Energy Harvesting by Waste Acid/Base Neutralization via Bipolar Membrane Reverse Electrodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ion Exchange Membranes
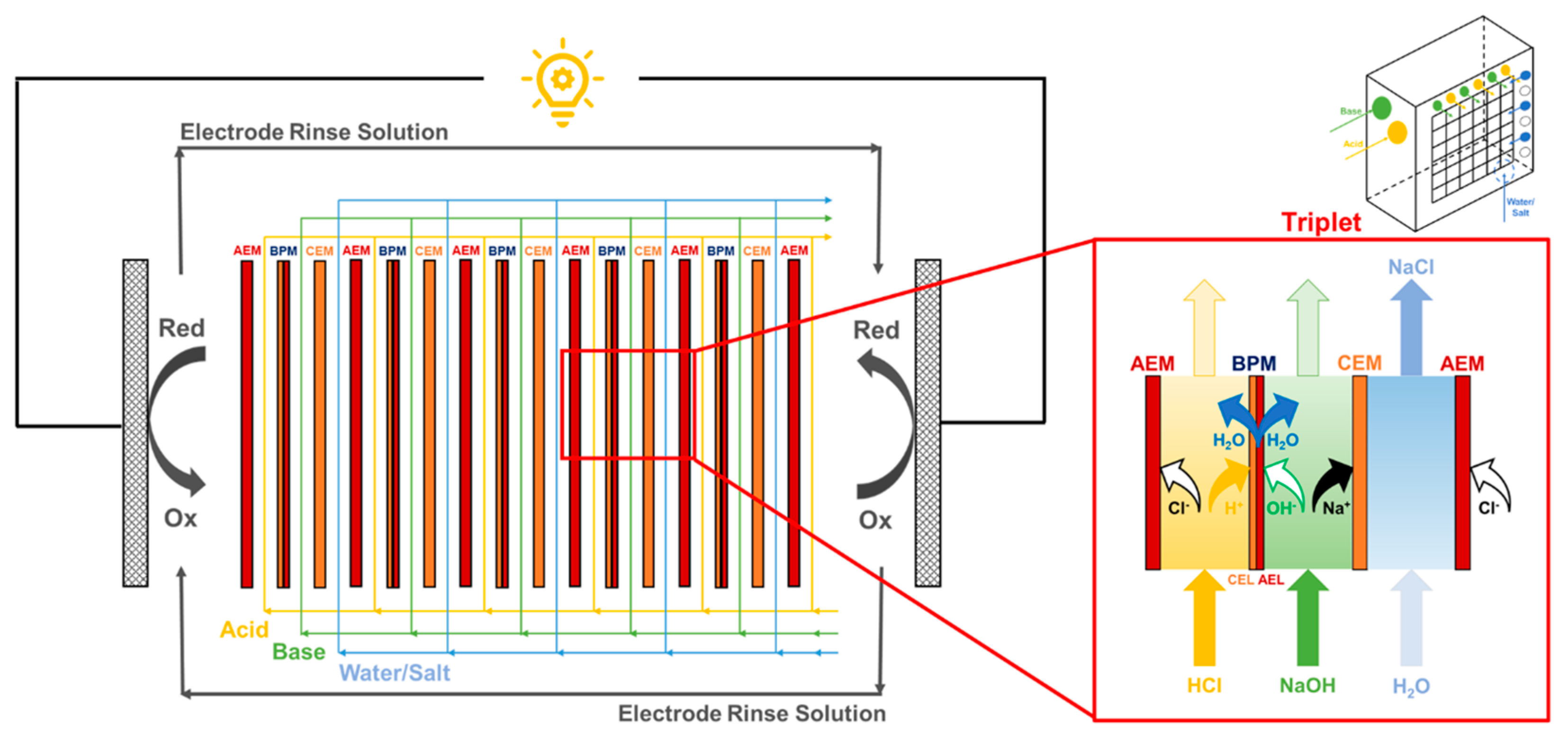
2.2. Stack and Solutions Characteristics
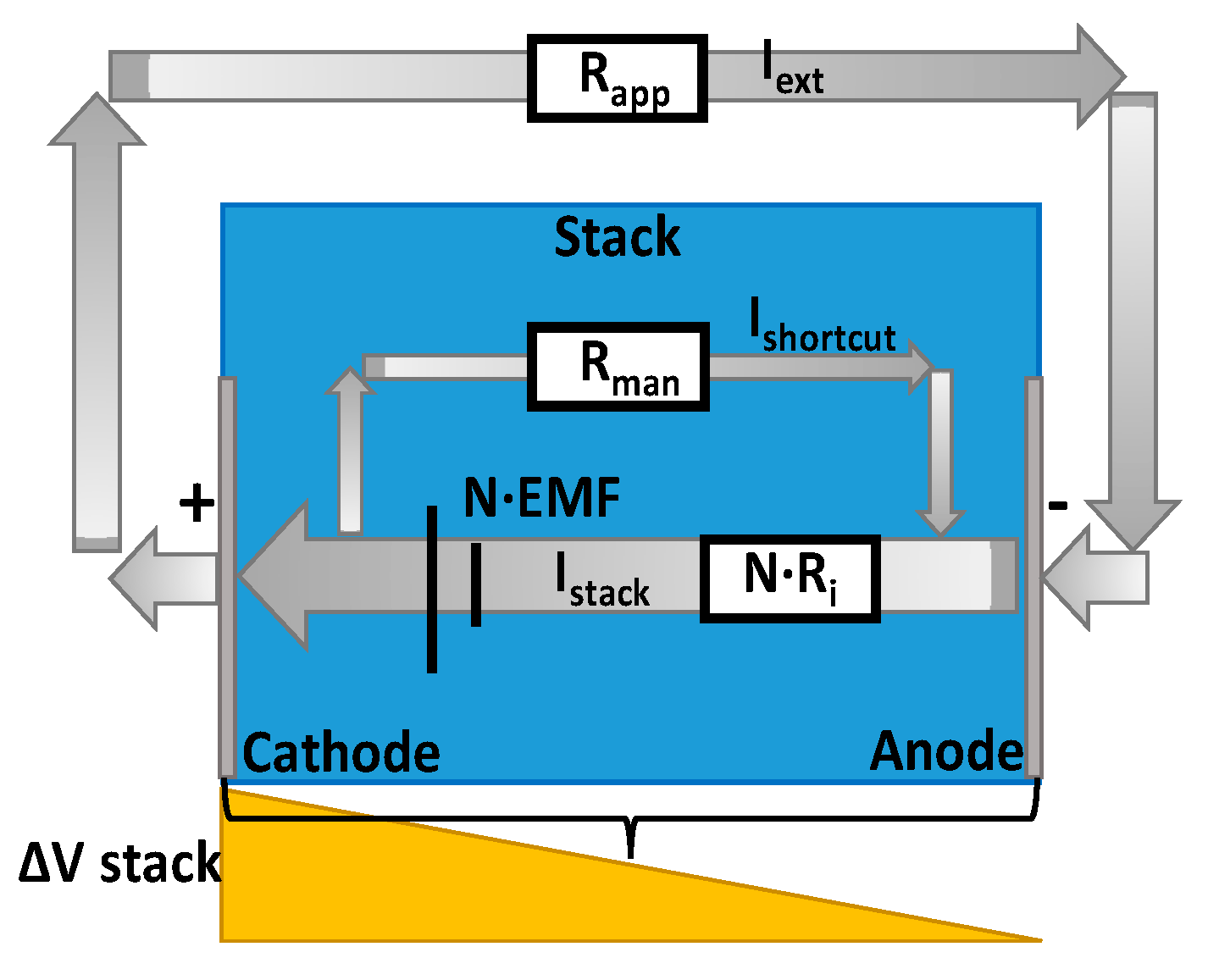
2.3. BMRED Module Characterization
3. Results and Discussion
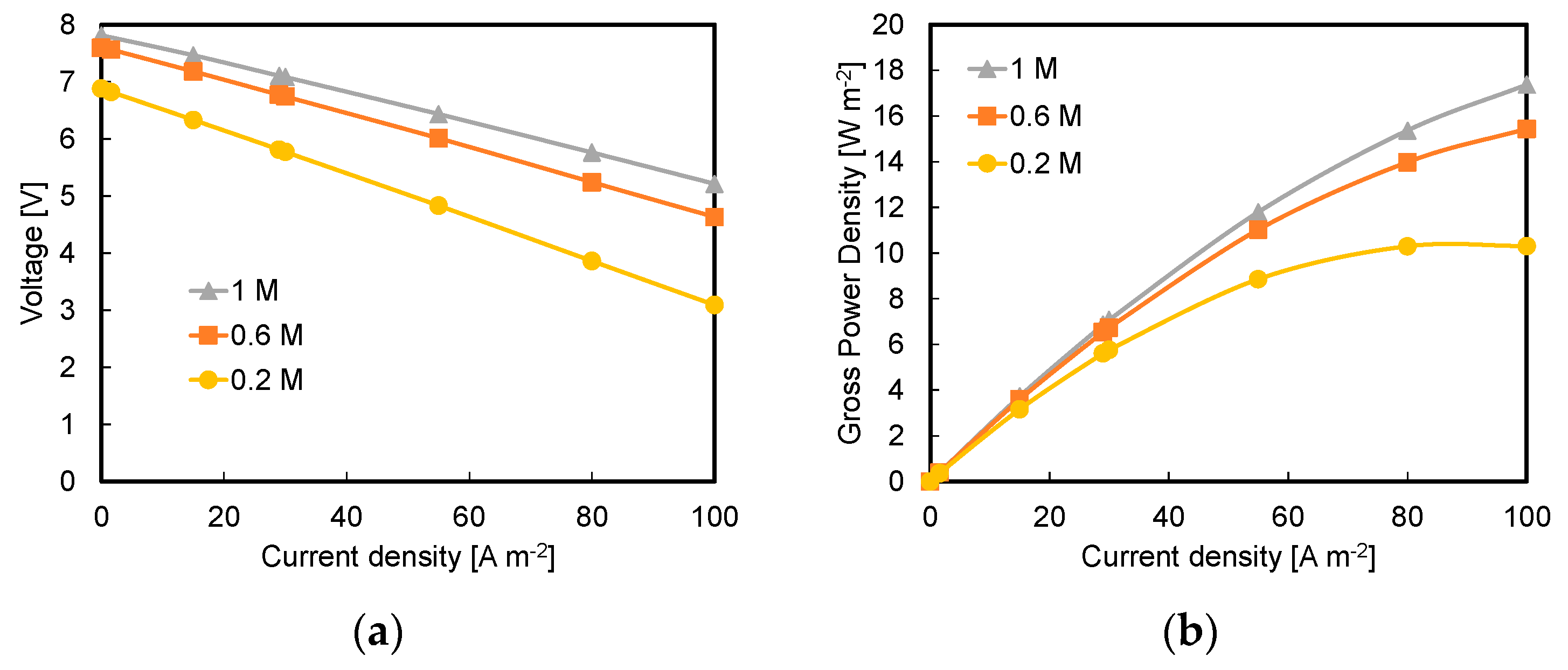
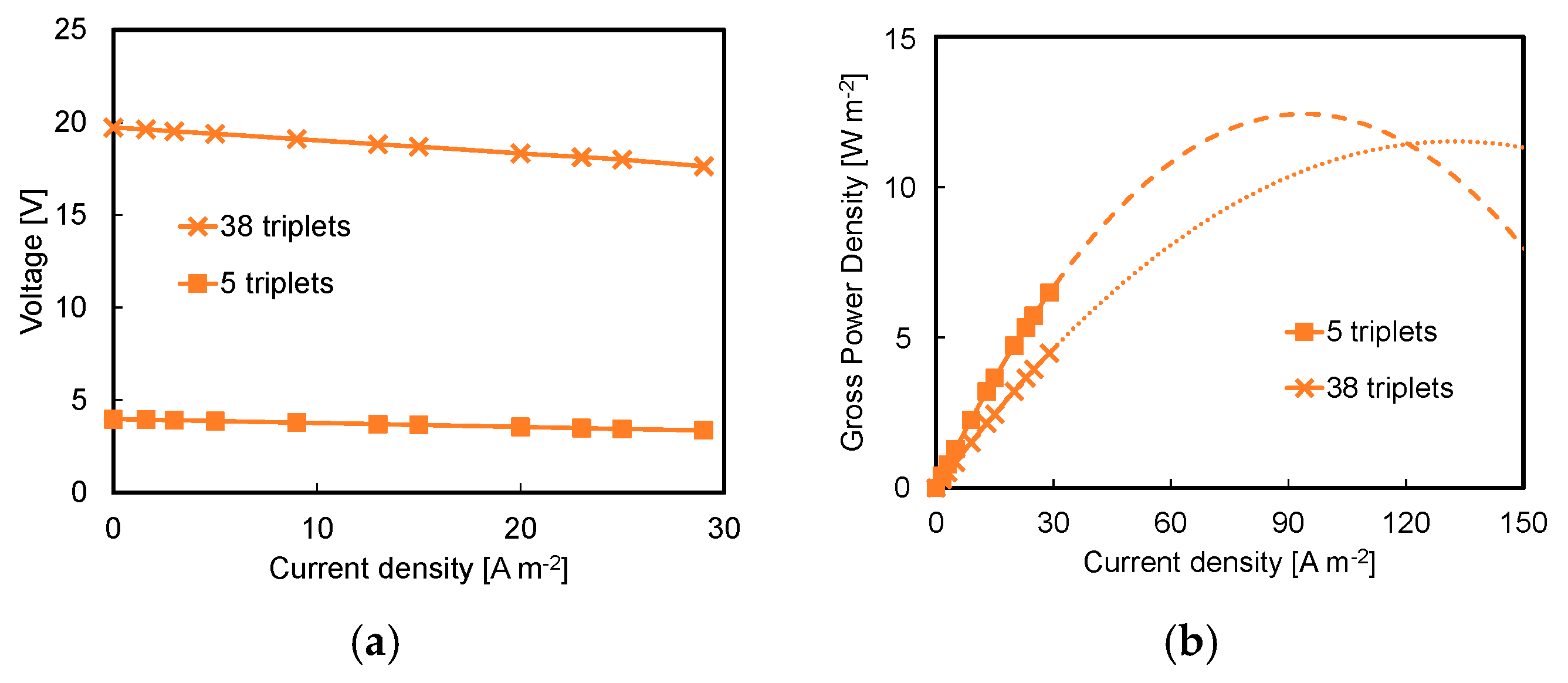
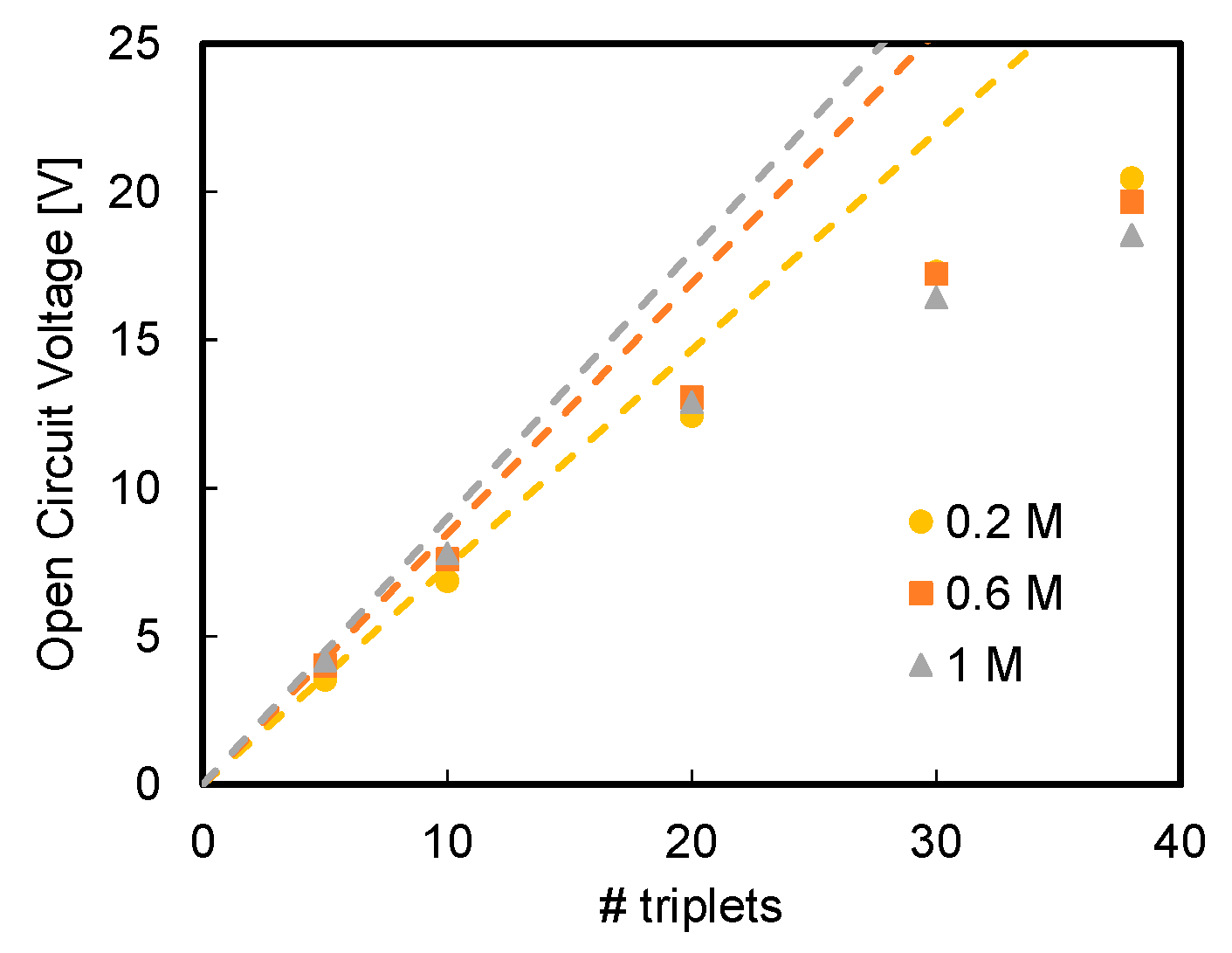
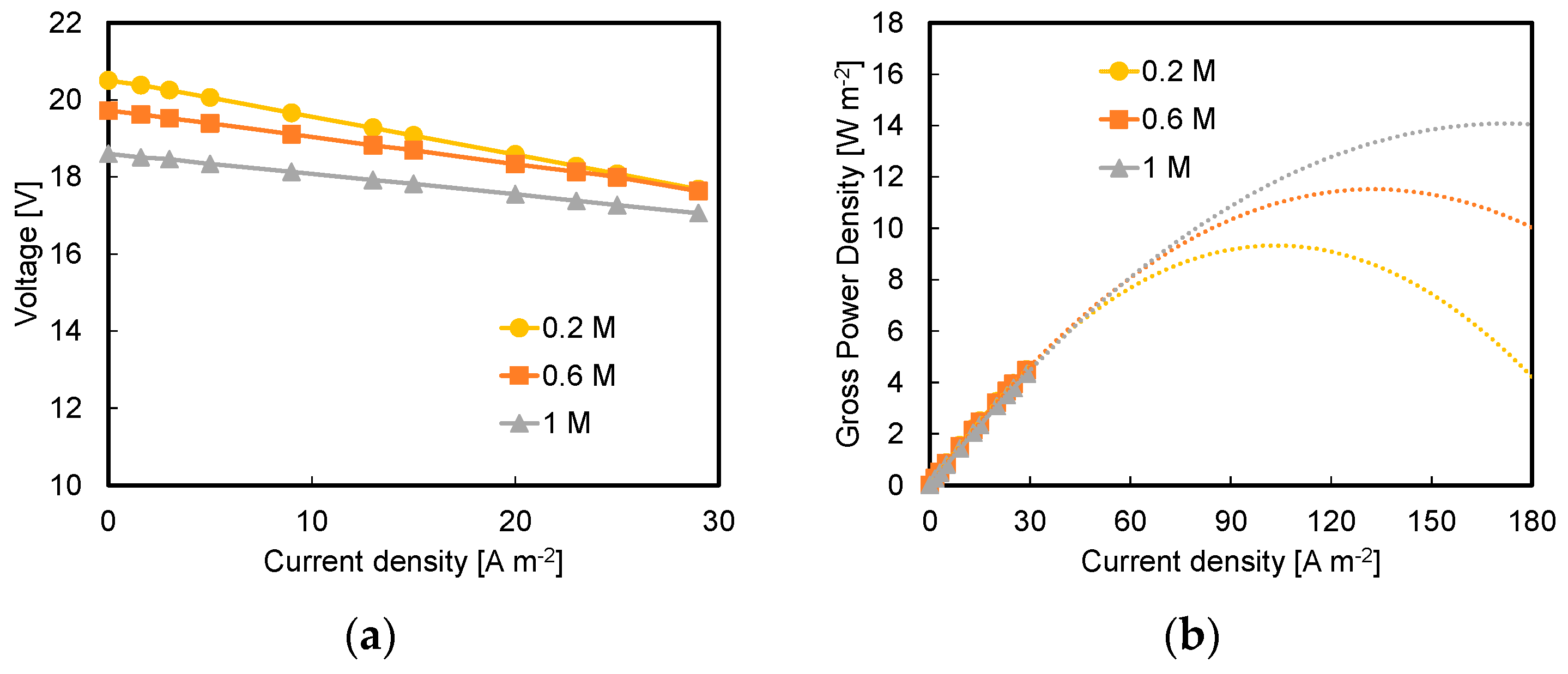
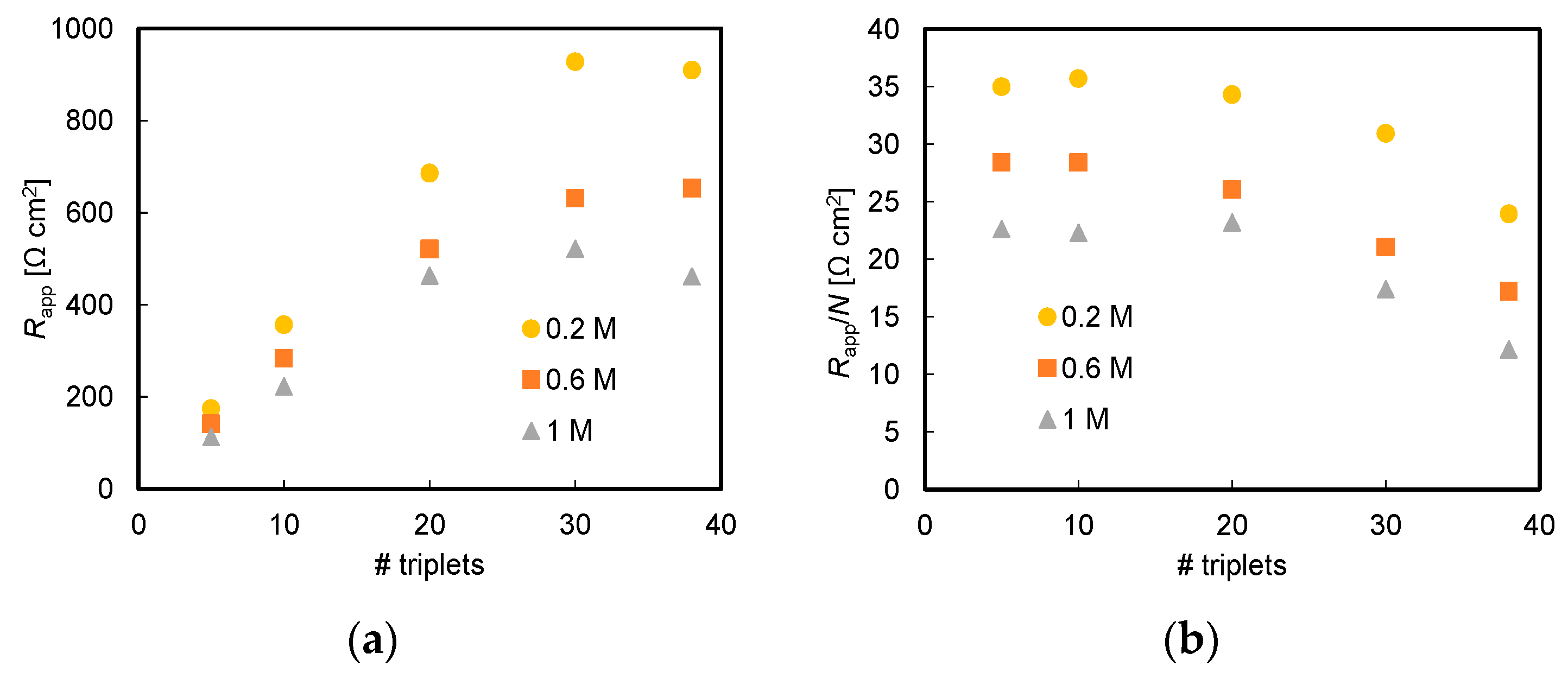
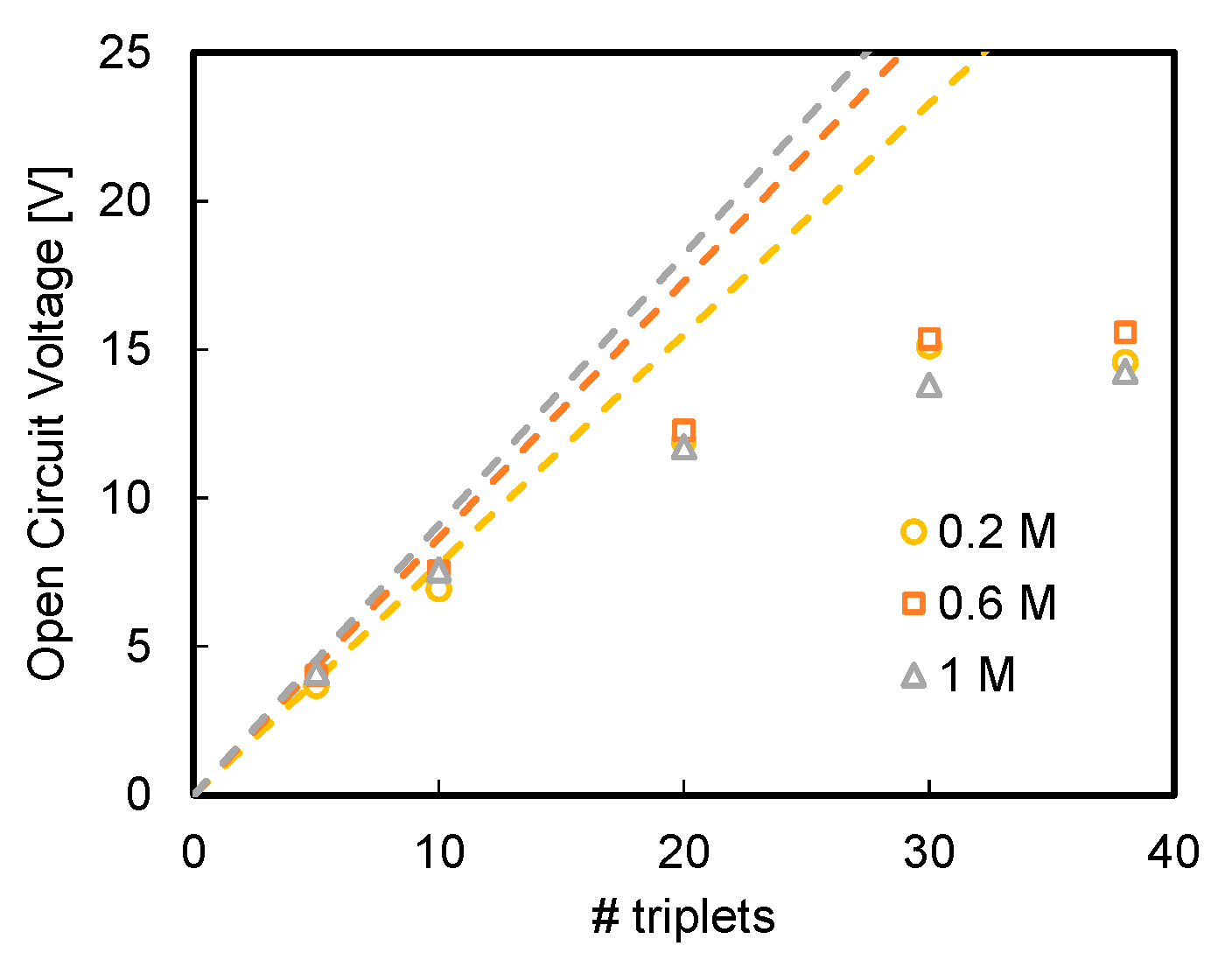
3.1. Effect of Acid and Base Concentration and of Number of Triplets
3.2. Effect of Flow Velocity
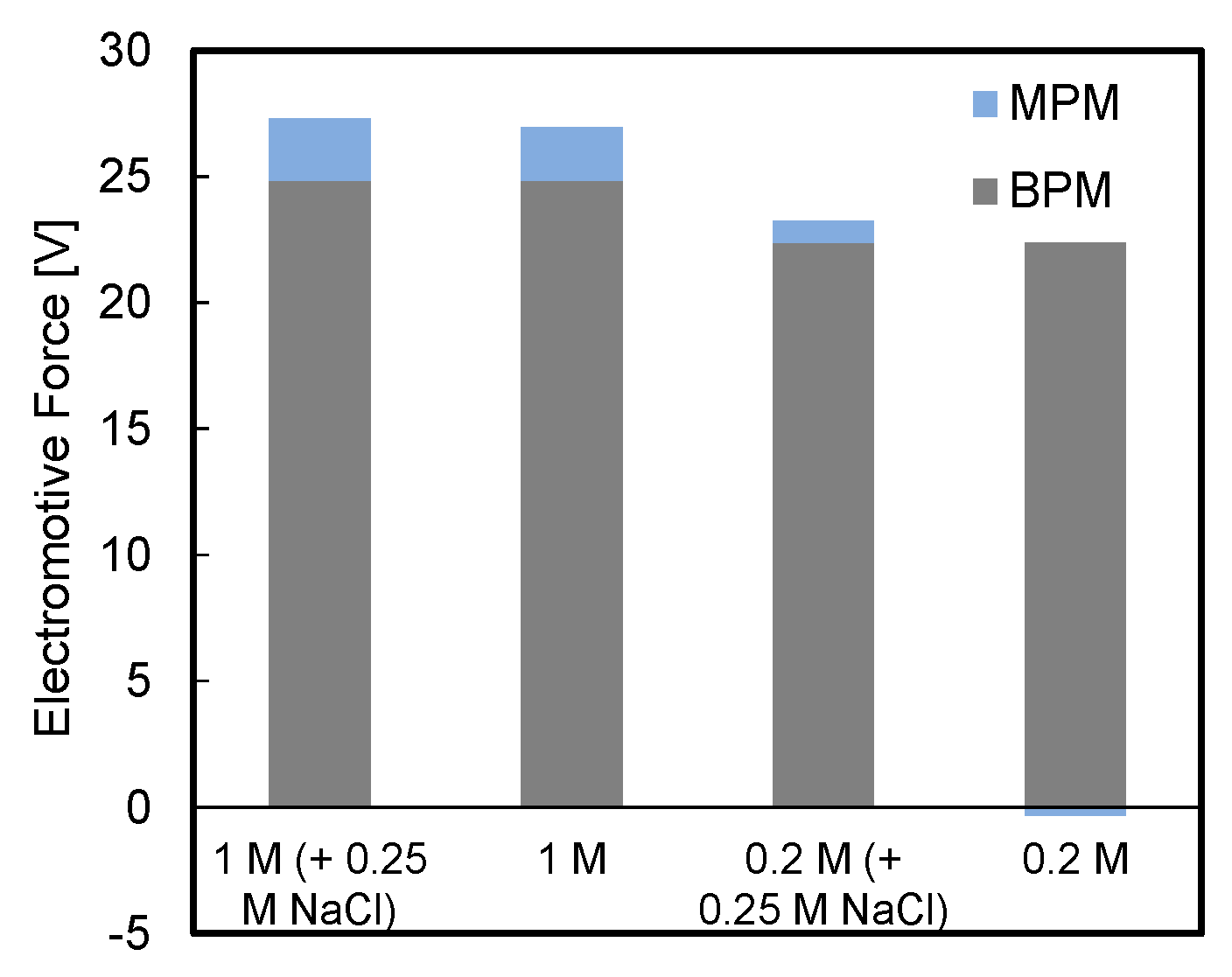
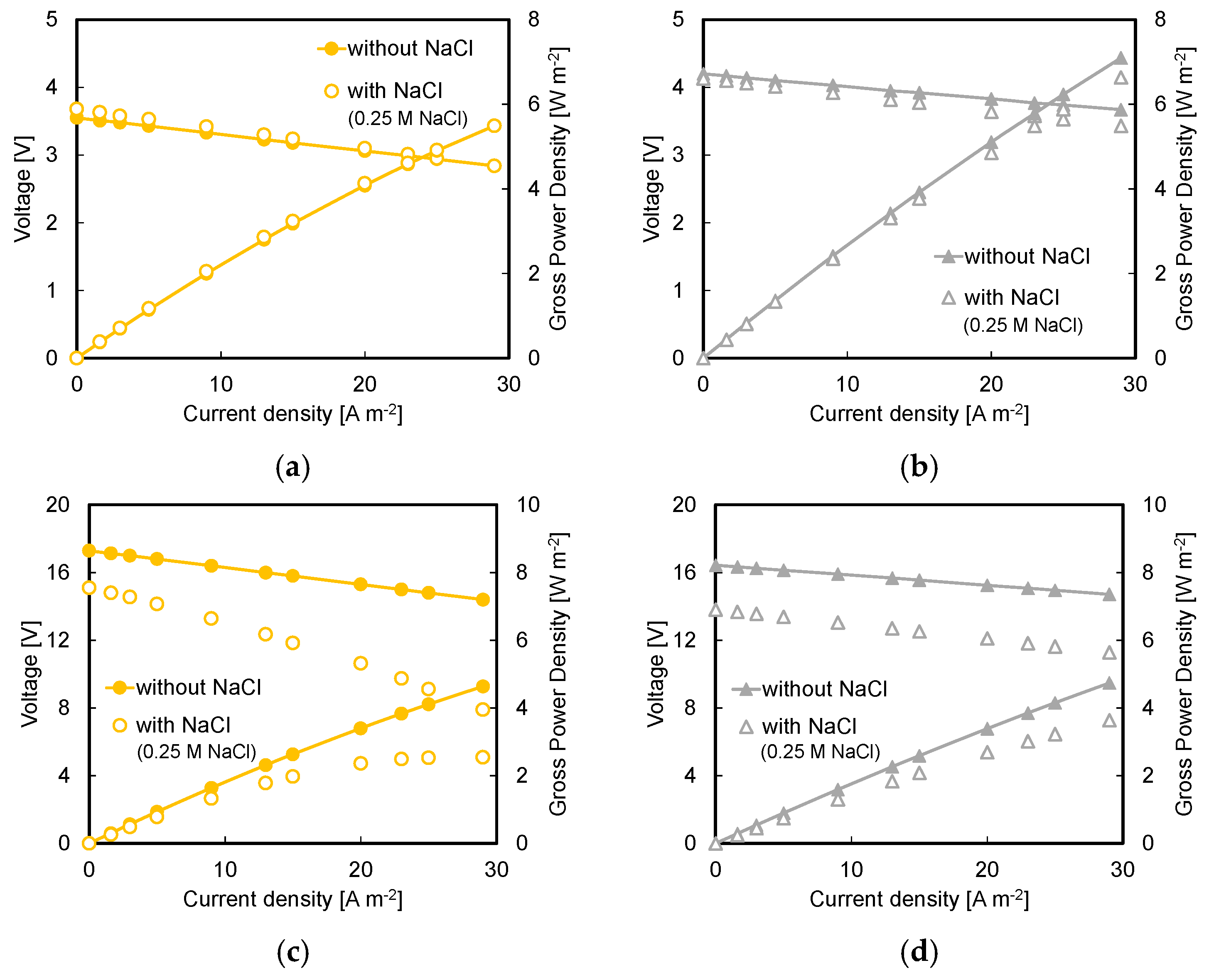
3.3. Effect of Background Salt in Acid and Base Streams
3.4. Energy Density and Perspective Cost Analysis
4. Conclusions
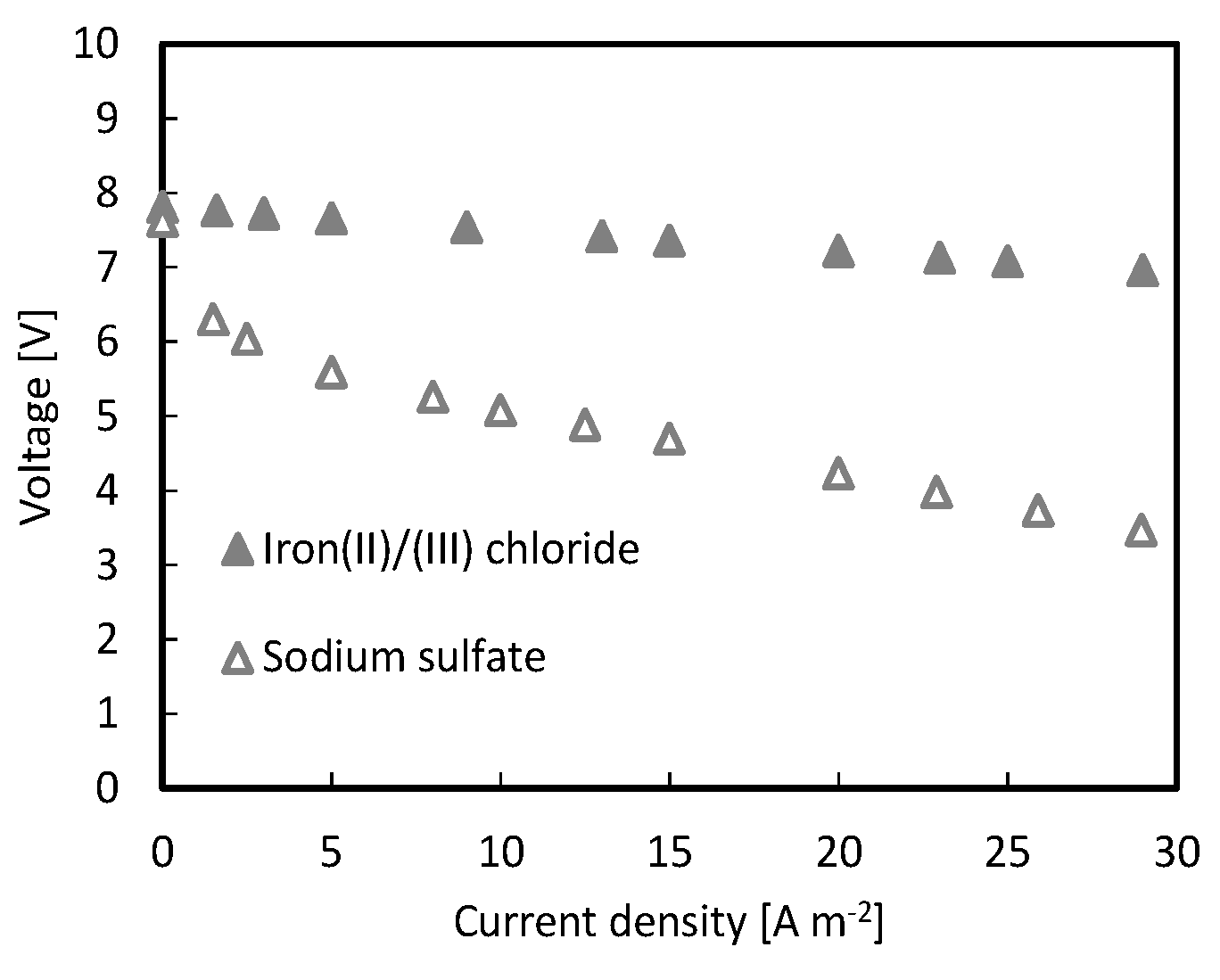
- Electrode rinse solution composed of FeCl2/FeCl3 was found to guarantee lower voltage losses compared to Na2SO4 (Appendix A);
- Higher available pH gradient allowed for higher power densities, due to the higher electromotive force and the lower stack resistance. Under the conservative value of current density of 29 A m−2, a power density of 7.1 W m−2 was reached for a stack with five repeating units fed by 1 M HCl and NaOH solutions;
- A maximum power density of 17.4 W m−2 was reached at higher values of current density, i.e., 100 A m−2, with a 10-triplet stack and a flow velocity of 1 cm s−1;
- Considering a current density value of 100 A m−2, a complete neutralization of the pH gradient would lead to an energy density higher than 10 kWh m−3 acid.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Symbols | |
| a [mol m−3] | Ion activity |
| A [m2] | Membrane area |
| C [mol l−1] | Concentration |
| EMF [V] | Electromotive Force |
| F [C mol−1] | Faraday constant |
| GED [kWh m−3] | Gross Energy Density |
| GPD [W m−2] | Gross Power Density |
| I [A] | Current |
| n | Number of stages |
| N | Number of triplets |
| NPD [W m−2] | Net Power Density |
| P [W] | Gross Power |
| Q [m3 s−1] | Flow rate |
| Rg [J mol−1 K−1] | Universal gas constant |
| R [Ω cm2] | Resistance |
| T [K] | Temperature |
| V [V] | Voltage |
| z | Ion oxidation number |
| Greek letters | |
| Δ | Difference |
| φ [V] | Electrical potential |
| α | Average apparent permselectivity |
| η | Efficiency |
| Subscripts/superscripts | |
| ac | Acidic solution/CEL interface |
| app | Apparent |
| ba | AEL interface/Base solution |
| blank | Blank |
| bp | Bipolar membrane interlayer |
| BPM | Bipolar membrane |
| ext | External |
| fin | Final |
| i | Cell triplet |
| in | Initial |
| inl | Inlet |
| man | Manifold |
| out | Outlet |
| par | Parasitic |
| Pump | Pumping |
| sa | IEM/salt solution interfaces |
| Stack | Stack |
| th | Theoretical |
| Acronyms/abbreviations | |
| AEL | Anion Exchange Layer |
| AEM | Anion Exchange Membrane |
| BMED | Bipolar Electrodialysis |
| BMRED | Bipolar Reverse Electrodialysis |
| BPM | Bipolar Membrane |
| CEL | Cation Exchange Layer |
| CEM | Cation Exchange Membrane |
| CFD | Computational Fluid Dynamics |
| DSA | Dimensionally Stable Anode |
| ED | Electrodialysis |
| EMF | Electromotive Force |
| ERS | Electrode Rinse Solution |
| GED | Gross Energy Density |
| GPD | Gross Power Density |
| IEC | Ion Exchange Capacity |
| IEM | Ion Exchange Membrane |
| MPM | Monopolar Membrane |
| OCV | Open Circuit Voltage |
| RED | Reverse Electrodialysis |
| SGE | Salinity Gradient Energy |
Appendix A
Effect of Electrode Rinse Solution Composition
References
- Goel, R.K.; Flora, J.R.V.; Chen, J.P. Flow Equalization and Neutralization. In Handbook of Environmental Engineering, Volume 3: Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; The Humana Press Inc.: Totowa, NJ, USA, 2005; pp. 21–45. [Google Scholar]
- Kesieme, U.; Chrysanthou, A.; Catulli, M.; Cheng, C.Y. A review of acid recovery from acidic mining waste solutions using solvent extraction. J. Chem. Technol. Biotechnol. 2018, 93, 3374–3385. [Google Scholar] [CrossRef]
- Culcasi, A.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Micale, G. Design of a novel membrane-integrated waste acid recovery process from pickling solution. J. Clean. Prod. 2019, 236, 117623. [Google Scholar] [CrossRef]
- Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis for wastewater treatment—Part II: Industrial effluents. In Current Trends and Future Developments in Bio-Membranes: Membrane Technology for Water and Wastewater Treatment—Advances and Emerging Processes; Basile, A., Comite, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–241. [Google Scholar]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Tansens, P.; Rodal, A.T.; Machado, C.M.; Soares, H.M. Recycling of aluminum and caustic soda solution from waste effluents generated during the cleaning of the extruder matrixes of the aluminum industry. J. Hazard. Mater. 2011, 187, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Aprea, P.; De Gennaro, B.; Colella, C. An unconventional method for the recovery of caustic soda from spent Al-rich pickling solutions. J. Environ. Manag. 2011, 92, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Khan, N.E. Study of an Evaporation System for Sodium Hydroxide Solution. J. Chem. Eng. 2006, 24, 35–36. [Google Scholar] [CrossRef][Green Version]
- Ben Hariz, I.; Halleb, A.; Adhoum, N.; Monser, L. Treatment of petroleum refinery sulfidic spent caustic wastes by electrocoagulation. Sep. Purif. Technol. 2013, 107, 150–157. [Google Scholar] [CrossRef]
- Imran, B.; Khan, S.J.; Qazi, I.A.; Arshad, M. Removal and recovery of sodium hydroxide (NaOH) from industrial wastewater by two-stage diffusion dialysis (DD) and electrodialysis (ED) processes. Desalin. Water Treat. 2016, 57, 7926–7932. [Google Scholar] [CrossRef]
- Sarkar, S.; Sengupta, A.K.; Greenleaf, J.E.; El-Moselhy, M. Energy Recovery from Acid–Base Neutralization Process through pH-Sensitive Polymeric Ion Exchangers. Ind. Eng. Chem. Res. 2011, 50, 12293–12298. [Google Scholar] [CrossRef]
- German, M.; Sengupta, A.K.; Greenleaf, J. Hydrogen Ion (H+) in Waste Acid as a Driver for Environmentally Sustainable Processes: Opportunities and Challenges. Environ. Sci. Technol. 2013, 47, 2145–2150. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W.; Hatzell, M.C.; Logan, B.E. Energy Recovery from Solutions with Different Salinities Based on Swelling and Shrinking of Hydrogels. Environ. Sci. Technol. 2014, 48, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Facci, T.; Gomes, W.J.A.S.; Bravin, B.; Araújo, D.M.; Huguenin, F. Proton Electroinsertion in Self-Assembled Materials for Neutralization Pseudocapacitors. Langmuir 2014, 30, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Pattle, R.E. Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Alvarez-Silva, O.A.; Osorio, A.F.; Winter, C. Practical global salinity gradient energy potential. Renew. Sustain. Energy Rev. 2016, 60, 1387–1395. [Google Scholar] [CrossRef]
- Geise, G.M.; Curtis, A.J.; Hatzell, M.C.; Hickner, M.A.; Logan, B.E. Salt Concentration Differences Alter Membrane Resistance in Reverse Electrodialysis Stacks. Environ. Sci. Technol. Lett. 2014, 1, 36–39. [Google Scholar] [CrossRef]
- Daniilidis, A.; Vermaas, D.A.; Herber, R.; Nijmeijer, K. Experimentally obtainable energy from mixing river water, seawater or brines with reverse electrodialysis. Renew. Energy 2014, 64, 123–131. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Micale, G. Towards 1 kW power production in a reverse electrodialysis pilot plant with saline waters and concentrated brines. J. Membr. Sci. 2017, 522, 226–236. [Google Scholar] [CrossRef]
- Avci, A.H.; Tufa, R.A.; Fontananova, E.; Di Profio, G.; Curcio, E. Reverse Electrodialysis for energy production from natural river water and seawater. Energy 2018, 165, 512–521. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Tristán, C.; Pérez, G.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. A comprehensive study on the effects of operation variables on reverse electrodialysis performance. Desalination 2020, 482, 114389. [Google Scholar] [CrossRef]
- Long, R.; Kuang, Z.; Liu, Z.; Liu, W. Ionic thermal up-diffusion in nanofluidic salinity-gradient energy harvesting. Natl. Sci. Rev. 2019, 6, 1266–1273. [Google Scholar] [CrossRef]
- Long, R.; Luo, Z.; Kuang, Z.; Liu, Z.; Liu, W. Effects of heat transfer and the membrane thermal conductivity on the thermally nanofluidic salinity gradient energy conversion. Nano Energy 2020, 67, 104284. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Electrical Power from Sea and River Water by Reverse Electrodialysis: A First Step from the Laboratory to a Real Power Plant. Environ. Sci. Technol. 2010, 44, 9207–9212. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, D.A.; Veerman, J.; Yip, N.Y.; Elimelech, M.; Saakes, M.; Nijmeijer, K. High Efficiency in Energy Generation from Salinity Gradients with Reverse Electrodialysis. ACS Sustain. Chem. Eng. 2013, 1, 1295–1302. [Google Scholar] [CrossRef]
- Hu, J.; Xu, S.; Wu, X.; Wu, D.; Jin, D.; Wang, P.; Leng, Q. Multi-stage reverse electrodialysis: Strategies to harvest salinity gradient energy. Energy Convers. Manag. 2019, 183, 803–815. [Google Scholar] [CrossRef]
- Giacalone, F.; Vassallo, F.; Scargiali, F.; Tamburini, A.; Cipollina, A.; Micale, G. The first operating thermolytic reverse electrodialysis heat engine. J. Membr. Sci. 2020, 595, 117522. [Google Scholar] [CrossRef]
- Veerman, J.; De Jong, R.M.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Comparison of six commercial membrane pairs on the thermodynamic efficiency and power density. J. Membr. Sci. 2009, 343, 7–15. [Google Scholar] [CrossRef]
- Długołęcki, P.; Ogonowski, P.; Metz, S.J.; Saakes, M.; Nijmeijer, K.; Wessling, M. On the resistances of membrane, diffusion boundary layer and double layer in ion exchange membrane transport. J. Membr. Sci. 2010, 349, 369–379. [Google Scholar] [CrossRef]
- Nikonenko, V.; Nebavsky, A.; Mareev, S.; Kovalenko, A.; Urtenov, M.; Pourcelly, G. Modelling of Ion Transport in Electromembrane Systems: Impacts of Membrane Bulk and Surface Heterogeneity. Appl. Sci. 2018, 9, 25. [Google Scholar] [CrossRef]
- Kozaderova, O.A.; Kim, K.B.; Gadzhiyeva, C.S.; Niftaliev, S.I. Electrochemical characteristics of thin heterogeneous ion exchange membranes. J. Membr. Sci. 2020, 604, 118081. [Google Scholar] [CrossRef]
- Ma, P.; Hao, X.; Galia, A.; Scialdone, O. Development of a process for the treatment of synthetic wastewater without energy inputs using the salinity gradient of wastewaters and a reverse electrodialysis stack. Chemosphere 2020, 248, 125994. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, J.L.; Cosenza, A.; Tamburini, A.; Micale, G.; Cipollina, A. Long-run operation of a reverse electrodialysis system fed with wastewaters. J. Environ. Manag. 2018, 217, 871–887. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Liu, F.; Zhu, S.; Boggs, C.; Armstrong, M.D.; Call, D.F.; Coronell, O. Impact of natural organic matter and inorganic solutes on energy recovery from five real salinity gradients using reverse electrodialysis. J. Membr. Sci. 2017, 541, 621–632. [Google Scholar] [CrossRef]
- Hatzell, M.C.; Zhu, X.; Logan, B.E. Simultaneous Hydrogen Generation and Waste Acid Neutralization in a Reverse Electrodialysis System. ACS Sustain. Chem. Eng. 2014, 2, 2211–2216. [Google Scholar] [CrossRef]
- Güler, E.; Zhang, Y.; Saakes, M.; Nijmeijer, K. Tailor-Made Anion-Exchange Membranes for Salinity Gradient Power Generation Using Reverse Electrodialysis. ChemSusChem 2012, 5, 2262–2270. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Coma, L.; Ortiz-Martínez, V.M.; Carmona, J.; Palacio, L.; Prádanos, P.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Modeling the influence of divalent ions on membrane resistance and electric power in reverse electrodialysis. J. Membr. Sci. 2019, 592, 117385. [Google Scholar] [CrossRef]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes 2019, 10, 7. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Kotoka, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Characterization of Poly (Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED. Membranes 2020, 10, 134. [Google Scholar] [CrossRef]
- Kotoka, F.; Merino-Garcia, I.; Velizarov, S. Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review. Membranes 2020, 10, 160. [Google Scholar] [CrossRef]
- Mei, Y.; Liu, L.; Lu, Y.-C.; Tang, C.Y. Reverse Electrodialysis Chemical Cell for Energy Harvesting from Controlled Acid–Base Neutralization. Environ. Sci. Technol. 2019, 53, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, T. Electrodialysis with Bipolar Membranes for Sustainable Development. Environ. Sci. Technol. 2006, 40, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Thiel, G.P.; Kumar, A.; Gómez-González, A.; Lienhard, J.H. Utilization of Desalination Brine for Sodium Hydroxide Production: Technologies, Engineering Principles, Recovery Limits, and Future Directions. ACS Sustain. Chem. Eng. 2017, 5, 11147–11162. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chen, Q.; Lv, Y.; Han, X.; Wei, Y.; Xu, T. Batch Preparation of High Basicity Polyferric Sulfate by Hydroxide Substitution from Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2017, 5, 2292–2301. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Ramírez, P.; Rapp, H.-J.; Mafé, S.; Bauer, B. Bipolar membranes under forward and reverse bias conditions. Theory vs. experiment. J. Electroanal. Chem. 1994, 375, 101–108. [Google Scholar] [CrossRef]
- Pretz, J.; Staude, E. Reverse electrodialysis (RED) with bipolar membranes, an energy storage system. Ber. Bunsenges. Phys. Chem. 1998, 102, 676–685. [Google Scholar] [CrossRef]
- Zholkovskij, E.K.; Müller, M.C.; Staude, E. The storage battery with bipolar membranes. J. Membr. Sci. 1998, 141, 231–243. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Maurya, S.; Shin, S.-H.; Lee, J.Y.; Chang, I.S.; Moon, S.-H. Proof-of-concept experiments of an acid-base junction flow battery by reverse bipolar electrodialysis for an energy conversion system. Electrochem. Commun. 2016, 72, 157–161. [Google Scholar] [CrossRef]
- Van Egmond, W.J.; Saakes, M.; Noor, I.; Porada, S.; Buisman, C.J.N.N.; Hamelers, H.V.M. Performance of an environmentally benign acid base flow battery at high energy density. Int. J. Energy Res. 2018, 42, 1524–1535. [Google Scholar] [CrossRef]
- Xia, J.; Eigenberger, G.; Strathmann, H.; Nieken, U. Flow battery based on reverse electrodialysis with bipolar membranes: Single cell experiments. J. Membr. Sci. 2018, 565, 157–168. [Google Scholar] [CrossRef]
- Xia, J.; Eigenberger, G.; Strathmann, H.; Nieken, U. Acid-Base Flow Battery, Based on Reverse Electrodialysis with Bi-Polar Membranes: Stack Experiments. Processes 2020, 8, 99. [Google Scholar] [CrossRef]
- Culcasi, A.; Gurreri, L.; Zaffora, A.; Cosenza, A.; Tamburini, A.; Micale, G. On the modelling of an Acid/Base Flow Battery: An innovative electrical energy storage device based on pH and salinity gradients. Appl. Energy 2020, 277, 115576. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Pressure drop at low Reynolds numbers in woven-spacer-filled channels for membrane processes: CFD prediction and experimental validation. Desalin. Water Treat. 2017, 61, 170–182. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Evaluation of suitable electrode systems. J. Appl. Electrochem. 2010, 40, 1461–1474. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef]
- Culcasi, A.; Gurreri, L.; Zaffora, A.; Cosenza, A.; Tamburini, A.; Cipollina, A.; Micale, G. Ionic shortcut currents via manifolds in reverse electrodialysis stacks. Desalination 2020, 485, 114450. [Google Scholar] [CrossRef]
- Rubinstein, I.; Pretz, J.; Staude, E. Open circuit voltage in a reverse electrodialysis cell. Phys. Chem. Chem. Phys. 2001, 3, 1666–1667. [Google Scholar] [CrossRef]
- Veerman, J.; Post, J.W.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reducing power losses caused by ionic shortcut currents in reverse electrodialysis stacks by a validated model. J. Membr. Sci. 2008, 310, 418–430. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Bogle, I.D.L.; Micale, G. A simulation tool for analysis and design of reverse electrodialysis using concentrated brines. Chem. Eng. Res. Des. 2015, 93, 441–456. [Google Scholar] [CrossRef]
- Ciofalo, M.; La Cerva, M.; Di Liberto, M.; Gurreri, L.; Cipollina, A.; Micale, G. Optimization of net power density in Reverse Electrodialysis. Energy 2019, 181, 576–588. [Google Scholar] [CrossRef]
- Nam, J.-Y.; Hwang, K.-S.; Kim, H.-C.; Jeong, H.; Kim, H.; Jwa, E.; Yang, S.C.; Choi, J.; Kim, C.-S.; Han, J.-H.; et al. Assessing the behavior of the feed-water constituents of a pilot-scale 1000-cell-pair reverse electrodialysis with seawater and municipal wastewater effluent. Water Res. 2019, 148, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Performance of a stack with 50 cells on the mixing of sea and river water. J. Membr. Sci. 2009, 327, 136–144. [Google Scholar] [CrossRef]
- Giacalone, F.; Papapetrou, M.; Kosmadakis, G.; Tamburini, A.; Micale, G.; Cipollina, A. Application of reverse electrodialysis to site-specific types of saline solutions: A techno-economic assessment. Energy 2019, 181, 532–547. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9. [Google Scholar]
- Scialdone, O.; Albanese, A.; D’Angelo, A.; Galia, A.; Guarisco, C. Investigation of electrode material—Redox couple systems for reverse electrodialysis processes. Part II: Experiments in a stack with 10–50 cell pairs. J. Electroanal. Chem. 2013, 704, 1–9. [Google Scholar] [CrossRef]

| Authors | Number of Repeating Units | Acid/Base Concentration Range | Energy | Power | Refs. |
|---|---|---|---|---|---|
| Pretz and Staude | 2–20 | 0.1–1 M | - | 10.9 W m−2 | [51] |
| Zholkovskij et al. | 4 | 0.03 M | 0.1 Wh kg−1 | 0.005 W kg−1 | [52] |
| Kim et al. | 1 | 0.1–0.7 M | - | 6.6–11.6 W m−2 | [53] |
| Van Egmond et al. | 1 | 0–1 M | 2.9 Wh kg−1 | 3.7 W m−2 | [54] |
| Xia et al. | 1–20 | 0–1 M | - | 16 W m−2 | [55,56] |
| Name | Type | Reinforcement | Thickness (µm) | IEC (meq g−1) | Selectivity (%) | Areal Resistance (Ω cm2) | pH Stability |
|---|---|---|---|---|---|---|---|
| FAB | Anion | PEEK | 100–130 | 1.0–1.1 | 94–97 | 4–7 | 1–14 |
| FKB | Cation | PEEK | 100–130 | 1.2–1.3 | 98–99 | 4–6 | 1–14 |
| FBM | Bipolar | PEEK | 180–200 | n.p. | n.p. | n.p. | 1–14 |
| i (A m−2) | ΔCinl-out | b1 | b2 | b3 | |
|---|---|---|---|---|---|
| 29 | 0.02 M | with salt | −1.01 | 1.76 | 1.11 |
| 29 | 0.017 M | without salt | 0 | 0.50 | 1.51 |
| 100 | 0.022 M | without salt | −3.00 | 6.25 | 1.96 |
| Items | Value | Notes |
|---|---|---|
| BMRED system | ||
| Fluid velocity | 1 cm s−1 | |
| Membrane area | 0.1 × 0.1 m2 | |
| Channel thickness | 500 μm | |
| Number of triplets per stage | 10 | |
| Number of stages | 45 | |
| GED (average GPD) | 10.3 kWh m−3 (13.7 W m−2) | |
| Working hours | 8000 h y−1 | It corresponds to a capacity factor of ~90% [68] |
| Membrane life-time | 3 y | |
| Investment and operating cost | ||
| AEM/CEM cost | 4 € m−2 | [68] |
| BPM cost | 20 € m−2 | 5 × AEM/CEM cost |
| Total membrane cost | 126 € | |
| Cost of stacks (including membranes) | 189 € | 1.5 × total membrane cost [69] |
| Cost of peripherals | 95 € | 0.5 × stacks cost [69] |
| Capital cost | 284 € | Stacks + peripheral costs [69] |
| Maintenance | 28.4 € y−1 | 0.1 × capital cost [69] |
| Economic parameters | ||
| Discount rate | 5% | [68] |
| Outcome | ||
| LCOE | 0.09 € kWh−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaffora, A.; Culcasi, A.; Gurreri, L.; Cosenza, A.; Tamburini, A.; Santamaria, M.; Micale, G. Energy Harvesting by Waste Acid/Base Neutralization via Bipolar Membrane Reverse Electrodialysis. Energies 2020, 13, 5510. https://doi.org/10.3390/en13205510
Zaffora A, Culcasi A, Gurreri L, Cosenza A, Tamburini A, Santamaria M, Micale G. Energy Harvesting by Waste Acid/Base Neutralization via Bipolar Membrane Reverse Electrodialysis. Energies. 2020; 13(20):5510. https://doi.org/10.3390/en13205510
Chicago/Turabian StyleZaffora, Andrea, Andrea Culcasi, Luigi Gurreri, Alessandro Cosenza, Alessandro Tamburini, Monica Santamaria, and Giorgio Micale. 2020. "Energy Harvesting by Waste Acid/Base Neutralization via Bipolar Membrane Reverse Electrodialysis" Energies 13, no. 20: 5510. https://doi.org/10.3390/en13205510
APA StyleZaffora, A., Culcasi, A., Gurreri, L., Cosenza, A., Tamburini, A., Santamaria, M., & Micale, G. (2020). Energy Harvesting by Waste Acid/Base Neutralization via Bipolar Membrane Reverse Electrodialysis. Energies, 13(20), 5510. https://doi.org/10.3390/en13205510










