Reverse Water–Gas Shift Chemical Looping Using a Core–Shell Structured Perovskite Oxygen Carrier
Abstract
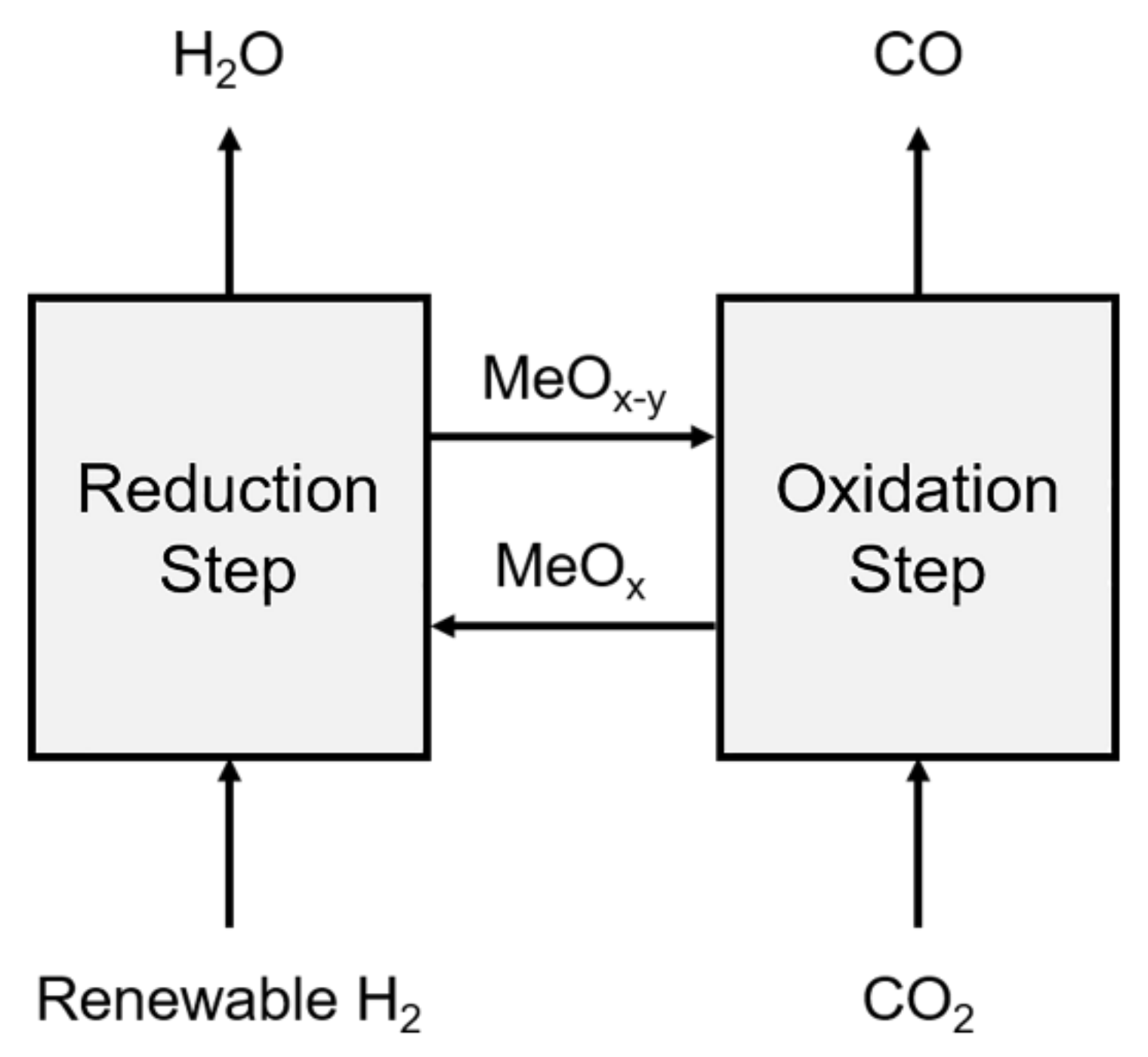
1. Introduction
2. Experimental Section
2.1. Oxygen Carrier Preparation
2.2. Characterization of Oxygen Carriers
2.3. RWGS-CL Experiments
2.4. Temperature-Programmed Processes
3. Results and Discussion
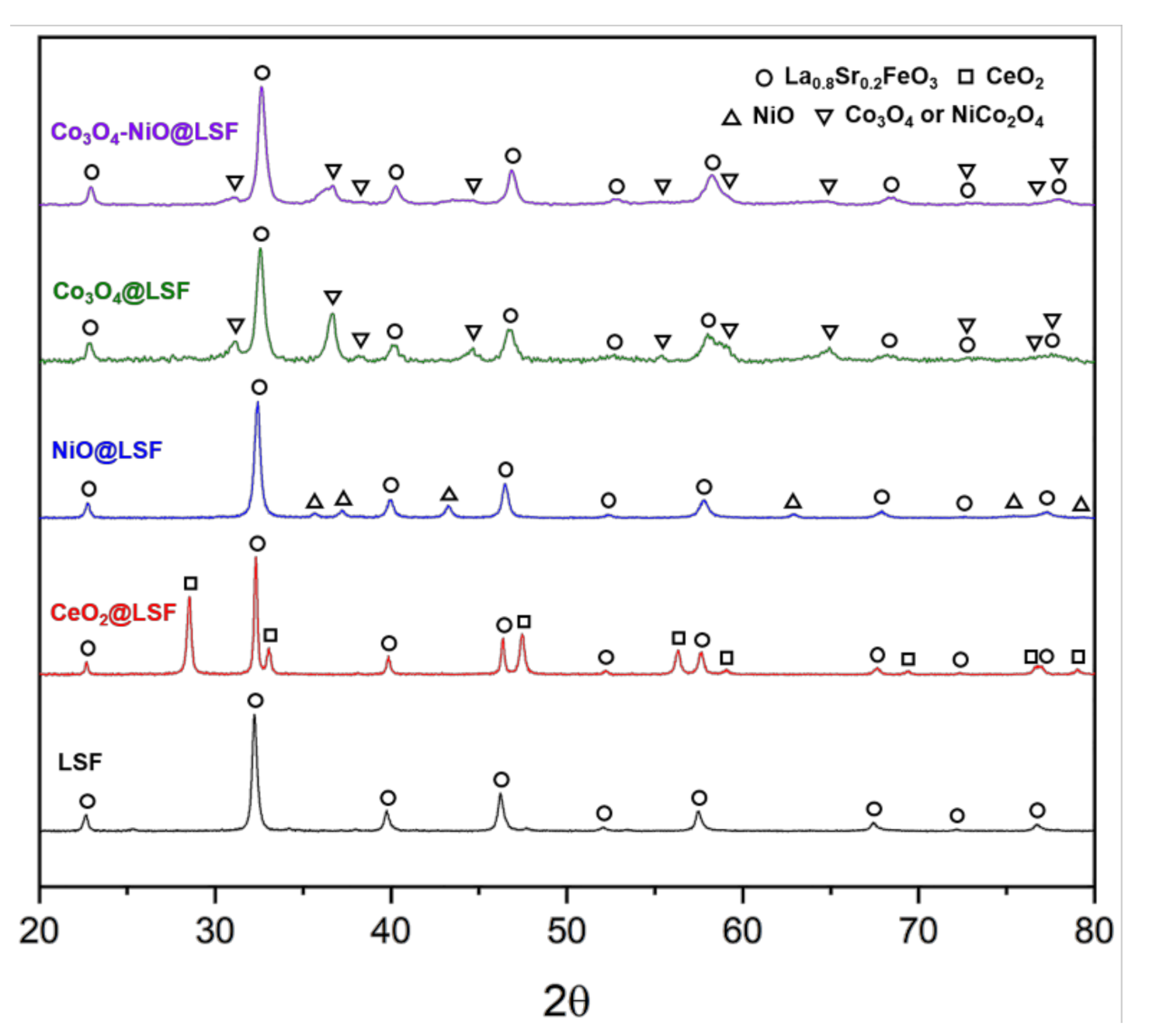
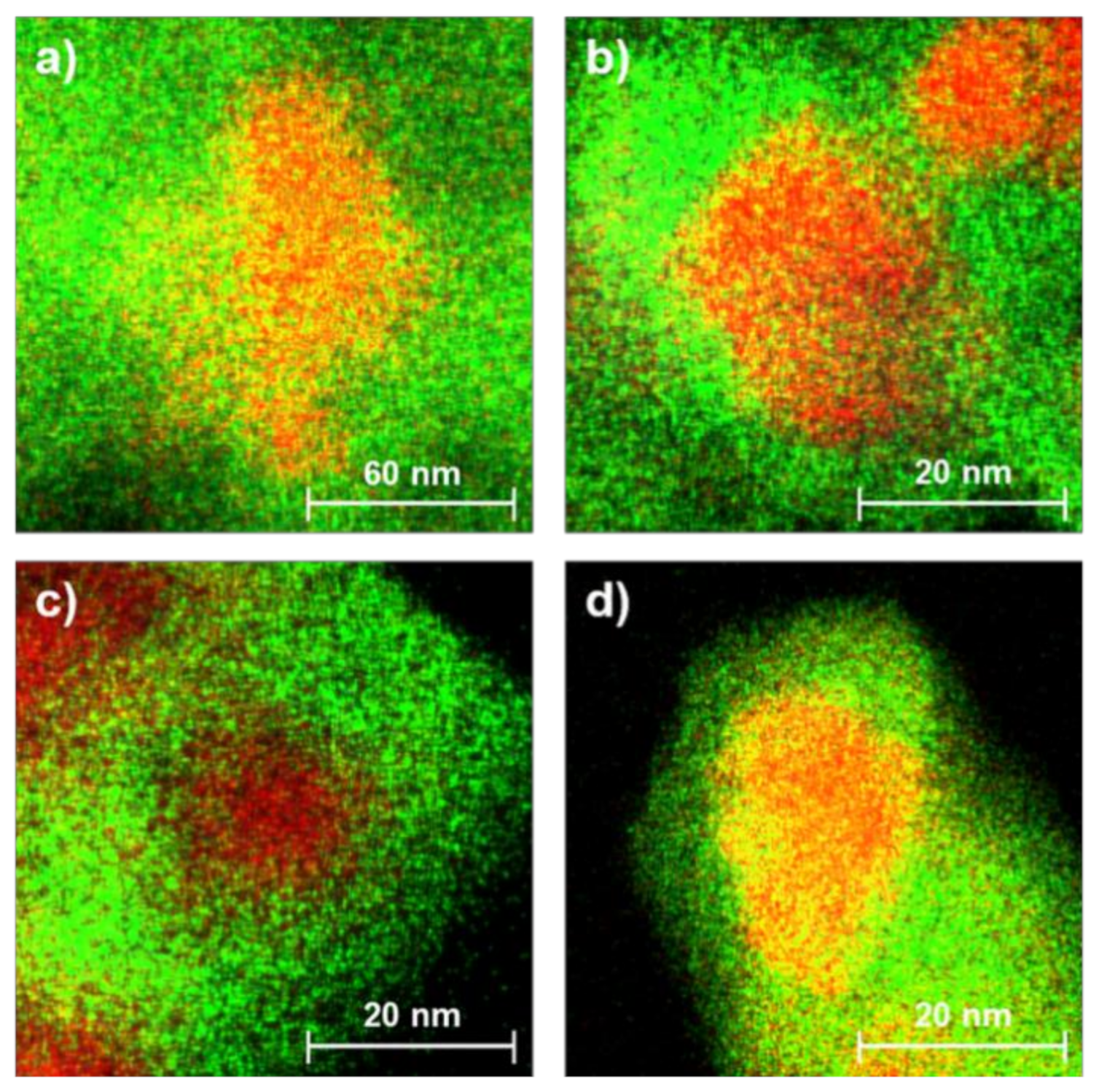
3.1. Structure of the Oxygen Carriers
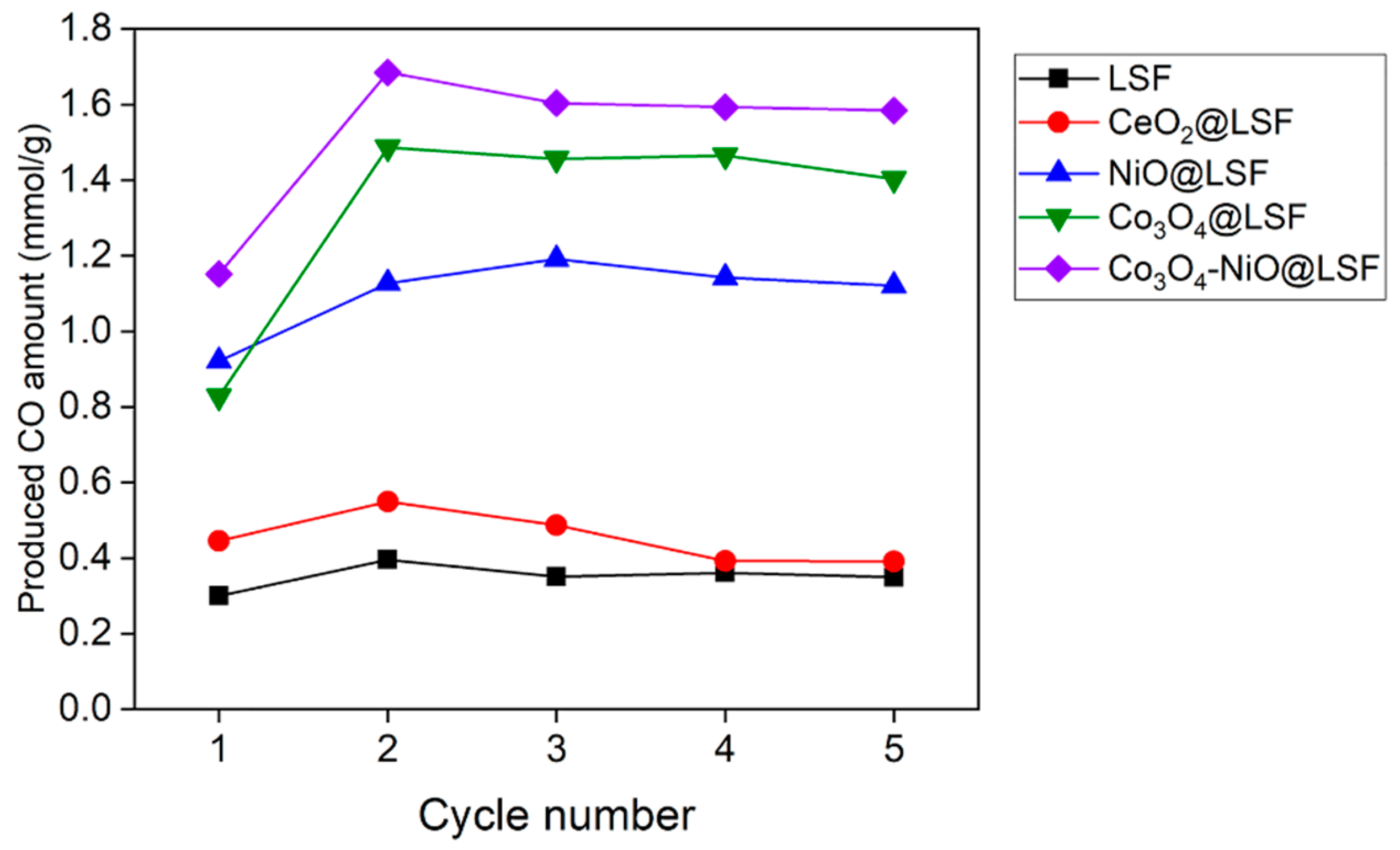
3.2. RWGS-CL Performance of the Oxygen Carriers
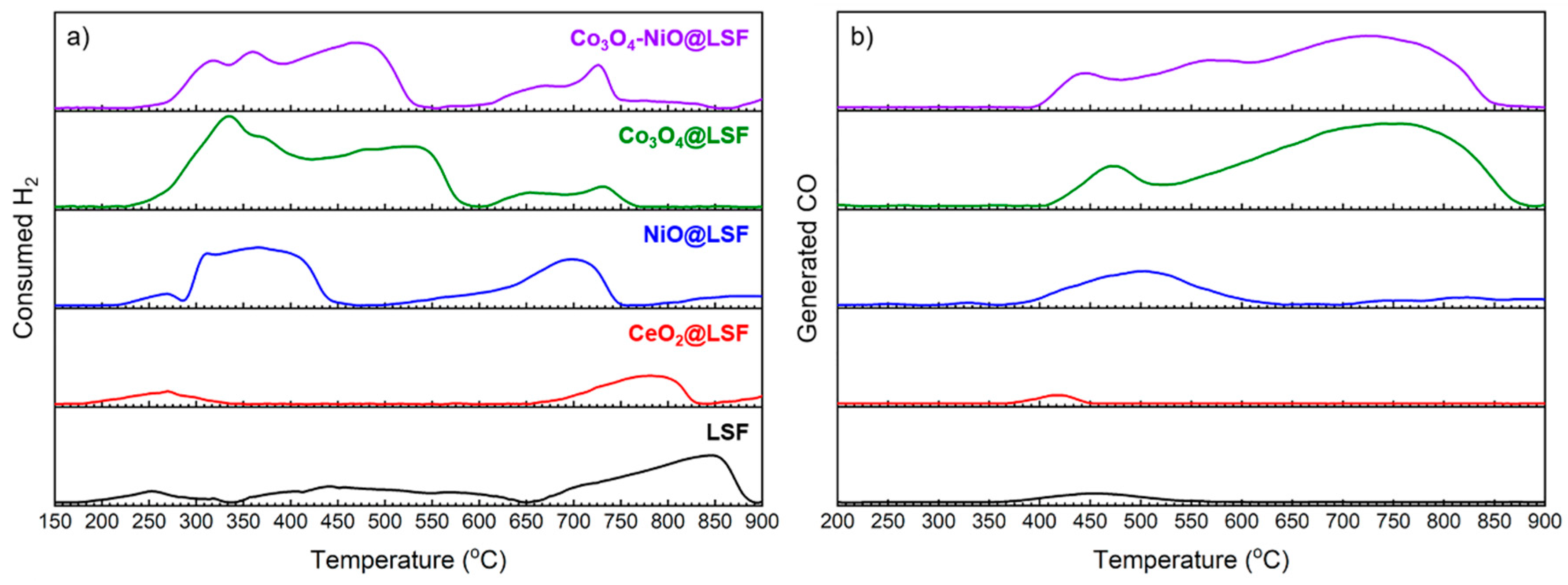
3.3. Temperature-Programmed Processes
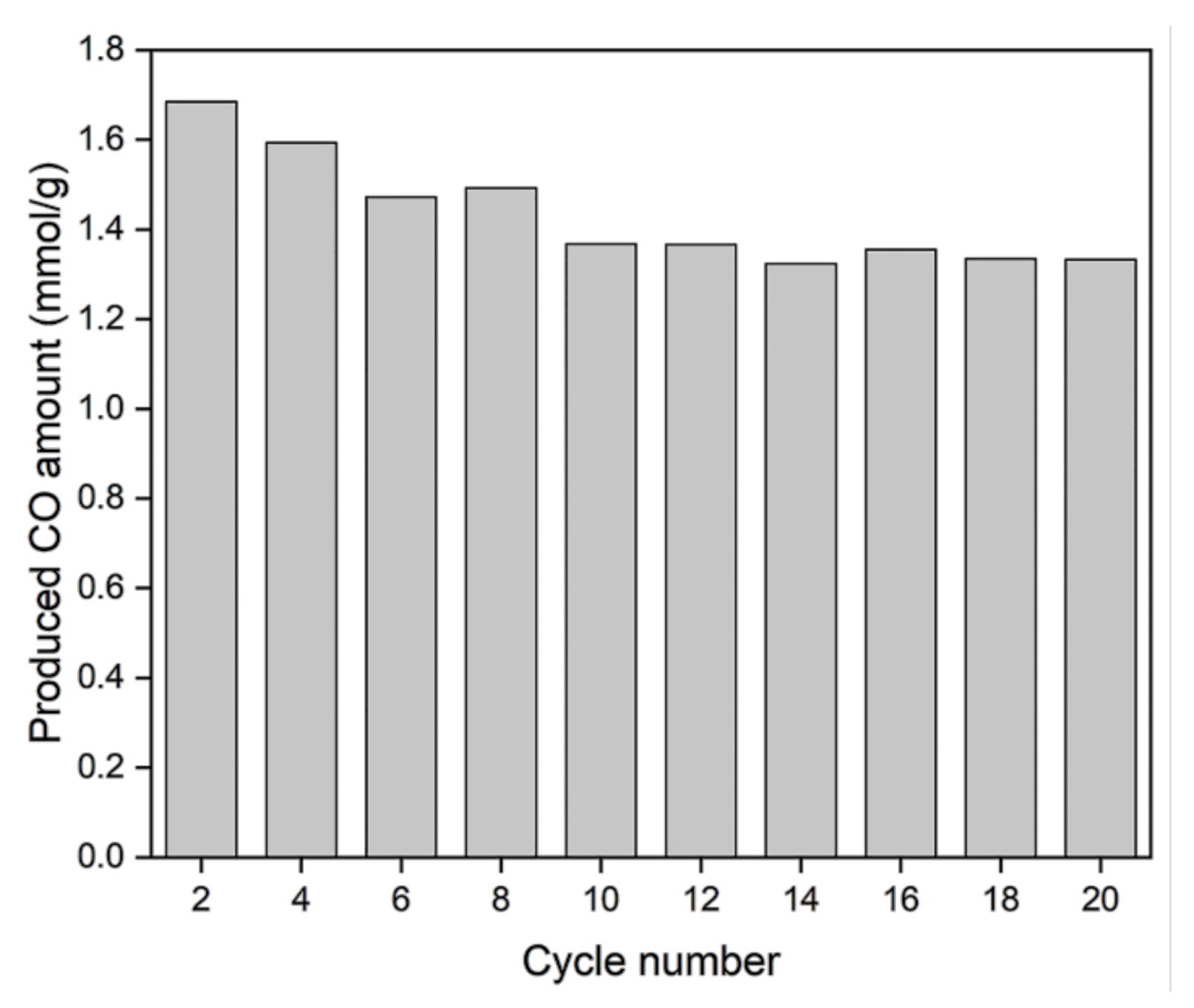
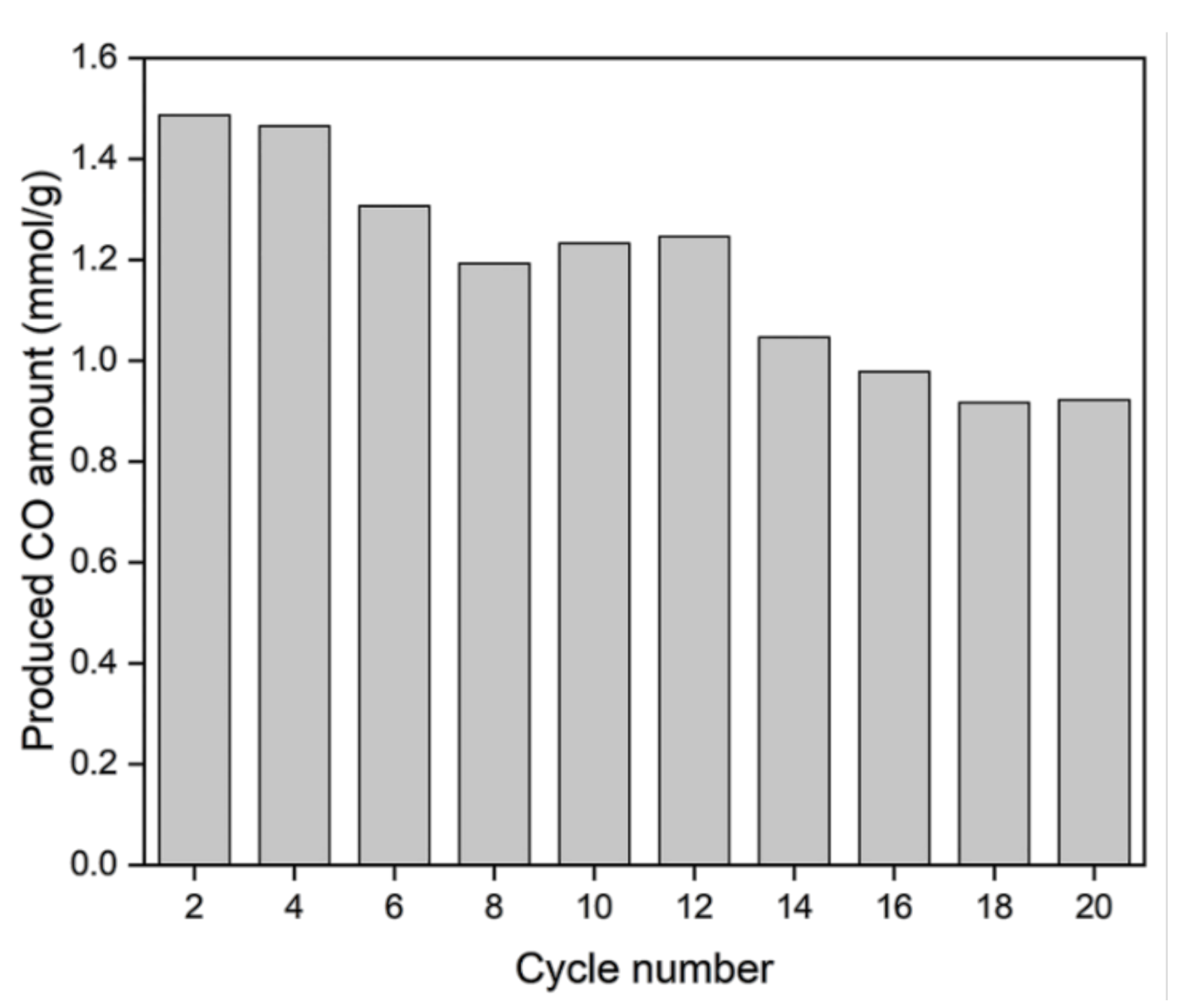
3.4. Long-Term Stability Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Byeon, A.; Baik, S.; Lee, J.W. Enhanced electrocatalytic reduction of oxygen at CO2-derived FeNB-doped porous carbon. J. CO2 Util. 2018, 26, 28–35. [Google Scholar] [CrossRef]
- Tucki, K.; Orynycz, O.; Mitoraj-Wojtanek, M. Perspectives for Mitigation of CO2 Emission due to Development of Electromobility in Several Countries. Energies 2020, 13, 4127. [Google Scholar] [CrossRef]
- Qiu, H.-H.; Liu, L.-G. A Study on the Evolution of Carbon Capture and Storage Technology Based on Knowledge Mapping. Energies 2018, 11, 1103. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J.A.H.W. Trends in Global CO2 Emissions; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2015. [Google Scholar]
- Kim, H.J.; Chun, Y.N. Conversion of Biogas to Renewable Energy by Microwave Reforming. Energies 2020, 13, 4093. [Google Scholar] [CrossRef]
- Olabi, A.G. Renewable energy and energy storage systems. Energy 2017, 136, 1–6. [Google Scholar] [CrossRef]
- Deshmukh, M.K.; Deshmukh, S.S. Modeling of hybrid renewable energy systems. Renew. Sustain. Energy Rev. 2008, 12, 235–249. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Ramani, S.; Ramos, A.E.; Bhethanabotla, V.R.; Kuhn, J.N. Thermochemical conversion of carbon dioxide by reverse water-gas shift chemical looping using supported perovskite oxides. Catal. Today 2019, 323, 225–232. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, G.M.; Lee, J.W. Highly porous N-doped carbons impregnated with sodium for efficient CO2 capture. J. Mater. Chem. A 2015, 3, 10919–10927. [Google Scholar] [CrossRef]
- Siefert, N.S.; Narburgh, S.; Chen, Y. Comprehensive Exergy Analysis of Three IGCC Power Plant Configurations with CO2 Capture. Energies 2016, 9, 669. [Google Scholar] [CrossRef]
- Daza, Y.A.; Maiti, D.; Kent, R.A.; Bhethanabotla, V.R.; Kuhn, J.N. Isothermal reverse water gas shift chemical looping on La0.75Sr0.25Co(1−Y)FeYO3 perovskite-type oxides. Catal. Today 2015, 258, 691–698. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- McDaniel, A.H.; Miller, E.C.; Arifin, D.; Ambrosini, A.; Coker, E.N.; O’Hayre, R.; Chueh, W.C.; Tong, J. Sr- and Mn-doped LaAlO3−δ for solar thermochemical H2 and CO production. Energy Environ. Sci. 2013, 6, 2424–2428. [Google Scholar] [CrossRef]
- Kang, D.; Lim, H.S.; Lee, M.; Lee, J.W. Syngas production on a Ni-enhanced Fe2O3/Al2O3 oxygen carrier via chemical looping partial oxidation with dry reforming of methane. Appl. Energy 2018, 211, 174–186. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.S.; Lee, M.; Lee, J.W. Ni-Fe-Al mixed oxide for combined dry reforming and decomposition of methane with CO2 utilization. Catal. Today 2020. [Google Scholar] [CrossRef]
- Tuci, G.; Filippi, J.; Rossin, A.; Luconi, L.; Pham-Huu, C.; Yakhvarov, D.; Vizza, F.; Giambastiani, G. CO2 Electrochemical Reduction by Exohedral N-Pyridine Decorated Metal-Free Carbon Nanotubes. Energies 2020, 13, 2703. [Google Scholar] [CrossRef]
- Kang, D.; Lee, M.; Lim, H.S.; Lee, J.W. Chemical looping partial oxidation of methane with CO2 utilization on the ceria-enhanced mesoporous Fe2O3 oxygen carrier. Fuel 2018, 215, 787–798. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Herron, J.A.; Kim, J.; Upadhye, A.A.; Huber, G.W.; Maravelias, C.T. A general framework for the assessment of solar fuel technologies. Energy Environ. Sci. 2015, 8, 126–157. [Google Scholar] [CrossRef]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria. Science 2010, 330, 1797. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Daza, Y.A.; Bhethanabotla, V.R.; Kuhn, J.N. Enhanced CO2 Conversion to CO by Silica-Supported Perovskite Oxides at Low Temperatures. ACS Catal. 2018, 8, 3021–3029. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Chen, X.; Duan, H.; Liang, B.; Liu, Q.; Liu, X.; Ren, Y.; Huang, Y.; Zhang, T. Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl. Catal. B Environ. 2017, 216, 95–105. [Google Scholar] [CrossRef]
- Haribal, V.P.; Wang, X.; Dudek, R.; Paulus, C.; Turk, B.; Gupta, R.; Li, F. Modified Ceria for “Low-Temperature” CO2 Utilization: A Chemical Looping Route to Exploit Industrial Waste Heat. Adv. Energy Mater. 2019, 9, 1901963. [Google Scholar] [CrossRef]
- Maiti, D.; Hare, B.J.; Daza, Y.A.; Ramos, A.E.; Kuhn, J.N.; Bhethanabotla, V.R. Earth abundant perovskite oxides for low temperature CO2 conversion. Energy Environ. Sci. 2018, 11, 648–659. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, Y.; Li, M.; Cui, D.; Zhang, S.; Zeng, D.; Xiao, R. Spinel-Structured Ternary Ferrites as Effective Agents for Chemical Looping CO2 Splitting. Ind. Eng. Chem. Res. 2020, 59, 6924–6930. [Google Scholar] [CrossRef]
- Wenzel, M.; Rihko-Struckmann, L.; Sundmacher, K. Thermodynamic analysis and optimization of RWGS processes for solar syngas production from CO2. Aiche J. 2017, 63, 15–22. [Google Scholar] [CrossRef]
- Wenzel, M.; Aditya Dharanipragada, N.V.R.; Galvita, V.V.; Poelman, H.; Marin, G.B.; Rihko-Struckmann, L.; Sundmacher, K. CO production from CO2 via reverse water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide. J. CO2 Util. 2017, 17, 60–68. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; Wei, G.; He, F.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Exploration of Reaction Mechanisms on Hydrogen Production through Chemical Looping Steam Reforming Using NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 11621–11632. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, M.; Kang, D.; Lee, J.W. Role of transition metal in perovskites for enhancing selectivity of methane to syngas. Int. J. Hydrog. Energy 2018, 43, 20580–20590. [Google Scholar] [CrossRef]
- Lee, M.; Lim, H.S.; Kim, Y.; Lee, J.W. Enhancement of highly-concentrated hydrogen productivity in chemical looping steam methane reforming using Fe-substituted LaCoO3. Energy Convers. Manag. 2020, 207, 112507. [Google Scholar] [CrossRef]
- Neal, L.M.; Shafiefarhood, A.; Li, F. Dynamic Methane Partial Oxidation Using a Fe2O3@La0.8Sr0.2FeO3-δ Core–Shell Redox Catalyst in the Absence of Gaseous Oxygen. ACS Catal. 2014, 4, 3560–3569. [Google Scholar] [CrossRef]
- Shafiefarhood, A.; Galinsky, N.; Huang, Y.; Chen, Y.; Li, F. Fe2O3@LaxSr1−xFeO3 Core–Shell Redox Catalyst for Methane Partial Oxidation. ChemCatChem 2014, 6, 790–799. [Google Scholar] [CrossRef]
- Neal, L.; Shafiefarhood, A.; Li, F. Effect of core and shell compositions on MeOx@LaySr1−yFeO3 Core–Shell redox catalysts for chemical looping reforming of methane. Appl. Energy 2015, 157, 391–398. [Google Scholar] [CrossRef]
- Lim, H.S.; Kang, D.; Lee, J.W. Phase transition of Fe2O3–NiO to NiFe2O4 in perovskite catalytic particles for enhanced methane chemical looping reforming-decomposition with CO2 conversion. Appl. Catal. B Environ. 2017, 202, 175–183. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon Dioxide Conversion by Reverse Water–Gas Shift Chemical Looping on Perovskite-Type Oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Rihko-Struckmann, L.K.; Datta, P.; Wenzel, M.; Sundmacher, K.; Dharanipragada, N.V.R.A.; Poelman, H.; Galvita, V.V.; Marin, G.B. Hydrogen and Carbon Monoxide Production by Chemical Looping over Iron-Aluminium Oxides. Energy Technol. 2016, 4, 304–313. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. CeO2-Modified Fe2O3 for CO2 Utilization via Chemical Looping. Ind. Eng. Chem. Res. 2013, 52, 8416–8426. [Google Scholar] [CrossRef]

| Oxygen Carrier | Cations of Metal Oxide | Cations of Perovskite | ||
|---|---|---|---|---|
| ICP-MS | XPS | ICP-MS | XPS | |
| CeO2@LSF | 0.31 | 0.17 | 0.69 | 0.83 |
| NiO@LSF | 0.31 | 0.13 | 0.69 | 0.87 |
| Co3O4@LSF | 0.58 | 0.23 | 0.42 | 0.77 |
| Co3O4-NiO@LSF | 0.53 | 0.29 | 0.47 | 0.71 |
| Oxygen Carrier | Consumed H2 (mmol/gOC) | Generated CO (mmol/gOC) | ||
|---|---|---|---|---|
| Until 600 °C | Until 900 °C | Until 600 °C | Until 900 °C | |
| LSF | 1.75 | 4.72 | 0.28 | 0.29 |
| CeO2@LSF | 0.47 | 1.86 | 0.11 | 0.11 |
| NiO@LSF | 3.79 | 6.14 | 1.27 | 1.58 |
| Co3O4@LSF | 8.18 | 9.06 | 1.48 | 6.26 |
| Co3O4-NiO@LSF | 5.68 | 7.42 | 1.89 | 5.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Kim, Y.; Lim, H.S.; Jo, A.; Kang, D.; Lee, J.W. Reverse Water–Gas Shift Chemical Looping Using a Core–Shell Structured Perovskite Oxygen Carrier. Energies 2020, 13, 5324. https://doi.org/10.3390/en13205324
Lee M, Kim Y, Lim HS, Jo A, Kang D, Lee JW. Reverse Water–Gas Shift Chemical Looping Using a Core–Shell Structured Perovskite Oxygen Carrier. Energies. 2020; 13(20):5324. https://doi.org/10.3390/en13205324
Chicago/Turabian StyleLee, Minbeom, Yikyeom Kim, Hyun Suk Lim, Ayeong Jo, Dohyung Kang, and Jae W. Lee. 2020. "Reverse Water–Gas Shift Chemical Looping Using a Core–Shell Structured Perovskite Oxygen Carrier" Energies 13, no. 20: 5324. https://doi.org/10.3390/en13205324
APA StyleLee, M., Kim, Y., Lim, H. S., Jo, A., Kang, D., & Lee, J. W. (2020). Reverse Water–Gas Shift Chemical Looping Using a Core–Shell Structured Perovskite Oxygen Carrier. Energies, 13(20), 5324. https://doi.org/10.3390/en13205324





