Chemical Free Two-Step Hydrothermal Pretreatment to Improve Sugar Yields from Energy Cane
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Liquid Hot Water Pretreatment
2.3. Disk Milling/Mechanical Refining
2.4. Chemical Composition of Raw and Pretreated Energy Cane Biomass
2.5. Enzymatic Hydrolysis
2.6. Sugar Recovery and Hydrolysis Efficiency
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Compositional of Raw and Pretreated Energycane Bagasse
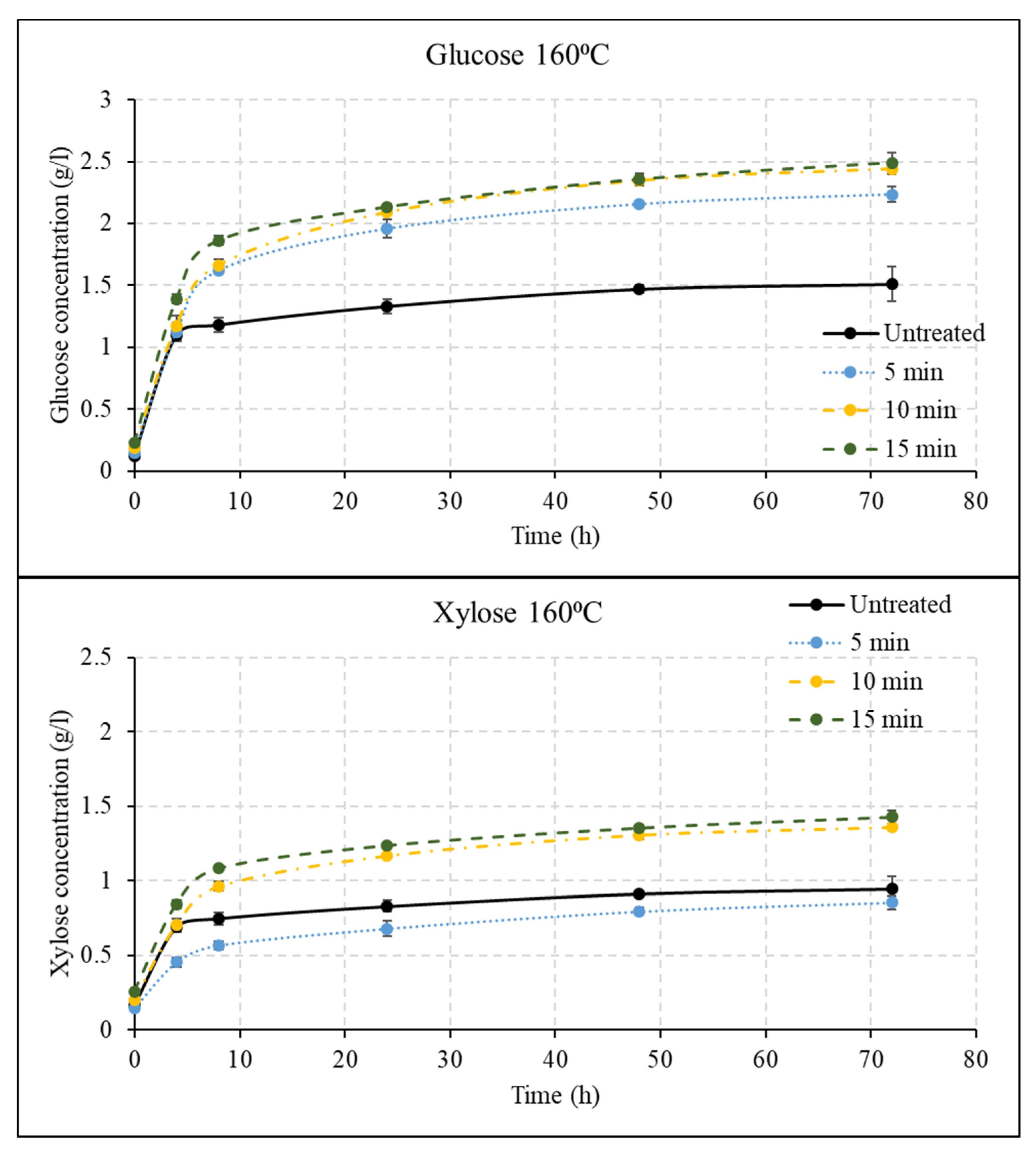
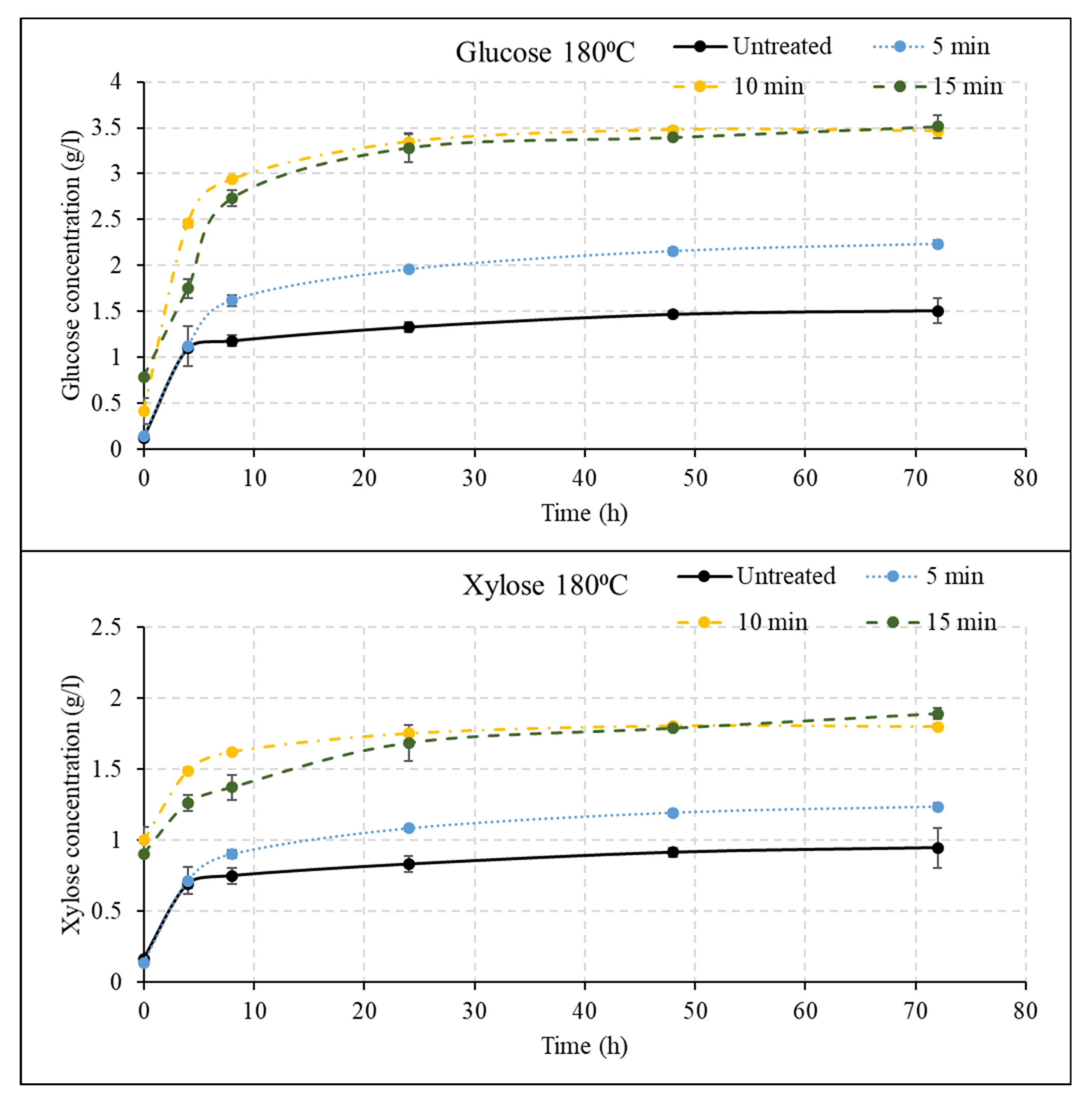
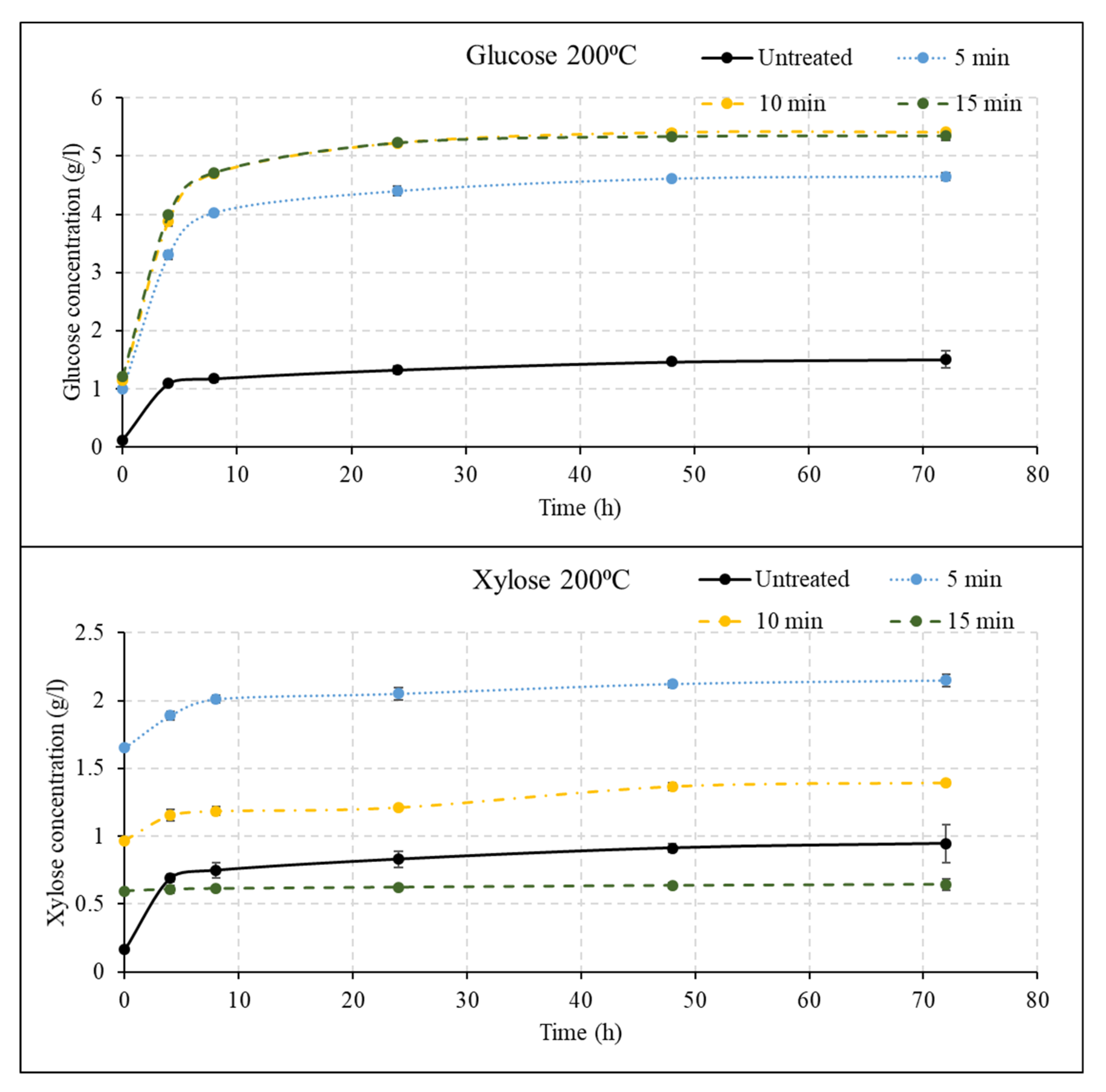
3.2. Effect of Liquid Hot Water Pretreatment Followed by Disk Milling on Sugar Yields
3.3. Effect of Disk Milling on Sugar Yields
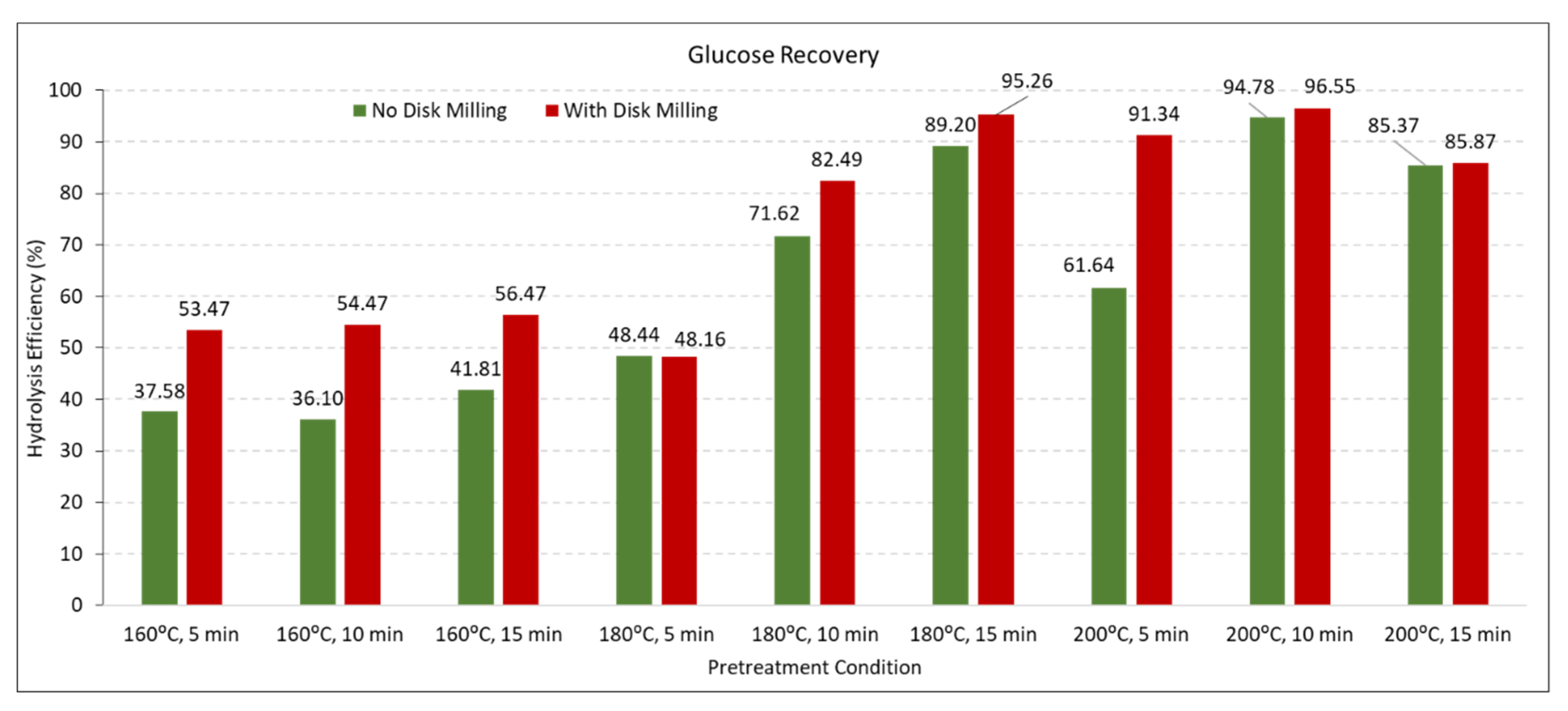
3.4. Hydrolysis Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matsuoka, S.; Kennedy, A.J.; Santos, E.G.D.D. Energy cane: Its concept, development, characteristics, and prospects. Adv. Bot. 2014, 2014, 597275. [Google Scholar] [CrossRef]
- Aragon, D.; Lu, S.; Kochergin, V. Conversion of energy cane and sweet sorghum into biofuels and chemicals: A modeling approach. In Proceedings of the Joint Annual Meeting of the Association for the Advancement of Industrial Crops and the USDA National Institute of Food and Agriculture, Washington, DC, USA, 12–16 October 2013. [Google Scholar]
- Qiu, Z.; Aita, G.M.; Walker, M.S. Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour. Technol. 2012, 117, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Suhardi, V.S.H.; Prasai, B.; Samaha, D.; Boopathy, R. Evaluation of pretreatment methods for lignocellulosic ethanol production from energy cane variety L 79-1002. Int. Biodeterior. Biodegrad. 2013, 85, 683–687. [Google Scholar] [CrossRef]
- Deng, F.; Aita, G.M. Detoxification of dilute ammonia pretreated energy cane bagasse enzymatic hydrolysate by soluble polyelectrolyte flocculants. Ind. Crop. Prod. 2018, 112, 681–690. [Google Scholar] [CrossRef]
- Aita, G.; Salvi, D.; Walker, M. Enzyme hydrolysis and ethanol fermentation of dilute ammonia pretreated energy cane. Bioresour. Technol. 2011, 102, 4444–4448. [Google Scholar] [CrossRef]
- Oladi, S.; Aita, G.M. Interactive effect of enzymes and surfactant on the cellulose digestibility of un-washed and washed dilute ammonia pretreated energy cane bagasse. Biomass Bioenergy 2018, 109, 221–230. [Google Scholar] [CrossRef]
- Qiu, Z.; Aita, G.M.; Mahalaxmi, S. Optimization by response surface methodology of processing conditions for the ionic liquid pretreatment of energy cane bagasse. J. Chem. Technol. Biotechnol. 2014, 89, 682–689. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.S. Fundamentals of aqueous pretreatment of biomass. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Wiley: West Sussex, UK, 2013; pp. 129–143. [Google Scholar]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A short overview on the hydrogen production via aqueous phase reforming (APR) of cellulose, C6-C5 sugars and polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Singh, V. Promise of combined hydrothermal/chemical and mechanical refining for pretreatment of woody and herbaceous biomass. Biotechnol. Biofuels 2016, 9, 97. [Google Scholar] [CrossRef]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Fermentation of undetoxified sugarcane bagasse hydrolyzates using a two stage hydrothermal and mechanical refining pretreatment. Bioresour. Technol. 2018, 261, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-H.; Wang, Z.; Dien, B.S.; Slininger, P.J.; Singh, V. Economic analysis of cellulosic ethanol production from sugarcane bagasse using a sequential deacetylation, hot water and disk-refining pretreatment. Processes 2019, 7, 642. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, J.Y.; Gleisner, R.; Pan, X.J. On energy consumption for size-reduction and yields from subsequent enzymatic saccharification of pretreated lodgepole pine. Bioresour. Technol. 2010, 101, 2782–2792. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Ertas, M.; Han, Q.; Jameel, H.; Chang, H.M. Enzymatic hydrolysis of autohydrolyzed wheat straw followed by refining to produce fermentable sugars. Bioresour. Technol. 2014, 152, 259–266. [Google Scholar] [CrossRef]
- Yang, B.; Tao, L.; Wyman, C.E. Strengths, challenges, and opportunities for hydrothermal pretreatment in lignocellulosic biorefineries. Biofuel Bioprod. Biorefining 2018, 12, 125–138. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Z.; Xu, G.; Qin, Y.; Deng, J.; Yang, J. Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour. Technol. 2019, 274, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Norrrahim, M.N.F.; Hirata, S.; Hassan, M.A. Hydrothermal and wet disk milling pretreatment for high conversion of biosugars from oil palm mesocarp fiber. Bioresour. Technol. 2015, 181, 263–269. [Google Scholar] [CrossRef]
- Lynd, L.R.; Elander, R.T.; Wyman, C.E. Likely features and costs of mature biomass ethanol technology. Appl. Biochem. Biotechnol. 1996, 57, 741–761. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Leu, S.-Y.; Zhu, J. Substrate-related factors affecting enzymatic saccharification of lignocelluloses: Our recent understanding. Bioenergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Pretreatments and enzymatic hydrolysis of grass straws for ethanol production in the Pacific Northwest US. Biol. Eng. Trans. 2011, 3, 97–110. [Google Scholar] [CrossRef]
- de Barros, R.d.R.O.; de Sousa Paredes, R.; Endo, T.; da Silva Bon, E.P.; Lee, S.H. Association of wet disk milling and ozonolysis as pretreatment for enzymatic saccharification of sugarcane bagasse and straw. Bioresour. Technol. 2013, 136, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Hendrickson, R.; Ho, N.; Sedlak, M.; Ladisch, M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef] [PubMed]


| Pretreatment Temp (°C) | Pretreatment Time (min) | Extractives (%, db) | Glucans (%, db) | Xylans (%, db) | Insoluble Lignin (%, db) | Soluble Lignin (%, db) |
|---|---|---|---|---|---|---|
| Untreated | 18.62 | 34.77 | 20.16 | 15.37 | 2.08 | |
| 160 | 5 | 15.70 | 32.32 | 16.32 | 14.06 | 1.78 |
| 160 | 10 | 18.04 | 33.71 | 15.88 | 13.96 | 1.60 |
| 160 | 15 | 36.33 | 32.04 | 10.39 | 9.22 | 1.00 |
| 180 | 5 | 18.40 | 34.31 | 16.70 | 13.48 | 1.58 |
| 180 | 10 | 33.69 | 31.48 | 8.56 | 11.70 | 0.57 |
| 180 | 15 | 29.78 | 32.73 | 9.07 | 12.54 | 0.67 |
| 200 | 5 | 41.54 | 34.15 | 4.40 | 10.32 | 0.73 |
| 200 | 10 | 45.16 | 37.07 | 0.00 | 10.78 | 0.38 |
| 200 | 15 | 45.51 | 41.58 | 0.00 | 10.23 | 0.37 |
| Xylose Recovery, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basis of Calculation | 160 °C, 5 min | 160 °C, 10 min | 160 °C, 15 min | 180 °C, 5 min | 180 °C, 10 min | 180 °C, 15 min | 200 °C, 5 min | 200 °C, 10 min | 200 °C, 15 min | |
| Raw biomass composition | No DM* | 28.36 | 45.48 | 45.239 | 41.9 | 32.88 | 41.16 | 20.48 | 17.31 | 2.058 |
| DM* | 34.14 | 53.53 | 55.155 | 47.15 | 74.08 | 78.9 | 88.67 | 56.61 | 26.86 | |
| Pretreated biomass composition | No DM* | 35.04 | 57.72 | 87.8 | 50.59 | 77.44 | 91.47 | 93.83 | - | - |
| DM* | 80.42 | 119 | 66.6 | 58.26 | 94.01 | 153.1 | 406.2 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juneja, A.; Kumar, D.; Singh, V.K.; Yadvika; Singh, V. Chemical Free Two-Step Hydrothermal Pretreatment to Improve Sugar Yields from Energy Cane. Energies 2020, 13, 5805. https://doi.org/10.3390/en13215805
Juneja A, Kumar D, Singh VK, Yadvika, Singh V. Chemical Free Two-Step Hydrothermal Pretreatment to Improve Sugar Yields from Energy Cane. Energies. 2020; 13(21):5805. https://doi.org/10.3390/en13215805
Chicago/Turabian StyleJuneja, Ankita, Deepak Kumar, Vijay Kumar Singh, Yadvika, and Vijay Singh. 2020. "Chemical Free Two-Step Hydrothermal Pretreatment to Improve Sugar Yields from Energy Cane" Energies 13, no. 21: 5805. https://doi.org/10.3390/en13215805
APA StyleJuneja, A., Kumar, D., Singh, V. K., Yadvika, & Singh, V. (2020). Chemical Free Two-Step Hydrothermal Pretreatment to Improve Sugar Yields from Energy Cane. Energies, 13(21), 5805. https://doi.org/10.3390/en13215805








