Comparative analysis for the production of fatty acid alkyl esterase using whole cell biocatalyst and purified enzyme from Rhizopus oryzae on waste cooking oil (sunflower oil). Waste Manag. 2012, 32, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sharma, M.P. Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 2016, 180, 164–174. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 2005, 28, 601–605. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Kalam, M.A.; Masjuki, H.H.; Wakil, M.A. Biodiesel production, characterization, engine performance, and emission characteristics of Malaysian Alexandrian laurel oil. RSC Adv. 2014, 4, 17787–17796. [Google Scholar] [CrossRef]
- Palash, S.M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Rizwanul Fattah, I.M.; Sanjid, A. Biodiesel production, characterization, diesel engine performance, and emission characteristics of methyl esters from Aphanamixis polystachya oil of Bangladesh. Energy Convers. Manag. 2015, 91, 149–157. [Google Scholar] [CrossRef]






| Properties | Units | Values |
|---|---|---|
| Density at 15 °C | kg/m3 | 910.30 |
| Acid Value | mg KOH/g | 7.80 |
| Free fatty acid | % | 3.90 |
| Molecular weight | g/mol | 860.56 |
| Viscosity at 40 °C | mm2/s | 6.80 |
| Operating Parameters | Unit | Ranges |
|---|---|---|
| Temperature | °C | 50–65 |
| Catalyst Concentration | w/w | 0.25–1.75 |
| Reaction Time | h | 1–3 |
| Reaction Speed | rpm | 400–800 |
| Methanol to oil ratio | - | 5:1–12:1 |
| Description | Specifications |
|---|---|
| No. of cylinders | 6 |
| Displacement (cm3) | 2825 |
| Bore (mm) | 85 |
| Stroke (mm) | 83 |
| Cooling system | Water-cooled |
| Compression ratio | 22:4 |
| Maximum power | 73.55 kW/4800 rpm |
| Maximum torque | 178.48 Nm/3000 rpm |
| Dynamometer | Hydraulic |
| Injection system | Indirect |
| Properties | Diesel | B100 | B10 | B20 | B30 | B40 | B50 |
|---|---|---|---|---|---|---|---|
| Density at 15 °C (kg/m3) | 831 | 892 | 833.5 | 837.5 | 842.5 | 851.5 | 859.5 |
| Viscosity at 40 °C (mm2/s) | 3.9016 | 5.69 | 3.993 | 3.58 | 4.163 | 4.648 | 4.933 |
| Acid value (mg KOH/g) | <0.247 | 0.6732 | 0.25 | 0.265 | 0.269 | 0.276 | 0.285 |
| Flashpoint (°C) | 79 | 140 | 135.78 | 133.34 | 130 | 124.56 | 120 |
| Pour point (°C) | 7 | 1.2 | 1.5 | 1.8 | 2 | 2.5 | 3 |
| Lower Heating Value (MJ/kg) | 44.2 | 38.753 | 42.209 | 41.987 | 41.632 | 40.098 | 39.456 |
| Common Name | Structure | WCOME |
|---|---|---|
| Methyl Palmitate | C16:0 | 6 |
| Methyl Palmitoleate | C16:1 | 0.6 |
| Methyl Eicosenoate | C20:1 | 0.8 |
| Methyl Behenate | C22:0 | 0.9 |
| Methyl Arachidate | C20:0 | 0.9 |
| Methyl Stearate | C18:0 | 0.8 |
| Methyl Oleate | C18:1 | 54.0 |
| Methyl Linoleate | C18:2 | 25.7 |
| Methyl Erucate | C22:1 | 2.2 |
| Methyl Linolenate | C18:3 | 8.1 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 2401.89 | 20 | 120.09 | 2.7 | 0.0100 a |
| A-Temperature | 0.39 | 1 | 0.39 | 8.78 × 10−3 | 0.9261 b |
| B-Catalyst concentration | 456.89 | 1 | 456.89 | 10.27 | 0.0037 a |
| C-Methanol/Oil | 922.64 | 1 | 922.64 | 20.74 | 0.0001 a |
| D-stirring speed | 189.06 | 1 | 189.06 | 4.25 | 0.0498 a |
| E-Time | 15.02 | 1 | 15.02 | 0.34 | 0.5664 b |
| AB | 64 | 1 | 64 | 1.44 | 0.2416 b |
| AC | 12.25 | 1 | 12.25 | 0.28 | 0.6043 b |
| AD | 121 | 1 | 121 | 2.72 | 0.1116 b |
| AE | 18.06 | 1 | 18.06 | 0.41 | 0.5297 b |
| BC | 162.56 | 1 | 162.56 | 3.66 | 0.0674 a |
| BD | 0.56 | 1 | 0.56 | 0.013 | 0.9114 b |
| BE | 0.063 | 1 | 0.063 | 1.41 × 10−3 | 0.9704 b |
| CD | 0.56 | 1 | 0.56 | 0.013 | 0.9114 b |
| CE | 10.56 | 1 | 10.56 | 0.24 | 0.6303 b |
| DE | 1 | 1 | 1 | 0.022 | 0.8820 b |
| A2 | 57.31 | 1 | 57.31 | 1.29 | 0.2671 b |
| B2 | 37.13 | 1 | 37.13 | 0.83 | 0.3696 b |
| C2 | 405.03 | 1 | 405.03 | 9.11 | 0.0058 a |
| D2 | 12.31 | 1 | 12.31 | 0.28 | 0.6035 b |
| E2 | 73.19 | 1 | 73.19 | 1.65 | 0.2113 b |
| Residual | 1111.92 | 25 | 44.48 | ||
| Lack of Fit | 981.58 | 20 | 49.08 | 1.88 | 0.2498 b |
| Pure Error | 130.33 | 5 | 26.07 | ||
| Cor Total | 3513.8 | 45 |
| Trail No | Temperature (A) | Catalyst Concertation (B) | Methanol-Oil Ratio (C) | Stirring Speed (D) | Time (E) | Biodiesel Yield |
|---|---|---|---|---|---|---|
| 1 | 65 | 1.75 | 8.5 | 600 | 2 | 76 |
| 2 | 57.5 | 1 | 5 | 600 | 3 | 84 |
| 3 | 65 | 1 | 8.5 | 600 | 3 | 77 |
| 4 | 57.5 | 1 | 5 | 800 | 2 | 90 |
| 5 | 57.5 | 0.25 | 12 | 600 | 2 | 63.5 |
| 6 | 57.5 | 1 | 8.5 | 800 | 3 | 85 |
| 7 | 57.5 | 1 | 12 | 800 | 2 | 76 |
| 8 | 65 | 1 | 5 | 600 | 2 | 86.5 |
| 9 | 65 | 1 | 12 | 600 | 2 | 77.5 |
| 10 | 57.5 | 1 | 8.5 | 600 | 2 | 84.5 |
| 11 | 57.5 | 1 | 12 | 600 | 3 | 65.5 |
| 12 | 50 | 1 | 8.5 | 800 | 2 | 84 |
| 13 | 57.5 | 1 | 8.5 | 600 | 2 | 91.5 |
| 14 | 57.5 | 1 | 5 | 400 | 2 | 75 |
| 15 | 57.5 | 1 | 5 | 600 | 1 | 86.5 |
| 16 | 57.5 | 1 | 8.5 | 800 | 1 | 74 |
| 17 | 57.5 | 1 | 8.5 | 600 | 2 | 75.5 |
| 18 | 57.5 | 0.25 | 8.5 | 600 | 1 | 91 |
| 19 | 65 | 1 | 8.5 | 800 | 2 | 92.5 |
| 20 | 50 | 1 | 8.5 | 600 | 3 | 76 |
| 21 | 57.5 | 0.25 | 5 | 600 | 2 | 89.5 |
| 22 | 57.5 | 1.75 | 12 | 600 | 2 | 72.5 |
| 23 | 65 | 1 | 8.5 | 400 | 2 | 71 |
| 24 | 57.5 | 1 | 12 | 600 | 1 | 61.5 |
| 25 | 57.5 | 1.75 | 8.5 | 800 | 2 | 71 |
| 26 | 50 | 1 | 12 | 600 | 2 | 63 |
| 27 | 57.5 | 0.25 | 8.5 | 600 | 3 | 93 |
| 28 | 50 | 1 | 8.5 | 600 | 1 | 85 |
| 29 | 57.5 | 1 | 8.5 | 400 | 1 | 70 |
| 30 | 57.5 | 1 | 8.5 | 400 | 3 | 79 |
| 31 | 57.5 | 0.25 | 8.5 | 400 | 2 | 93 |
| 32 | 65 | 1 | 8.5 | 600 | 1 | 77.5 |
| 33 | 57.5 | 1.75 | 8.5 | 600 | 1 | 73 |
| 34 | 57.5 | 1 | 8.5 | 600 | 2 | 85 |
| 35 | 57.5 | 1 | 8.5 | 600 | 2 | 85 |
| 36 | 57.5 | 1.75 | 8.5 | 400 | 2 | 74 |
| 37 | 57.5 | 1.75 | 8.5 | 600 | 3 | 74.5 |
| 38 | 57.5 | 1 | 12 | 400 | 2 | 62.5 |
| 39 | 57.5 | 0.25 | 8.5 | 800 | 2 | 91.5 |
| 40 | 50 | 0.25 | 8.5 | 600 | 2 | 83.5 |
| 41 | 50 | 1 | 8.5 | 400 | 2 | 84.5 |
| 42 | 50 | 1.75 | 8.5 | 600 | 2 | 74.5 |
| 43 | 57.5 | 1.75 | 5 | 600 | 2 | 73 |
| 44 | 65 | 0.25 | 8.5 | 600 | 2 | 69 |
| 45 | 57.5 | 1 | 8.5 | 600 | 2 | 84.5 |
| 46 | 50 | 1 | 5 | 600 | 2 | 79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaq, L.; Imran, S.; Anwar, Z.; Farooq, M.; Abbas, M.M.; Mehmood Khan, H.; Asif, T.; Amjad, M.; Soudagar, M.E.M.; Shaukat, N.; et al. Maximising Yield and Engine Efficiency Using Optimised Waste Cooking Oil Biodiesel. Energies 2020, 13, 5941. https://doi.org/10.3390/en13225941
Razzaq L, Imran S, Anwar Z, Farooq M, Abbas MM, Mehmood Khan H, Asif T, Amjad M, Soudagar MEM, Shaukat N, et al. Maximising Yield and Engine Efficiency Using Optimised Waste Cooking Oil Biodiesel. Energies. 2020; 13(22):5941. https://doi.org/10.3390/en13225941
Chicago/Turabian StyleRazzaq, Luqman, Shahid Imran, Zahid Anwar, Muhammad Farooq, Muhammad Mujtaba Abbas, Haris Mehmood Khan, Tahir Asif, Muhammad Amjad, Manzoore Elahi M. Soudagar, Nabeel Shaukat, and et al. 2020. "Maximising Yield and Engine Efficiency Using Optimised Waste Cooking Oil Biodiesel" Energies 13, no. 22: 5941. https://doi.org/10.3390/en13225941
APA StyleRazzaq, L., Imran, S., Anwar, Z., Farooq, M., Abbas, M. M., Mehmood Khan, H., Asif, T., Amjad, M., Soudagar, M. E. M., Shaukat, N., Rizwanul Fattah, I. M., & Rahman, S. M. A. (2020). Maximising Yield and Engine Efficiency Using Optimised Waste Cooking Oil Biodiesel. Energies, 13(22), 5941. https://doi.org/10.3390/en13225941













