Cation-Exchange Capacity Distribution within Hydrothermal Systems and Its Relation to the Alteration Mineralogy and Electrical Resistivity
Abstract
1. Introduction
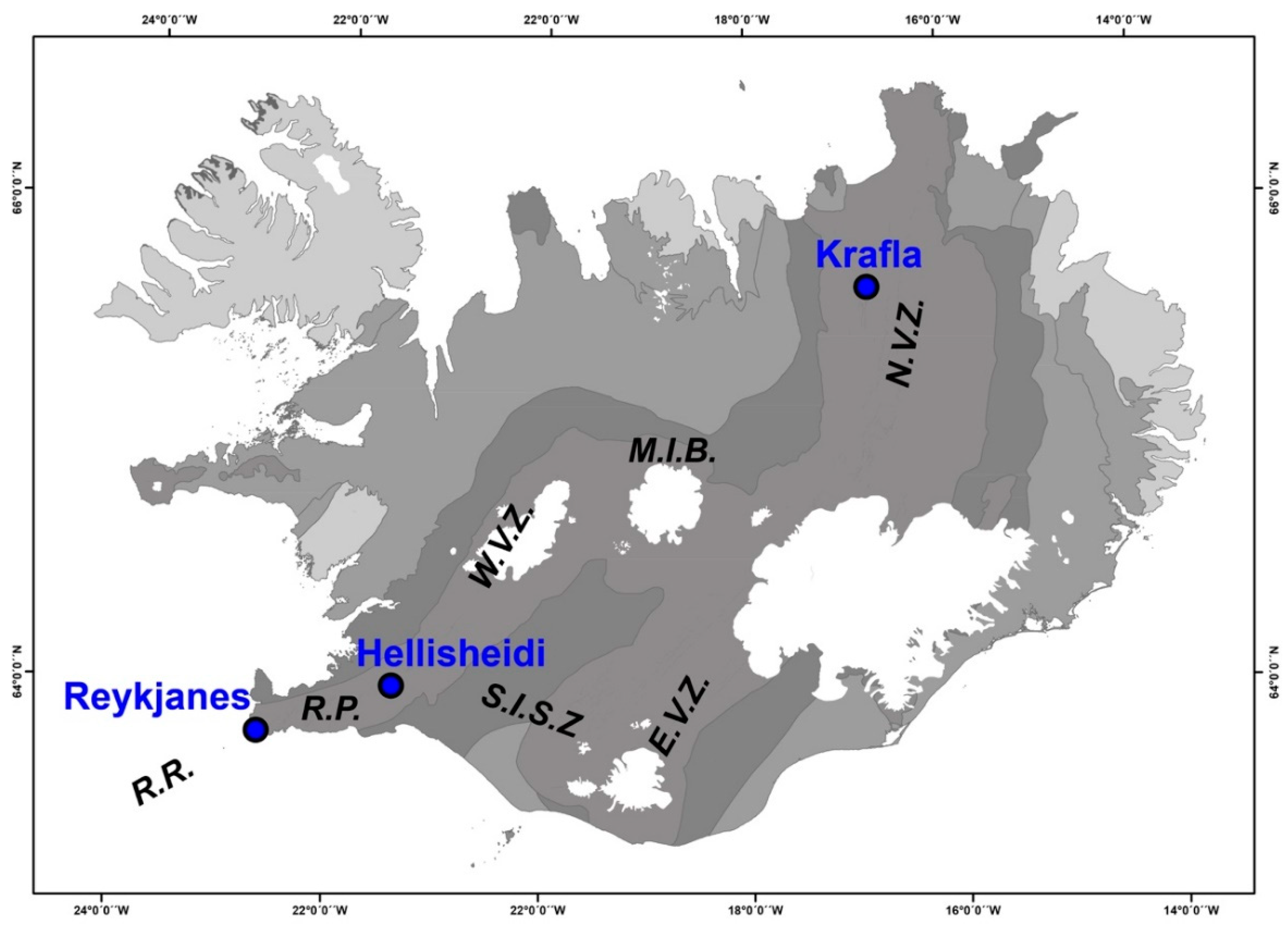
2. Regional Setting
2.1. Krafla Geothermal System
2.2. Hellisheidi Geothermal System
2.3. Reykjanes Geothermal System
3. Methods
4. Results
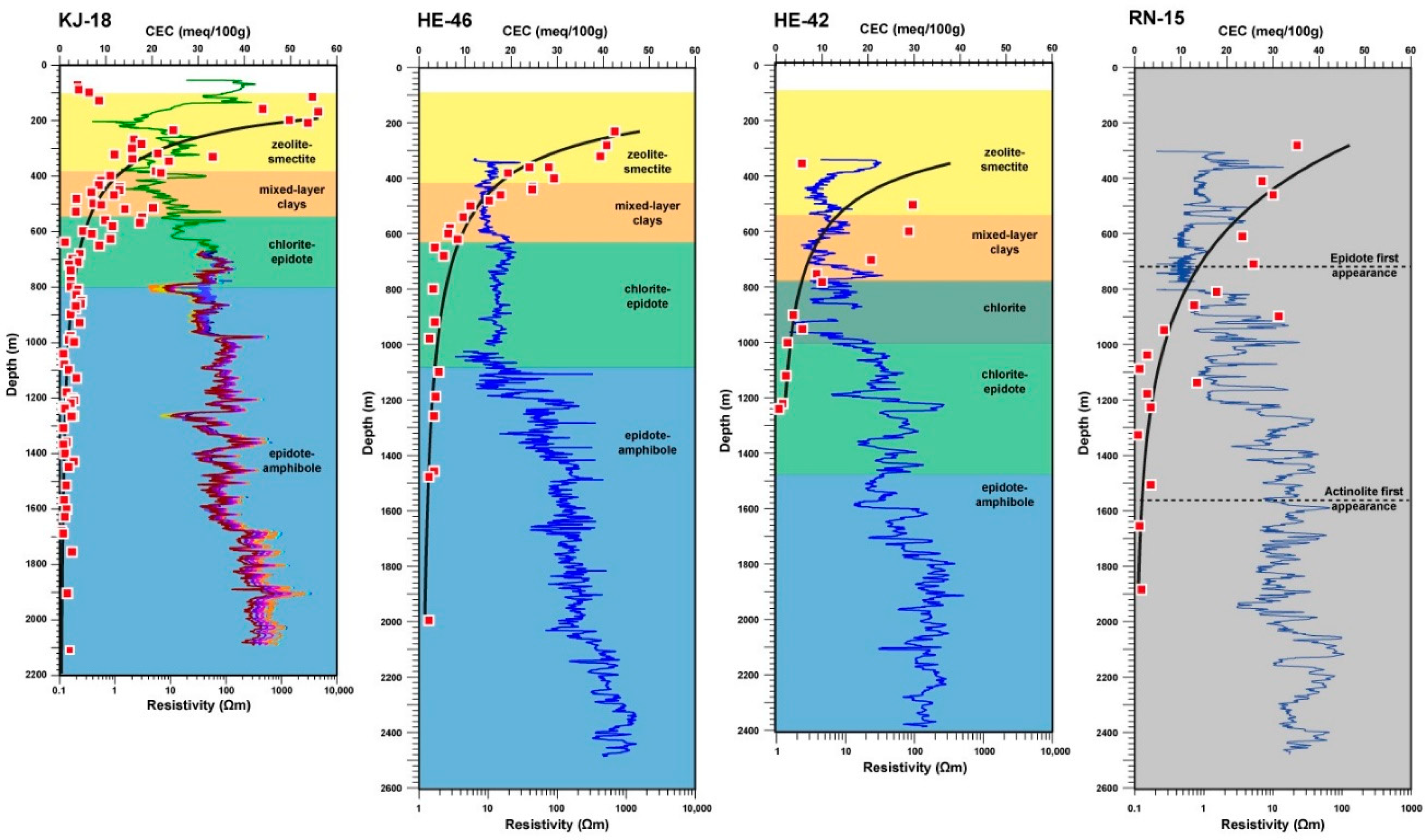
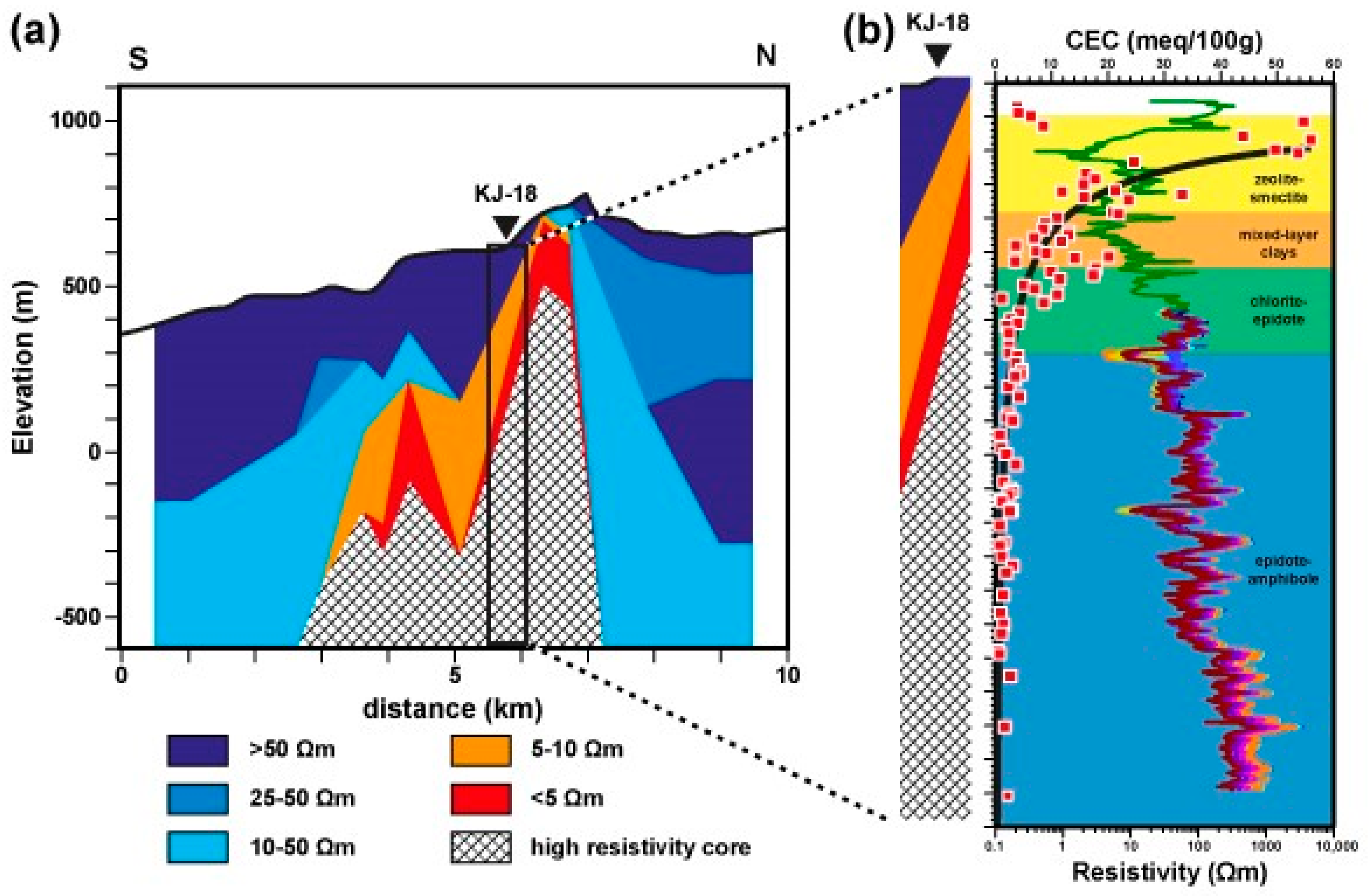
4.1. KJ-18
4.2. HE-42 and HE-46
4.3. RN-15
4.4. Core Samples
5. Discussion
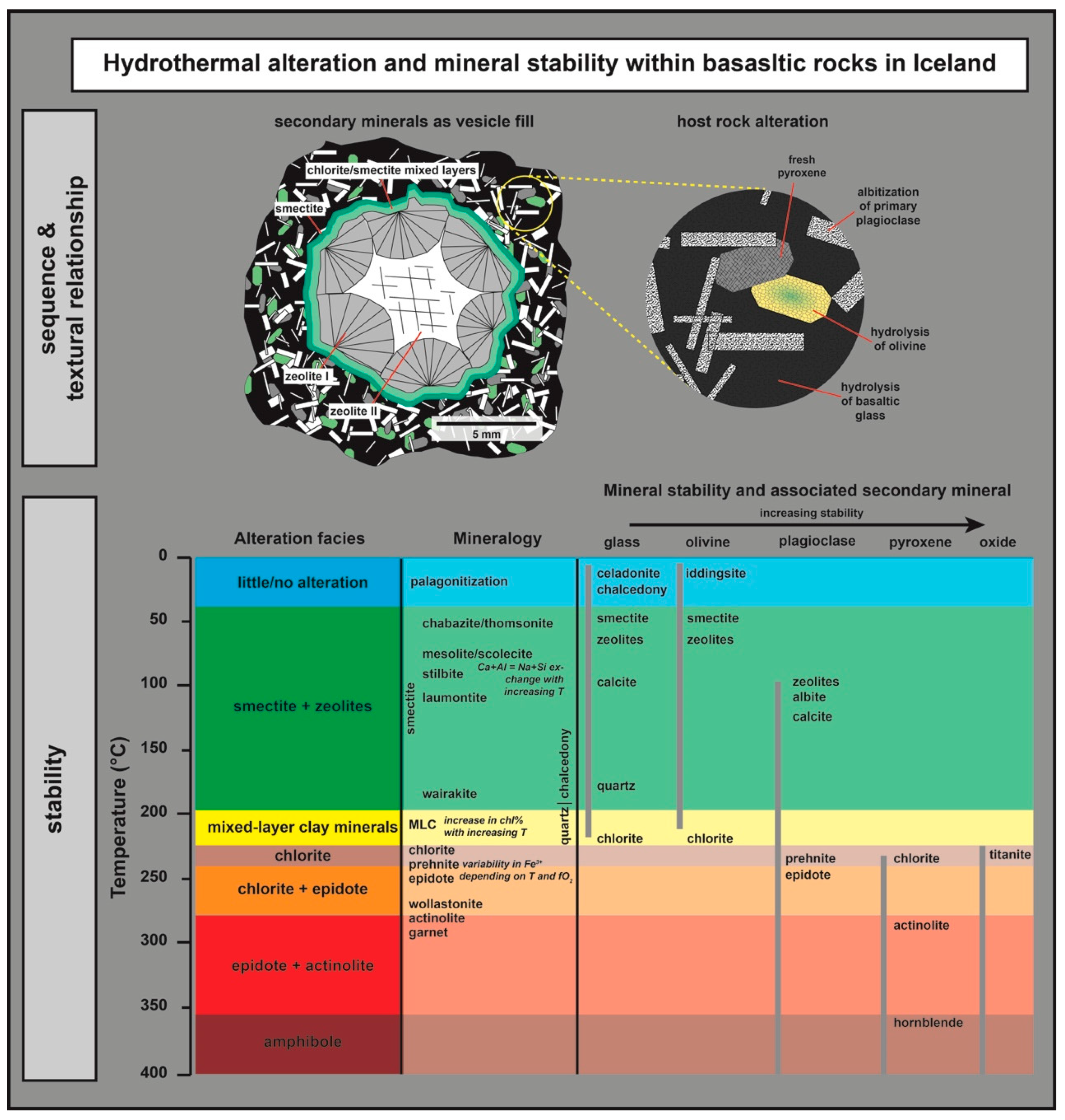
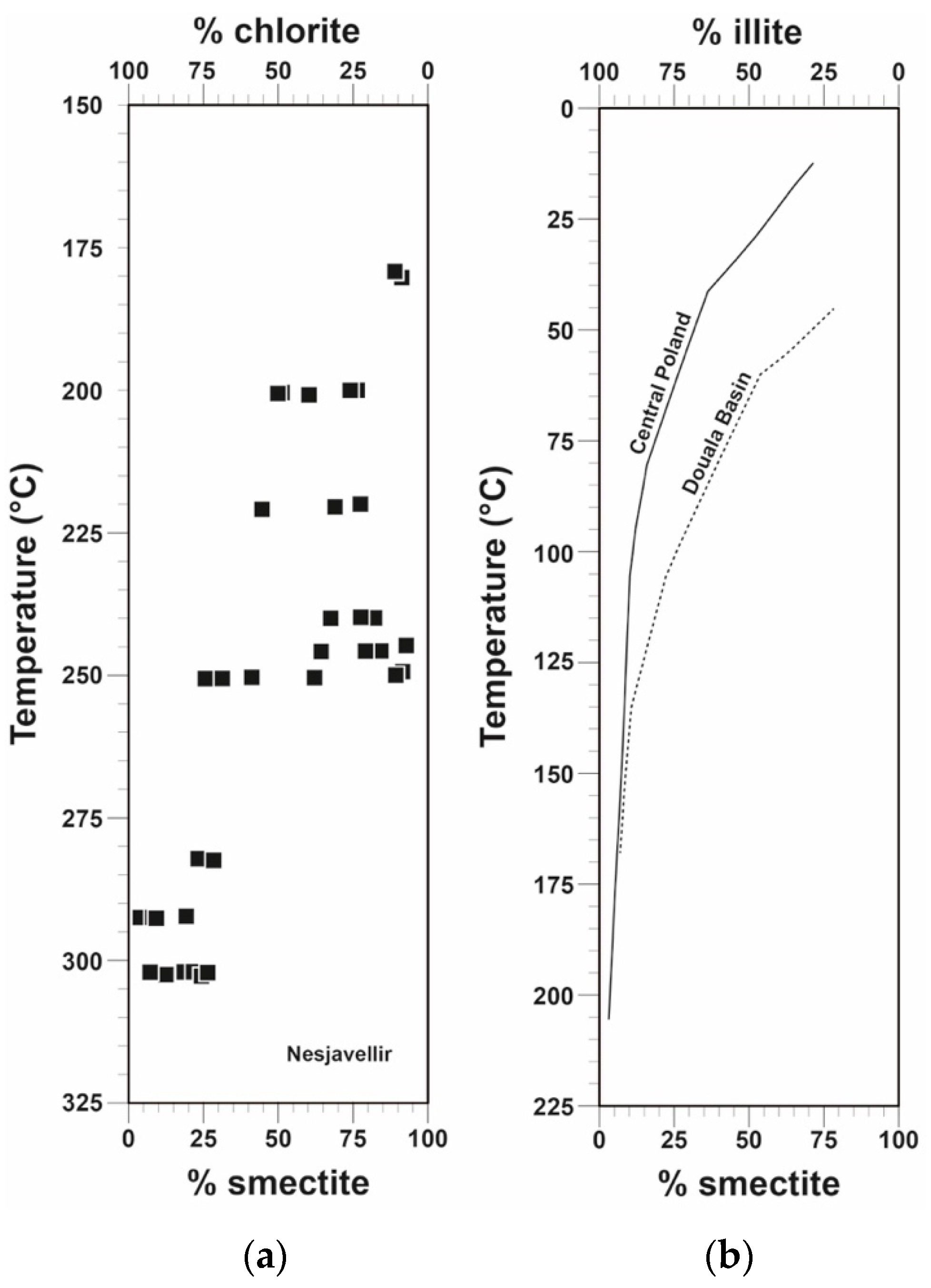
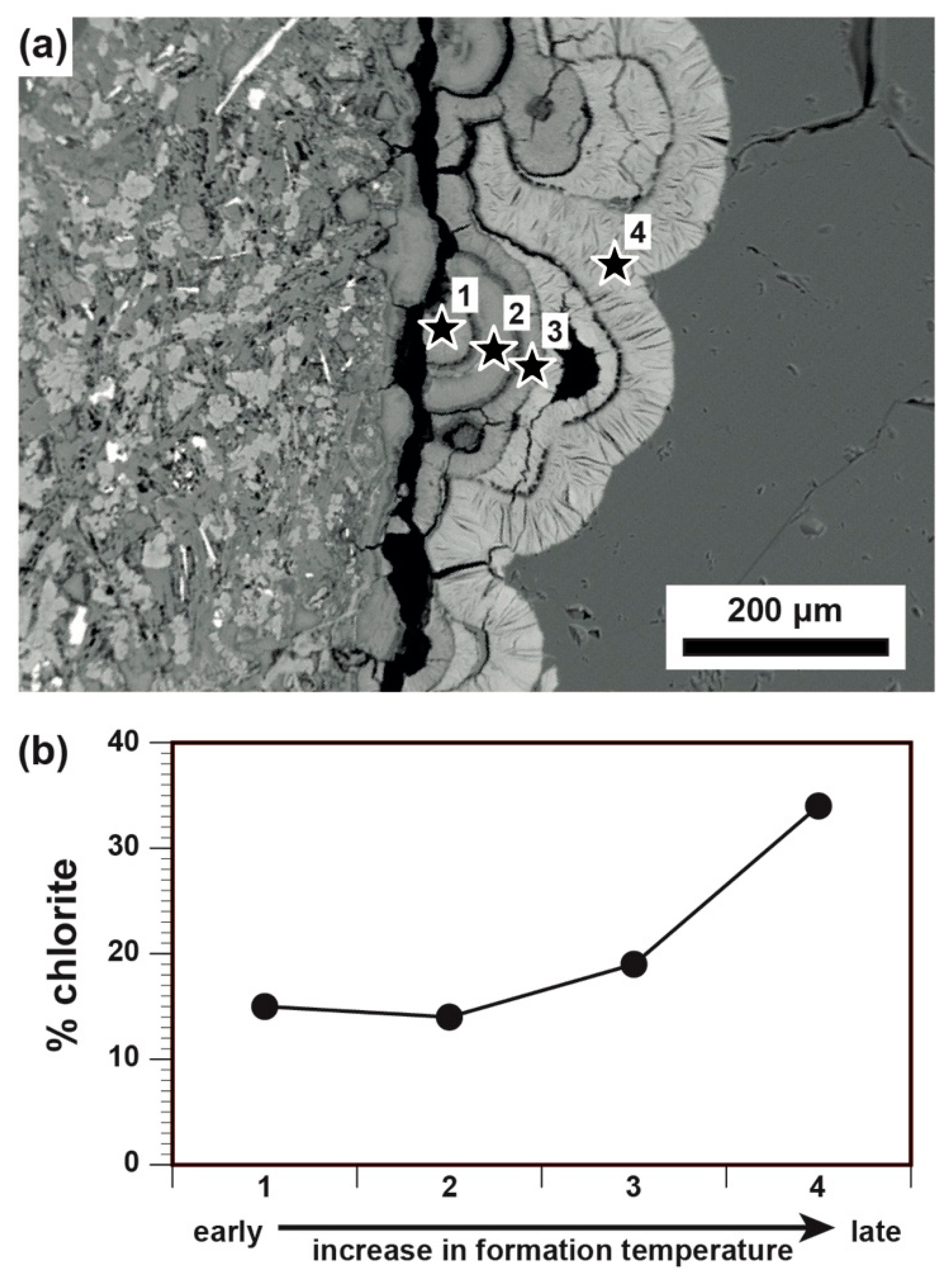
5.1. CEC and Relation to Alteration Mineralogy
5.2. CEC vs. Electrical Resistivity
5.3. Resistivity Structure of High-Temperature Fields and Relation to the CEC
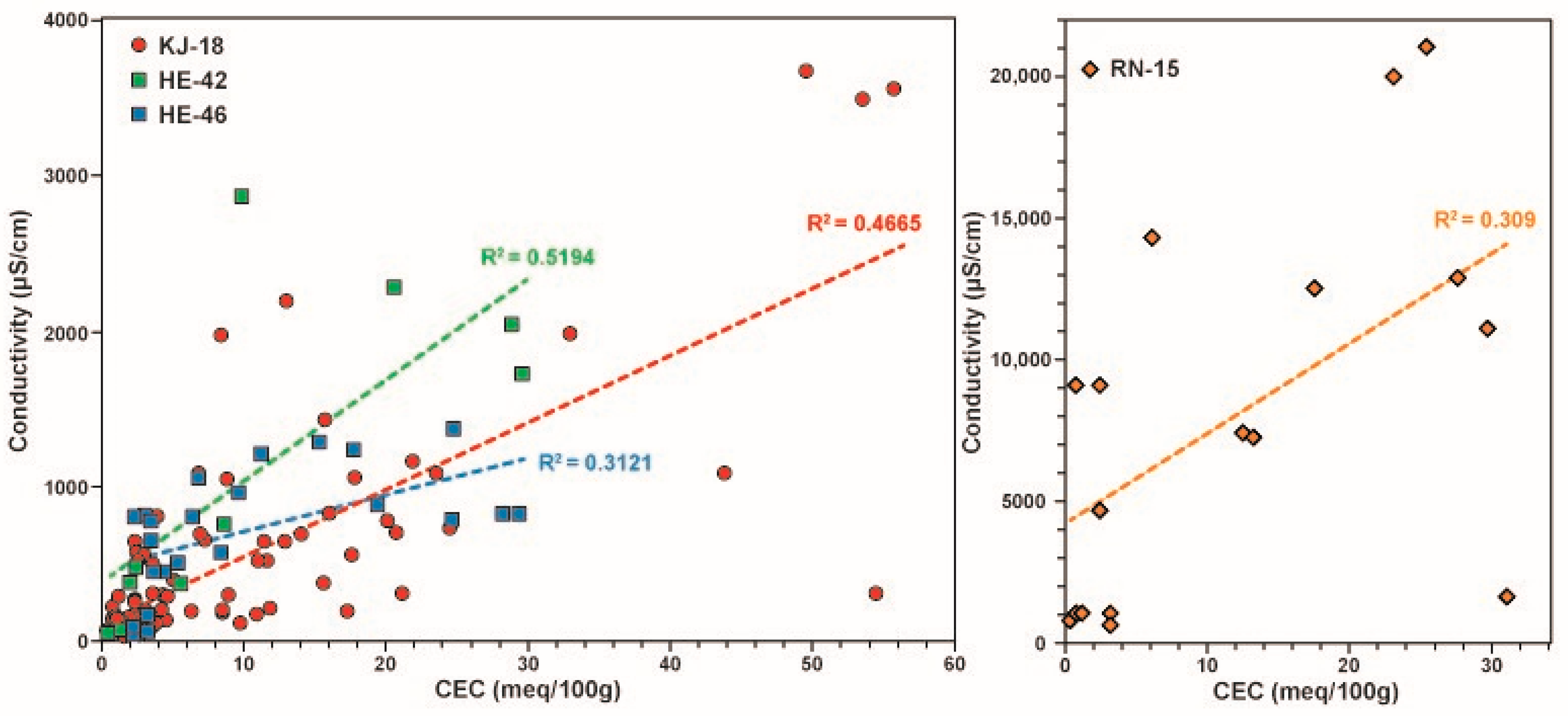
5.4. Relationship between Sample Conductivity, Fluid Conductivity and CEC
6. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Árnason, K.; Haraldsson, G.I.; Johnsen, G.V.; Þorbergsson, G.; Hersir, G.P.; Sæmundsson, K.; Georgsson, L.S.; Snorrason, S.P. Nesjavellir. Jarðfræði- og Jarðeðlisfræðileg Könnun 1985; Report OS-86014/JHD-02; Orkustofnun: Reykjavík, Iceland, 1986. [Google Scholar]
- Árnason, K.; Flóvenz, Ó.G.; Georgsson, L.S.; Hersir, G.P. Resistivity structure of high temperature geothermal systems in Iceland. In Proceedings of the International Union of Geodesy and Geophysics (IUGG) XIX General Assembly, Vancouver, BC, Canada, 9–22 August 1987. Abstract V. [Google Scholar]
- Árnason, K.; Flóvenz, Ó.G. Evaluation of physical methods in geothermal exploration of rifted volcanic crust. GRC Trans. 1992, 16, 207–214. [Google Scholar]
- Flóvenz, Ó.G.; Hersir, G.P.; Saemundsson, K.; Ármannsson, H.; Friðriksson, Þ. 7.03-Geothermal Energy Exploration Techniques. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012; Volume 7, pp. 51–95. [Google Scholar] [CrossRef]
- Schiffman, P.; Fridleifsson, G.Ó. The smectite-chlorite transition in drillhole NJ-15, Nesjavellir geothermal field, Iceland: XRD, BSE and electron microprobe investigations. J. Metamorph. Geol. 1991, 9, 679–696. [Google Scholar] [CrossRef]
- Neuhoff, P.S.; Fridriksson, T.; Arnorsson, S.; Bird, D.K. Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, eastern Iceland. Am. J. Sci. 1999, 299, 467–501. [Google Scholar] [CrossRef]
- Weisenberger, T.; Selbekk, R.S. Multi-stage zeolite facies mineralization in the Hvalfjördur area, Iceland. Int. J. Earth Sci. 2009, 98, 985–999. [Google Scholar] [CrossRef]
- Kristmannsdóttir, H. Types of Clay Minerals in Hydrothermally Altered Basaltic Rocks, Reykjanes, Iceland. Jökull 1979, 26, 30–39. [Google Scholar]
- Alt, J.C.; Honnorez, J.; Laverne, C.; Emmermann, R. Hydrothermal Alteration of a 1 km Section Through the Upper Oceanic Crust, Deep Sea Drilling Project Hole 504B: Mineralogy, Chemistry and Evolution of Seawater-Basalt Interactions. J. Geophys. Res. Solid Earth 1986, 91, 10309–10335. [Google Scholar] [CrossRef]
- Evarts, R.C.; Schiffman, P. Submarine hydrothermal metamorphism of the Del Puerto ophiolite, California. Am. J. Sci. 1983, 283, 289–340. [Google Scholar] [CrossRef]
- Kaufhold, S.; Grissemann, C.; Dohrmann, R.; Klinkenberg, M.; Decher, A. Comparison of three small-scale devices for the investigation of the electrical conductivity/resistivity of swelling and other clays. Clays Clay Miner. 2014, 62, 1–12. [Google Scholar] [CrossRef]
- Flóvenz, Ó.G.; Georgsson, L.S.; Árnason, K. Resistivity structure of the upper crust in Iceland. J. Geophys. Res. Solid Earth 1985, 90, 10136–10150. [Google Scholar] [CrossRef]
- Revil, A.; Glover, P.W.J. Nature of surface electrical conductivity in natural sands, sandstones, and clays. Geophys. Res. Lett. 1998, 25, 691–694. [Google Scholar] [CrossRef]
- Pezard, P. Electrical properties of mid-ocean ridge basalt and implications for the structure of the upper oceanic crust in hole 504B. J. Geophys. Res. 1990, 95, 9237–9264. [Google Scholar] [CrossRef]
- Flóvenz, Ó.G.; Spangenberg, E.; Kulenkampff, J.; Árnason, K.; Karlsdóttir, R.; Huenges, E. The role of electrical interface conduction in geothermal exploration. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Kulenkampff, J.; Spangenberg, E.; Flóvenz, Ó.G.; Raab, S.; Huenges, E. Petrophysical parameters of rocks saturated with liquid water at high temperature geothermal reservoir conditions. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Hjartarson, Á.; Saemundsson, K. Geological Map of Iceland: Bedrock, 1:600000; GeoSurvey: Reykjavík, Iceland, 2014. [Google Scholar]
- Saemundsson, K. Geology of Krafla volcanic system. In Náttúra Mývatns; Garðarsson, A., Einarsson, Á., Eds.; Hið Íslenska Náttúrufræðifélag: Reykjavík, Iceland, 1991; pp. 25–95. [Google Scholar]
- Saemundsson, K. Krafla, Geological Map, 1:25000; Landsvirkjun and ÍSOR: Reykjavík, Iceland, 2008. [Google Scholar]
- Jónasson, K. Rhyolite volcanism in the Krafla central volcano, north-east Iceland. Bull. Volcanol. 1994, 56, 516–528. [Google Scholar] [CrossRef]
- Pope, E.C.; Bird, D.K.; Arnórsson, S.; Giroud, N. Hydrogeology of the Krafla geothermal system, northeast Iceland. Geofluids 2016, 16, 175–197. [Google Scholar] [CrossRef]
- Gudmundsson, Á. An expansion of the Krafla Power Plant from 30 to 60 MWe. Geothermal Considerations. GRC Trans. 2001, 25, 741–746. [Google Scholar]
- Ármannsson, H. The fluid geochemistry of Icelandic high temperature geothermal areas. Appl. Geochem. 2016, 66, 14–64. [Google Scholar] [CrossRef]
- Weisenberger, T.B.; Axelsson, G.; Arnaldsson, A.; Blischke, A.; Óskarsson, F.; Ármannsson, H.; Blanck, H.; Helgadóttir, H.M.; Berthet, J.-C.C.; Árnason, K.; et al. Revision of the Conceptual Model of the Krafla Geothermal System; ÍSOR-2015/012; LV-2015-040; Iceland GeoSurvey: Reykjavík, Iceland, 2015. [Google Scholar]
- Stefánsson, V.; Guðmundsson, Á.; Steingrímsson, B.; Guðmundsson, G.; Friðleifsson, G.Ó.; Axelsson, G.; Ármannsson, H.; Sigvaldason, H.; Benjamínsson, J.; Sigurðsson, Ó. Krafla, holur KJ-16, 17 og 18–Rannsóknir samhliða borun og vinnslueiginleikar. Orkustofnun 1983. Unpublished Manuscript. [Google Scholar]
- Blischke, A.; Árnadóttir, S.; Helgadóttir, H.M.; Millett, J. Well K-18 in the Krafla High-Temperature Field, NE-Iceland. Review of Wireline and Televiewer Log Data, and Electronic Facies Log (EFL). Preliminaries; ÍSOR-2016/021; Iceland GeoSurvey: Reykjavík, Iceland, 2016. [Google Scholar]
- Vilhjálmsson, A.M.; Hersir, G.P.; Flóvenz, Ó.G. IMAGE Task 3.3–Physical Properties of Rock at Reservoir Conditions. Resistivity vs. Temperature during Heating up of Well KJ-18 in Krafla, NE-Iceland; ÍSOR-2016/045; Iceland GeoSurvey: Reykjavík, Iceland, 2016. [Google Scholar]
- Millett, J.M.; Planke, S.; Kästner, F.; Blischke, A.; Hersir, G.P.; Halldórsdóttir, S.; Flóvenz, Ó.G.; Árnadóttir, S.; Helgadóttir, H.M.; Vakulenko, S.; et al. Sub-surface geology and velocity structure of the Krafla high temperature geothermal field, Iceland: Integrated ditch cuttings, wireline and zero offset vertical seismic profile analysis. J. Volcanol. Geotherm. Res. 2020, 391, 106342. [Google Scholar] [CrossRef]
- Saemundsson, K. Vulkanismus und Tektonik des Hengill-Gebietes in Südwest Island. Acta Nat. Isl. 1967, 2, 1–105. [Google Scholar]
- Saemundsson, K. Hellisheidi power plant: Geological conditions within the utilization area. In Iceland GeoSurvey Short Report; Orkustofnun: Reykjavík, Iceland, 2003; KS 03/02. [Google Scholar]
- Jakobsson, S.P.; Jónsson, J.; Shido, F. Petrology of the Western Reykjanes Peninsula, Iceland. J. Petrol. 1978, 19, 669–705. [Google Scholar] [CrossRef]
- Hardarson, B.S.; Einarsson, G.M.; Kristjánsson, B.R.; Gunnarsson, G.; Helgadóttir, H.M.; Franzson, H.; Árnason, K.; Ágústsson, K.; Gunnlaugsson, E. Geothermal Reinjection at the Hengill Triple Junction, SW Iceland. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010. [Google Scholar]
- Árnason, K.; Eysteinsson, H.; Hersir, G.P. Joint 1D inversion of TEM and MT data and 3D inversion of MT data in the Hengill area, SW Iceland. Geothermics 2010, 39, 13–34. [Google Scholar] [CrossRef]
- Saemundsson, K. Hengill, Geological Map (Bedrock) 1:50.000; National Energy Authority and Iceland Geodetic Survey: Reykjavík, Iceland, 1995. [Google Scholar]
- Franzson, H.; Kristjánsson, B.R.; Gunnarsson, G.; Björnsson, G.; Hjartarson, A.; Steingrímsson, B.; Gunnlaugsson, E.; Gíslason, G. The Hengill–Hellisheiði geothermal field. Development of a conceptual geothermal model. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Haraldsdóttir, S.H.; Franzson, H. Viðnám á Suðurhluta Hengilssvæðis. Samanburður Borholumælinga, Niðurstaðna úr TEM-MT og Ummyndunar; ÍSOR-2011/075; Iceland GeoSurvey: Reykjavík, Iceland, 2011. [Google Scholar]
- Snaebjörnsdóttir, S.Ó. Jarðfræði og Jarðhitaummyndun við Vesturjaðar Sigdældar Hengils. Master’s Thesis, University of Iceland, Reykjavík, Iceland, 2011. [Google Scholar]
- Gunnarsdóttir, S.H. Jarðfræði og Ummyndun í Nágrenni Reykjafells á Hellisheiði. Master’s Thesis, University of Iceland, Reykjavík, Iceland, 2012. [Google Scholar]
- Saemundsson, K.; Sigurgeirsson, M.Á.; Hjartarson, Á.; Kaldal, I.; Kristinsson, S.G.; Víkingsson, S. Geological Map of Southwest Iceland, 1:100000, 2nd ed.; Iceland GeoSurvey: Reykjavík, Iceland, 2016. [Google Scholar]
- Weisenberger, T.B.; Einarsson, G.M.; Hardaarson, B.S.; Níelsson, S. Lithostratigraphic Model of the Reykjanes Geothermal Field. In Iceland GeoSurvey Short Report; ÍSOR-16076; Orkustofnun: Reykjavík, Iceland, 2016. [Google Scholar]
- Franzson, H.; Thordarson, S.; Bjornsson, G.; Gudlaugsson, S.T.; Richter, B.; Fridleifsson, G.O.; Thorhallsson, S. Reykjanes high-temperature field, SW-Iceland. Geology and hydrothermal alteration of well RN-10. In Proceedings of the 27th Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 28–30 January 2002. SGP-TR-171. [Google Scholar]
- Tómasson, J.; Kristmannsdóttir, H. High temperature alteration minerals and thermal brine, Reykjanes, Iceland. Contrib. Miner. Petrol. 1972, 36, 123–134. [Google Scholar] [CrossRef]
- Marks, N.; Schiffman, P.; Zierenberg, R.A. High-grade contact metamorphism in the Reykjanes geothermal system: Implications for fluid-rock interactions at mid-oceanic ridge spreading centers. Geochem. Geophy. Geosy. 2011, 12, Q08007. [Google Scholar] [CrossRef]
- Arnórsson, S. Major element chemistry of the geothermal sea-water at Reykjanes and Svartsengi, Iceland. Miner. Mag. 1978, 42, 209–220. [Google Scholar] [CrossRef]
- Arnórsson, S. Geothermal systems in Iceland: Structure and conceptual models–I. High temperature areas. Geothermics 1995, 24, 561–602. [Google Scholar] [CrossRef]
- Ólafsson, J.; Riley, J.P. Geochemical studies on the thermal brine from Reykjanes. Chem. Geol. 1978, 21, 219–237. [Google Scholar] [CrossRef]
- Óskarsson, F.; Friðriksson, Þ.; Þorbjörnsson, D. Geochemical monitoring of the Reykjanes geothermal reservoir 2003 to 2013. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 16–24 April 2015. [Google Scholar]
- Jónsson, S.S.; Sigurgeirsson, M.Á.; Sigurðsson, Ó.; Ingólfsson, H. Reykjanes–Hola RN-15, 3. áfangi: Borun Vinnsluhluta frá 804 m í 2507 m Dýpi; ÍSOR-2010/050; Iceland GeoSurvey: Reykjavík, Iceland, 2010. [Google Scholar]
- Fridleifsson, G.Ó.; Elders, W.A.; Zierenberg, R.A.; Stefánsson, A.; Fowler, A.P.G.; Weisenberger, T.B.; Harðarson, B.S.; Mesfin, K.G. The Iceland Deep Drilling Project 4.5km deep well, IDDP-2, in the seawater-recharged Reykjanes geothermal field in SW Iceland has successfully reached its supercritical target. Sci. Drill. 2017, 23, 1–12. [Google Scholar] [CrossRef]
- Fridleifsson, G.Ó.; Elders, W.A.; Zierenberg, R.A.; Fowler, A.P.G.; Weisenberger, T.B.; Mesfin, K.G.; Sigurðsson, Ó.; Níelsson, S.; Einarsson, G.; Óskarsson, F.; et al. The Iceland Deep Drilling Project at Reykjanes: Drilling into the root zone of a black smoker analog. J. Volcanol. Geotherm. Res. 2020, 391, 106435. [Google Scholar] [CrossRef]
- Weisenberger, T.B.; Harðarson, B.S.; Kästner, F.; Gunnarsdóttir, S.H.; Tulinius, H.; Guðmundsdóttir, H.; Einarsson, G.M.; Pétursson, F.; Vilhjálmsson, S.; Stefánsson, H.Ö.; et al. Well Report–RN-15/IDDP-2, Drilling in Reykjanes–Phase 4 and 5; ÍSOR-2017/016; Iceland GeoSurvey: Reykjavík, Iceland, 2017. [Google Scholar]
- Haraldsdótt ir, S.H. Processing Lithological Well Logs from Svartensgi and Reykjanes; ÍSOR-2016/005; Iceland GeoSurvey: Reykjavík, Iceland, 2016. [Google Scholar]
- Christidis, G.E. Industrial clays. EMU Notes Miner. 2011, 9, 341–414. [Google Scholar] [CrossRef]
- Ammann, L.; Bergaya, F.; Lagalym, G. Determination of the cation exchange capacity of clays with copper complexes revisited. Clay Miner. 2005, 40, 441–453. [Google Scholar] [CrossRef]
- Bergaya, F.; Vayer, M. CEC of clays: Measurement by adsorption of a copper ethylenediamine complex. Appl. Clay Sci. 1997, 12, 275–280. [Google Scholar] [CrossRef]
- Meier, L.P.; Kahr, G. Determination of the Cation Exchange Capacity (CEC) of Clay Minerals Using the Complexes of Copper(II) Ion with Triethylenetetramine and Tetraethylenepentamine. Clays Clay Miner. 1999, 47, 386–388. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G.; Vayer, M. Chapter 12.10 Cation and Anion Exchange. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 979–1001. [Google Scholar] [CrossRef]
- Ciesielski, H.; Sterckeman, T. Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agronomie 1997, 17, 1–7. [Google Scholar] [CrossRef]
- Dohrmann, R.; Kaufhold, S. Three new, quick CEC methods for determining the amounts of exchangeable calcium cations in calcareous clays. Clays Clay Miner. 2009, 57, 338–352. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. Beyond the Methylene Blue Method: Determination of the Smectite Content using the Cutriene Method. Z. Angew. Geol. 2003, 49, 13–17. [Google Scholar]
- Steudel, A.; Weidler, P.; Schuhmann, R.; Emmerich, K. Cation exchange reactions of vermiculite with Cu-triethylenetetramine as affected by mechanical and chemical pretreatment. Clays Clay Miner. 2009, 57, 405–412. [Google Scholar] [CrossRef]
- Dohrmann, R.; Genske, D.; Karnland, O.; Kaufhold, S.; Kiviranta, L.; Olsson, S.; Plötze, M.; Sandén, T.; Sellin, P.; Svensson, D.; et al. Interlaboratory CEC and exchangeable cation study of betonite buffer materials: I. Cu (II)-triethylenetetramine method. Clays Clay Miner. 2012, 60, 162–175. [Google Scholar] [CrossRef]
- Kaufhold, S. Untersuchungen zur Eignung von Natürlich Alterierten Sowie mit Oxalsäure Aktivierten Bentoniten als Bleicherde für Pflanzenöle. Ph.D. Thesis, RWTH, Aachen, Germany, 2001. [Google Scholar]
- Kousehlar, M.; Weisenberger, T.B.; Tutti, F.; Mirnejad, H. Fluid control on low-temperature mineral formation in volcanic rocks of Kahrizak, Iran. Geofluids 2012, 12, 295–311. [Google Scholar] [CrossRef]
- Carroll, D. Ion exchange in clays and other minerals. Geol. Soc. Am. Bull. 1959, 70, 749–779. [Google Scholar] [CrossRef]
- Kristmannsdóttir, H.; Tómasson, J. Zeolites zones in geothermal areas in Iceland. In Natural Zeolites; Occurrence, Properties, Use; Sand, L.B., Mumpton, F.A., Eds.; Pergamon Press: New York, NY, USA, 1978; pp. 277–284. [Google Scholar]
- Neuhoff, P.S.; Fridriksson, T.; Bird, D.K. Zeolite parageneses in the North Atlantic Igneous Provinces: Implications for geotectonics and groundwater quality of basaltic crust. Int. Geol. Rev. 2000, 42, 15–44. [Google Scholar] [CrossRef]
- Weisenberger, T.; Bucher, K. Mass transfer and porosity evolution during low temperature water-rock interaction in gneisses of the Simano nappe: Arvigo, Val Calanca, Swiss Alps. Contrib. Miner. Petrol. 2011, 162, 61–81. [Google Scholar] [CrossRef]
- Weisenberger, T.; Spürgin, S. Zeolites in alkaline rocks of the Kaiserstuhl volcanic complex, SW Germany-new micropobe investigation and their relationship to the host rock. Geol. Belg. 2009, 12, 75–91. [Google Scholar]
- Schiffman, P.; Southard, R.J. Cation exchange capacity of layer silicates and palagonitized glass in mafic volcanic rocks; a comparative study of bulk extraction and in situ techniques. Clays Clay Miner. 1996, 44, 624–634. [Google Scholar] [CrossRef]
- Meunier, A. Clays; Springer: Berlin, Germany, 2005; p. 472. [Google Scholar]
- Dunoyer de Segonzac, G. Les mineraux argileux dans la diagenese. Passage au metamorphisme. Mém. Serv Carte Géol. Als. Lorr. 1969, 29, 1–320. [Google Scholar]
- Srodon, J. X-ray powder diffraction of randomly interstratified illite/smectite in mixtures with discrete illite. Clays Clay Miner. 1984, 16, 297–304. [Google Scholar] [CrossRef]
- Hower, J.; Mowatt, T.C. The mineralogy of illites and mixed-layer illite/montmorillonites. Am. Min. 1966, 51, 825–854. [Google Scholar]
- Hower, J.; Eslinger, E.V.; Hower, M.E.; Perry, E.A. Mechanism of burial metamorphism of argillaceous sediments: 1. Mineralogical and chemical evidence. Geol. Soc. Am. Bull. 1976, 87, 725–737. [Google Scholar] [CrossRef]
- Boles, J.R.; Franks, S.G. Clay diagenesis in Wilcox sandstones of Southwest Texas; implications of smectite diagenesis on sandstone cementation. J. Sediment. Res. 1979, 49, 55–70. [Google Scholar] [CrossRef]
- Srodon, J.; Eberl, D.D. Illite. Rev. Miner. Geochem. 1984, 13, 495–544. [Google Scholar]
- Flóvenz, Ó.G.; Karlsdóttir, R. TEM-resistivity image of a geothermal field in N-Iceland and the relation of the resistivity with lithology and temperature. In Proceedings of the World Geothermal Congress, Kyushu-Tohoku, Japan, 28 May–10 June 2000. [Google Scholar]
- Árnason, K.; Magnússon, I.Þ. Niðurstöður Viðnámsmælinga í Kröflu; Report OS-2001/062; Orkustofnun: Reykjavík, Iceland, 2001. [Google Scholar]
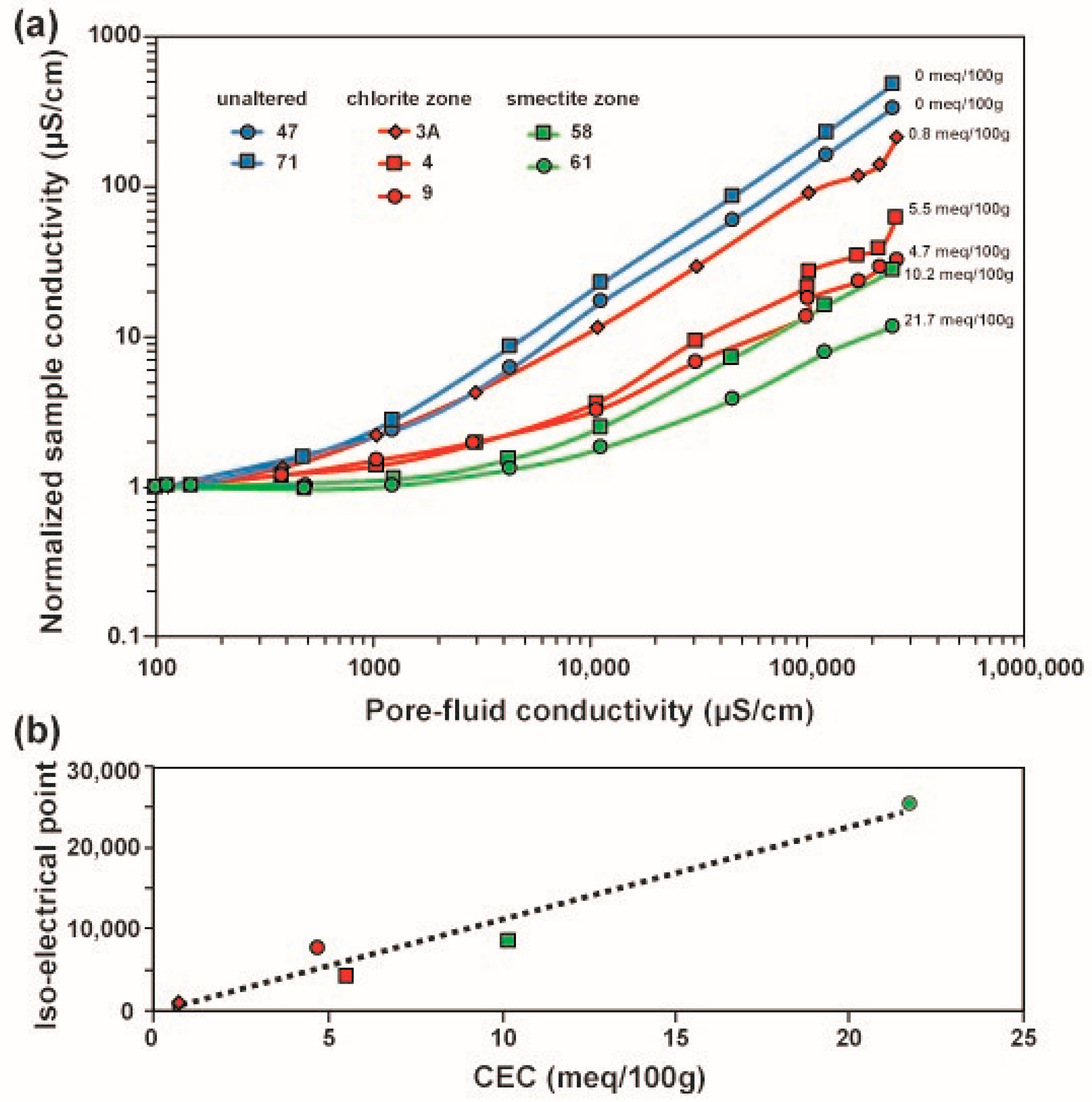
| Sample No | Mass (g) | Water Content (%) | CCu-trien (mol) | Absorbance (Blank) | Absorbance (Supernatant Solution) | CEC (meq/100 g) | Alteration Zone |
|---|---|---|---|---|---|---|---|
| 3a | 0.2004 | 2.05 | 0.0091 | 0.234 | 0.232 | 0.8 | chlorite |
| 4 | 0.2006 | 0.83 | 0.0091 | 0.234 | 0.220 | 5.5 | chlorite |
| 9 | 0.2000 | 0.63 | 0.0091 | 0.234 | 0.222 | 4.7 | chlorite |
| 47 | 0.2007 | 0.45 | 0.0091 | 0.234 | 0.235 | 0 | unaltered |
| 58 | 0.2006 | 1.25 | 0.0091 | 0.234 | 0.208 | 10.2 | smectite |
| 61 | 0.2005 | 3.26 | 0.0091 | 0.234 | 0.180 | 21.7 | smectite |
| 71 | 0.2007 | 0.06 | 0.0091 | 0.234 | 0.237 | 0 | unaltered |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weisenberger, T.B.; Ingimarsson, H.; Hersir, G.P.; Flóvenz, Ó.G. Cation-Exchange Capacity Distribution within Hydrothermal Systems and Its Relation to the Alteration Mineralogy and Electrical Resistivity. Energies 2020, 13, 5730. https://doi.org/10.3390/en13215730
Weisenberger TB, Ingimarsson H, Hersir GP, Flóvenz ÓG. Cation-Exchange Capacity Distribution within Hydrothermal Systems and Its Relation to the Alteration Mineralogy and Electrical Resistivity. Energies. 2020; 13(21):5730. https://doi.org/10.3390/en13215730
Chicago/Turabian StyleWeisenberger, Tobias Björn, Heimir Ingimarsson, Gylfi Páll Hersir, and Ólafur G. Flóvenz. 2020. "Cation-Exchange Capacity Distribution within Hydrothermal Systems and Its Relation to the Alteration Mineralogy and Electrical Resistivity" Energies 13, no. 21: 5730. https://doi.org/10.3390/en13215730
APA StyleWeisenberger, T. B., Ingimarsson, H., Hersir, G. P., & Flóvenz, Ó. G. (2020). Cation-Exchange Capacity Distribution within Hydrothermal Systems and Its Relation to the Alteration Mineralogy and Electrical Resistivity. Energies, 13(21), 5730. https://doi.org/10.3390/en13215730






