Abstract
Pretreatment to improve the enzymatic digestibility of highly crystallized lignocellulosic biomass is essential in biorefinery processes. This study investigates the combination of lignocellulose pretreatment with continuous alkaline single-screw extrusion and ultrasonication for biosugar production. Miscanthus sacchariflorus was used because it is a promising bioenergy crop. The results show that ultrasonication with continuous alkaline pretreatment increased the enzymatic digestibility of carbohydrates and reduced the use of chemicals during pretreatment. An hour of ultrasonication following 0.2 M NaOH (2.25 mol-NaOH/kg-biomass) continuous alkaline pretreatment resulted in a 6.7% increase in total biosugar production (83.1% of theoretical yield), a decrease of up to 26.1% in chemical usage, and a 17.0% increase in lignin removal compared with the case without ultrasonication. The developed method can be considered an effective and eco-friendly approach to the production of bio-based materials.
1. Introduction
Lignocellulosic biomass is the most abundant biomass available on earth, and is a source of value-added products that can be converted into fuels, power, hydrogen, and chemicals [1,2]. In this context, fermentable sugars (also known as biosugars) from non-edible lignocellulose have been of interest for several decades as platform chemicals that can replace or complement present fossil-based materials and fuels [3,4,5]. However, the annual production of bio-based plastics represents only 0.59% of the total global plastic production of 359 million tons [6,7]. Accordingly, bio-based fuel production accounts for only approximately 3.0% of the total fuel for road transportation globally [8]. Therefore, the development of biorefineries for the production of green materials and chemicals from renewable carbon sources is essential to address the gap between bio- and fossil-based fuels.
The biorefinery process for biosugar recovery from lignocellulose has three main operation steps: pretreatment, saccharification, and separation. The pretreatment step increases the accessibility of carbohydrates to biodegradation, and is the most energy-demanding and expensive process [9,10]. Despite being extensively studied, several pretreatment methods for the conversion of lignocellulosic biomass to biosugars are controversial because of their advantages and disadvantages [11]. For example, acid pretreatments are commonly used to increase the carbohydrate digestibility toward enzymatic saccharification. Although acids cannot separate lignin, they can produce inhibitory compounds and cause corrosion in the reactors. Hydrothermal and steam explosion methods can solubilize the hemicellulose fraction but do not decrease cellulose crystallinity. Among the various pretreatments, alkaline pretreatment with sodium hydroxide (NaOH) is the most efficient conventional method because it results in less sugar degradation and lower formation of inhibitors than acid and thermal pretreatments [12]. The alkaline pretreatment with NaOH used as a limited swelling agent results in the cleavage of intermolecular ester and C-C bonds in lignin molecules, thereby loosening the crystalline structure of cellulose, increasing the accessible amorphous regions of carbohydrates, disintegrating lignin, and facilitating enzyme penetration into the cellulose and hemicellulose [13]. In addition, alkaline pretreatment can be applied to selectively fractionate sulfur-free lignin from biomass. However, the difficulties of recovering and reducing the use of alkaline chemicals that cause environmental pollution and increase the amount of salts incorporated into carbohydrates have emerged as drawbacks that need to be overcome. In this context, ultrasonication treatment uses sound energy in the high-frequency range of 10 to 20 MHz to provide biomass with high physical energy by cavitation. As the ultrasonic energy spreads through the liquid phase, microbubbles containing solvent vapors are generated and migrate while growing. The collapse of bubbles in the biomass surface results in high temperature and pressure, and extremely strong shear forces, thus loosening or breaking down the carbohydrate-lignin matrix. Therefore, some studies have utilized ultrasonication pretreatment to reduce particle size, decrease crystallinity, and change the morphology of lignocellulosic biomass [14,15]. However, the drawbacks of ultrasonication pretreatment include low lignin removal efficiency and safety concerns.
In this work, to effectively increase biosugar production and reduce chemical dosage, the combination of continuous alkaline single-screw extrusion with ultrasonication for the pretreatment of Miscanthus sacchariflorus biomass was investigated. The enzymatic saccharification of biomass pretreated by different alkaline concentrations (NaOH) and ultrasonication times were separately conducted to determine their digestibility. The effects of combining ultrasonication and continuous alkaline pretreatment on chemical composition, sugar production, chemical consumption, and morphological changes were examined and are discussed.
2. Materials and Methods
2.1. Raw Materials
Miscanthus sacchariflorus strain Goedae 1 (hereafter, M. sacchariflorus G1) was cultivated and harvested in the Bioenergy Crop Research Institute, National Institute of Crop Science, Rural Development Administration (Muan Jeonnam, Korea) [16]. M. sacchariflorus G1 was sliced into chunks of approximately 5 cm using a cutting mill (Sechang Machine, Co., Ltd., Daegu, Korea). It was then finely ground and sieved to obtain 3 mm particles using a pulverizer (Korea Pulverizing Machinery Co., Ltd., Incheon, Korea). The moisture content of the pulverized biomass was 10.1 ± 0.5 wt.%. Approximately 300 kg of pulverized raw biomass was prepared and stored in bulk bags in a cool and dry place, out of direct sunlight, for future use.
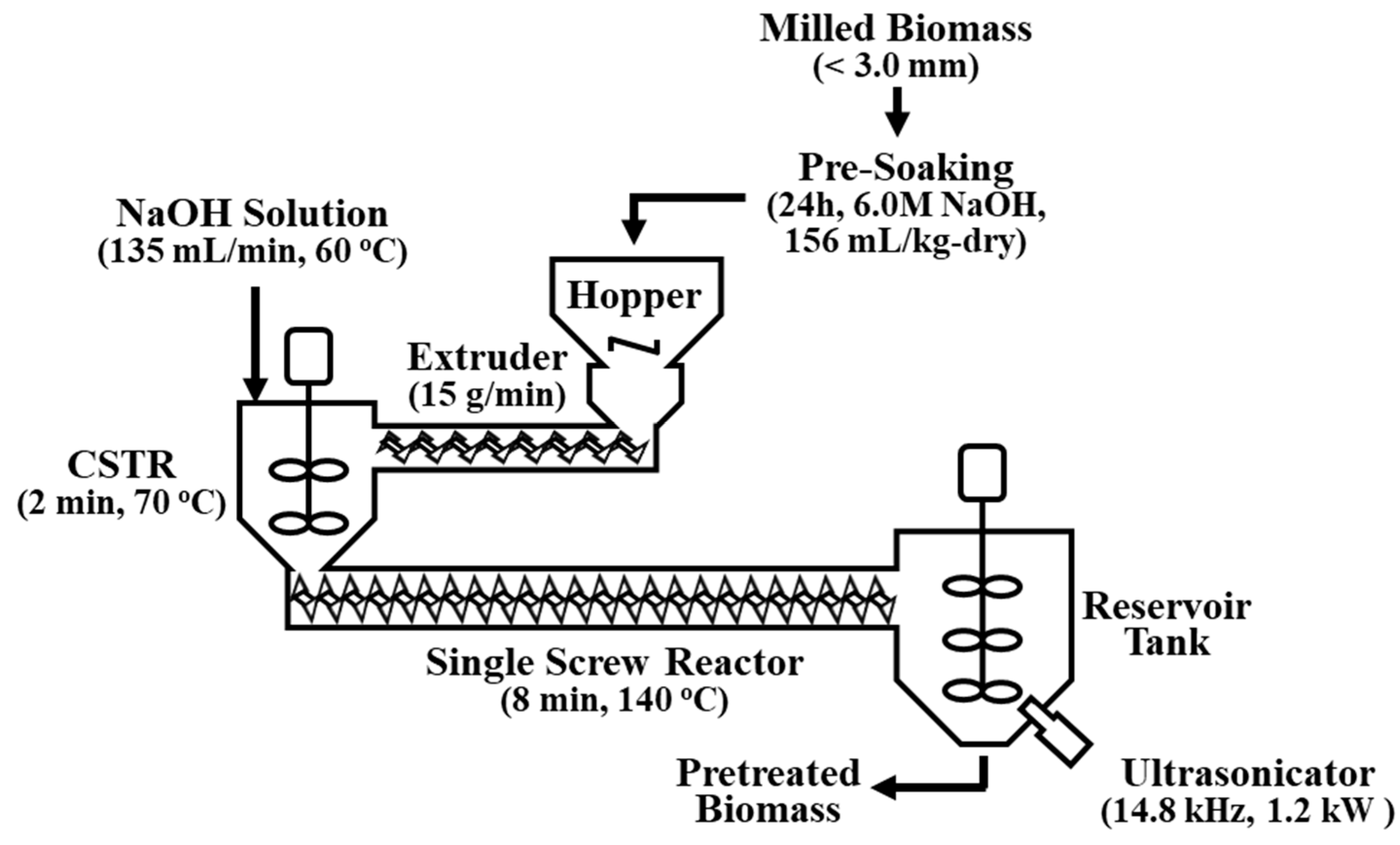
2.2. Alkaline Pretreatment by Using a Continuous Single-Screw Reactor
A continuous single-screw reactor fabricated in a previous study [17] was modified to combine the alkaline pretreatment with ultrasonication (Figure 1). The final structure consisted of seven components: hopper, screw extruder, solvent tank, continuous stirred tank reactor (CSTR), main single-screw reactor (plug flow reactor, PFR), reservoir tank, and ultrasonicator. Prior to the continuous alkaline pretreatment, a pre-soaking step was applied to achieve a more effective biomass pretreatment. Pre-soaking biomass is known to effectively increase the enzymatic digestibility of biomass because it allows adequate time for chemical and water molecules to penetrate the cell walls, thus softening the lignocellulose structure. The volume of the pre-soaking solution was 156 mL to maintain a 20.0% biomass moisture content, which was optimal for the extruder operation. The alkaline concentration of the pre-soaking solution was determined to be 6.0 M NaOH, which was equivalent to a reduction of approximately 0.1 M of alkaline concentration in the main continuous pretreatment process. The pulverized raw biomass was well-mixed with the pre-soaking solution and then kept in a sealed container at room temperature for 24 h. The presoaked M. sacchariflorus G1 was fed continuously to the CSTR through the extruder at a feeding rate of 15 g/min, thus generating compressive and shear stresses. Further alkaline soaking occurred for 2 min at 70 °C in the CSTR, where the extruded biomass was mixed with 60 °C pre-heated NaOH solution fed at a flow rate of 135 mL/min. The main alkaline pretreatment occurred in the PFR single-screw reactor for 8 min at 140 °C. The pretreatment time was set by regulating the screw rotating speed to 30 rpm, and the heat was supplied from a jacket around the reactor filled with hot oil. Alkaline concentrations of 0.1, 0.2, and 0.3 M NaOH solution were used in the alkaline pretreatment. The pretreated biomass was then collected in the reservoir tank in which the ultrasonication reaction occurred with the remaining heat.
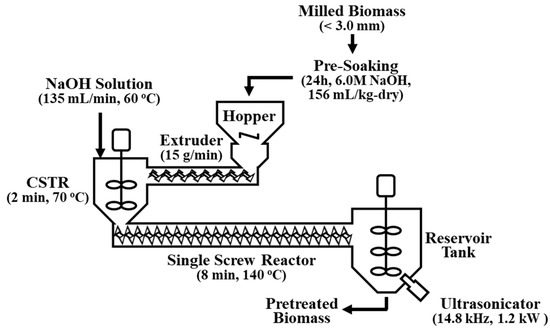
Figure 1.
Schematic of the ultrasonication-assisted single-screw alkaline pretreatment system modified from Cha et al. (2015) (CSTR: continuous stirred tank reactor).
2.3. Ultrasonic Reactor
The main aim of this work was to apply ultrasonication to alkaline-pretreated biomass to enhance its enzymatic digestibility by reducing the use of chemicals. The single-screw reactor based on extrusion pretreatment was modified by placing an ultrasonicator on the reservoir tank (Figure 1). The ultrasonication treatment was conducted using a titanium horn-type ultrasonicator (D60-45/170L, Daelim Ultrasonic Company, Seoul, Korea). As illustrated in Figure 1, an ultrasonic horn was built on the reservoir tank in which the pretreated biomass was collected. An external water-cooling loop was installed to prevent overheating. The operating frequency and power of the ultrasonicator were 14.8 ± 0.1 kHz and 1.2 kW, respectively. The amplitude was maintained at 100%. The pretreatment experiments with 0, 1, and 2 h ultrasonication (PCU-0h, PCU-1h, PCU-2h) were performed in triplicate. After the ultrasonication treatment, the samples were centrifuged at 10,000 rpm (15,344× g) for 20 min (Avanti J-E, Beckman Coulter, Brea, CA, USA) to obtain solids, which were then fully washed 6–7 times with water until the pH was 7–8. The solid residue was dried at 60 °C overnight and stored in a sealed container until use.
2.4. Analytical Methods
To determine the structural carbohydrates and lignin in the samples according to the NREL/TP-510-42618 guidelines issued by the National Renewable Energy Laboratory [18], the ground samples were passed through a 1.0 mm sieve and collected on a 0.5 mm sieve (Chunggye Sieve, Seoul, Korea). After two steps of acid hydrolysis, the sugar (glucose, xylose, and arabinose) concentration in the sample mixture was determined by high-performance liquid chromatography (HPLC) (e2695, Waters, Milford, MA, USA) using a Biorad Aminex HPX-87H 300 × 8.7 mm column (Bio-Rad, Hercules, CA, USA) equipped with a micro-guard Cation H 30 × 4.6 mm guard column. The column temperature was maintained at 65 °C inside a column heater module (WAT038040, Waters, Milford, MA, USA), and a refractive index detector (2414, Waters, Milford, MA, US) was used at 30 °C. In addition, 0.5 mM H2SO4 was used as the mobile phase at a flow rate of 0.5 mL/min. Peaks were quantified according to a calibration curve. The lignin content was determined by the sum of the acid-soluble and acid-insoluble lignin. The acid-soluble lignin content was measured at 205 nm using a UV-Visible spectrophotometer (Libra S22, Biochrom Ltd., Cambridge, UK), and the acid-insoluble lignin was determined by treating the residue filtered from acid hydrolysis solution at 575 °C for 3 h.
To determine the amount of dissolved material in the pretreated liquor, the pretreated biomass was centrifuged at 10,000 rpm (15,344× g) for 30 min and the supernatants passed through a 0.2 um syringe filter. Approximately 15.0 g aliquot of the filtered supernatant was dried in a conviction oven at 60 °C until a constant weight was reached. The weight ratio of oven-dried materials was noted as the dissolved material in the pretreated liquor.
Scanning electron microscopy (SEM) morphological analysis was conducted to further understand the impact of ultrasonication on the surface of the alkaline-pretreated biomass. SEM images were obtained using a Hitachi TM 1000 (Hitachi High-Tech Corp., Tokyo, Japan) tabletop microscope equipped with energy dispersive spectroscopy. The SEM images were taken at a magnification of ×1000 and ×5000 to demonstrate the microstructures of the biomass surface.
2.5. Enzymatic Saccharification
To evaluate the influence of the ultrasonication treatment on the alkaline pretreatment, the ultrasonicated and non-ultrasonicated samples were subjected to enzymatic saccharification according to the NREL/TP-5100-63351 guidelines issued by NREL [19]. Cellic® CTec2 and Cellic® HTec2 enzymes used in the saccharification were purchased from Novozymes (Bagsvaerd, Denmark). The filter paper units of Cellic® CTec2 were determined as 136 FPU/mL according to the NREL/TP-510-42628 [20]. The dried pretreated biomass sample equivalent to 3.0 wt.% glucan was placed in a 500 mL sterilized flask with 150 mL of 50 mM citrate buffer (pH 4.8). The enzyme loading was 30 FPU cellulase/g-glucan and 0.1/0.9 of hemicellulose/cellulase (mL/mL). The hydrolysis was conducted at 50 °C for 72 h in an incubator at 150 rpm. Aliquots of 1.0 mL were removed periodically and immediately boiled for 5 min to deactivate the enzyme. The supernatant was then obtained after centrifugation at 13,000 rpm (15,871× g) for 10 min and filtered through a 0.20 μm porosity syringe filter into a vial to be analyzed by HPLC as described earlier. Experiments were performed in duplicate. Mean values with standard deviation and p-values (t-test) were obtained.
3. Results and Discussions
3.1. Compositional Analysis for M. sacchariflorus G1
The chemical composition of the biomass can significantly affect its biosugar production efficiency. The composition of the raw M. sacchariflorus G1 biomass presented in Table 1 is similar to that noted in a previous study [17]; it contained 40.3 ± 0.55 wt.% glucan, 24.1 ± 0.07 wt.% hemicellulose (xylan and arabinan), and 24.1 ± 0.02 wt.% lignin. The slight difference between the studies was attributed to the different cultivation years. M. sacchariflorus, commonly known as elephant grass, is of interest in the biorefinery industry as a perennial non-edible raw biomass owing to its high carbohydrate content, high productivity, and active growth rate, even on marginal lands in transitional temperate climates [21,22,23,24,25]. In other recent studies, M. sacchariflorus has different compositions. For instance, samples cultivated in west-central Poland were composed of 43–45% cellulose, 31% hemicellulose, and 20–22% lignin [26]. Native M. sacchariflorus harvested in west China was reported to consist of 38.2% cellulose, 31.0% hemicellulose, and 20.3% lignin [27]. Composition analyses confirm that these slight differences for the same M. sacchariflorus genotype may be attributed to soil conditions, temperature fluctuations, precipitation levels, and other regional characteristics.

Table 1.
Compositional analysis of alkaline-ultrasonic pretreated M. sacchariflorus G1 (wt.%).
3.2. Alkaline Pretreatment of M. sacchariflorus G1 in the Continuous Single-Screw Reactor
The analysis of alkaline pretreatments revealed that the large fraction of lignin decreased by up to 8.34 ± 1.02 wt.%, as the NaOH concentration increased to 0.3 M (Table 1). As the lignin was removed, the glucan content in the pretreated solid increased, but the change in hemicellulose (xylan and arabinan) content was negligible. This occurred because a small fraction of the hemicellulose is also solubilized as pentose oligomers under alkaline pretreatment conditions [28]. Compared with Cha et al. [17], the glucan content (59.58 ± 1.03 wt.%, Table 1) after 0.3 M NaOH pretreatment with the pre-soaking step was similar to that after 0.4 M NaOH pretreatment without the pre-soaking step, whereas the hemicellulose content increased by an average of approximately 2.84%. In Cha et al. [17] the pre-soaking step was not employed, and at alkaline concentrations of 0.4 M NaOH or higher, the amount of lignin in the pretreated solids no longer decreased and the hemicellulose content began to decrease. Thus, the total NaOH dosage in this study was determined to be less than 0.4 M NaOH, which was used for pretreatment without the pre-soaking step. For the pretreatments of 0.3 M NaOH with 6.0 M NaOH pre-soaking and 0.4 M NaOH without pre-soaking, 4.31 and 4.50 moles of NaOH per kg of dry biomass were used, respectively. The results indicate that the additional dosage of NaOH in the pre-soaking step can be offset by the reduced NaOH concentration required in the main pretreatment step.
3.3. Combination of Ultrasonication with Continuous Alkaline Single-Screw Pretreatment of M. sacchariflorus G1
3.3.1. Compositional Changes in the Pretreated Solid
The results shown in Table 1 confirm an increasing trend in the delignification as the ultrasonication time increased (p-value = 0.004). The 0.2 M NaOH PCU-1h reduced the lignin content from 23.10 ± 0.38 to 8.26 ± 0.03 wt.%. Compared to PCU-0h, it increased lignin removal by 17.0%. Therefore, the ultrasonication generated cellulose-rich materials that have high biosugar concentration. No significant change in the hemicellulose (xylose + arabinose) content occurred after 1 h ultrasonication (p-value = 0.469). For example, under 0.2 M NaOH pretreatment, 1 h ultrasonication decreased the hemicellulose content by 0.69%. However, a decrease in the hemicellulose content was observed when the ultrasonication time increased to 2 h (p = 0.009). These results confirm that there is a threshold level for the ultrasonication time to rapidly increase the degree of hemicellulose loss. Moreover, the glucan content in the pretreated solid significantly increased as the ultrasonication time increased (p-value = 0.021).
Han et al. [29] used approximately 4.91 moles of NaOH per kg of dry biomass (barley straw) to obtain an optimal glucan yield of approximately 53.0 wt.% by a continuous twin-screw alkaline pretreatment, which is similar to the process in the present study. Accordingly, approximately 58.38 ± 0.20 wt.% of glucan in the pretreated solid was obtained through the 0.3 M NaOH PCU-1h process, in which the NaOH dosage was reduced to 3.18 moles of NaOH per kg of dry biomass. The comparative results indicate that the application of ultrasonication in the continuous alkaline pretreatment process is effective for carbohydrate recovery and lignin removal with lower chemical usage.
3.3.2. Dissolved Materials in Pretreated Liquor
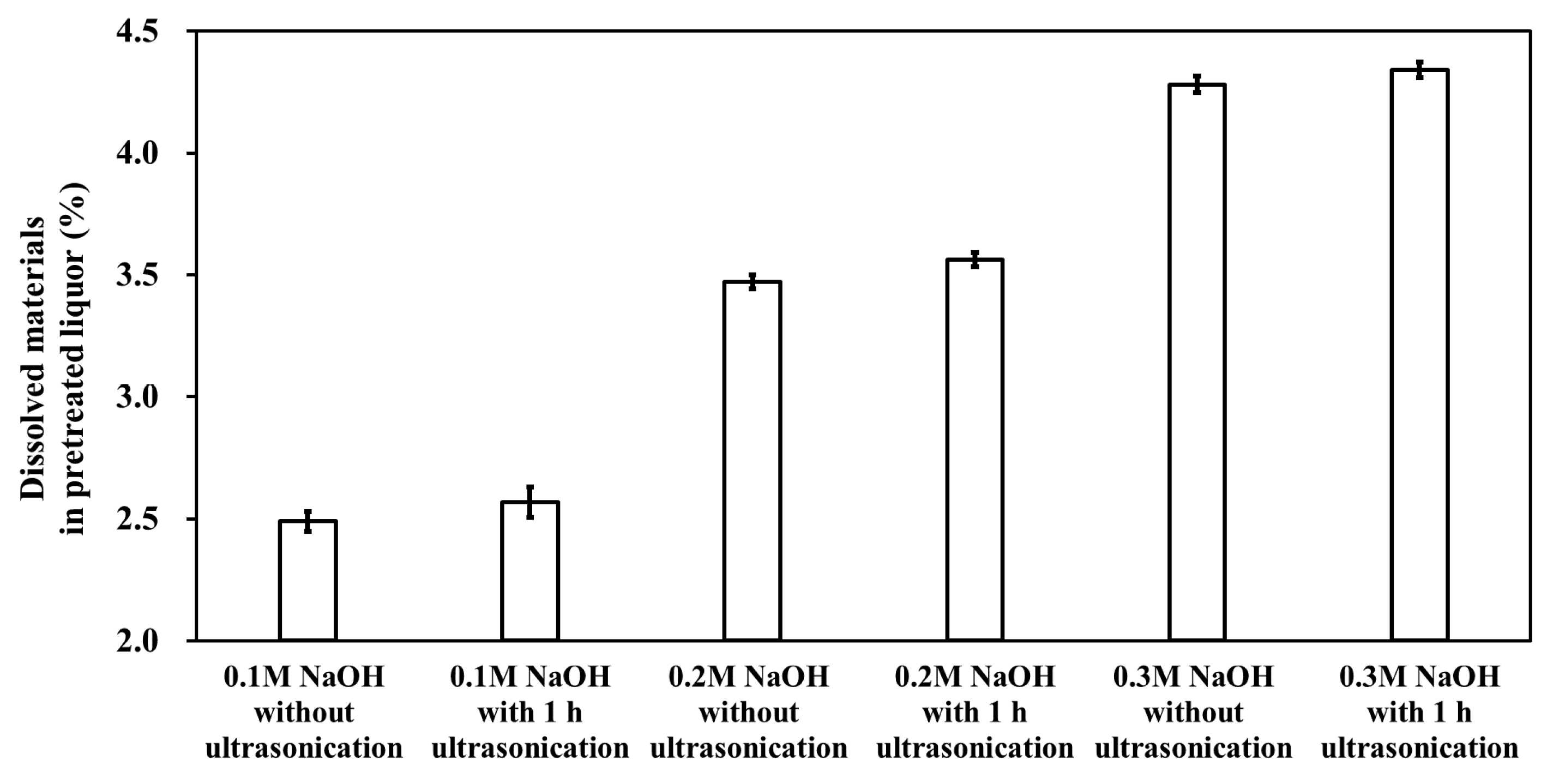
The dissolved materials in the liquid fraction of the pretreated biomass include lignin and oligo-, di-, and mono-saccharides. The extent of biomass dissolution due to ultrasonication was examined. An increasing trend in the amount of dissolved materials in the pretreated liquor was observed when the ultrasonication treatment was applied (Figure 2). The incremental increase in the dissolved material of the liquid fraction was linearly correlated with the NaOH dose. One hour of ultrasonication caused a 3.22, 2.62, and 1.41% increase in the level of dissolved material at 0.1, 0.2, and 0.3 M NaOH biomass pretreatment, respectively. This result indicates that the increasing extent of the dissolved materials was more significant as the pretreatment concentration decreased. However, this tendency was not correlated with the changes in the lignin content of the pretreated solids. For example, under 1 h ultrasonication, the greatest extent of lignin decrease was observed at 0.2 M NaOH PCU (Table 1), whereas the extent of dissolved materials was the highest for 0.1 M NaOH PCU. Although additional lignin disintegration and subsequent delignification occurred during ultrasonication, the dissolution of lignin into the pretreated liquor did not occur.
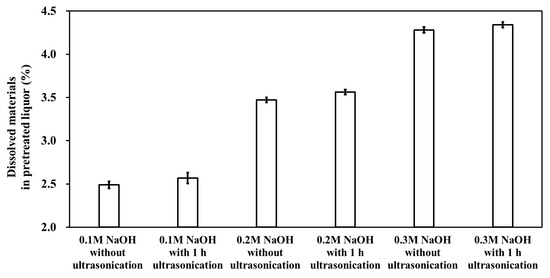
Figure 2.
Dissolved materials in the pretreated liquor of M. sacchariflorus G1.
The additional HPLC analysis of the supernatant in the samples revealed that no inhibitory product chemicals were detected. The formation of inhibitory products such as lactic acid, acetic acid, succinic acid, levulinic acid, glycerol, furfural, and 5-hydroxymethylfural (HMF) can hamper enzymatic saccharification and microbial fermentation [30,31,32]. Consistent with the results reported by Jönsson and Martín [33], the alkaline pretreatment in this study led to a negligible formation of inhibitory compounds. Furthermore, no inhibitory compounds were observed even after ultrasonication. This result also confirms that ultrasonication as a mechanical treatment does not directly induce chemical reactions that produce inhibitors.
3.3.3. Scanning Electron Microscopy (SEM) Analysis
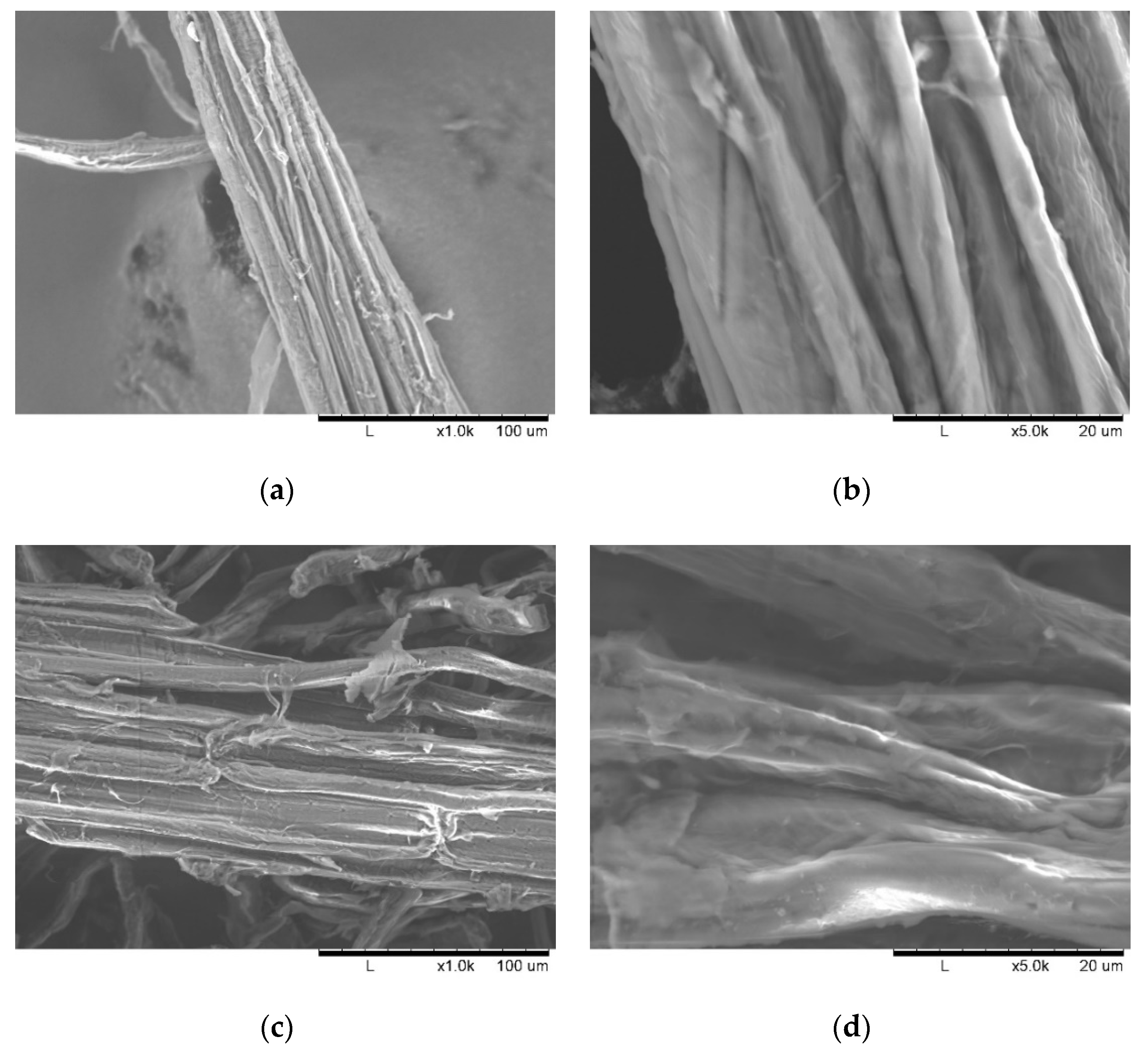
As illustrated in Figure 3, the fibrous residues in the pretreated biomass became untied and untangled, resulting in single or bundled short fibers. The SEM images show differences in the morphology of the plant cell wall surfaces before and after the ultrasonication. The images show that the morphology of the PCU-1h samples (Figure 3c) is more heterogeneous, consisting of cracks and torsions, whereas the PCU-0h samples (Figure 3a) are more homogeneous and uniform. However, the formation of pores on the biomass fiber structure was not observed. Figure 3d shows that the peeling of the layers and disruption of the fiber bundles occurred mainly because of the collapse of microbubbles in the biomass surface. The reduction of biomass crystallinity, mainly due to ultrasonication, was expected to increase the accessibility of enzymes to the biomass, thus resulting in higher hydrolysis efficiency in the further enzymatic saccharification step.
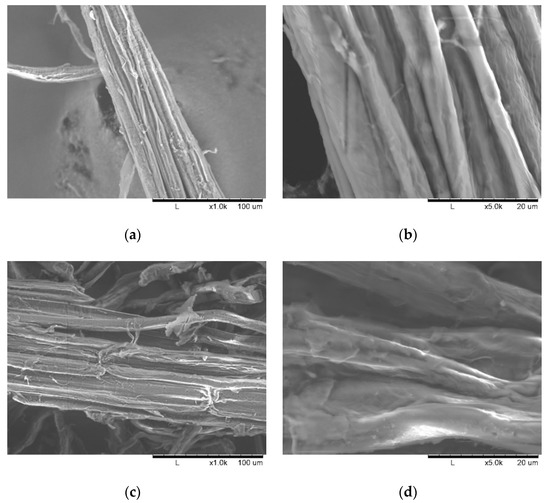
Figure 3.
SEM images of M. sacchariflorus G1 pretreated with 0.3 M NaOH at magnification: (a) ×1000 and (b) ×5000 without ultrasonication treatment; and at magnification: (c) ×1000 and (d) ×5000 with 1 h ultrasonication treatment.
3.4. Enzymatic Saccharification
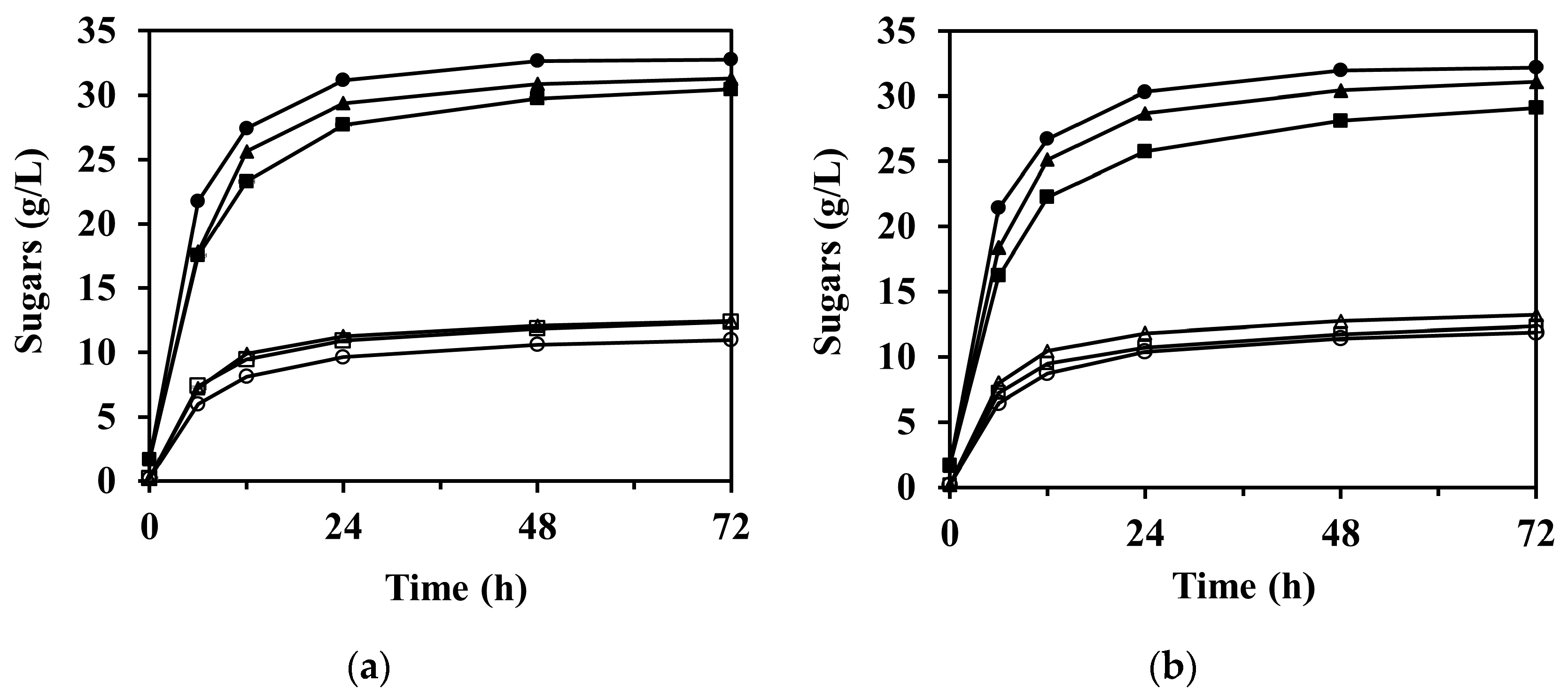
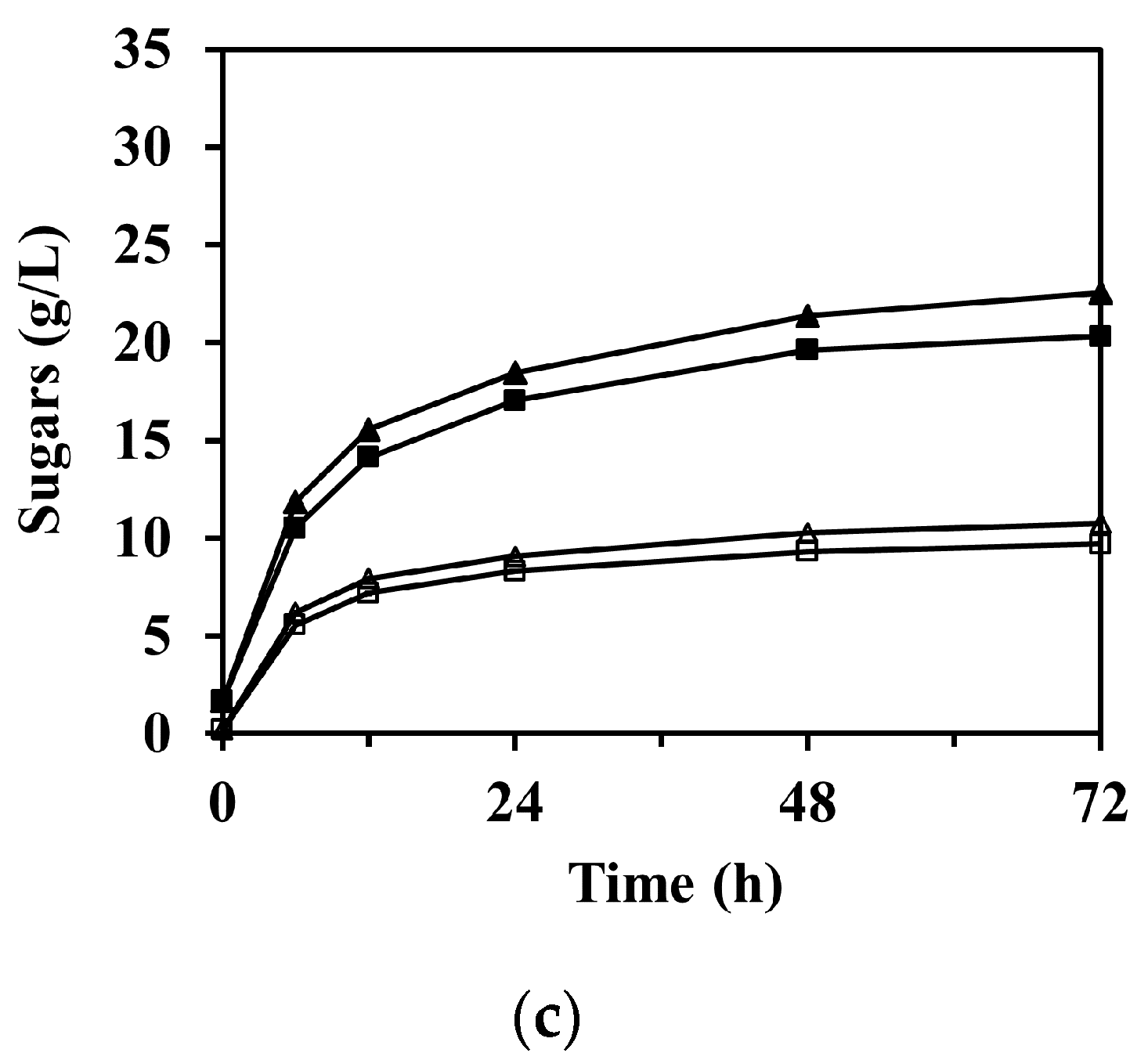
The sugar concentration through the enzymatic saccharification according to reaction time is shown in Figure 4, in which the concentration of arabinose is not shown due to its relatively low concentration compared to glucose and xylose. As expected, the formation of sugars was significantly faster at the beginning of the reaction and then gradually began to stabilize after 12 h in all experimental cases.
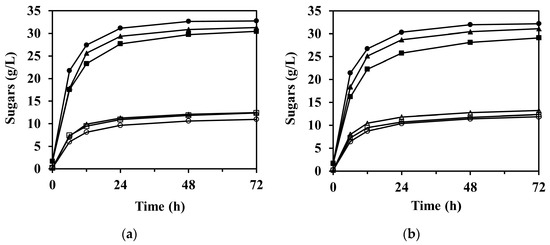
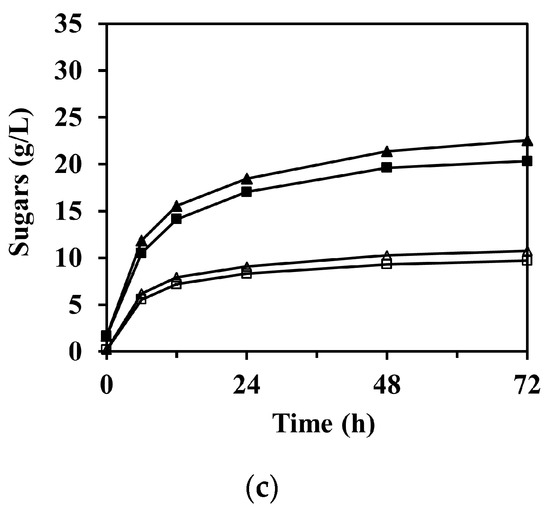
Figure 4.
Enzymatic saccharification of M. sacchariflorus G1 pretreated with (a) 0.3 M, (b) 0.2 M, and (c) 0.1 M NaOH, with/without ultrasonication treatment. Symbols: glucose (filled) and xylose (blank); 0 h (square), 1 h (triangle), and 2 h (circle) ultrasonication. Results are the means of duplicate experiments (standard deviations <0.25 g/L).
3.4.1. Glucose Production
The enzymatic saccharification of PCU-1h and PCU-2h substrates significantly increased the concentration of glucose in the hydrolysate compared to that of PCU-0h (p < 0.001). For PCU-1h, the glucose concentration after 72 h of enzymatic saccharification reached 22.55 ± 0.11, 31.09 ± 0.01, and 31.29 ± 0.14 g/L for 0.1, 0.2, and 0.3 M NaOH, whereas those for PCU-0h were 20.33 ± 0.09, 29.11 ± 0.23, and 30.46 ± 0.13 g/L, respectively (Figure 4). This increasing trend was attributed mostly to the high enzyme accessibility on the cellulose surface, which was damaged by the cavitation energy of the ultrasonication. This increasing trend of glucose concentration was still predominant for PCU-2h. The highest concentration of glucose (32.76 ± 0.06 g/L, 93.2% of theoretical yield) was obtained after 72 h of enzyme hydrolysis loading for the 0.3 M NaOH PCU-2h substrate, which closely reached the theoretical maximum and represented 3.0 wt.% of glucan loading. Thus, the effectiveness of glucose production in the enzymatic saccharification appeared to increase with increasing ultrasonication time. However, the effect of alkaline concentration increases from 0.2 to 0.3 M NaOH on the concentration of glucose after 72 h of enzymatic saccharification (p-value = 0.174) was insignificant. Moreover, the glucose concentration (31.09 ± 0.01 g/L) for 0.2 M NaOH PCU-1h was similar to that for 0.3 M NaOH PCU-0h (30.46 ± 0.13 g/L). In these cases, the amount of NaOH added in the 0.2 and 0.3 M NaOH pretreatment processes was 172.3 and 127.3 g/kg-dry biomass, respectively. This glucose trend was also observed for 0.2 M NaOH PCU-2h (32.20 ± 0.09 g/L) and 0.3 M NaOH PCU-1h (31.29 ± 0.14 g/L). Therefore, this analysis shows that ultrasonication can compensate for a reduction in the amount of used chemicals by up to 26.1% in the alkaline pretreatment process.
3.4.2. Xylose Production
Unlike glucose, which increased in all experimental cases, there was no significant difference in the concentration of xylose in the enzymatic saccharification of the substrates between 0 and 1 h ultrasonication under 0.3 M NaOH pretreatment (Figure 4a, p-value = 0.099). However, in the 0.1 and 0.2 M NaOH pretreatments (Figure 4b and Figure 3c), 1 h ultrasonication resulted in a slight increase in the xylose concentrations (p-value = 0.001). For PCU-2h, the xylose concentrations were even lower than those for PCU-0h and PCU-1h. For instance, the xylose concentration after 72 h of enzyme saccharification for 0.2 M NaOH PCU-2h was 11.87 ± 0.03 g/L, and was 12.34 ± 0.08 and 13.21 ± 0.01 g/L for PCU-0h and PCU-1h, respectively. Under ultrasonication of 2 h or longer and/or NaOH concentration of 0.3 M or higher, the ultrasonication treatment negatively affected the xylose concentration. This may be attributed to the increased hemicellulose loss, which caused the difference in the initial amount of hemicellulose loading into the reaction mixture for enzymatic saccharification. As mentioned earlier, the solid substrate loading on enzymatic saccharification was based on 3.0 wt.% of glucan. Thus, the amount of hemicellulose loading was unavoidably different for each reaction mixture. For example, based on 3.0 wt.% of glucan loading, the hemicellulose loadings into the reaction mixture were 1.53 and 1.40 wt.% for the 0.2 and 0.3 M NaOH PCU-1h substrates, respectively. This difference could theoretically be converted into xylose to a maximum concentration of ~1.44 g/L. Consequently, the lowered xylose concentration in the enzyme hydrolysate was attributed to the loss of hemicellulose under harsh pretreatment conditions.
3.4.3. Total Biosugars
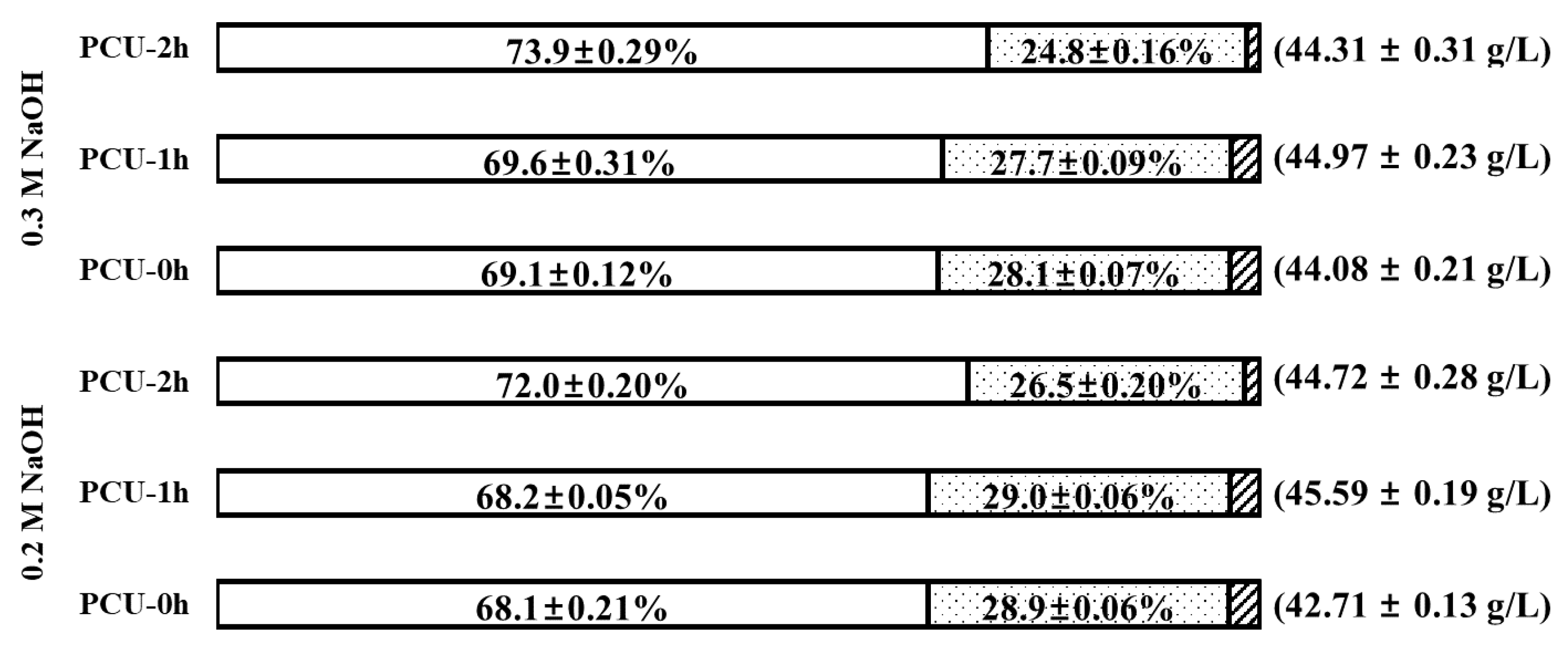
After 72 h of enzyme saccharification, the percent distribution of individual sugars (glucose, xylose, and arabinose) compared to the total biosugar was examined (Figure 5). The goal of this analysis was to examine the effect of ultrasonication on the distribution of individual sugar components. In both PCU-0h and PCU-1h, the percent contribution of individual sugar was insignificant (p-value = 0.777). However, distinct changes in the distribution of individual sugars were observed after 2 h of ultrasonication. This behavior can also be attributed to the loss of hemicellulose due to its disintegration at relatively long ultrasonication times, which led to low concentrations of xylose and arabinose in the enzyme hydrolysate. Finally, for this reason, even though the glucose concentration was the highest at 0.3 M NaOH PCU-2h, the highest amount of total biosugar (45.59 ± 0.19 g/L, 83.1% of theoretical yield) was obtained for 0.2 M NaOH PCU-1h, in which a 26.1% lower NaOH usage and 1 h shorter ultrasonication time was applied. The yield of total biosugar increased by 6.7% compared to the 0.2 M NaOH PCU-0h (42.71 ± 0.13 g/L, 77.7% yield).
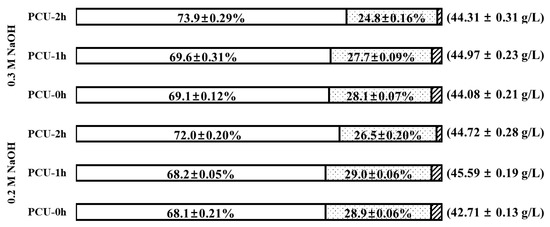
Figure 5.
Percent distribution of individual sugars (glucose, xylose, and arabinose) and total sugar after 72 h enzymatic saccharification for the pretreatment combined with ultrasonication (PCU). The numbers in parentheses indicate the concentration of total sugars. Symbols: glucose (blank), xylose (dots), and arabinose (diagonal).
4. Conclusions
The combination of ultrasonication and continuous alkaline single-screw extrusion pretreatment of M. sacchariflorus lignocellulose was investigated. This process was proven effective by compositional, SEM, and enzymatic saccharification analyses. The developed process resulted in a 6.7% increase in biosugar production and a 17.0% increase in lignin removal. The ultrasonication compensated for the reduction of chemical use in the continuous alkaline pretreatment process by up to 26.1%. The reduction of biomass crystallinity by ultrasonication is considered to increase the accessibility of enzymes to the biomass, resulting in higher hydrolysis efficiency in the subsequent enzymatic saccharification step. Thus, the developed treatment is environmentally friendly because it can reduce the dependence on harsh chemicals, thus contributing to the sustainability of large-scale biorefineries for chemical production.
Author Contributions
Conceptualization, J.B. and Y.-L.C.; methodology, J.B. and Y.-L.C.; software, J.B.; validation, J.B. and Y.-L.C.; formal analysis, J.B. and S.-M.P.; investigation, K.-S.K. and J.-E.L.; resources, Y.-L.C.; data curation, J.B. and S.-M.P.; writing—original draft preparation, J.B.; writing—review and editing, J.B. and Y.-L.C.; visualization, J.B. and S.-M.P.; supervision, Y.-L.C.; project administration, Y.-G.K.; funding acquisition, Y.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ012575012020), Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis Goes Bio: From Carbohydrates and Sugar Alcohols to Platform Chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Wilkins, M.; Widmer, W.W.; Grohmann, K.; Cameron, R. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour. Technol. 2007, 98, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nat. Cell Biol. 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Quintero, R.F.C.; Rico-Cruz, C.J.; Stansfield, K.E.; Colmenares, J.C. Assessment of biofuels production in Colombia. Cogent Eng. 2020, 7, 1740041. [Google Scholar] [CrossRef]
- Ullah, K.; Sharma, V.K.; Ahmad, M.; Lv, P.; Krahl, J.; Wang, Z. Sofia The insight views of advanced technologies and its application in bio-origin fuel synthesis from lignocellulose biomasses waste, a review. Renew. Sustain. Energy Rev. 2018, 82, 3992–4008. [Google Scholar] [CrossRef]
- Vergara, P.; Garcia-Ochoa, F.; Ladero, M.; Gutiérrez, S.; Villar, J.C. Liquor re-use strategy in lignocellulosic biomass fractionation with ethanol-water mixtures. Bioresour. Technol. 2019, 280, 396–403. [Google Scholar] [CrossRef]
- Gu, T.; Held, M.A.; Faik, A. Supercritical CO2 and ionic liquids for the pretreatment of lignocellulosic biomass in bioethanol production. Environ. Technol. 2013, 34, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Dien, B.S.; Ferrer, B.; Hespell, R.B.; Dale, B.E.; Ingram, L.O.; Bothast, R.J. Ethanol production from AFEX pretreated corn fiber by recombinant bacteria. Biotechnol. Lett. 1996, 18, 985–990. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Ultrasonic and high-temperature pretreatment, enzymatic hydrolysis and fermentation of lignocellulosic sweet sorghum to bio-ethanol. Int. J. Ambient. Energy 2012, 33, 152–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Benoit, M.; Vigier, K.D.O.; Barrault, J.; Jégou, G.; Philippe, M.; Jérôme, F. Pretreatment of microcrystalline cellulose by ultrasounds: Effect of particle size in the heterogeneously-catalyzed hydrolysis of cellulose to glucose. Green Chem. 2013, 15, 963–969. [Google Scholar] [CrossRef]
- Moon, Y.; Koo, B.; Choi, Y.; Ahn, S.; Bark, S.; Cha, Y.; An, G.; Kim, J.; Suh, S. Development of ‘‘Miscanthus’’ the promising bioenergy crop. Korean J. Weed Sci. 2010, 30, 330–339. [Google Scholar] [CrossRef]
- Cha, Y.-L.; Yang, J.; Park, Y.; An, G.H.; Ahn, J.-W.; Moon, Y.-H.; Yoon, Y.-M.; Yu, G.-D.; Choi, I.-H. Continuous alkaline pretreatment of Miscanthus sacchariflorus using a bench-scale single screw reactor. Bioresour. Technol. 2015, 181, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—Laboratory Analytical Procedure (LAP), NREL/TP-510-42618. 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 16 October 2020).
- Resch, M.; Baker, J.; Decker, S. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass—Laboratory Analytical Procedure (LAP), NREL/TP-5100-63351. 2015. Available online: https://www.nrel.gov/docs/fy15osti/63351.pdf (accessed on 16 October 2020).
- Adney, B.; Baker, J. Measurement of Cellulase Activities—Laboratory Analytical Procedure (LAP), NREL/TP-510-42628. 1996. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 16 October 2020).
- Ben Fradj, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European bio-economy: A network analysis. Ind. Crop. Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef]
- Sharma, B.; Brandt, C.; McCullough-Amal, D.; Langholtz, M.; Webb, E. Assessment of the feedstock supply for siting single- and multiple-feedstock biorefineries in the USA and identification of prevalent feedstocks. Biofuels Bioprod. Biorefining 2020, 14, 578–593. [Google Scholar] [CrossRef]
- Shepherd, A.; Clifton-Brown, J.; Kam, J.; Buckby, S.; Hastings, A. Commercial experience with miscanthus crops: Establishment, yields and environmental observations. GCB Bioenergy 2020, 12, 510–523. [Google Scholar] [CrossRef]
- Shepherd, A.; Littleton, E.; Clifton-Brown, J.; Martin, M.; Hastings, A. Projections of global and UK bioenergy potential from Miscanthus × giganteus-Feedstock yield, carbon cycling and electricity generation in the 21st century. GCB Bioenergy 2020, 12, 287–305. [Google Scholar] [CrossRef]
- Cerazy-Waliszewska, J.; Jeżowski, S.; Łysakowski, P.; Waliszewska, B.; Zborowska, M.; Sobańska, K.; Ślusarkiewicz-Jarzina, A.; Białas, W.; Pniewski, T. Potential of bioethanol production from biomass of various Miscanthus genotypes cultivated in three-year plantations in west-central Poland. Ind. Crop. Prod. 2019, 141, 111790. [Google Scholar] [CrossRef]
- Xue, Y.; Li, Q.; Gu, Y.; Yu, H.; Zhang, Y.; Zhou, X. Improving biodegradability and biogas production of miscanthus using a combination of hydrothermal and alkaline pretreatment. Ind. Crop. Prod. 2020, 144, 111985. [Google Scholar] [CrossRef]
- MacDonald, D.G.; Bakhshi, N.N.; Mathews, J.F.; Roychowdhury, A.; Bajpai, P.; Moo-Young, M. Alkali treatment of corn stover to improve sugar production by enzymatic hydrolysis. Biotechnol. Bioeng. 1983, 25, 2067–2076. [Google Scholar] [CrossRef]
- Han, M.; Kang, K.E.; Kim, Y.; Choi, G.-W. High efficiency bioethanol production from barley straw using a continuous pretreatment reactor. Process. Biochem. 2013, 48, 488–495. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef]
- Wang, X.; Jin, M.; Balan, V.; Jones, A.D.; Li, X.; Li, B.-Z.; Dale, B.; Yuan, Y. Comparative metabolic profiling revealed limitations in xylose-fermenting yeast during co-fermentation of glucose and xylose in the presence of inhibitors. Biotechnol. Bioeng. 2013, 111, 152–164. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).