A Laser-Based Heating System for Studying the Morphological Stability of Porous Ceria and Porous La0.6Sr0.4MnO3 Perovskite during Solar Thermochemical Redox Cycling
Abstract
1. Introduction
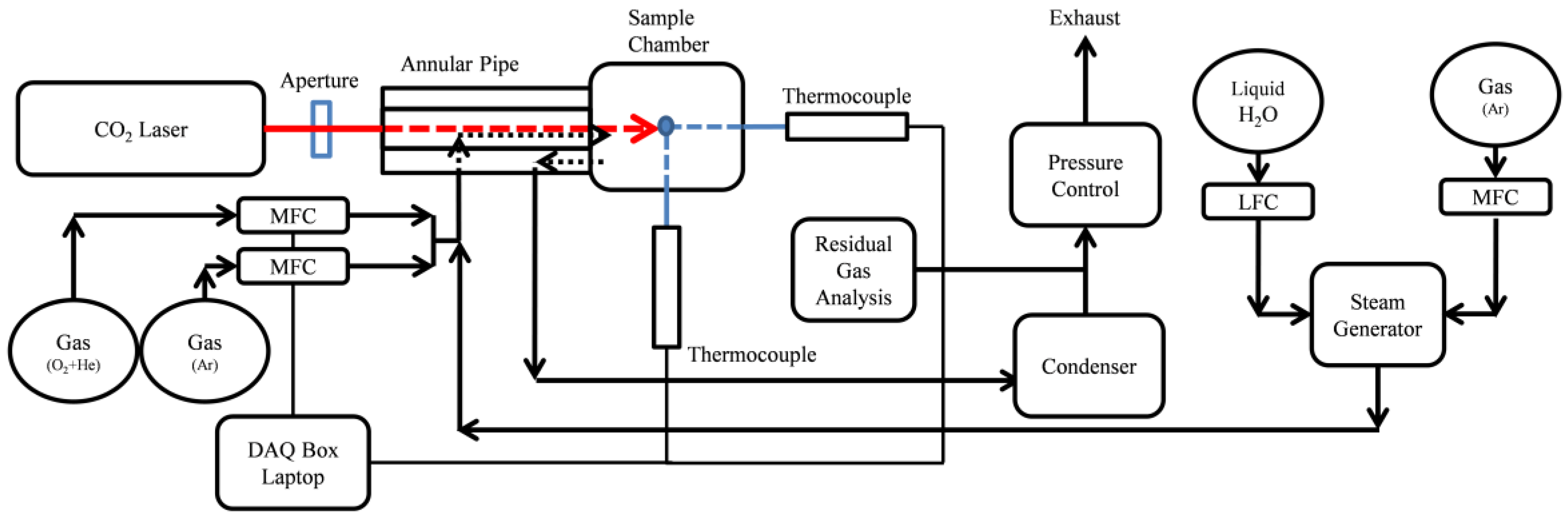
2. Experimental Methods
3. Results and Discussion
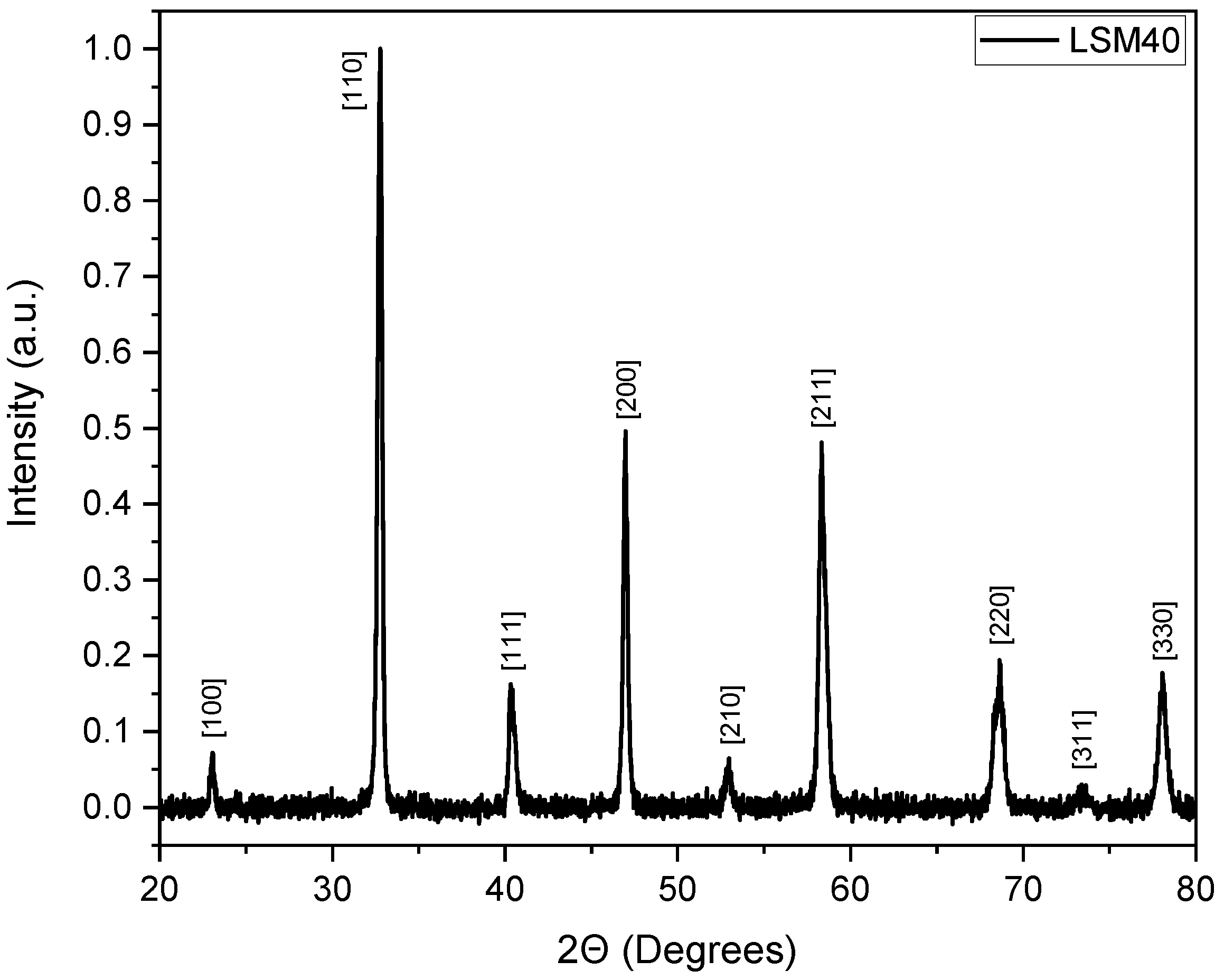
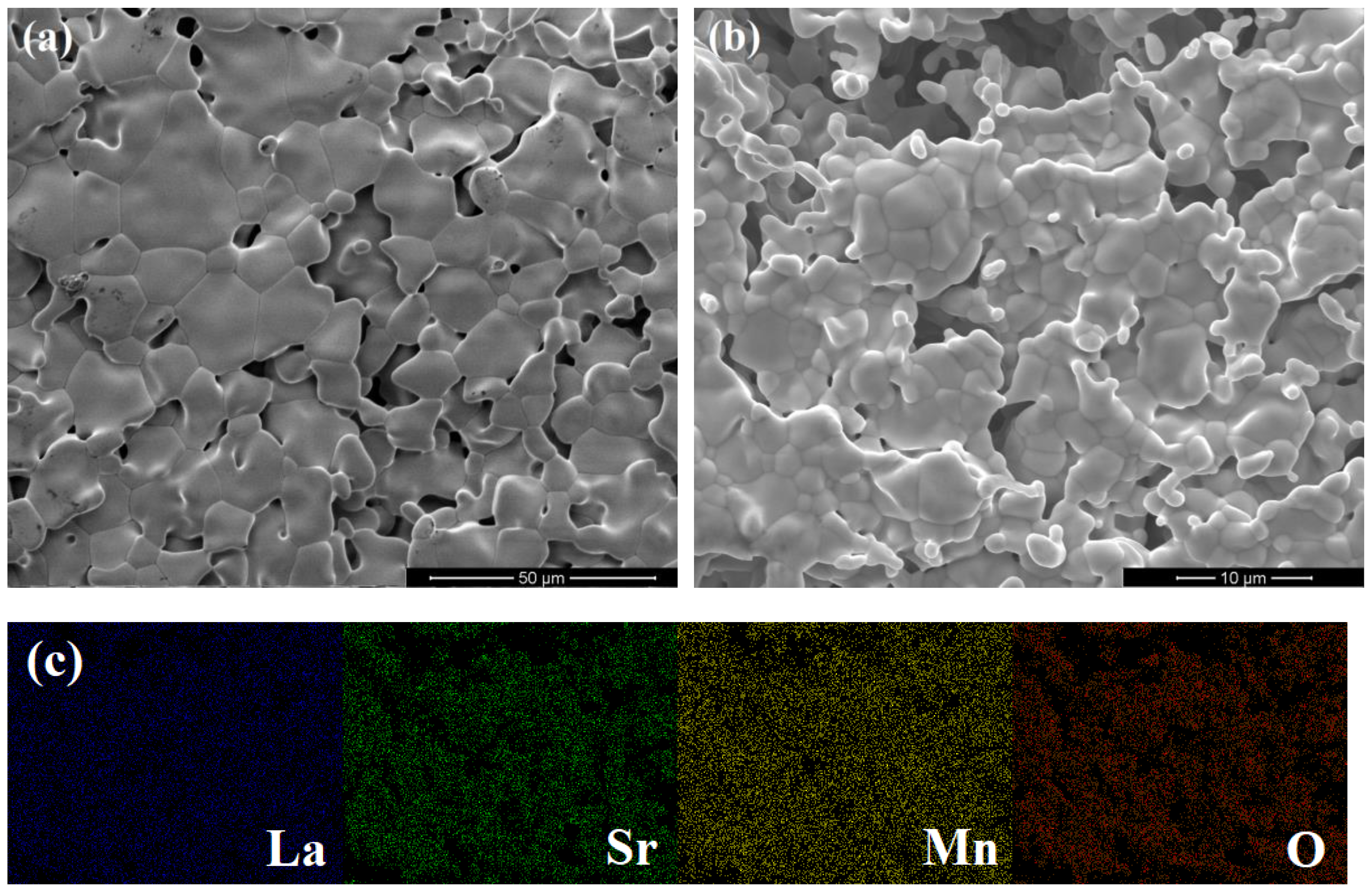
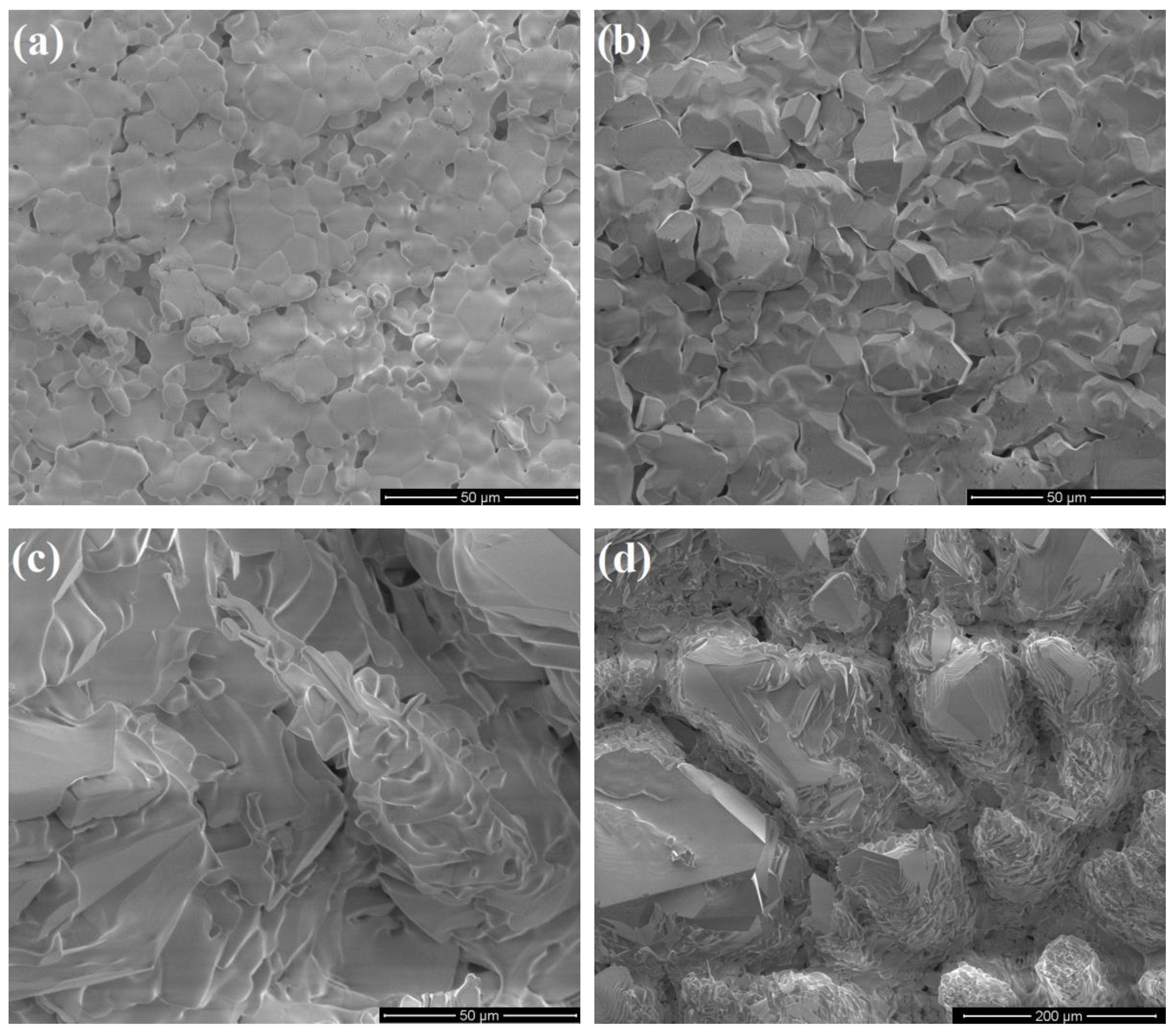
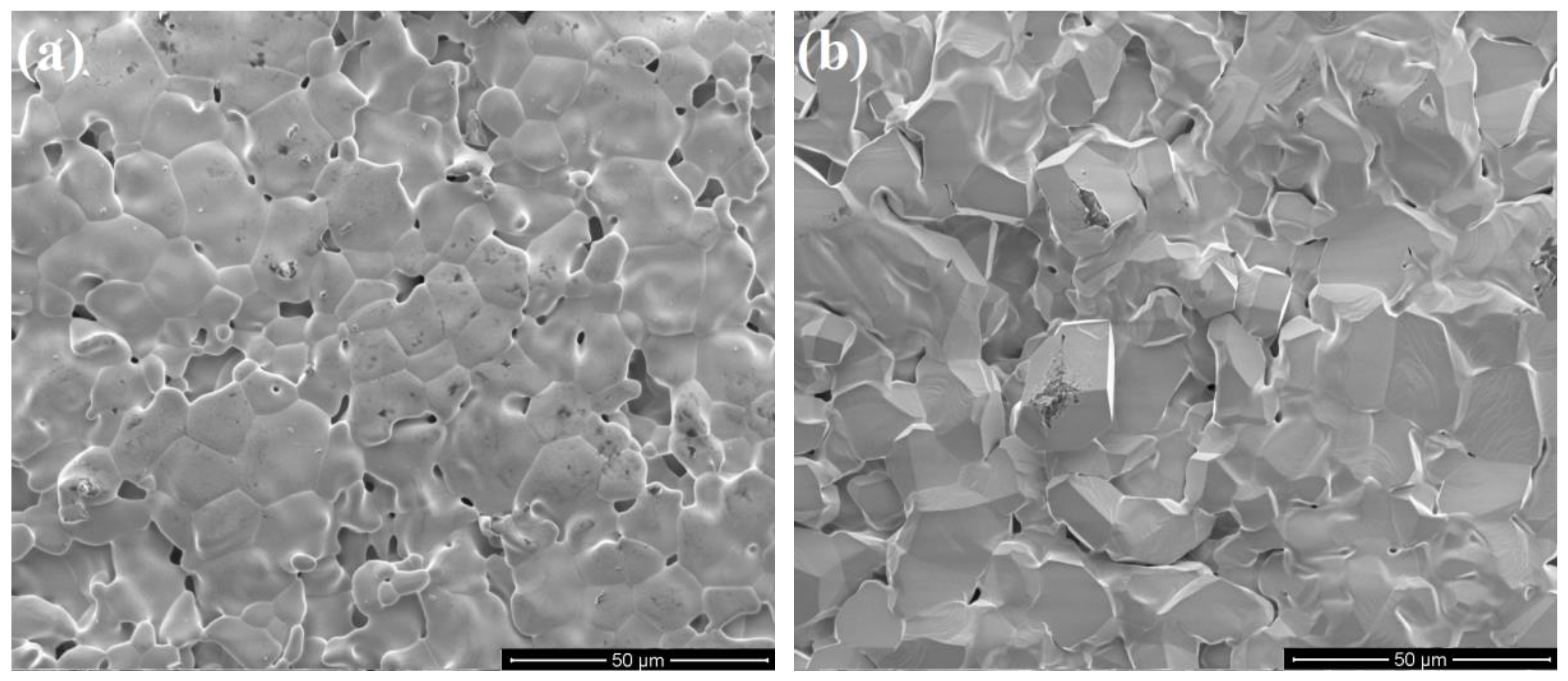
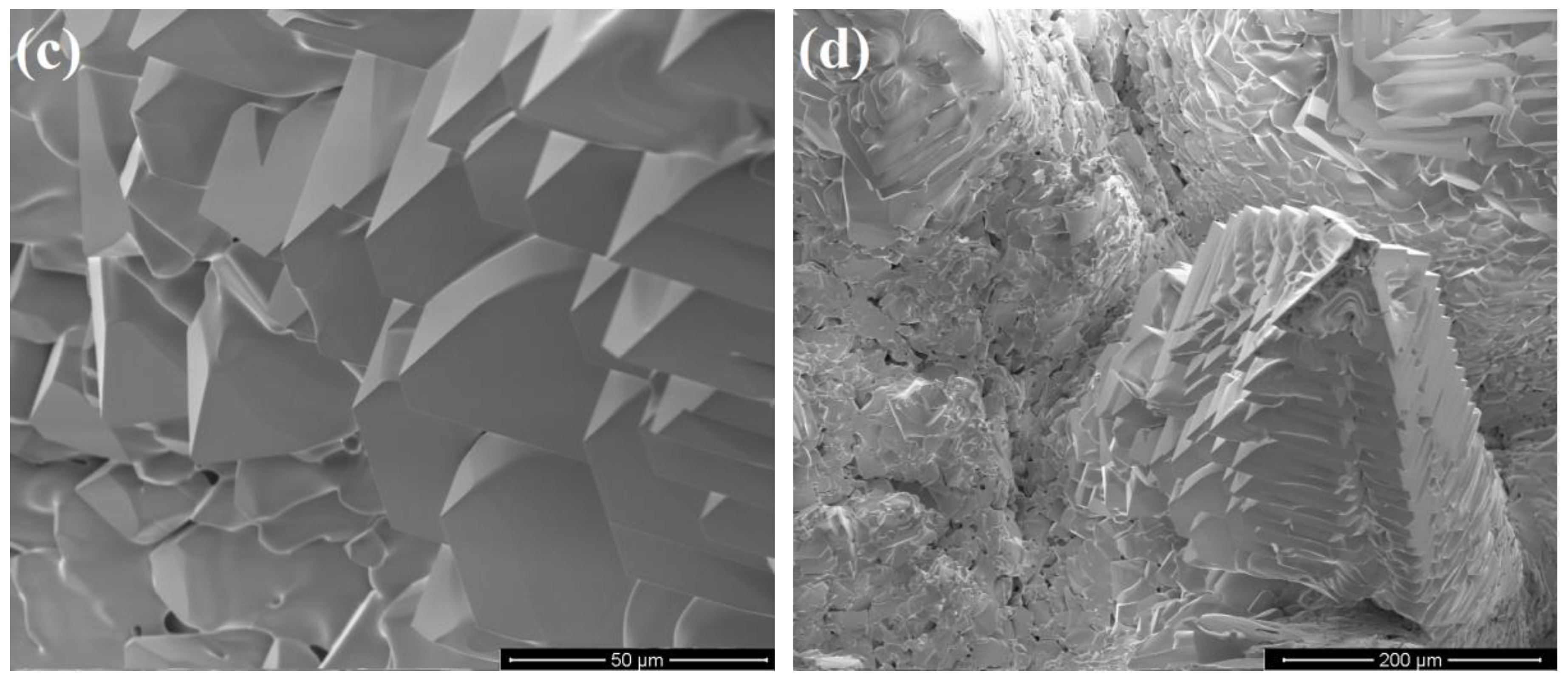
3.1. Characterization of Porous Ceria and Porous LSM40
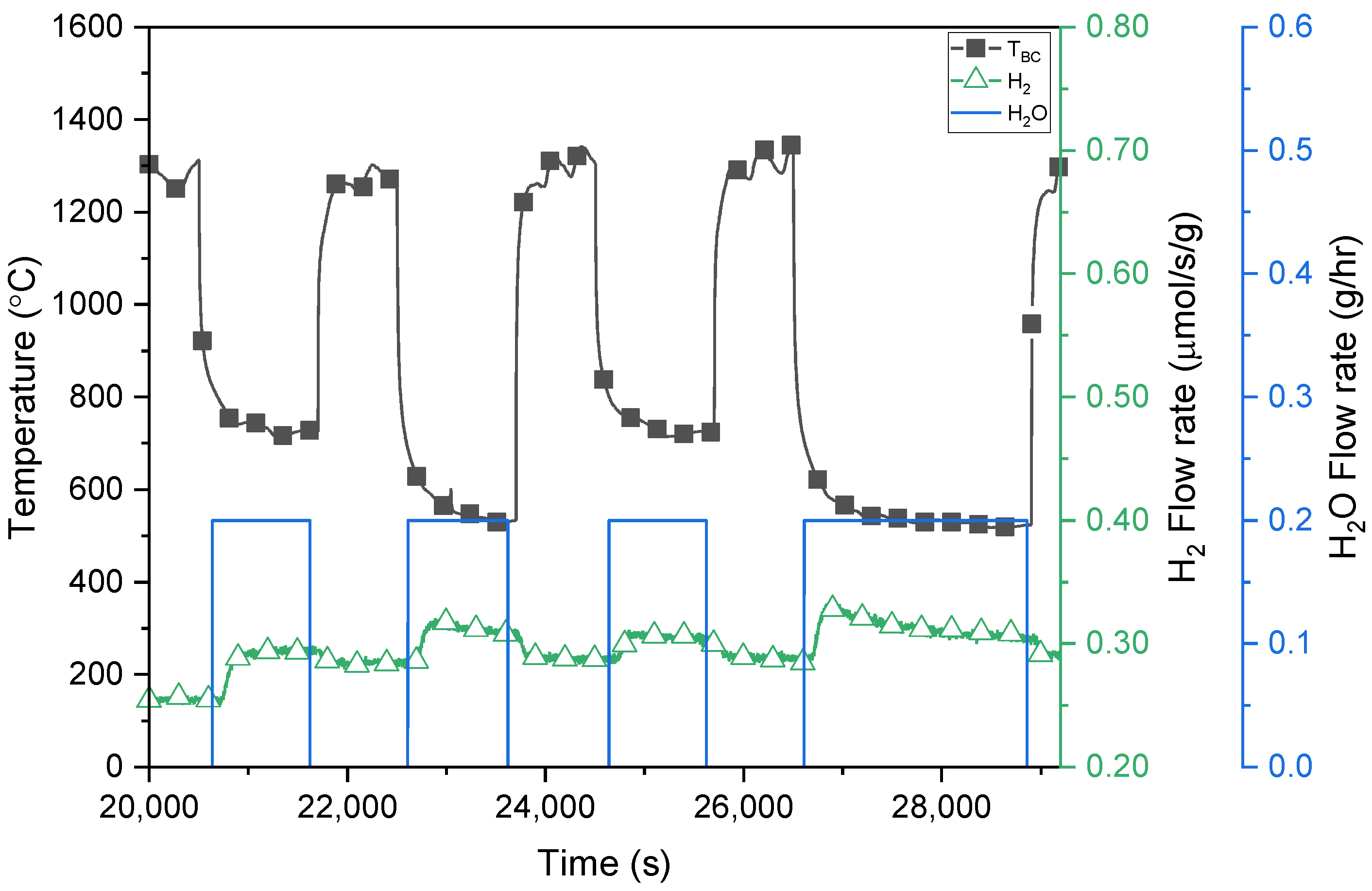
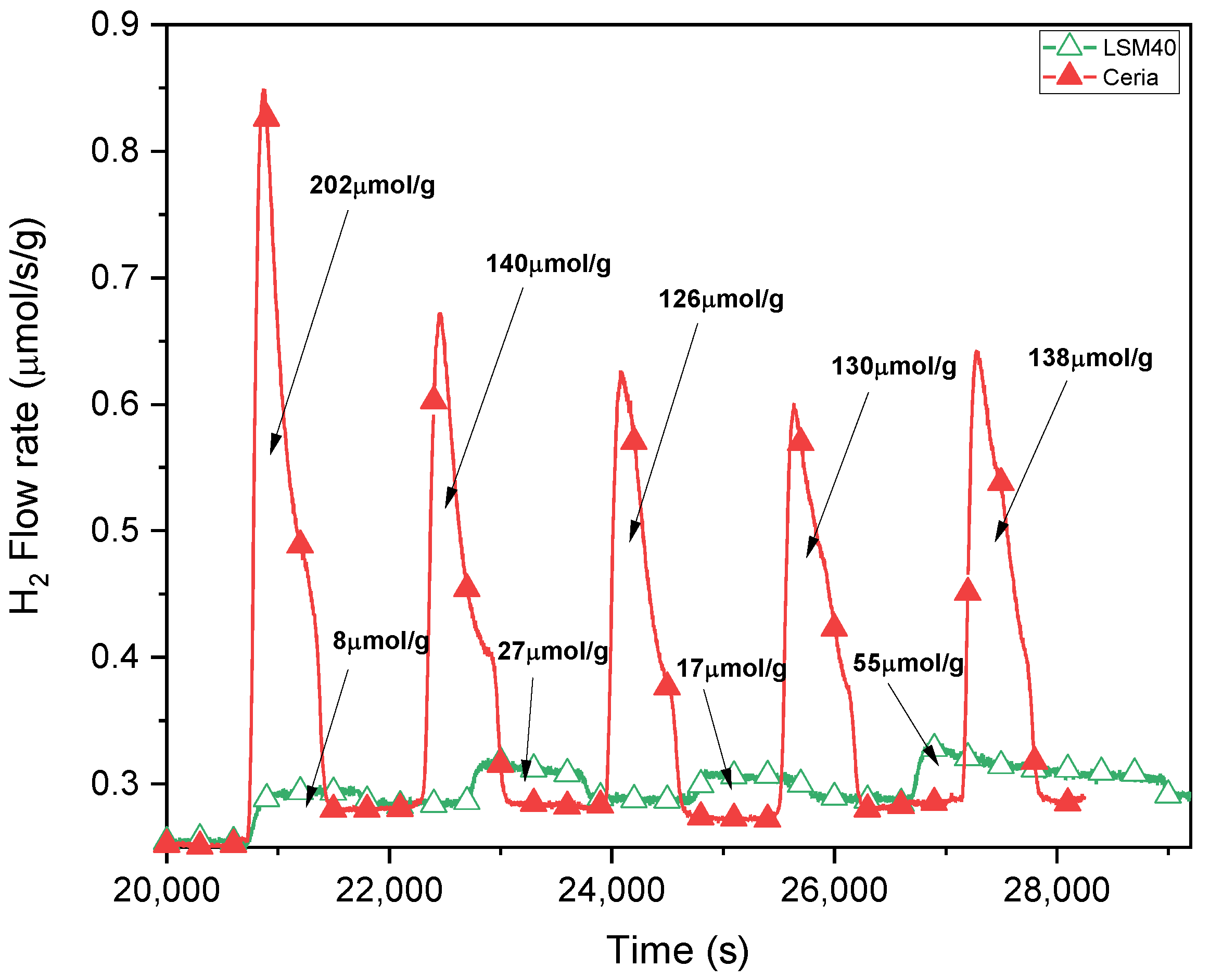
3.2. H2O Splitting Temperature-Swing Cycling Using Porous Ceria and LSM40
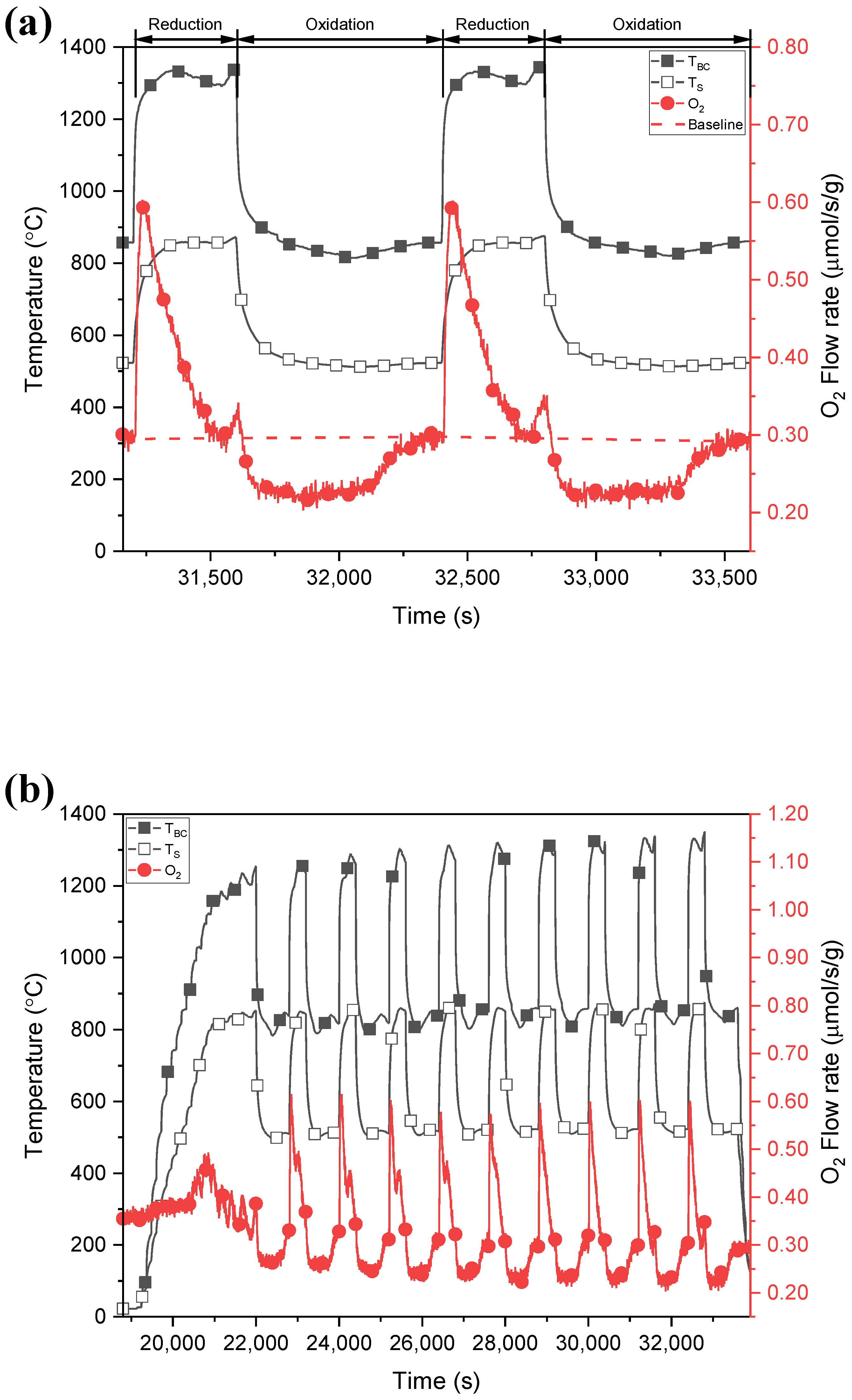
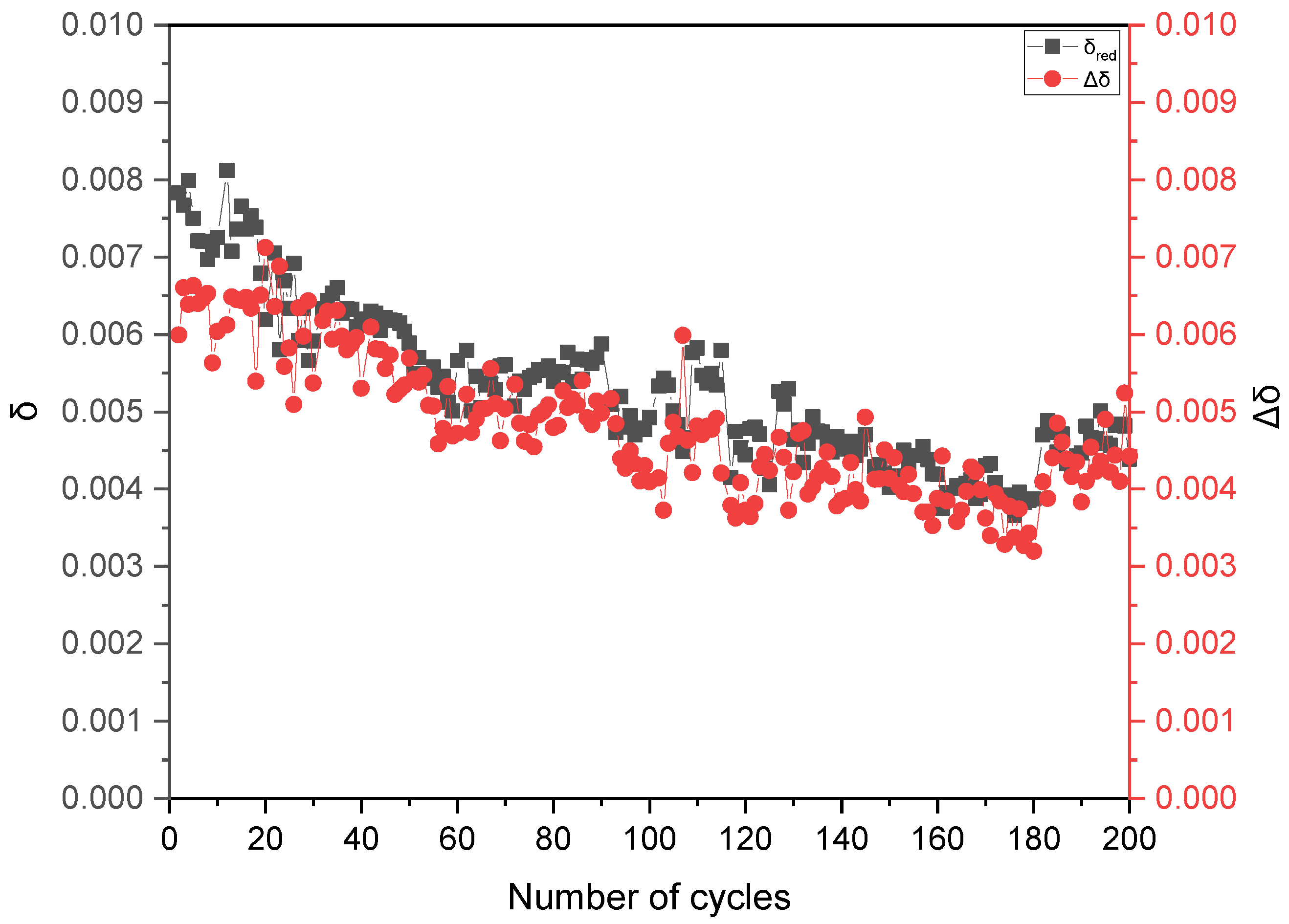
3.3. Temperature-Swing Cycling of Porous Ceria at Constant pO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Scheffe, J.R.; Steinfeld, A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: A review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Romero, M.; Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 2012, 5, 9234–9245. [Google Scholar] [CrossRef]
- Smestad, G.P.; Steinfeld, A. Review: Photochemical and Thermochemical Production of Solar Fuels from H2O and CO2 Using Metal Oxide Catalysts. Ind. Eng. Chem. Res. 2012, 51, 11828–11840. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. Ceria as a Thermochemical Reaction Medium for Selectively Generating Syngas or Methane from H2O and CO2. ChemSusChem 2009, 2, 735–739. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. A thermochemical study of ceria: Exploiting an old material for new modes of energy conversion and CO 2 mitigation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3269–3294. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Scheffe, J.R. Advances and trends in redox materials for solar thermochemical fuel production. Sol. Energy 2017, 156, 3–20. [Google Scholar] [CrossRef]
- Marxer, D.; Furler, P.; Takacs, M.; Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 2017, 10, 1142–1149. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Welte, M.; Steinfeld, A. Thermal Reduction of Ceria within an Aerosol Reactor for H2O and CO2Splitting. Ind. Eng. Chem. Res. 2014, 53, 2175–2182. [Google Scholar] [CrossRef]
- Sørensen, O. Thermodynamic studies of the phase relationships of nonstoichiometric cerium oxides at higher temperatures. J. Solid State Chem. 1976, 18, 217–233. [Google Scholar] [CrossRef]
- Ackermann, S.; Scheffe, J.R.; Steinfeld, A. Diffusion of Oxygen in Ceria at Elevated Temperatures and Its Application to H2O/CO2 Splitting Thermochemical Redox Cycles. J. Phys. Chem. C 2014, 118, 5216–5225. [Google Scholar] [CrossRef]
- Chueh, W.C.; McDaniel, A.H.; Grass, M.E.; Hao, Y.; Jabeen, N.; Liu, Z.; Haile, S.M.; Mccarty, K.F.; Bluhm, H.; El Gabaly, F. Highly Enhanced Concentration and Stability of Reactive Ce3+ on Doped CeO2 Surface Revealed In Operando. Chem. Mater. 2012, 24, 1876–1882. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Weibel, D.; Steinfeld, A. Lanthanum–Strontium–Manganese Perovskites as Redox Materials for Solar Thermochemical Splitting of H2O and CO2. Energy Fuels 2013, 27, 4250–4257. [Google Scholar] [CrossRef]
- Evdou, A.; Zaspalis, V.T.; Nalbandian, L. La(1−x)SrxMnO3−δ perovskites as redox materials for the production of high purity hydrogen. Int. J. Hydrogen Energy 2008, 33, 5554–5562. [Google Scholar] [CrossRef]
- McDaniel, A.H.; Miller, E.C.; Arifin, D.; Ambrosini, A.; Coker, E.N.; O’Hayre, R.; Chueh, W.C.; Tong, J. Sr- and Mn-doped LaAlO3−δ for solar thermochemical H2 and CO production. Energy Environ. Sci. 2013, 6, 2424–2428. [Google Scholar] [CrossRef]
- Takacs, M.; Hoes, M.; Caduff, M.; Cooper, T.; Scheffe, J.R.; Steinfeld, A. Oxygen nonstoichiometry, defect equilibria, and thermodynamic characterization of LaMnO3 perovskites with Ca/Sr A-site and Al B-site doping. Acta Mater. 2016, 103, 700–710. [Google Scholar] [CrossRef]
- Ezbiri, M.; Takacs, M.; Theiler, D.; Michalsky, R.; Steinfeld, A. Tunable thermodynamic activity of LaxSr1−xMnyAl1−yO3−δ (0 ≤ x ≤ 1, 0 ≤ y ≤ 1) perovskites for solar thermochemical fuel synthesis. J. Mater. Chem. A 2017, 5, 4172–4182. [Google Scholar] [CrossRef] [PubMed]
- Gladen, A.C.; Davidson, J.H. The morphological stability and fuel production of commercial fibrous ceria particles for solar thermochemical redox cycling. Sol. Energy 2016, 139, 524–532. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Chandran, R.B.; Gladen, A.C.; Chase, T.R.; Davidson, J.H. Demonstration of a Solar Reactor for Carbon Dioxide Splitting via the Isothermal Ceria Redox Cycle and Practical Implications. Energy Fuels 2016, 30, 6654–6661. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Bork, A.H.; Moser, T.; Sediva, E.; Hood, Z.D.; Rupp, J.L.M. Modifying La0.6Sr0.4MnO3 Perovskites with Cr Incorporation for Fast Isothermal CO2--Splitting Kinetics in Solar--Driven Thermochemical Cycles. Adv. Energy Mater. 2019, 9, 1803886:1–1803886:13. [Google Scholar] [CrossRef]
- Lee, K.; Scheffe, J.R. Characterization of a Laser-Based Heating System Coupled With In Operando Raman Spectroscopy for Studying Solar Thermochemical Redox Cycles. J. Sol. Energy Eng. 2019, 141, 021013:1–021013:7. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to form a Capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Cooper, T.; Scheffe, J.R.; Galvez, M.E.; Jacot, R.; Patzke, G.R.; Steinfeld, A. Lanthanum Manganite Perovskites with Ca/Sr A-site and Al B-site Doping as Effective Oxygen Exchange Materials for Solar Thermochemical Fuel Production. Energy Technol. 2015, 3, 1130–1142. [Google Scholar] [CrossRef]
- Khafizov, M.; Park, I.-W.; Chernatynskiy, A.; He, L.-F.; Lin, J.; Moore, J.J.; Swank, D.; Lillo, T.; Phillpot, S.R.; El-Azab, A.; et al. Thermal Conductivity in Nanocrystalline Ceria Thin Films. J. Am. Ceram. Soc. 2013, 97, 562–569. [Google Scholar] [CrossRef]
- Ackermann, S.; Scheffe, J.R.; Duss, J.; Steinfeld, A. Morphological Characterization and Effective Thermal Conductivity of Dual-Scale Reticulated Porous Structures. Materials 2014, 7, 7173–7195. [Google Scholar] [CrossRef]
- Yang, C.-K.; Yamazaki, Y.; Aydin, A.; Haile, S.M. Thermodynamic and kinetic assessments of strontium-doped lanthanum manganite perovskites for two-step thermochemical water splitting. J. Mater. Chem. A 2014, 2, 13612–13623. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Jacot, R.; Scheffe, J.R.; Cooper, T.; Patzke, G.R.; Steinfeld, A. Physico-chemical changes in Ca, Sr and Al-doped La–Mn–O perovskites upon thermochemical splitting of CO2via redox cycling. Phys. Chem. Chem. Phys. 2015, 17, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Mizusaki, J.; Tagawa, H. Nonstoichiometry and thermochemical stability of the perovskite-type La1−xSrxMnO3−δ. Solid State Ion. 1991, 49, 111–118. [Google Scholar] [CrossRef]
- Panlener, R.; Blumenthal, R.; Garnier, J. A thermodynamic study of nonstoichiometric cerium dioxide. J. Phys. Chem. Solids 1975, 36, 1213–1222. [Google Scholar] [CrossRef]
- Mizusaki, J. Oxygen nonstoichiometry and defect equilibrium in the perovskite-type oxides La1−xSrxMnO3+d. Solid State Ion. 2000, 129, 163–177. [Google Scholar] [CrossRef]
- Abanades, S.; Legal, A.; Cordier, A.; Peraudeau, G.; Flamant, G.; Julbe, A. Investigation of reactive cerium-based oxides for H2 production by thermochemical two-step water-splitting. J. Mater. Sci. 2010, 45, 4163–4173. [Google Scholar] [CrossRef]
- Furler, P.; Scheffe, J.R.; Steinfeld, A. Syngas production by simultaneous splitting of H2O and CO2via ceria redox reactions in a high-temperature solar reactor. Energy Environ. Sci. 2012, 5, 6098–6103. [Google Scholar] [CrossRef]
- Riess, I.; Ricken, M.; No¨lting, J. On the specific heat of nonstoichiometric ceria. J. Solid State Chem. 1985, 57, 314–322. [Google Scholar] [CrossRef]
- Hayashi, H. Thermal expansion of Gd-doped ceria and reduced ceria. Solid State Ion. 2000, 132, 227–233. [Google Scholar] [CrossRef]
- Bishop, S.; Duncan, K.; Wachsman, E. Defect equilibria and chemical expansion in non-stoichiometric undoped and gadolinium-doped cerium oxide. Electrochim. Acta 2009, 54, 1436–1443. [Google Scholar] [CrossRef]
- Lim, G.; Steen, W. Measurement of the temporal and spatial power distribution of a high-power CO2 laser beam. Opt. Laser Technol. 1982, 14, 149–153. [Google Scholar] [CrossRef]
- Furler, P.; Scheffe, J.R.; Marxer, D.; Gorbar, M.; Bonk, A.; Vogt, U.; Steinfeld, A. Thermochemical CO2 splitting via redox cycling of ceria reticulated foam structures with dual-scale porosities. Phys. Chem. Chem. Phys. 2014, 16, 10503–10511. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Sauvin, L.; Castiglioni, R.; Rupp, J.L.M.; Scheffe, J.R.; Steinfeld, A. Kinetics of CO2 Reduction over Nonstoichiometric Ceria. J. Phys. Chem. C 2015, 119, 16452–16461. [Google Scholar] [CrossRef] [PubMed]
- Furler, P.; Scheffe, J.; Gorbar, M.; Moes, L.; Vogt, U.; Steinfeld, A. Solar Thermochemical CO2 Splitting Utilizing a Reticulated Porous Ceria Redox System. Energy Fuels 2012, 26, 7051–7059. [Google Scholar] [CrossRef]
- Bârcă, E.; Filipescu, M.; Luculescu, C.; Birjega, R.; Ion, V.; Dumitru, M.; Nistor, L.; Stanciu, G.; Abrudeanu, M.; Munteanu, C.; et al. Pyramidal growth of ceria nanostructures by pulsed laser deposition. Appl. Surf. Sci. 2016, 363, 245–251. [Google Scholar] [CrossRef]
- Mansilla, C.; Holgado, J.P.; Espinós, J.P.; González-Elipe, A.; Yubero, F. Microstructure and transport properties of ceria and samaria doped ceria thin films prepared by EBE–IBAD. Surf. Coat. Technol. 2007, 202, 1256–1261. [Google Scholar] [CrossRef]
- Pryds, N.H.; Rodrigo, K.; Linderoth, S.; Schou, J. On the growth of gadolinia-doped ceria by pulsed laser deposition. Appl. Surf. Sci. 2009, 255, 5232–5235. [Google Scholar] [CrossRef]
- Takacs, M.; Ackermann, S.; Bonk, A.; Puttkamer, M.N.-V.; Haueter, P.; Scheffe, J.R.; Vogt, U.F.; Steinfeld, A. Splitting CO2with a ceria-based redox cycle in a solar-driven thermogravimetric analyzer. AIChE J. 2016, 63, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Abanades, S.; Flamant, G. Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
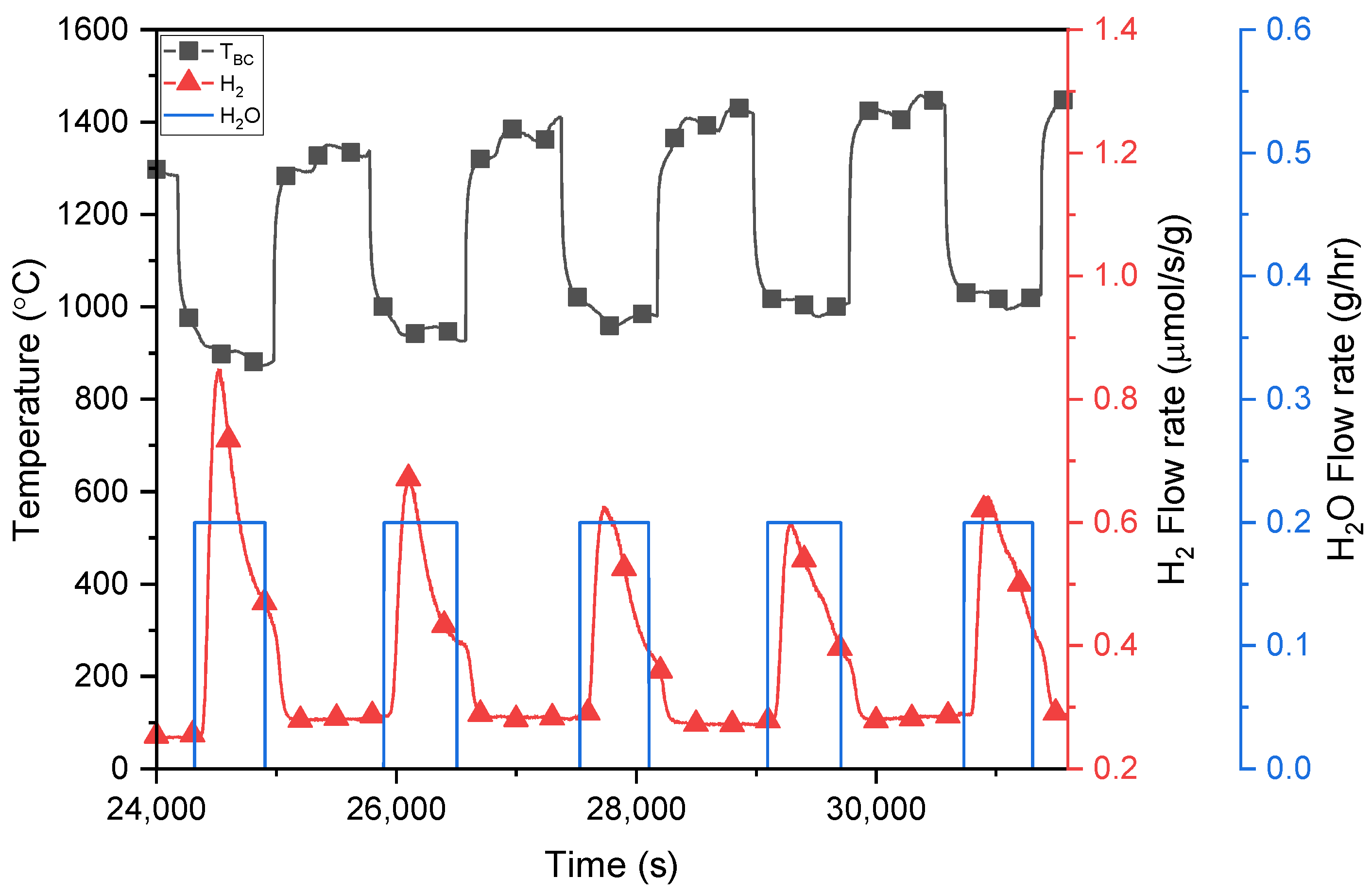
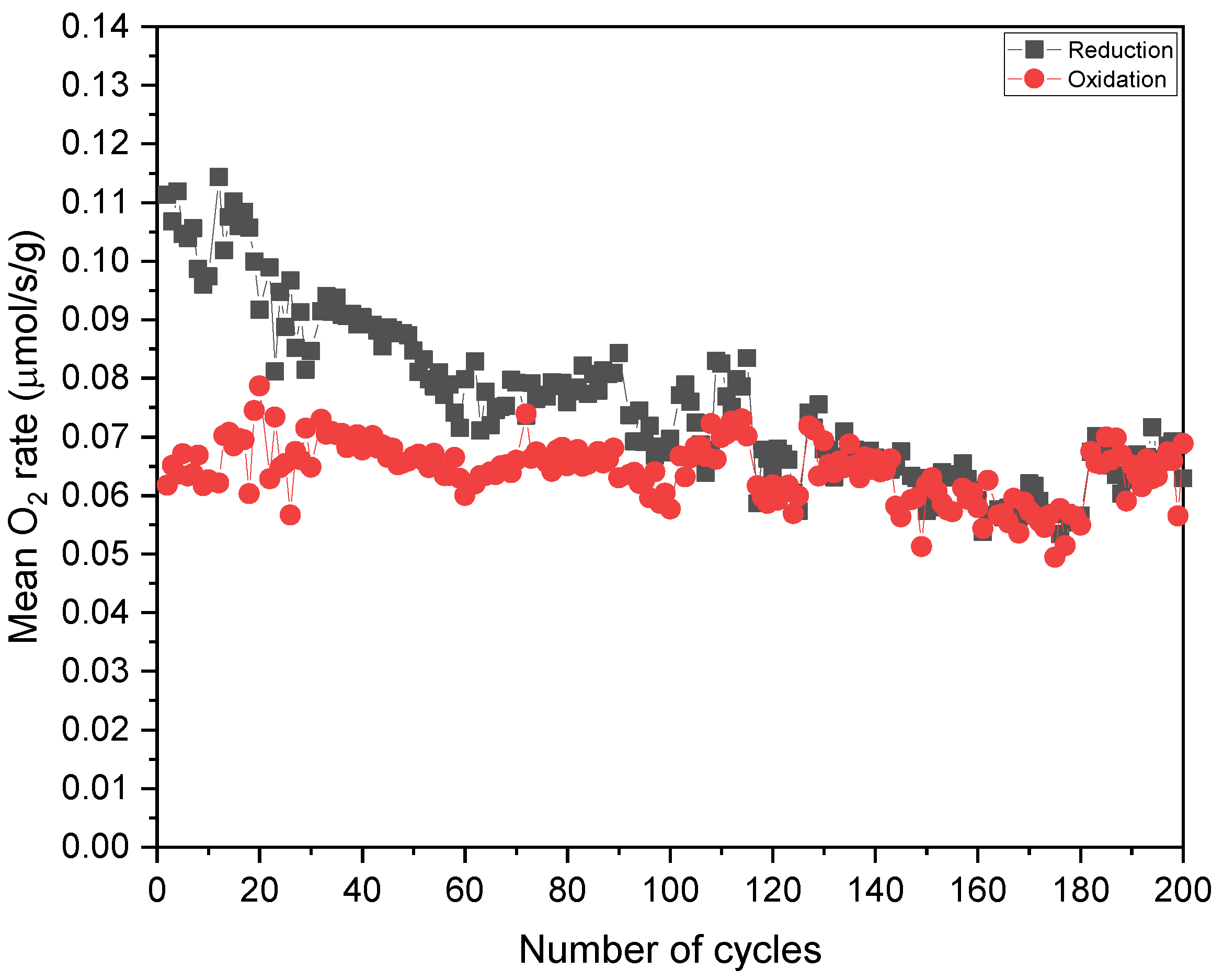
| Ncycle | O2,red (μmol/g) | O2,ox (μmol/g) | δred | Δδ | Ratered (μmol/s/g) | Rateox (μmol/s/g) |
|---|---|---|---|---|---|---|
| 1 | 45.67 | 38.67 | 0.0079 | 0.0067 | 0.0399 | 0.0579 |
| 2 | 44.50 | 36.54 | 0.0077 | 0.0063 | 0.1113 | 0.0617 |
| 3 | 43.77 | 40.42 | 0.0075 | 0.0070 | 0.1068 | 0.0651 |
| 4 | 45.19 | 40.26 | 0.0078 | 0.0069 | 0.1119 | 0.0637 |
| 5 | 42.02 | 40.24 | 0.0072 | 0.0069 | 0.1046 | 0.0671 |
| 6 | 41.54 | 37.49 | 0.0072 | 0.0065 | 0.1039 | 0.0633 |
| 7 | 41.80 | 37.64 | 0.0072 | 0.0065 | 0.1056 | 0.0637 |
| 8 | 40.23 | 40.10 | 0.0069 | 0.0069 | 0.0987 | 0.0669 |
| 9 | 39.72 | 36.01 | 0.0068 | 0.0062 | 0.0960 | 0.0616 |
| 10 | 41.09 | 35.54 | 0.0071 | 0.0061 | 0.0974 | 0.0626 |
| Material | Pore Volumes (cc/g) | SSA (m2/g) | Effective Porosity |
|---|---|---|---|
| Ceria (before cycling) | 0.0764 | 18.6778 | 0.5509 |
| Ceria (after 10 cycles) | 0.0625 | 4.2121 | 0.4519 |
| Ceria (after 200 cycles) | 0.0518 | 0.0507 | 0.3742 |
| Material | Pore Volumes (cc/g) | SSA (m2/g) | Effective Porosity |
|---|---|---|---|
| Ceria (before cycling) | 0.1226 | 0.0508 | 0.8838 |
| Ceria (after 10 cycles) | 0.0796 | 0.0355 | 0.5752 |
| Ceria (after 200 cycles) | 0.0778 | 0.0264 | 0.5615 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Scheffe, J.R. A Laser-Based Heating System for Studying the Morphological Stability of Porous Ceria and Porous La0.6Sr0.4MnO3 Perovskite during Solar Thermochemical Redox Cycling. Energies 2020, 13, 5935. https://doi.org/10.3390/en13225935
Lee K, Scheffe JR. A Laser-Based Heating System for Studying the Morphological Stability of Porous Ceria and Porous La0.6Sr0.4MnO3 Perovskite during Solar Thermochemical Redox Cycling. Energies. 2020; 13(22):5935. https://doi.org/10.3390/en13225935
Chicago/Turabian StyleLee, Kangjae, and Jonathan R. Scheffe. 2020. "A Laser-Based Heating System for Studying the Morphological Stability of Porous Ceria and Porous La0.6Sr0.4MnO3 Perovskite during Solar Thermochemical Redox Cycling" Energies 13, no. 22: 5935. https://doi.org/10.3390/en13225935
APA StyleLee, K., & Scheffe, J. R. (2020). A Laser-Based Heating System for Studying the Morphological Stability of Porous Ceria and Porous La0.6Sr0.4MnO3 Perovskite during Solar Thermochemical Redox Cycling. Energies, 13(22), 5935. https://doi.org/10.3390/en13225935







