New Insights on a µm-Scale into the Transformation Process of CH4 Hydrates to CO2-Rich Mixed Hydrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Procedures
2.3. Data Processing
3. Results
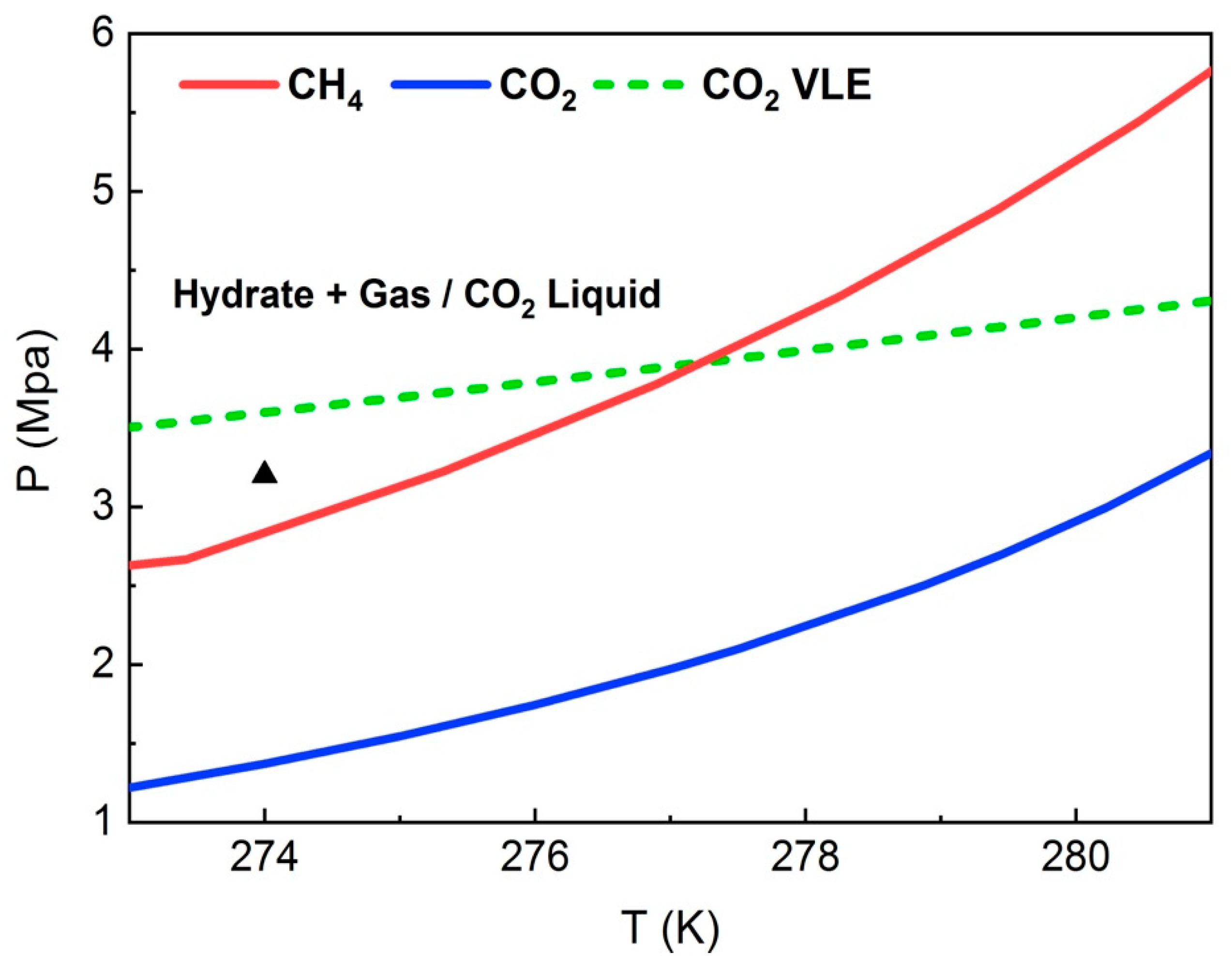
3.1. CH4 Hydrate Formation
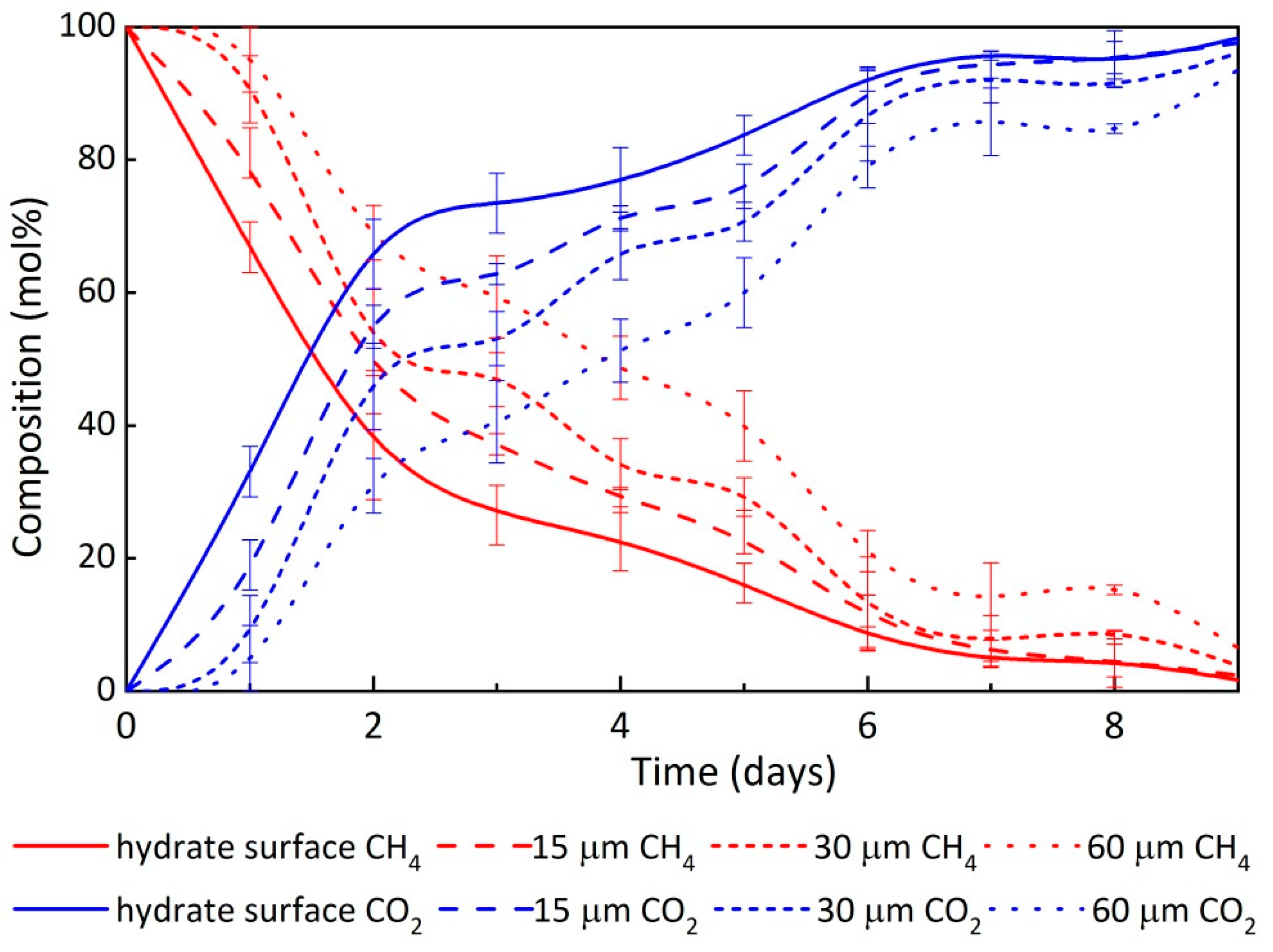
3.2. Changes in Composition of the Initial Gas and Hydrate Phase When Exposed to CO2
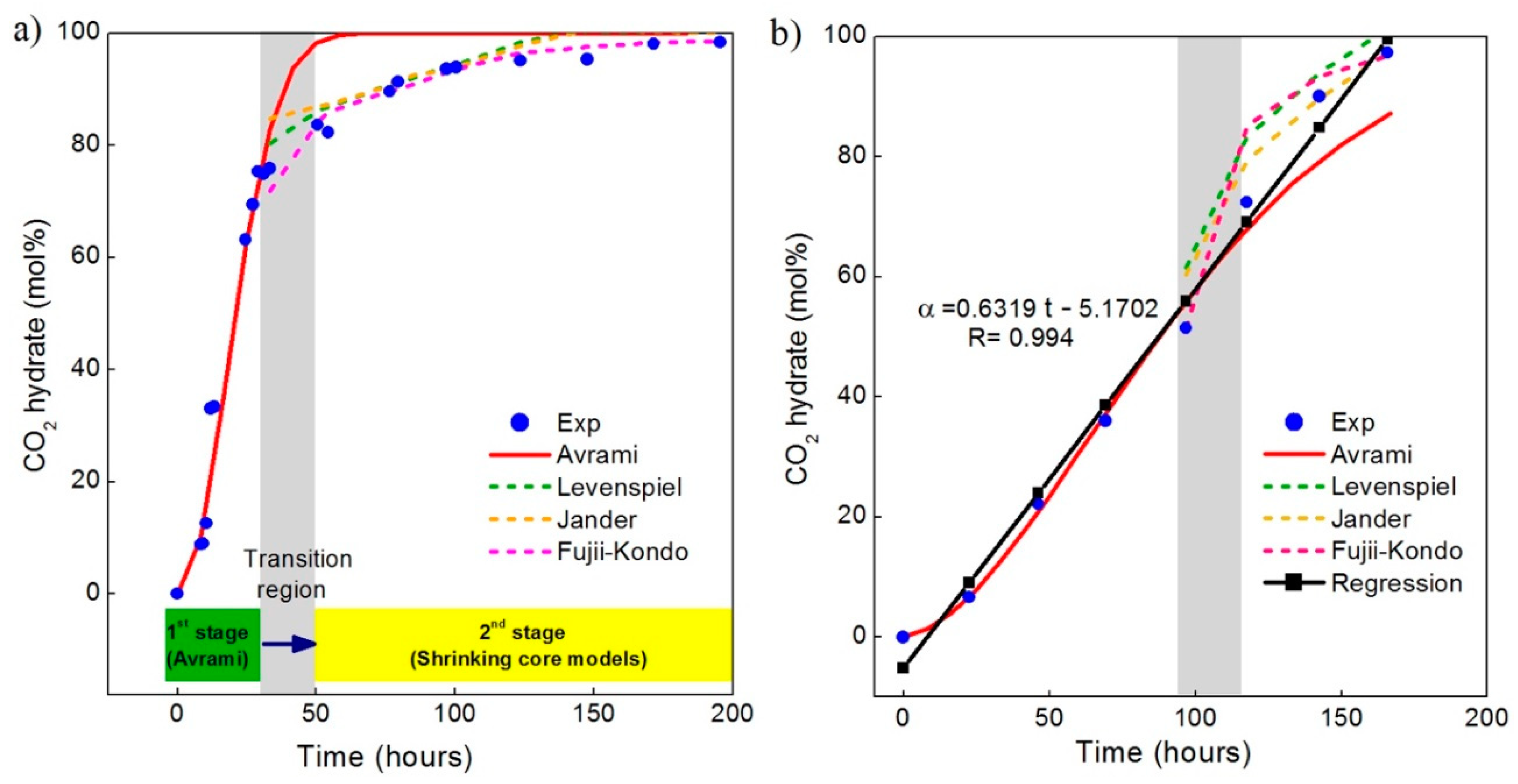
3.3. Changes in Morphology of Hydrate Crystals When Exposed to CO2
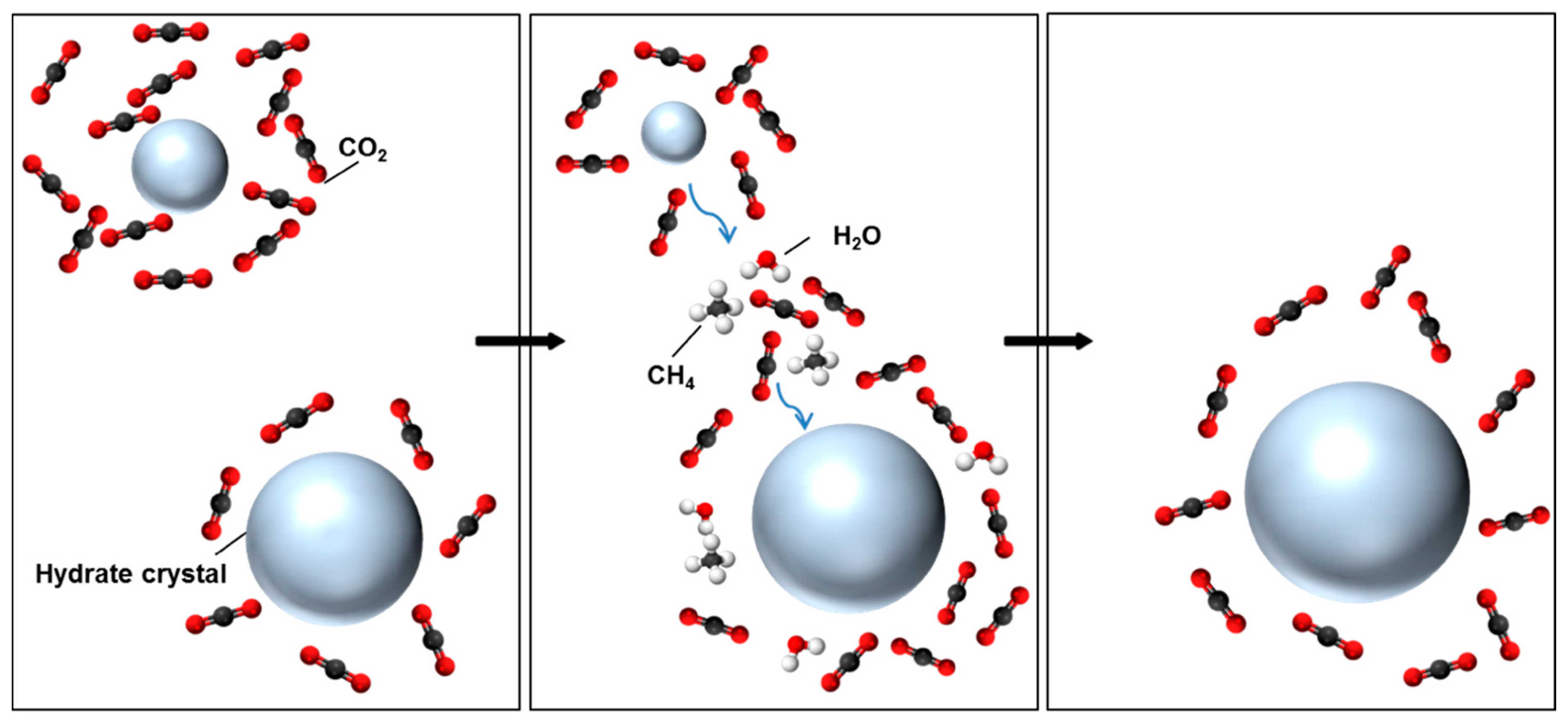
4. Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- von Stackelberg, M. Feste Gashydrate. Naturwissenschaften 1949, 36, 327–333. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kvenvolden, K.A. Gas hydrates—Geological perspective and global change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Hunter, R.B.; Collett, T.S.; Boswell, R.; Anderson, B.J.; Digert, S.A.; Pospisil, G.; Baker, R.; Weeks, M. Mount Elbert Gas Hydrate Stratigraphic Test Well, Alaska North Slope: Overview of scientific and technical program. Mar. Pet. Geol. 2011, 28, 295–310. [Google Scholar] [CrossRef]
- Kurihara, M.; Sato, A.; Funatsu, K.; Ouchi, H.; Yamamoto, K.; Numasawa, M.; Ebinuma, T.; Narita, H.; Masuda, Y.; Dallimore, S.R.; et al. Analysis of production data for 2007/2008 mallik gas hydrate production tests in Canada. In Proceedings of the International oil and Gas Conference and Exhibition in China, Society of Petroleum Engineers, Beijing, China, 8–10 June 2010; pp. 2908–2931. [Google Scholar]
- Yamamoto, K.; Terao, Y.; Fujii, T.; Ikawa, T.; Seki, M.; Matsuzawa, M.; Kanno, T. Operational overview of the first offshore production test of methane hydrates in the Eastern Nankai Trough. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 8 May 2014; pp. 1–15. [Google Scholar]
- Moridis, G.; Kowalsky, M.B.; Pruess, K. Depressurization-Induced Gas Production From Class 1 Hydrate Deposits. Soc. Pet. Eng. 2007, 10, 458–481. [Google Scholar] [CrossRef]
- Schicks, J.M.; Spangenberg, E.; Giese, R.; Luzi-Helbing, M.; Priegnitz, M.; Beeskow-Strauch, B. A counter-current heat-exchange reactor for the thermal stimulation of hydrate-bearing sediments. Energies 2013, 6, 3002–3016. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liang, J.; Li, K.; Wang, H.B.; Li, X.M.; Wu, J.X. Research Progress of Drilling Coring and Mining on the Permafrost Natural Gas Hydrates in China. In Proceedings of the Geological Engineering Drilling Technology Conference (IGEDTC), Chengdu, China, 20–22 June 2014; Volume 73, pp. 362–367. [Google Scholar]
- Fitzgerald, G.C.; Castaldi, M.J. Thermal Stimulation Based Methane Production from Hydrate Bearing Quartz Sediment. Ind. Eng. Chem. Res. 2013, 52, 6571–6581. [Google Scholar] [CrossRef]
- Kamath, V.A.; Mutalik, P.N.; Sira, J.H.; Patil, S.L. Experimental study of brine injection and depressurization methods for dissociation of gas hydrates. SPE Form. Eval. 1991, 6, 477–484. [Google Scholar] [CrossRef]
- Li, X.S.; Wan, L.H.; Li, G.; Li, Q.P.; Chen, Z.Y.; Yan, K.F. Experimental Investigation into the Production Behavior of Methane Hydrate in Porous Sediment with Hot Brine Stimulation. Ind. Eng. Chem. Res. 2008, 47, 9696–9702. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Collett, T.S. Summary and implications of the Mallik 2002 Gas Hydrate Production ResearchWell Program. In Scientific Results from the Mallik 2002 Gas Hydrate Production Research Well Program, Mackenzie Delta, Northwest Territories, Canada; Dallimore, S.R., Collett, T.S., Eds.; Geological Survey of Canada: Ottawa, ON, Canada, 2005; pp. 1–36. [Google Scholar]
- Falser, S.; Uchida, S.; Palmer, A.C.; Soga, K.; Tan, T.S. Increased gas production from hydrates by combining depressurization with heating of the wellbore. Energy Fuels 2012, 26, 6259–6267. [Google Scholar] [CrossRef]
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Koh, C.A.; Sloan, E.D. Toward production from gas hydrates: Current status, assessment of resources, and simulation-based evaluation of technology and potential. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef]
- Gunn, D.A.; Nelder, L.M.; Rochelle, C.A.; Bateman, K.; Jackson, P.D.; Lovell, M.A.; Hobbs, P.R.N.; Long, D.; Rees, J.G.; Schultheiss, P.; et al. Towards improved ground models for slope instability evaluations through better characterization of sediment-hosted gas-hydrates. Terra Nov. 2002, 14, 443–451. [Google Scholar] [CrossRef]
- Ersland, G.; Husebø, J.; Graue, A.; Kvamme, B. Transport and storage of CO2 in natural gas hydrate reservoirs. Energy Procedia 2009, 1, 3477–3484. [Google Scholar] [CrossRef]
- Everett, S.M.; Rawn, C.J.; Chakoumakos, B.C.; Keffer, D.J.; Huq, A.; Phelps, T.J. Insights into the structure of mixed CO2/CH4 in gas hydrates. Am. Mineral. 2015, 100, 1203–1208. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.-Y.; Lee, J.-W.J.; Huh, D.-G.; Park, K.-P.; Lee, J.-W.J.; Lee, H. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. Proc. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef]
- Khlebnikov, V.N.; Antonov, S.V.; Mishin, A.S.; Bakulin, D.A.; Khamidullina, I.V.; Liang, M.; Vinokurov, V.A.; Gushchin, P.A. A new method for the replacement of CH4 with CO2 in natural gas hydrate production. Nat. Gas Ind. B 2016, 3, 445–451. [Google Scholar] [CrossRef]
- Lee, H.; Seo, Y.; Seo, Y.T.; Moudrakovski, I.L.; Ripmeester, J.A. Recovering Methane from Solid Methane Hydrate with Carbon Dioxide. Angew. Chem. Int. Ed. 2003, 42, 5048–5051. [Google Scholar] [CrossRef]
- Schicks, J.M.; Luzi, M.; Beeskow-Strauch, B. The Conversion Process of Hydrocarbon Hydrates into CO2 Hydrates and Vice Versa: Thermodynamic Considerations. J. Phys. Chem. A 2011, 115, 13324–13331. [Google Scholar] [CrossRef]
- Hirohama, S.; Shimoyama, Y.; Wakabayashi, A.; Tatsuta, S.; Nishida, N. Conversion of CH4-hydrate to CO2-hydrate in liquid CO2. J. Chem. Eng. Jpn. 1996, 29, 1014–1020. [Google Scholar] [CrossRef]
- Ota, M.; Morohashi, K.; Abe, Y.; Watanabe, M.; Lee Smith, R.; Inomata, H. Replacement of CH4 in the hydrate by use of liquid CO2. Energy Convers. Manag. 2005, 46, 1680–1691. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, S.; Liang, D.; Du, J. Replacement of methane from quartz sand-bearing hydrate with carbon dioxide-in-water emulsion. Energy Fuels 2008, 22, 1759–1764. [Google Scholar] [CrossRef]
- Xiong, L.J.; Li, X.-S.; Zeng, Z.Y.; Li, G.; Chen, Z.Y.; Zhang, Y.; Li, Q.P. Experimental investigation into replacement of CH4 in hydrate in porous sediment with liquid CO2 injection. In Proceedings of the 7th International Conference on Gas Hydrate, Edinburgh, Scotland, UK, 17–21 July 2011. [Google Scholar]
- Deusner, C.; Bigalke, N.; Kossel, E.; Haeckel, M. Methane production from gas hydrate deposits through injection of supercritical CO2. Energies 2012, 5, 2112–2140. [Google Scholar] [CrossRef]
- Boswell, R.; Schoderbek, D.; Collett, T.S.; Ohtsuki, S.; White, M.; Anderson, B.J. The Iġnik Sikumi field experiment, Alaska North Slope: Design, operations, and implications for CO2-CH4 exchange in gas hydrate reservoirs. Energy Fuels 2017, 31, 140–153. [Google Scholar] [CrossRef]
- Schicks, J.M.; Strauch, B.; Heeschen, K.U.; Spangenberg, E.; Luzi-Helbing, M. From Microscale (400 μl) to Macroscale (425 L): Experimental Investigations of the CO2/N2-CH4 Exchange in Gas Hydrates Simulating the Iġnik Sikumi Field Trial. J. Geophys. Res. Solid Earth 2018, 123, 3608–3620. [Google Scholar] [CrossRef]
- Beeskow-Strauch, B.; Schicks, J.M. The Driving Forces of Guest Substitution in Gas Hydrates-A Laser Raman Study on CH4-CO2 Exchange in the Presence of Impurities. Energies 2012, 5, 420–437. [Google Scholar] [CrossRef]
- Falenty, A.; Murshed, M.M.; Salamatin, A.N.; Kuhs, W.F. Gas replacement in clathrate hydrates during CO2 injection—Kinetics and micro-structural mechanism. In Proceedings of the ISOPE Ocean Mining Symposium, Szczecin, Poland, 22–26 September 2013; pp. 109–115. [Google Scholar]
- Lee, B.R.; Koh, C.A.; Sum, A.K. Quantitative measurement and mechanisms for CH4 production from hydrates with the injection of liquid CO2. Phys. Chem. Chem. Phys. 2014, 16, 14922–14927. [Google Scholar] [CrossRef]
- Murshed, M.M.; Schmidt, B.C.; Kuhs, W.F. Kinetics of methane-ethane gas replacement in clathrate-hydrates studied by time-resolved neutron diffraction and Raman spectroscopy. J. Phys. Chem. A 2010, 114, 247–255. [Google Scholar] [CrossRef]
- Ota, M.; Abe, Y.; Watanabe, M.; Smith, R.L.; Inomata, H. Methane recovery from methane hydrate using pressurized CO2. Fluid Phase Equilib. 2005, 228, 553–559. [Google Scholar] [CrossRef]
- Kvamme, B.; Graue, A.; Buanes, T.; Kuznetsova, T.; Ersland, G. Storage of CO2 in natural gas hydrate reservoirs and the effect of hydrate as an extra sealing in cold aquifers. Int. J. Greenh. Gas Control 2007, 1, 236–246. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kawamura, T.; Yamamoto, Y.; Komai, T. Transformation of methane hydrate to carbon dioxide hydrate: In situ Raman spectroscopic observations. J. Phys. Chem. A 2004, 108, 5057–5059. [Google Scholar] [CrossRef]
- Mok, J.; Choi, W.; Seo, Y. Time-dependent observation of a cage-specific guest exchange in sI hydrates for CH4 recovery and CO2 sequestration. Chem. Eng. J. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Falenty, A.; Qin, J.; Salamatin, A.N.; Yang, L.; Kuhs, W.F. Fluid Composition and Kinetics of the in Situ Replacement in CH4–CO2 Hydrate System. J. Phys. Chem. C 2016, 120, 27159–27172. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, F.; Liang, D. Multiscale Analysis on CH4-CO2 Swapping Phenomenon Occurred in Hydrates. J. Phys. Chem. C 2016, 120, 25668–25677. [Google Scholar] [CrossRef]
- Kang, H.; Koh, D.Y.; Lee, H. Nondestructive natural gas hydrate recovery driven by air and carbon dioxide. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Park, Y.; Cha, M.; Park, K.P.; Huh, D.G.; Lee, J.; Kim, S.J.; Lee, H. Swapping phenomena occurring in deep-sea gas hydrates. Energy Fuels 2008, 22, 3160–3163. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Lee, H.; Moudrakovski, I.L.; Ripmeester, J.A.; Seo, Y. Kinetics of methane hydrate replacement with carbon dioxide and nitrogen gas mixture using in situ NMR spectroscopy. Environ. Sci. Technol. 2015, 49, 1964–1971. [Google Scholar] [CrossRef]
- Salamatin, A.N.; Falenty, A.; Kuhs, W.F. Diffusion Model for Gas Replacement in an Isostructural CH4-CO2 Hydrate System. J. Phys. Chem. C 2017, 121, 17603–17616. [Google Scholar] [CrossRef]
- Schicks, J.M.; Pan, M.; Giese, R.; Poser, M.; Aminatulmimi, N.I.; Luzi-Helbing, M.; Bleisteiner, B.; Lenz, C. A new high-pressure cell for systematic in situ investigations of micro-scale processes in gas hydrates using confocal micro-Raman spectroscopy. Rev. Sci. Instrum. 2020, 91, 115103. [Google Scholar] [CrossRef]
- Schicks, J.M.; Ripmeester, J.A. The coexistence of two different methane hydrate phases under moderate pressure and temperature conditions: Kinetic versus thermodynamic products. Angew. Chem. Int. Ed. 2004, 43, 3310–3313. [Google Scholar] [CrossRef]
- Aktins, P.; Jones, L. Chemical Principles: The Quest for Insight, 3rd ed.; W.H. Freeman: New York, NY, USA, 1940. [Google Scholar]
- Wedler, G.; Freund, H.-J. Lehrbuch der Physikalischen Chemie, 6th ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Beeskow-Strauch, B.; Schicks, J.M.; Spangenberg, E.; Erzinger, J. The influence of SO2 and NO2 impurities on CO2 gas hydrate formation and stability. Chem. A Eur. J. 2011, 17, 4376–4384. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.J.E. Raman microspectrometry of fluid inclusions. Lithos 2001, 55, 139–158. [Google Scholar] [CrossRef]
- Schrader, B. Infrared and Raman Spectroscopy: Methods and Applications; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1995. [Google Scholar]
- Schrötter, H.W.; Klöckner, H.W. Raman Scattering Cross Sections in Gases and Liquids. In Raman Spectroscopy of Gases and Liquids. Topics in Current Physics, Volume 11; Springer: Berlin/Heidelberg, Germany, 1979; pp. 123–166. [Google Scholar]
- Qin, J.; Kuhs, W.F. Quantitative analysis of gas hydrates using Raman spectroscopy. AIChE J. 2013, 59, 2155–2167. [Google Scholar] [CrossRef]
- Eaves, J.D.; Loparo, J.J.; Fecko, C.J.; Roberts, S.T.; Tokmakoff, A.; Geissler, P.L. Hydrogen bonds in liquid water are broken only fleetingly. Proc. Natl. Acad. Sci. USA 2005, 102, 13019–13022. [Google Scholar] [CrossRef]
- Pakoulev, A.; Wang, Z.; Dlott, D.D. Vibrational relaxation and spectral evolution following ultrafast OH stretch excitation of water. Chem. Phys. Lett. 2003, 371, 594–600. [Google Scholar] [CrossRef]
- Hester, K.C.; White, S.N.; Peltzer, E.T.; Brewer, P.G.; Sloan, E.D. Raman spectroscopic measurements of synthetic gas hydrates in the ocean. Mar. Chem. 2006, 98, 304–314. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I: General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Schmalzried, H. Chemical Kinetics of Solids; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Susilo, R.; Ripmeester, J.A.; Englezos, P. Methane conversion rate into structure H hydrate crystals from ice. AIChE J. 2007, 53, 2451–2460. [Google Scholar] [CrossRef]
- Wang, X.; Schultz, A.J.; Halpern, Y. Kinetics of methane hydrate formation from polycrystalline deuterated ice. J. Phys. Chem. A 2002, 106, 7304–7309. [Google Scholar] [CrossRef]
- Luzi, M.; Schicks, J.M.; Naumann, R.; Erzinger, J. Systematic kinetic studies on mixed gas hydrates by Raman spectroscopy and powder X-ray diffraction. J. Chem. Thermodyn. 2012, 48, 28–35. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Chen, X.; Fu, Z.; Liu, Y.; Song, Y. Experimental Study of Conditions for Methane Hydrate Productivity by the CO2 Swap Method. Energy Fuels 2015, 29, 6887–6895. [Google Scholar] [CrossRef]
- Jander, W. Reaktionen im festen Zustande bei höheren Temperaturen. Reaktionsgeschwindigkeiten endotherm verlaufender Umsetzungen. Zeitschrift für Anorg. Allg. Chem. 1927, 163, 1–30. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical reaction engineering. In Industrial and Engineering Chemistry Research; John Wiley & Sons: New York, NY, USA, 1999; pp. 4140–4143. [Google Scholar]
- Fujii, K.; Kondo, W. Kinetics of the Hydration of Tricalcium Silicate. J. Am. Ceram. Soc. 1974, 57, 492–497. [Google Scholar] [CrossRef]
- Chen, X.; Espinoza, D.N. Ostwald ripening changes the pore habit and spatial variability of clathrate hydrate. Fuel 2018, 214, 614–622. [Google Scholar] [CrossRef]
- Kuang, Y.; Feng, Y.; Yang, L.; Song, Y.; Zhao, J. Effects of micro-bubbles on the nucleation and morphology of gas hydrate crystals. Phys. Chem. Chem. Phys. 2019, 21, 23401–23407. [Google Scholar] [CrossRef]
- Jung, J.W.; Santamarina, J.C. Hydrate formation and growth in pores. J. Cryst. Growth 2012, 345, 61–68. [Google Scholar] [CrossRef]
- Uchida, T.; Kishi, D.; Shiga, T.; Nagayama, M.; Gohara, K. Sintering process observations on gas hydrates under hydrate-stable and self-preservation conditions. J. Chem. Eng. Data 2015, 60, 284–292. [Google Scholar] [CrossRef]
- Chaouachi, M.; Neher, S.H.; Falenty, A.; Kuhs, W.F. Time Resolved Coarsening of Clathrate Crystals: The Case of Gas Hydrates. Cryst. Growth Des. 2017, 17, 2458–2472. [Google Scholar] [CrossRef]
- Perez, M. Gibbs-Thomson effects in phase transformations. Scr. Mater. 2005, 52, 709–712. [Google Scholar] [CrossRef]
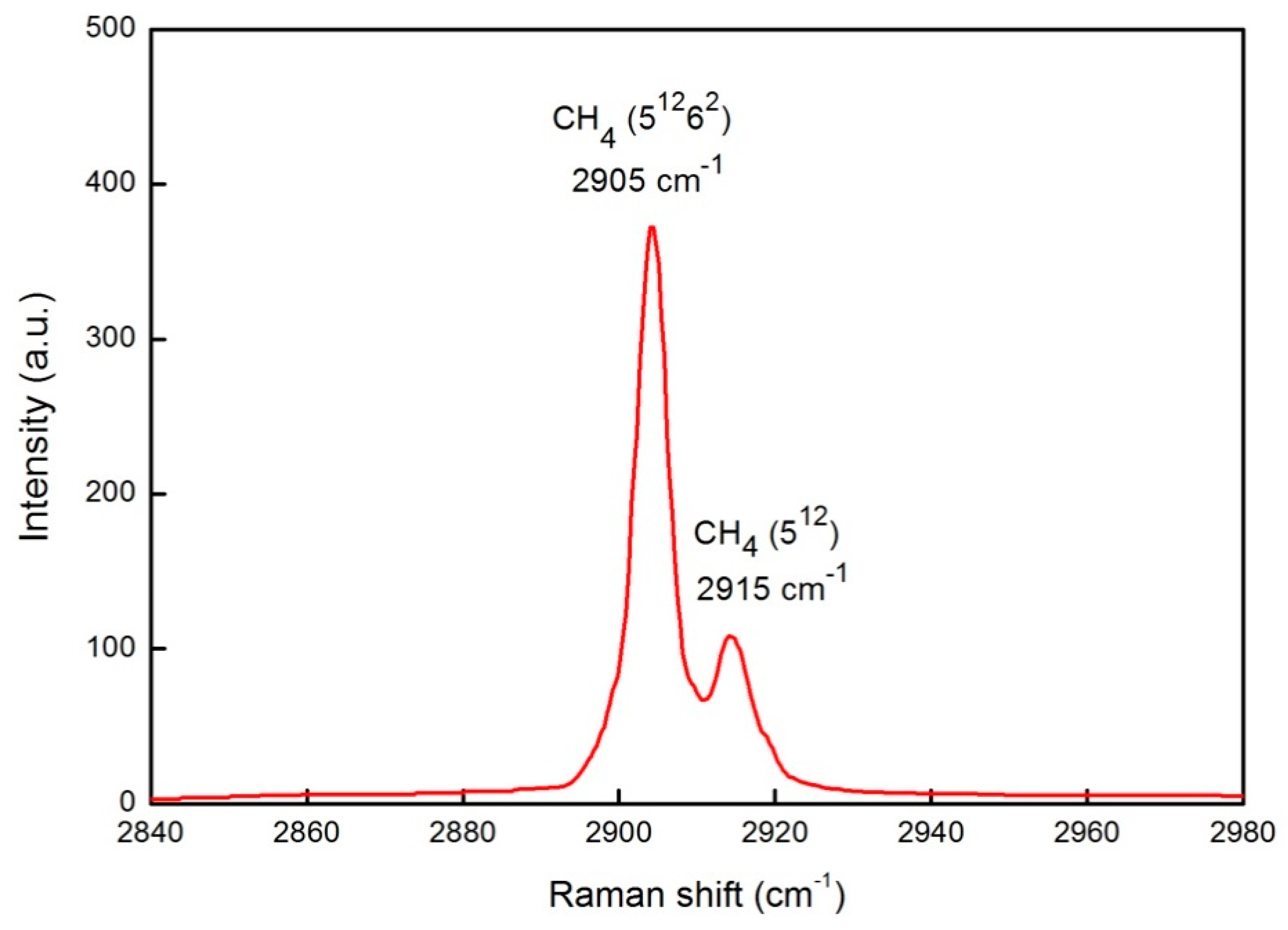








| Component | Vibrational Mode | Cavity Type/Gas Phase | νmeasured (cm−1) | νliterature (cm−1) | References |
|---|---|---|---|---|---|
| CH4 | ν1 C–H symmetric stretching | Gas phase | 2917 | 2917 | [50] |
| sI 512 | 2915 | 2915 | [51] | ||
| sI 51262 | 2905 | 2905 | [51] | ||
| CO2 | ν1 C–O symmetric stretching | Gas phase | 1285 | 1285 | [50] |
| sI 51262 | 1277 | 1276 | [32] | ||
| 2ν2 overtone of bending | Gas phase | 1388 | 1388 | [50] | |
| sI 51262 | 1382 | 1381 | [32] |
| Crystal Number | k/min−n | n | Experiment |
|---|---|---|---|
| 1 | 9.11 × 10−6 | 1.5829 | Run 1 |
| 2 | 9.31 × 10−5 | 1.2674 | Run 1 |
| 3 | 3.23 × 10−5 | 1.3221 | Run 2 |
| 4 | 5.28 × 10−7 | 1.8317 | Run 2 |
| 5 | 5.16 × 10−5 | 1.2594 | Run 3 |
| 6 | 3.55 × 10−7 | 2.0291 | Run 3 |
| Crystal Number | k/min−n | Radius/µm | Experiment |
|---|---|---|---|
| 1 | 4.20 × 10−3 | 46.2 | Run 1 |
| 2 | 1.59 × 10−2 | 45.5 | Run 1 |
| 3 | 7.63 × 10−3 | 30.2 | Run 2 |
| 4 | 2.50 × 10−2 | 43.4 | Run 2 |
| 5 | 2.08 × 10−2 | 51.0 | Run 3 |
| 6 | 1.69 × 10−2 | 44.9 | Run 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Ismail, N.A.; Luzi-Helbing, M.; Koh, C.A.; Schicks, J.M. New Insights on a µm-Scale into the Transformation Process of CH4 Hydrates to CO2-Rich Mixed Hydrates. Energies 2020, 13, 5908. https://doi.org/10.3390/en13225908
Pan M, Ismail NA, Luzi-Helbing M, Koh CA, Schicks JM. New Insights on a µm-Scale into the Transformation Process of CH4 Hydrates to CO2-Rich Mixed Hydrates. Energies. 2020; 13(22):5908. https://doi.org/10.3390/en13225908
Chicago/Turabian StylePan, Mengdi, Nur Aminatulmimi Ismail, Manja Luzi-Helbing, Carolyn A. Koh, and Judith M. Schicks. 2020. "New Insights on a µm-Scale into the Transformation Process of CH4 Hydrates to CO2-Rich Mixed Hydrates" Energies 13, no. 22: 5908. https://doi.org/10.3390/en13225908
APA StylePan, M., Ismail, N. A., Luzi-Helbing, M., Koh, C. A., & Schicks, J. M. (2020). New Insights on a µm-Scale into the Transformation Process of CH4 Hydrates to CO2-Rich Mixed Hydrates. Energies, 13(22), 5908. https://doi.org/10.3390/en13225908






