Characterization of Customized Encapsulant Polyvinyl Butyral Used in the Solar Industry and Its Impact on the Environment
Abstract
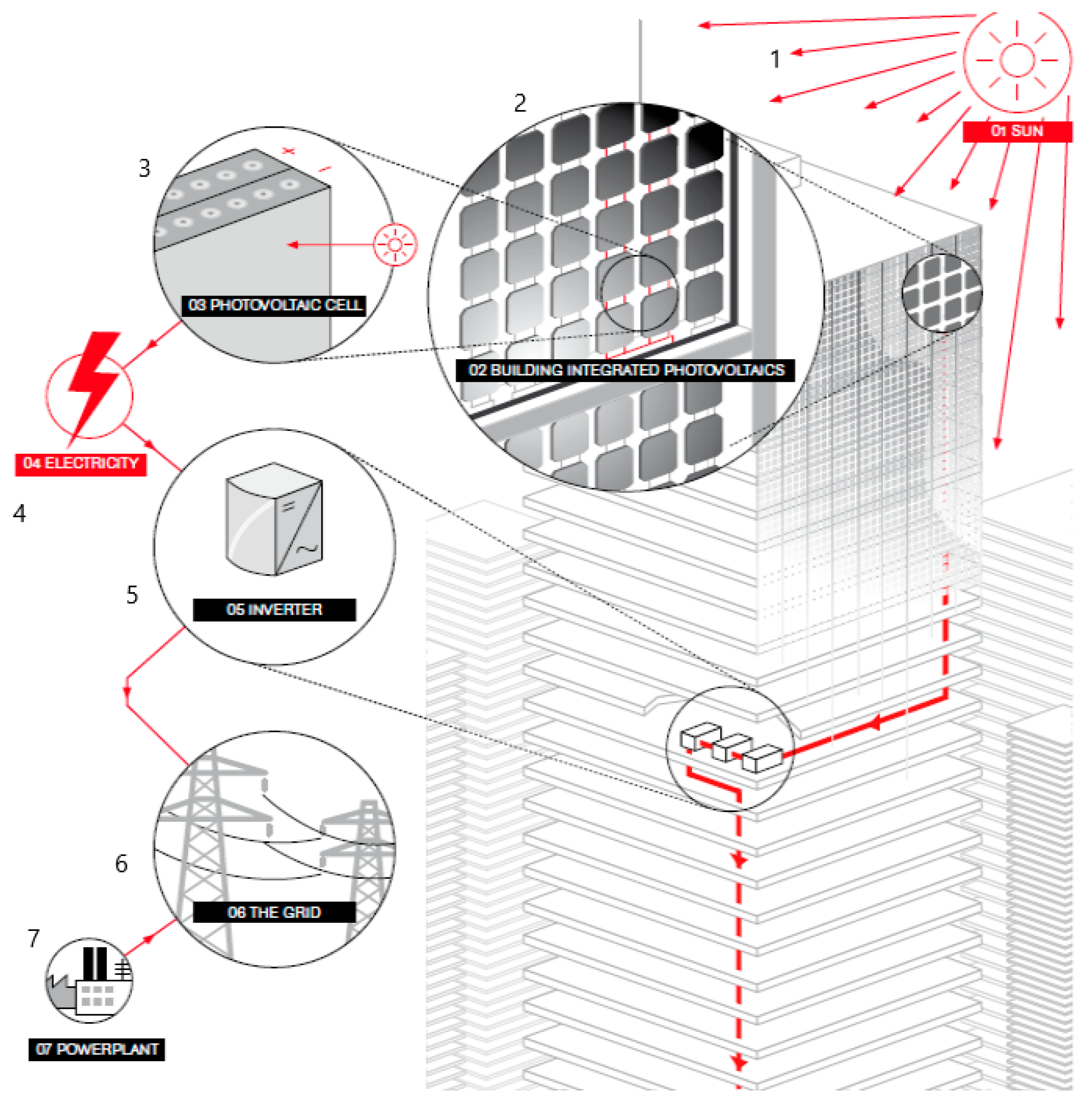
1. Introduction
- Mechanical cell protection;
- Weather protection;
- Electrical insulation;
- Resistance to external impact;
- Protection against oxygen and water vapor;
- Minimization of cell corrosion;
- High adhesion to other components of the module (glass, chamber, base foil, contacts, etc.);
- Maximum guarantee of transparency and high protection against ultraviolet (UV) radiation.
2. Material and Methods
2.1. Material Characterization
2.2. Homogenization and Moulding Process
- opening the mould,
- material filling,
- moulding process,
- moulding process,
- opening the mould,
- removing process,
- cooling and cleaning process.
2.3. Polyvinyl Butyral Testing Used Methods of Exposure to Laboratory Light Sources
3. Results and Discussion
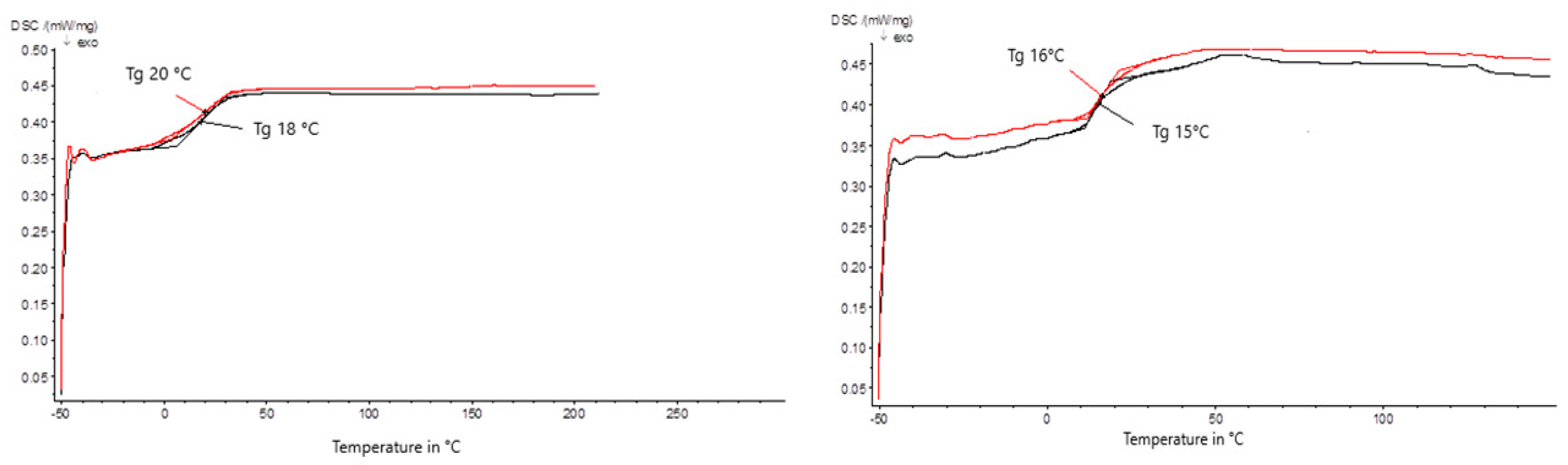
3.1. Differential Scanning Calorimetry Testing
3.2. Mechanical Testing (Tensile Test)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sunshine Energy. Available online: http://www.inforse.org/europe/fae/OEZ/slnko/slnko.html#TOP (accessed on 30 June 2020).
- Average Sunshine a Year at Cities in Europe—Current Results. Available online: https://www.currentresults.com/Weather/Europe/Cities/sunshine-annual-average.php (accessed on 6 August 2020).
- Possibilities of Using Solar Energy. Available online: http://www.aee-now.at/cms/fileadmin/downloads/projekte/Homepageuploads/Infosolar_ECB_Moznosti_vyuzivania_slnecnej_energie.pdf (accessed on 11 August 2020).
- Urtinger, K.; Beranovsky, J.; Tomeš, M. Fotovoltaika: Energies from the Sun; Vyd. 1; EkoWATT: Praha, Slovakia, 2007; p. 81. [Google Scholar]
- Sapa Solar Building. Available online: http://www.sapabuildingsystems.com/globalassets/sapa-building-systems-ab/pictures/brochures/solar_bipv_low.pdf?ts=636316455108630000 (accessed on 12 August 2020).
- GridEdge Solar. Available online: https://gridedgesolar.org/ (accessed on 8 July 2020).
- A PVB Film Helping to Make Photovoltaic Modules Lighter and Less Expensive. Available online: https://www.kuraray.com/news/2013/131002 (accessed on 13 August 2020).
- Transparency Market Research. Available online: http://www.transparencymarketresearch.com/polyvinyl-butyral-films-sheets.html (accessed on 10 August 2020).
- Solar Panels. Available online: https://www.quest.sk/solarne-kolektory/uspory-navratnost/ (accessed on 15 August 2020).
- Cutler, J.C.; Morris, C. Available online: https://www.elsevier.com/books/handbook-of-energy/cleveland/978-0-12-417013-1 (accessed on 10 August 2020).
- Polyvinyl Butyral. Available online: https://www.ihs.com/products/polyvinyl-butyral-chemical-economics-handbook.html (accessed on 28 July 2020).
- Polyvinyl Butyral. Available online: https://www.britannica.com/science/polyvinyl-butyral (accessed on 26 July 2020).
- Global Polyvinyl Butyral (PVB) Films Market Status (2015–2019) and Forecast (2020–2024) by Region, Product Type and End-Use. Available online: https://www.marketintellica.com/report/MI3819-global-polyvinyl-butyral-pvb-films-market (accessed on 10 August 2020).
- Kolcun, M.; Medveď, D.; Petráš, J.; Stolárik, R.; Vaško, Š. Research of Characteristics of Photovoltaic Possibilities for Potential Design of Solar Systems. Available online: http://people.tuke.sk/dusan.medved/Subory/VADIUM-Kniha.pdf (accessed on 21 July 2020).
- Standard EN ISO 4892 Plastics—Methods of Exposure to Laboratory Light Sources. Available online: https://www.iso.org/standard/60048.html (accessed on 21 August 2020).
- Agroui, K.; Koll, B.; Collins, G.; Salama, M.; Arab, A.H.; Belghachi, A.; Doulache, N.; Khemici, M.W. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/7048/70480G/Characterization-of-encapsulant-materials-for-photovoltaic-solar-energy-conversion/10.1117/12.794012.short?SSO=1. (accessed on 11 August 2020).
- Eisentraeger, J.; Naumenko, K.; Altenbach, H.; Meenen, J. A user-defined finite element for laminated glass panels and photovoltaic modules based on a layer-wise theory. Compos. Struct. 2015, 133, 265–277. [Google Scholar] [CrossRef]
- Drabczyk, K.; Sobik, P.; Starowicz, Z.; Gawlińska, K.; Pluta, A.; Drabczyk, B. Study of lamination of solar modules with PMMA front layer. Microelectron. Int. 2019, 36, 100–103. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Li, N. Carrier transport composites with suppressed glass-transition for stable planar perovskite solar cells. J. Mater. Chem. A 2020, 8, 14106–14113. [Google Scholar] [CrossRef]
- Heath, G.; Silverman, T.J.; Kempe, M.; Deceglie, M.G.; Ravikumar, D.; Remo, T.; Cui, H.; Sinha, P.; Libby, C.; Shaw, S.; et al. Research and development priorities for silicon photovoltaic module recycling to support a circular economy. Nat. Energy 2020, 5, 502–510. [Google Scholar] [CrossRef]
- Straka, M.; Khouri, S.; Rosova, A.; Cagáňová, D.; Culkova, K. Utilization of Computer Simulation for Waste Separation Design as a Logistics System. Int. J. Simul. Model. 2018, 17, 583–596. [Google Scholar] [CrossRef]
- Pujadas-Gispert, E.; Korevaar, C.; Alsailani, M.; Moonen, S. Linking constructive and energy innovations for a net zero-energy building. J. Green Build. 2020, 15, 153–184. [Google Scholar] [CrossRef]
- Tartakowski, Z.; Cimander, K.; Bursa, J. New polymer construction materials for applications in electrical engineering. In Proceedings of the Conference on Innovative Materials and Technologies in Electrical Engineering (i-MITEL), IEEE, Sulecin, Poland, 18–20 April 2018. [Google Scholar]
- Tanyildizi, H.; Asilturk, E. Performance of Phosphazene-Containing Polymer-Strengthened Concrete after Exposure to High Temperatures. J. Mater. Civ. Eng. 2018, 30, 04018329. [Google Scholar] [CrossRef]
- Mandičák, T.; Mesároš, P.; Tkáč, M. Impact of management decisions based on managerial competencies and skills developed trough BIM technology on performance of construction enterprises. Pollack Period. 2018, 13, 131–140. [Google Scholar] [CrossRef]
- Chen, S.; Zang, M.; Wang, D.; Yoshimura, S.; Yamada, T. Numerical analysis of impact failure of automotive laminated glass: A review. Compos. Part B Eng. 2017, 122, 47–60. [Google Scholar] [CrossRef]
- Liu, B.; Xu, J.; Li, Y. Constitutive Investigation on Viscoelasticity of PolyVinyl Butyral: Experiments Based on Dynamic Mechanical Analysis Method. Adv. Mater. Sci. Eng. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Zajac, J.; Dupláková, D.; Hatala, M.; Goldyniak, D.; Poklemba, R.; Šoltés, P. The Effect of Used Fillers on the Strength Characteristics of Polymer Concrete Test Bodies. TEM J. 2019, 8, 795–800. [Google Scholar]
- Moseley, J.; Miller, D.; Shah, Q.; Sakurai, K. Available online: https://www.nrel.gov/docs/fy12osti/52586.pdf (accessed on 17 July 2020).
- Straka, M.; Rosova, A.; Lenort, R.; Šaderová, J.; Besta, P. Principles of computer simulation designfor the needs of improvement of the raw materials combined transport system. Acta Montan. Slov. 2018, 23, 163–174. [Google Scholar]
- Mouritz, A.P.; Mathys, Z. Mechanical properties of fire-damaged glass-reinforced phenolic composites. Fire Mater. 2000, 24, 67–75. [Google Scholar] [CrossRef]
- Dodds, N.; Gibson, A.G.; Dewhurst, D.; Davies, J.M. Fire behavior of composite laminates. Compos. Part A 2000, 31, 689. [Google Scholar] [CrossRef]
- Niaki, M.H.; Fereidoon, A.; Ahangari, M.G. Experimental study on the mechanical and thermal properties of basalt fiber and nanoclay reinforced polymer concrete. Compos. Struct. 2018, 191, 231–238. [Google Scholar] [CrossRef]
- Weiheng, F.; Weirong, H.; Tianze, X.; Kim, Y.; Lyu, C.; Anqi, Z. Exploring the Interconnection of Greenhouse Gas Emission and Socio-economic Factors. SAR J. 2019, 2, 115–120. [Google Scholar] [CrossRef]





| Material | |
|---|---|
| Type | flakes form neutral |
| Coloring-matter | 20–30 mm |
| Flakes parameter | >98% |
| Purity | <% |
| Impurity content | ca. 2% |
| Residual amount of humidity | Less as 2% |
| Ratio of glass particle content | |
| Fire point | none |
| Glasstransition temperature | 130–170 °C |
| Viscosity (dynamic) | 100–175 m Pa*s |
| MVR (Melt VolumeRate) | (DIN 53015) |
| MFR (Melt Flow Rate) | 6–7 cm3/10 min 5–6 g/10 min |
| Moulding Parameters | Brabender W 350 |
|---|---|
| Moulding Temperature | 190 °C |
| Heating | 20 min (±2 min) |
| Moulding Time | 20 min (±2 min) |
| Cooling | 20 min (±2 min) |
| Pressure | 10 MPa (±0.1 MPa) |
| No. Cycle | Load Cycle | The Lamp Type | Radiation Intensity | Prescribed Temperature | Relative Humidity |
|---|---|---|---|---|---|
| 2 | 8 h Drying | Type 1 A (Ultraviolet (UV-A-340)) | 0.76 W.m–2.Nm–1 By 340 nm | (50 ± 3) °C | Unregulated |
| 0.15 h Water spray | - | Unregulated | Unregulated | ||
| 0.15 h Water spray | - | Unregulated | Unregulated | ||
| 0.15 h Water spray | - | Unregulated | Unregulated | ||
| 0.15 h Water spray | - | Unregulated | Unregulated | ||
| 2 h Condensation | Radiation off | (50 ± 3) °C | Unregulated |
| Equipment | Zwick Z020 Universalpruefungsmaschine |
|---|---|
| Max. Force | 20 kN |
| Testing Speed | 100 mm/min |
| Standard | DIN EN ISO 527 |
| The Statistic Variables | The Thickness of the Test Sample [mm] | Width of the Test Sample [mm] | E-Module [MPa] | Tensile Stress at Break [MPa] | Tensile Strain at Break [%] | Max. Tensile Stress [MPa] | Max. Tensile Strain [%] |
|---|---|---|---|---|---|---|---|
| x | 2.9701 | 6.0250 | 5.0000 | 17.2360 | 146.140 | 17.5182 | 145.9610 |
| s | 0.0294 | 0.0129 | 2.0000 | 1.4731 | 11.5940 | 1.6361 | 11.6471 |
| ν | 0.9900 | 0.2100 | 52.450 | 8.3401 | 7.9300 | 9.3400 | 7.9800 |
| The Statistic Variables | The Thickness of the Test Sample [mm] | Width of the Test Sample [mm] | E-Module [MPa] | Tensile Stress at Break [MPa] | Tensile Strain at Break [%] | Max. Tensile Stress [MPa] | Max. Tensile Strain [%] |
|---|---|---|---|---|---|---|---|
| x | 2.8901 | 6.0050 | 4.9850 | 17.1890 | 145.940 | 17.4981 | 144.8700 |
| s | 0.0199 | 0.0021 | 2.0030 | 1.3931 | 10.6840 | 1.5931 | 11.2561 |
| ν | 0.8900 | 0.1990 | 51.650 | 7.9501 | 7.8700 | 9.2910 | 7.8500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khouri, S.; Behun, M.; Knapcikova, L.; Behunova, A.; Sofranko, M.; Rosova, A. Characterization of Customized Encapsulant Polyvinyl Butyral Used in the Solar Industry and Its Impact on the Environment. Energies 2020, 13, 5391. https://doi.org/10.3390/en13205391
Khouri S, Behun M, Knapcikova L, Behunova A, Sofranko M, Rosova A. Characterization of Customized Encapsulant Polyvinyl Butyral Used in the Solar Industry and Its Impact on the Environment. Energies. 2020; 13(20):5391. https://doi.org/10.3390/en13205391
Chicago/Turabian StyleKhouri, Samer, Marcel Behun, Lucia Knapcikova, Annamaria Behunova, Marian Sofranko, and Andrea Rosova. 2020. "Characterization of Customized Encapsulant Polyvinyl Butyral Used in the Solar Industry and Its Impact on the Environment" Energies 13, no. 20: 5391. https://doi.org/10.3390/en13205391
APA StyleKhouri, S., Behun, M., Knapcikova, L., Behunova, A., Sofranko, M., & Rosova, A. (2020). Characterization of Customized Encapsulant Polyvinyl Butyral Used in the Solar Industry and Its Impact on the Environment. Energies, 13(20), 5391. https://doi.org/10.3390/en13205391










