4.1. The Propagation of the Isothermal Displacement Flow
The overtaking of the displaced water by n-hexanol, the separation of the water phase and the scattered water distribution are the three important characteristics of the isothermal displacement process in Group A. The analysis and discussion on the progress of isothermal displacement are carried out around the three characteristics.
4.1.1. The Overtaking of the Displaced Water by N-Hexanol
The overtaking starts right at the beginning of the displacement process. As the n-hexanol begins to push the displaced water, the pushing force is generally applied on the contact surface of the two fluids, which is supposed to generate a uniform acceleration effect on the displaced water. On the other hand, water flow in the near-wall region of the sighting tube is hindered by the wall friction. It leads to an approximate parabolic axial velocity profile with the maximum velocity in the centerline of the sighting tube of the displaced water, which is similar to that in Poiseuille flow. As a result, part of the displaced water is left behind and the n-hexanol would gradually overtake the water. It should be noted that, in the current tests, the notch on the sighting tube led to a non-uniform distribution of wall friction force on the displaced water. Water flow near the notch is not affected by the wall friction due to the absence of the tube wall. As the notch is on the top of the tube, the water in the upper portion would flow faster than the water in the lower portion during the displacement process, as depicted in
Figure 4.
The “overtaking rate” is used to quantitatively evaluate the rapidity of the overtaking process. It is defined as the change rate of the water column length (L) over time, which represents the relative velocity between the n-hexanol and the displaced water. The measured overtaking rates in the tests of Group A are listed in
Table 4.
Table 4 shows that, under the same n-hexanol flow rate, the overtaking rate would decrease along with the increase of the initial length of water column. When the initial length of water column in the flow channel is a constant, the overtaking rate would increase along with the flow rate of n-hexanol increasing.
The test results of Group A indicate that the pore water might be overtaken by the fracturing fluid during the actual cryogenic fracturing process. The initial blockage of the pore channel by water would be enhanced as the displacement process goes on. The consideration of the effect of fracturing fluid vaporization, wettability of water/fracturing fluid on the reservoir rock surface, micro pore in the reservoir, etc. are necessary in the future studies, which could further verify the occurrence of overtaking during cryogenic fracturing.
4.1.2. The Separation of the Displaced Water and the Scattered Distribution of Water Phase
The analysis of the overtaking phenomenon indicates that there is a non-uniform velocity distribution within the water region from the beginning of the displacement process, leading to a “stretched” state of the water phase throughout the entire displacement process. Separation would occur at the locations where the tensile limit is exceeded and the single water phase would split into two or more smaller pieces, as shown in
Figure 5. The scattered distribution of water shown in
Figure 6 is actually induced by the water separation during the displacement process.
Similar to the overtaking process, separation of water phase could also happen during the actual cryogenic fracturing process and would finally lead to a scattered distribution of pore water within the reservoir. The occurrence of the separation process should be carefully considered in the future studies as well.
4.2. The Propagation of the Freezing-Accompanied Displacement Flow
The analysis and discussion on the freezing-accompanied displacement process in Group B is carried out around the following aspects: water freezing, the influence of ice phase on the fluid flow (ice blockage) and heat transfer.
4.2.1. Freezing Process
The freezing process observed in Cases B1, B2 and Case B23 could be explained based on the classical nucleation theory (CNT) [
23,
24,
25,
26]. In CNT, a certain degree of energy barrier is required to be overcome to induce the nucleation/freezing process. By reducing the water temperature or adding nucleating agent into the water, the energy barrier could be lowered, which enhances the nucleation/freezing process. In Cases B1, B2 and B23, the displaced water was supercooled at the initial state with a supercooling degree of 5, 5 and 7 °C, which is not enough to induce the nucleation process. Thus, no ice is observed in the sighting tube at this moment. As the displacement process begins, according to the analysis in
Section 4.1, part of the displaced water at the near-wall region of the tube would stay at (or stay near) its initial position due to wall friction. Since the flow direction of n-hexanol is from left to right in the tube, the water distributed near the left end of the sighting tube could receive the cooling effect from the n-hexanol earlier than the water at other locations, which makes the temperature of water near the left end of the tube drop faster. Consequently, the water near the left end of the tube could firstly satisfy the temperature condition for nucleation, resulting in the formation of the primal ice phase. The appearance of the primal ice introduces new solid phase into the water region, which creates favorable conditions for heterogeneous nucleation. The supercooling degree for nucleation is then lowered in the water surrounding the primal ice. In other words, the newly-formed ice enhances the nucleation probability in the water vicinity, which leads to the subsequent continuous freezing (the recalescence stage of the freezing process). It should be noted that the occurrence of continuous freezing requires the entire water region reaching a certain supercooling degree as a premise. If the overall supercooling degree of the water region is not sufficient, continuous freezing could not be triggered even after the appearance of primal ice.
To further discuss the freezing process in the tests of Group B, two parameters i.e., trigger-time of freezing and freezing rate, are introduced. The trigger-time of freezing is defined as the time interval between the beginning of the displacement process and the initiation of the freezing of the displaced water. The trigger-time is closely related to the following moments: (1) the moment when the n-hexanol contacts the water in the tube; and (2) the moment when the freezing process initiates. The freezing rate is defined as the moving rate of the freezing front during the freezing process. The freezing front refers to the rightmost border between the frozen zone and the unfrozen zone of the displaced water, which is indicated with green arrows in
Figure 7,
Figure 10 and
Figure 12. The freezing rate of each test is obtained based on the locations of the freezing front at various moments. The freezing trigger-time and the average freezing rate obtained from the tests in Group B are listed in
Table 5 and
Table 6, respectively.
Table 5 shows that the trigger-time would decrease with the increase of n-hexanol flow rate and the decrease of the initial water temperature. Increasing the flow rate of n-hexanol could reduce the cold energy loss of n-hexanol before it enters the sighting tube. The n-hexanol thus contacts the water in the tube at an earlier timing and lower temperature, which can significantly improve the cooling efficiency. On the other hand, decreasing the initial water temperature would lower the energy barrier of nucleation, which also promotes the occurrence of freezing. The trigger-time of freezing is found to change irregularly with the initial length of water column: In the test groups that the n-hexanol flow rate is 39.7 mL/min and initial water temperature is −6 °C, the trigger-time of freezing decreases as the initial length of water column increases. In the tests that the n-hexanol flow rate is 24.9 mL/min and the initial water temperature is −7 °C, the minimum trigger-time of freezing is found in the test that the initial length of water column is set as 4 cm. However, in the tests that the n-hexanol flow rate is 54.1 mL/min and the initial water temperature is −5 °C, the maximum trigger time of freezing is found in the test that the initial length of water column is set as 4 cm. These changes indicate that there is a connection between the trigger-time of freezing and the initial length of water column. More tests could be conducted in the future to figure out this relationship.
Table 6 shows that the freezing rate would increase along with the decrease of initial water temperature, which is in consistent with the analysis based on classical nucleation theory (CNT) that a lower temperature (higher super-cooling degree) could accelerate the nucleation process.
The water distribution in the sighting tube is another influencing factor for the freezing process. It is found from the observation results (
Figure 7,
Figure 8,
Figure 10 and
Figure 12) that only a part of the water is finally frozen in Case B1, which is different from those in Cases B2 and B23. This disparity is mainly caused by the difference of water distribution when the freezing process initiates. At the moment when the primal ice appears, the water in Cases B2 and B23 still holds its continuous distribution state. However, the water in Case B1 is split into several separate parts by the displacement process. Consequently, the freezing process in Case B1 is interrupted due to the discontinuity of water distribution, leaving part of the water in the tube unfrozen. To make this unfrozen part of water solidify, further cooling (supply of cold n-hexanol) is necessary to satisfy the temperature condition for nucleation, which would take extra time. It indicates that the separation of water phase during the displacement process is actually obstructing the freezing process by destroying the continuity of water distribution.
It is thought that the continuous freezing process observed in the current study may influence the actual cryogenic fracturing process. When a wide range of pore water in the reservoir is connected together by the pore network and they are supercooled, a rapid freezing process could be triggered as the cryogenic fracturing fluid begins to cool down a portion of the pore water and the entire pore water zone could be frozen within a short period. The trigger conditions may be kind of rigorous, considering the complicated heat and mass transfer situation in the reservoir. Therefore, the possibility of the occurrence of the continuous freezing should not be discarded.
4.2.2. The Influence of the Ice Phase on the Flow State
As stated in
Section 3.2, two typical patterns of the ice blockage were observed in the tests of Group B, which are termed as “incomplete ice blockage” and “complete ice blockage”. It is seen that different blockage patterns could lead to different flow situations in the sighting tube. The analysis of these blockage patterns is introduced in the following.
Incomplete blockage refers to the pattern where the cross section of the flow channel is partly occupied by the ice phase after the freezing process, which is shown in
Figure 7,
Figure 8 and
Figure 10. It is noted that the ice phase shown in
Figure 7 (Case B1) and
Figure 10 (Case B2) is a range of thin ice layers attached to the inner wall of the sighting tube. The central part of the tube is free for the fluid to get through after the freezing process. As the flow channel is not totally blocked up, fluid flow is able to continue after the freezing process with the flow rate altered, which is the main feature of this blockage pattern.
Complete blockage refers to the pattern where the cross section of the flow channel is fully occupied by the ice phase after the freezing process, which is shown in
Figure 12 (the region of 7–10 cm in the tube is completely occupied by the ice). As the flow channel is totally blocked, fluid flow in the original direction is interrupted and fluids are forced to change their flow direction in order to continue the flow behavior, which is the main feature of this blockage pattern. Compared with the incomplete ice blockage, complete ice blockage is more significant for the flow situation since both the flow rate and the flow direction are altered.
The pattern of ice blockage closely depends on the final ice distribution in the tube. The final ice distribution in the tube is actually determined by the distribution of the water in the tube at the initial freezing stage. Since the water distribution changes with the displacement process, it can be found that both freezing process and the displacement process are key factors which directly determine the ice blockage pattern. The characterization of the occurrence of the two ice blockage patterns can be summarized as follows. For the incomplete ice blockage, the water in the upper portion of the tube is totally driven away (the displacement process steps into the later stage) by the moment that the freezing process initiates. For the complete ice blockage, there is still a certain amount of water in the upper portion of the tube (the earlier stage of the displacement process is continued) by the moment that the freezing process initiates. The overall blockage patterns occurred in the tests of Group B are listed in
Table 7.
The influencing factors for the displacement process and the freezing process can affect the occurrence of the ice blockage as well. Based on
Table 7 and the above analysis, the influence of the initial water column length, initial water temperature and the n-hexanol flow rate on the ice blockage pattern could be basically understood.
- (1)
The initial length of the water column
According to the analysis on the overtaking rate in
Section 4.1, the overtaking rate decreases with the increase of the initial length of water column, which extends the duration of the earlier stage of the displacement process and enhances the occurrence of complete blockage.
Table 7 shows that complete ice blockage preferably occurs in the cases that the initial water column length is 7 cm (six out of nine) compared to those with the initial water column of 4 cm (three out of nine) and 2 cm (two out of nine), indicating the initial water column length is one of the main influencing factor for the ice blockage.
- (2)
Initial water temperature
According to the analysis of the trigger-time of freezing and the freezing rate in the current section, initial water temperature could significantly affect the freezing process. The decrease of initial water temperature could lead to the decrease of the trigger-time of freezing and accelerate the freezing rate, which can further promote the occurrence of complete blockage.
Table 7 shows that the numbers of complete ice blockage in the cases of −7 °C are more than those in the cases of −5 and −6 °C, indicating a lower water temperature could facilitate the occurrence of complete ice blockage.
- (3)
N-hexanol flow rate
According to the analysis in
Section 4.1 and the current section, n-hexanol flow rate would play an important role on both displacement process and freezing process. The increase of n-hexanol flow rate leads to the increase of the overtaking rate and the decrease of the trigger-time of freezing, while the increase of overtaking rate could enhance the occurrence of incomplete ice blockage and the decrease of the trigger-time of freezing could enhance the occurrence of complete ice blockage. It suggests that the change of n-hexanol flow rate would bring two conflicting effects on the occurrence of ice blockage. Therefore, the influence of n-hexanol flow rate on ice blockage pattern depends on which of the above two effects dominates the displacement process. No definite link between n-hexanol flow rate and the occurrence of ice blockage was found from the results in
Table 7, indicating that the current result is not sufficient to draw a reliable conclusion on this point, which is expected to be further discussed and improved in the future work.
It is deduced that both incomplete ice blockage and complete ice blockage are important for the actual cryogenic fracturing process. The occurrence of complete ice blockage is supposed to significantly affect the cryogenic fracturing progress as it can largely alter the flow state (flow rate and flow direction) of the fracturing fluid within the reservoir, which also changes the transport of cold energy. The prediction of ice blockage is supposed to be a vital task in configuring the cryogenic fracturing process in the future, which requires a deeper understanding on the occurrence of the ice blockage pattern.
4.2.3. Heat Transfer
The estimated values of Biot number of the sighting tube in all the cases of Group B are less than 0.1, which means the lumped parameter method is valid in heat transfer analysis. Accordingly, the temperature of the tube outer wall measured by the thermocouples could reflect the temperature variations of the fluid in the tube.
The temperature variations in the tests of Group B are mainly controlled by the supplement of the cold n-hexanol and latent heat release due to water freezing. In Case B1, the n-hexanol passes Point 1 at 10 s, leading to the first intense temperature drop at Point 1 (
Figure 9). The sequent decrease of the wall temperature at the five measurement points is in agreement with the order of the n-hexanol passing through. As the temperature of n-hexanol rises while the n-hexanol is flowing in the tube, the heat exchange between the fluid and the surroundings is weakened, leading to a gentler temperature change at the points on the right side compared to the points on the left side. As the pump is turned off at 77 s, the cold n-hexanol supply is cut off and the temperatures of Points 1–5 stop decreasing. The significant temperature difference between the tube wall and its surroundings at Point 1 results in a fierce heat exchange, which induces the temperature of Point 1 to increase immediately after the cold energy supply stops. It is found that the influence of latent heat release is barely reflected in the temperature variations in Case B1, which can be explained as follows. As shown in
Figure 7, water freezing is limited in the region of 0–10 cm in the tube. Since the displaced water is initially set in the region of 2 cm, Point 2 is the only measurement point that can be affected by water freezing in Case B1. By the moment the freezing process begins, most of the water near Point 2 has been driven away. As a result, quite limited latent heat was released around Point 2, which is insufficient to raise the temperature at Point 2.
In Case B2, the temperature rise at Points 3–5 is induced by the latent heat release of water freezing. As shown in
Figure 11, the three curves show increasing trends (the freezing process begins) and then go down (the freezing process ends) in the order of Points 3–5, which is in accordance with the freezing trend (freezing proceeds from left to right) shown in
Figure 10. No temperature rise of Point 2 induced by freezing is observed, the reason for which is the same as that in Case B1.
In Case B23, as shown in
Figure 13, the influence of latent heat release is reflected in the temperature variation at Points 2 and 3. As the freezing process initiated soon after the displacement process began in this case, plenty of water froze into ice, which released considerable latent heat in the vicinity of Point 2, making the temperature of Point 2 rise. The temperature of Point 2 began to decrease again as the freezing process near the point finalized. On the other hand, the curve of Point 3 kept at a steady level after rising to a certain value (about −3.7 °C). It suggests a balance is reached between the cooling down of water by n-hexanol and the latent heat release due to water freezing. The temperature variation at Point 3 indicates that the freezing process at Point 3 continued throughout the entire test process of Case B23. Since the peristaltic pump was turned off at quite an early time and no fluid (either water or n-hexanol) reached these points during the test, no temperature changes were observed at Points 4 and 5 from beginning to end.
Temperature variation in the above three cases could reflect the general characteristics of heat transfer in the tests of Group B. Latent heat released during the freezing process could significantly affect the temperature distribution in the sighting tube. The measured temperature variation could provide reliable information to estimate the freezing process. As the amount of latent heat depends on amount of water solidifies, the water distribution could become another important influencing factor for temperature distribution during the displacement process.
The heat transfer results indicate that during the actual cryogenic fracturing process, pore water in the reservoir could remarkably influence the heat transfer progress. Temperature drop in the water-rich region lags behind that in dry region. As the water distribution and temperature distribution are closely related, high temperature gradient could be induced in the region where the water content difference is fierce, leading to thermal stress, which is desirable for the fracturing purpose.
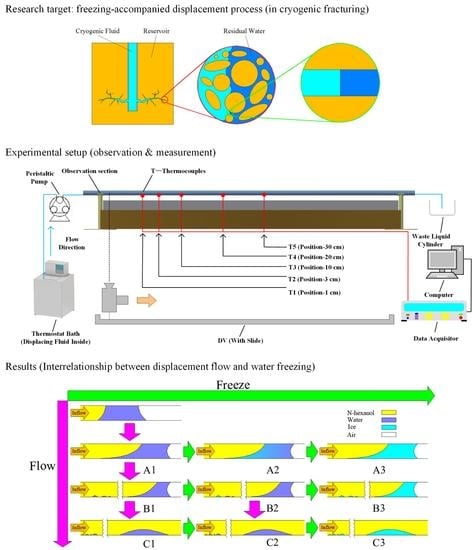
4.3. Interrelationship between Displacement Flow and Water Freezing
From the above discussions, the development of the displacement flow in company with water freezing could be schematically summarized in
Figure 14. The development of the flow situation is displayed by the images arranged along the vertical direction (from top to bottom), with the change trend indicated by purple arrows. Meanwhile, the development of the freezing situation is displayed by the images arranged along the horizontal direction (from left to right) with the change trend indicated by green arrows.
Based on
Figure 14, the interrelationship between displacement process and freezing process could be basically understood from the following three aspects.
(1) Displacement process alters the water distribution in the flow passage. In
Figure 14, the flow situation during the displacement process is divided into three phases according to the water distribution in the flow passage, which are marked as “A1”, “B1” and “C1”. It should be noted that this phase division plotted in
Figure 14 is different from that in
Section 3.1, because the separation of water phase is also regarded as a phase (B1) of the flow situation development here. As stated in
Section 4.1, the separation of water phase could occur anywhere in the water region, either before or after the displaced water is completely overtaken by the n-hexanol. In
Figure 14, the pattern where water separation occurs before the water is completely overtaken by the n-hexanol is displayed. The other similar patterns are omitted for simplicity. Details of the three phases are described as follows.
Phase A1: In this phase, the entire water region is still in a continuous state. With the proceeding of the displacement process, the initial water in the center region of the flow passage is gradually driven to the edge area. On the other hand, due to the effect of wall friction, water in the edge region of the flow passage is hard to be completely driven away, thus a part of the water in this region remains in the initial position during the displacement process.
Phase B1: In this phase, several smaller isolated water regions begin to form due to the separation of water phase.
Phase C1: In this phase, the water in the center region of the flow passage has been totally driven away. Moreover, the displaced water is also completely overtaken by the n-hexanol.
(2) A continuous freezing process could be triggered in a continuous water region with a certain overall supercooling degree. The continuous freezing process can lead the entire water region to be frozen in a short period. Discontinuity in water distribution, which is caused by the separation of water phase during the displacement process, would interrupt the continuous freezing process, slowing down the progress of water freezing. In
Figure 14, the freezing processes of “B1–B3” and “C1–C3” are supposed to take more time in comparison with those of “A1–A3”.
(3) Freezing process occurring at different moments in the displacement process (A1, B1 and C1) could result in different distributions of ice in the flow passage (A3, B3 and C3), which further leads to the occurrence of two typical ice blockage patterns, i.e., incomplete ice blockage (C3) and complete ice blockage (A3 and B3), in the flow passage. In the incomplete ice blockage pattern, fluid flow is allowed to continue after the freezing process. In the complete ice blockage pattern, however, flowing process in the original direction is cut off after the freezing process. The fluids are forced to change their flow direction. The ice blockage pattern is determined by both the displacement process and freezing process. Factors that affect these two physical processes, i.e. initial water column length, initial water temperature and n-hexanol flow rate, could also influence the occurrence of ice blockage.