Combustion Modeling and Simulation of Recycled Anode-off-Gas from Solid Oxide Fuel Cell
Abstract
1. Introduction
2. Model Development
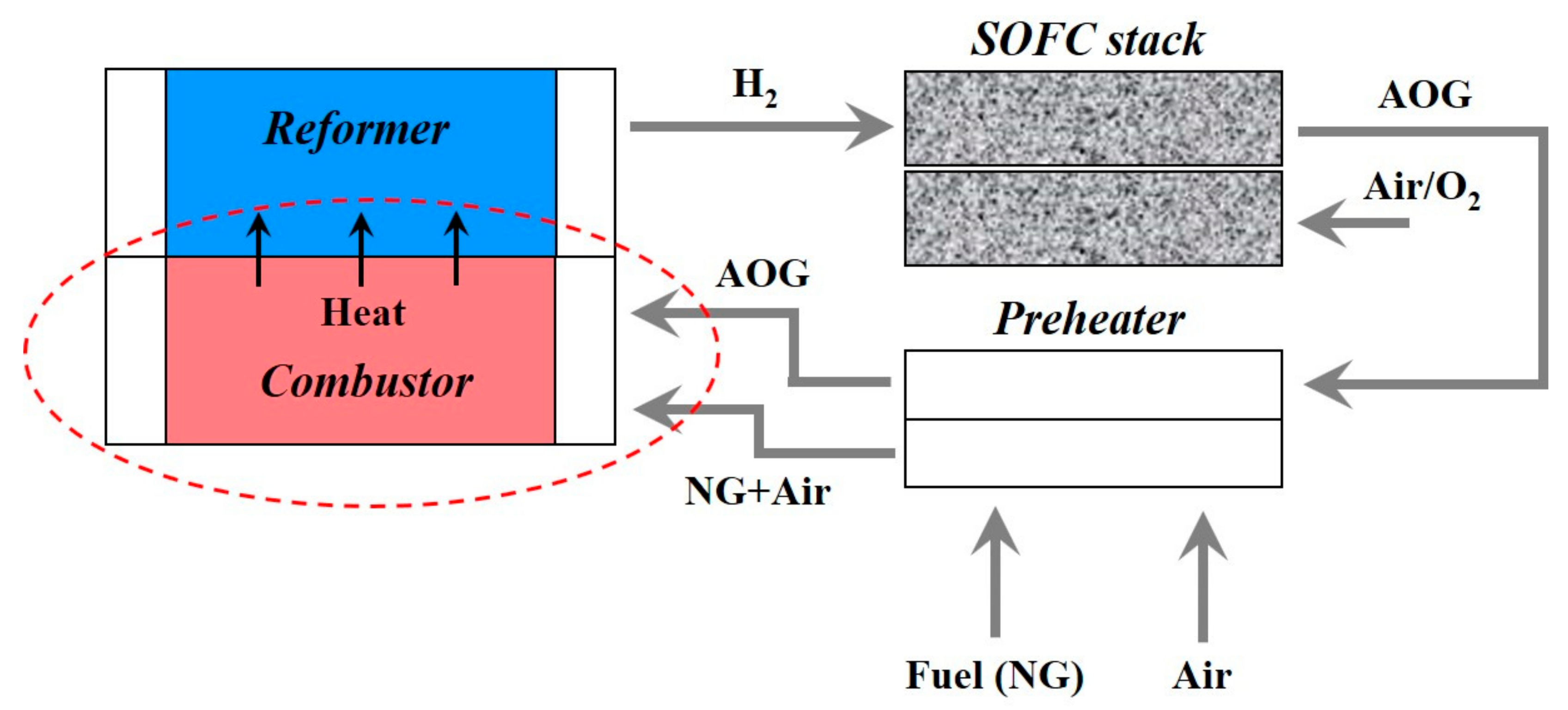
2.1. Integrated Combustor-Reformer Design
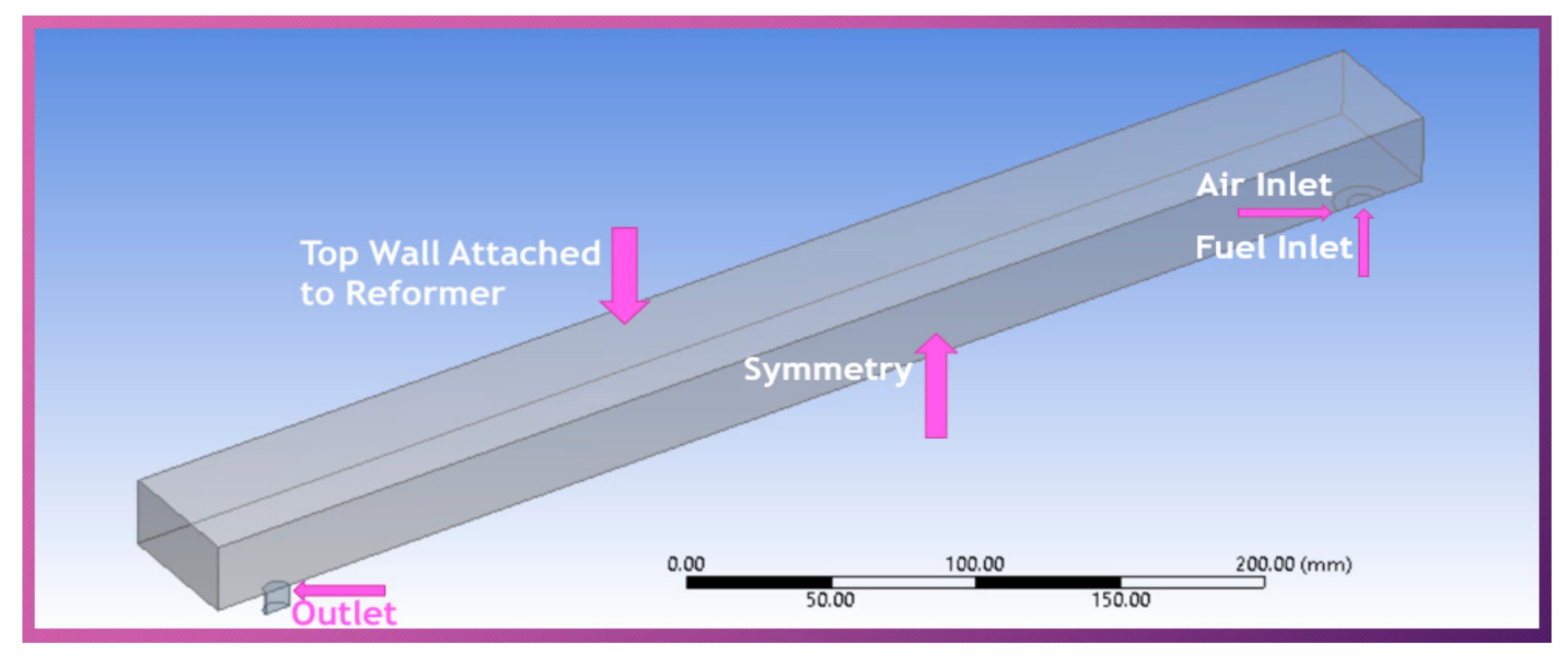
2.2. Geometry Model
2.3. Mathematical Model
2.3.1. Model Assumptions
- Steady state conditions are assumed ignoring temporal variations.
- Adiabatic conditions are assumed at all the boundaries of the combustor except for the wall adjacent to the reformer (top wall in the geometry).
- The heat transfer coefficient is applied to the top wall to draw the heat to an endothermic reforming process. Constant values of heat transfer coefficient and convective fluid temperature are assumed
- Non- catalytic combustion is assumed.
- Cathode off-gases are excluded from the analysis.
- All fluids are assumed to exhibit ideal gas behavior.
2.3.2. Governing Equations
2.3.3. Simulation Procedure
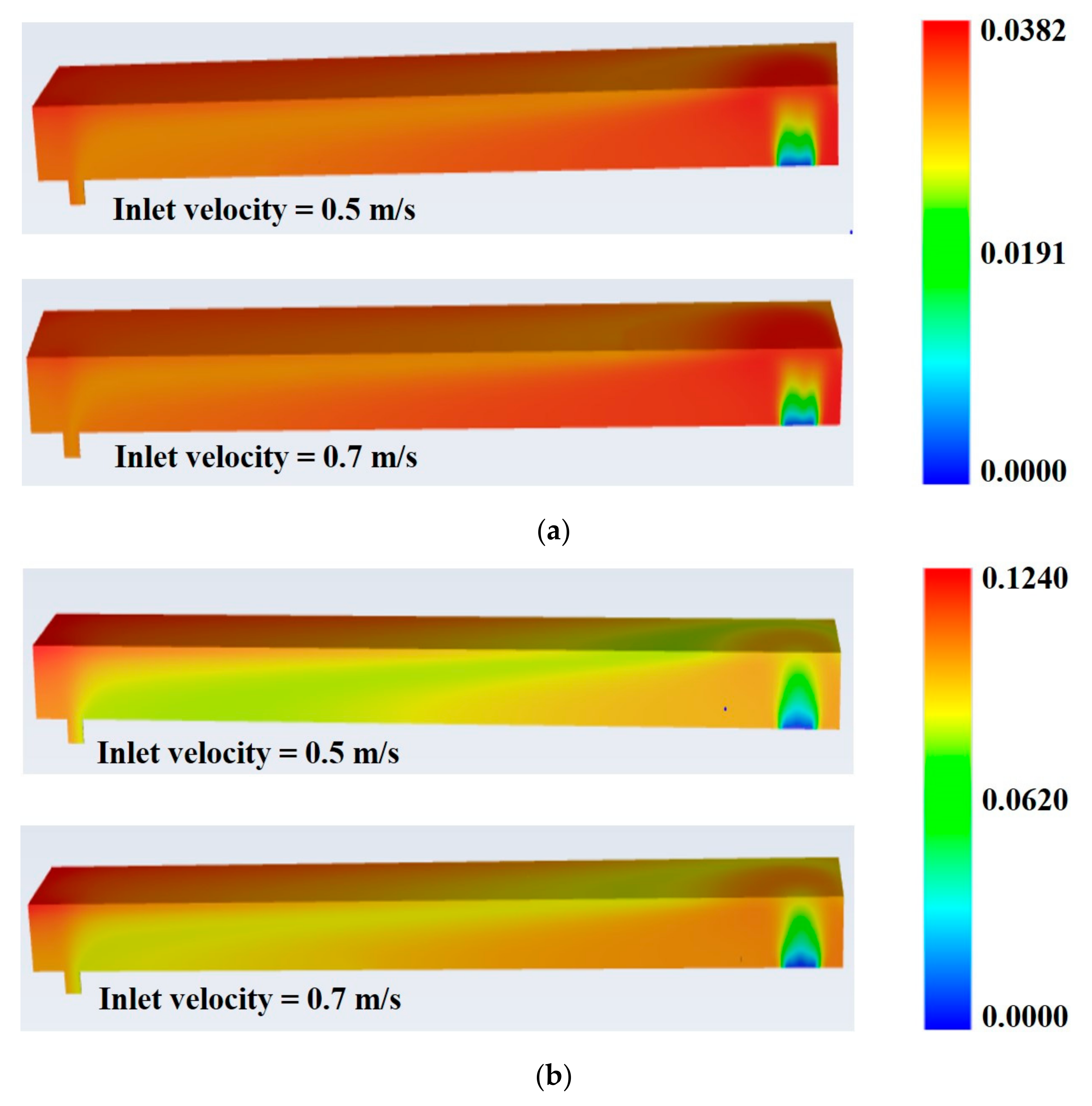
- Case I: Pure natural gas and air (NG + Air) were allowed to enter the combustor as a mixture of fuel and oxidizer, through both the inlets (fuel and air inlets) at 300 K with varying velocities of 0.5 m/s−1 and 0.7 m/s−1. Species fractions were selected based on A/F = 10/1. Intermediate or moderate inlet velocities (0.5 m/s−1 and 0.7 m/s−1) have been selected based on the combustor length (0.5 m). Too fast, and the reactants could not get enough time to get mixed and react within the combustor. Too slow, and the chances of flame out increase.
- Case II: AOG (mainly H2) is fed to the combustor through the fuel inlet while the mixture of NG + Air as an oxidizer through air inlet at 623 K and 573 K, respectively.
- Case III: In this case AOG containing H2O is allowed to enter the combustor at fuel inlet at 623 K while NG + Air enters through air inlet at 573 K.
2.3.4. Model Implementation
3. Results and Discussion
4. Conclusions and Outlook
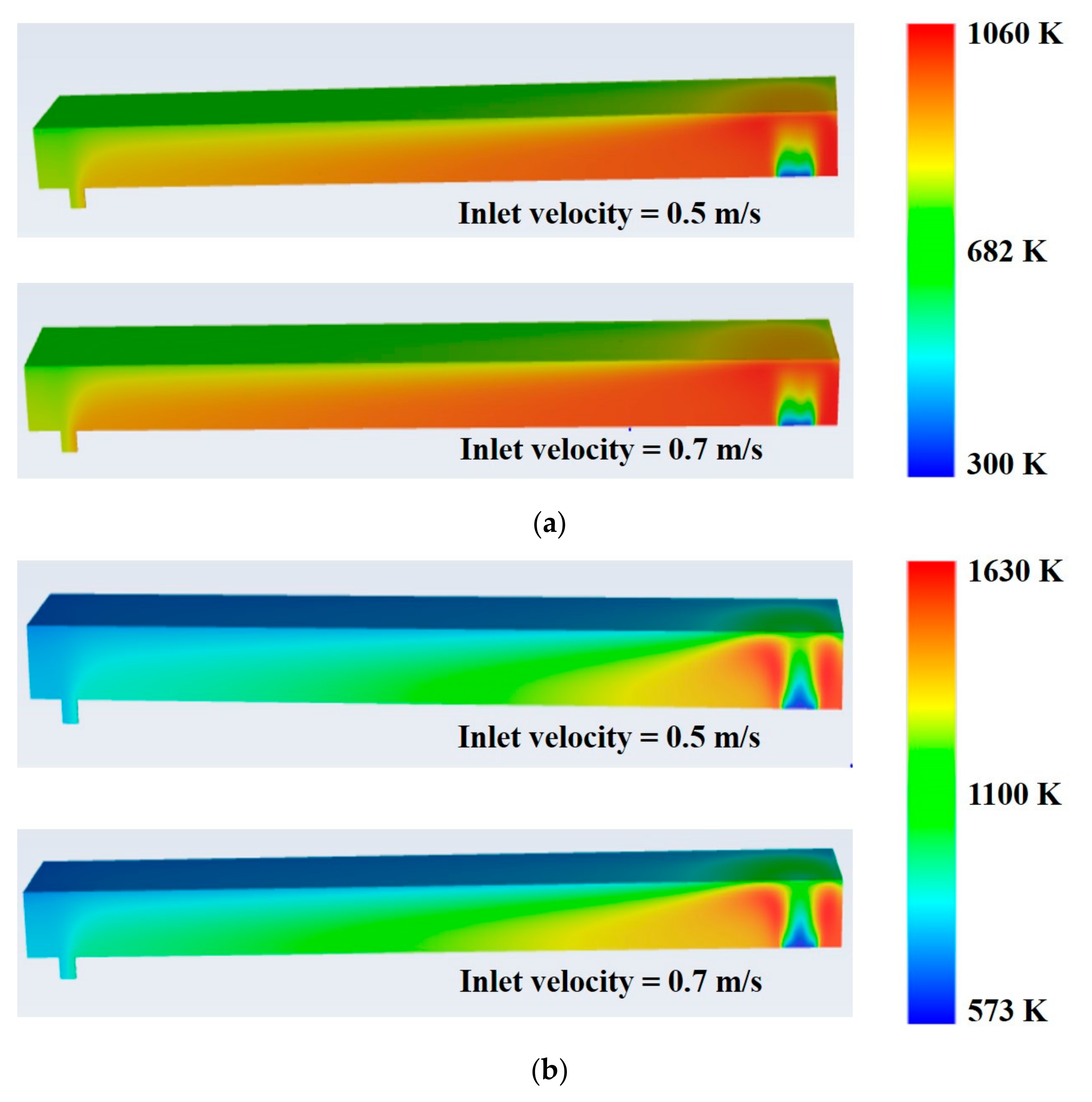
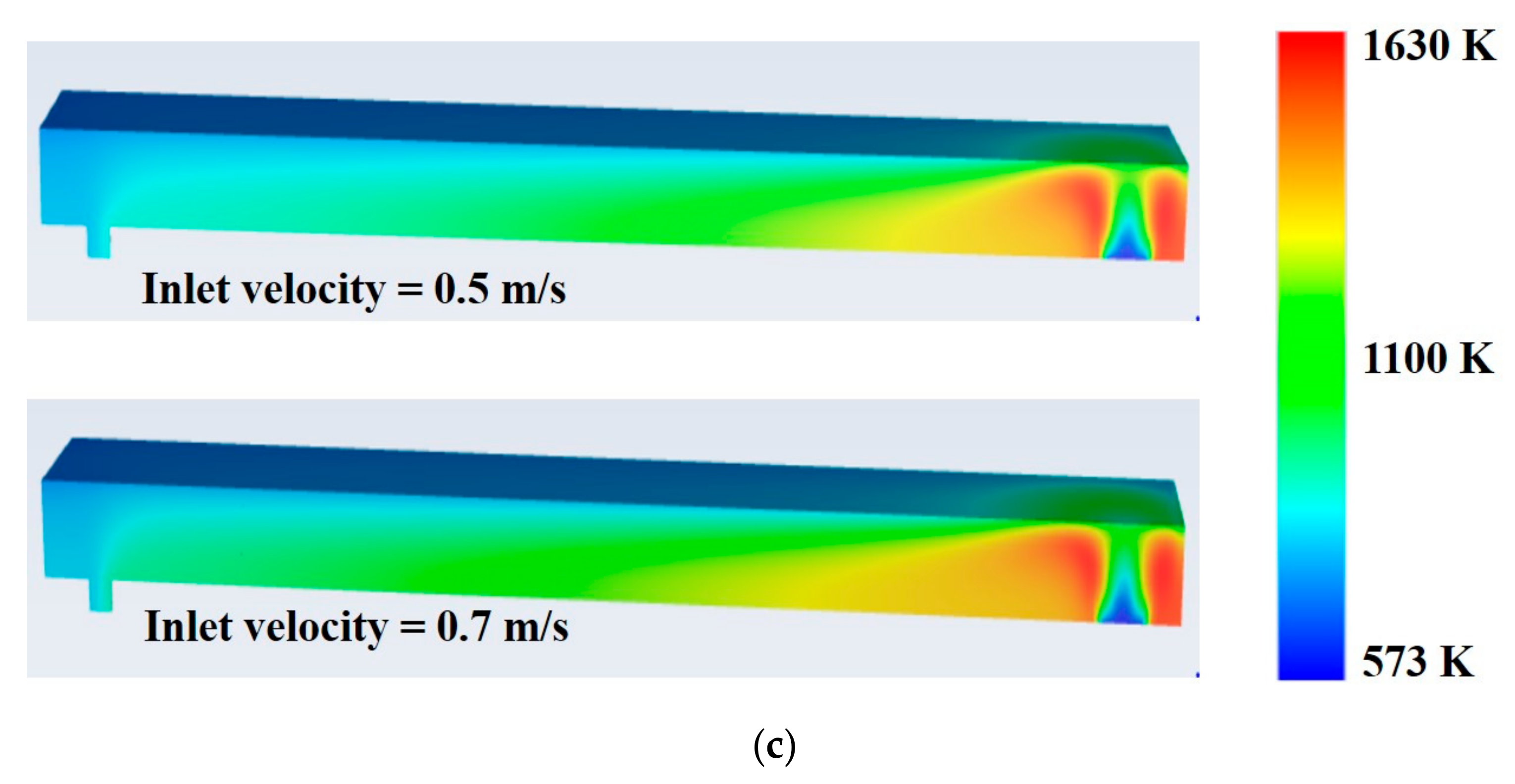
- Average temperature of the combustor is found to have increased by 130 K at 0.5 m/s inlet velocity and 164 K at 0.7 m/s inlet velocity with AOG utilization (case II).
- When increasing the feed temperature, combustion proceeds more efficiently.
- AOG utilization causes a 3% increase in maximum CO2 production and a 6.8% increase in water content.
- A saving of up to 18% in combustor fuel (NG) is affected by AOG utilization along with conventional fuel within the integrated combustor.
- A 38% and 39% increase in the heat flux from the top wall of the combustor attached to the reformer is observed at 0.5 m/s and 0.7 m/s inlet velocity, respectively.
- Maximum and average combustor temperatures slightly increase with increasing inlet velocity of the feed gases.
- Feed inlet velocity of 0.7 m/s is found to be superior to 0.5 m/s regarding improvement in thermal efficiency of the combustor.
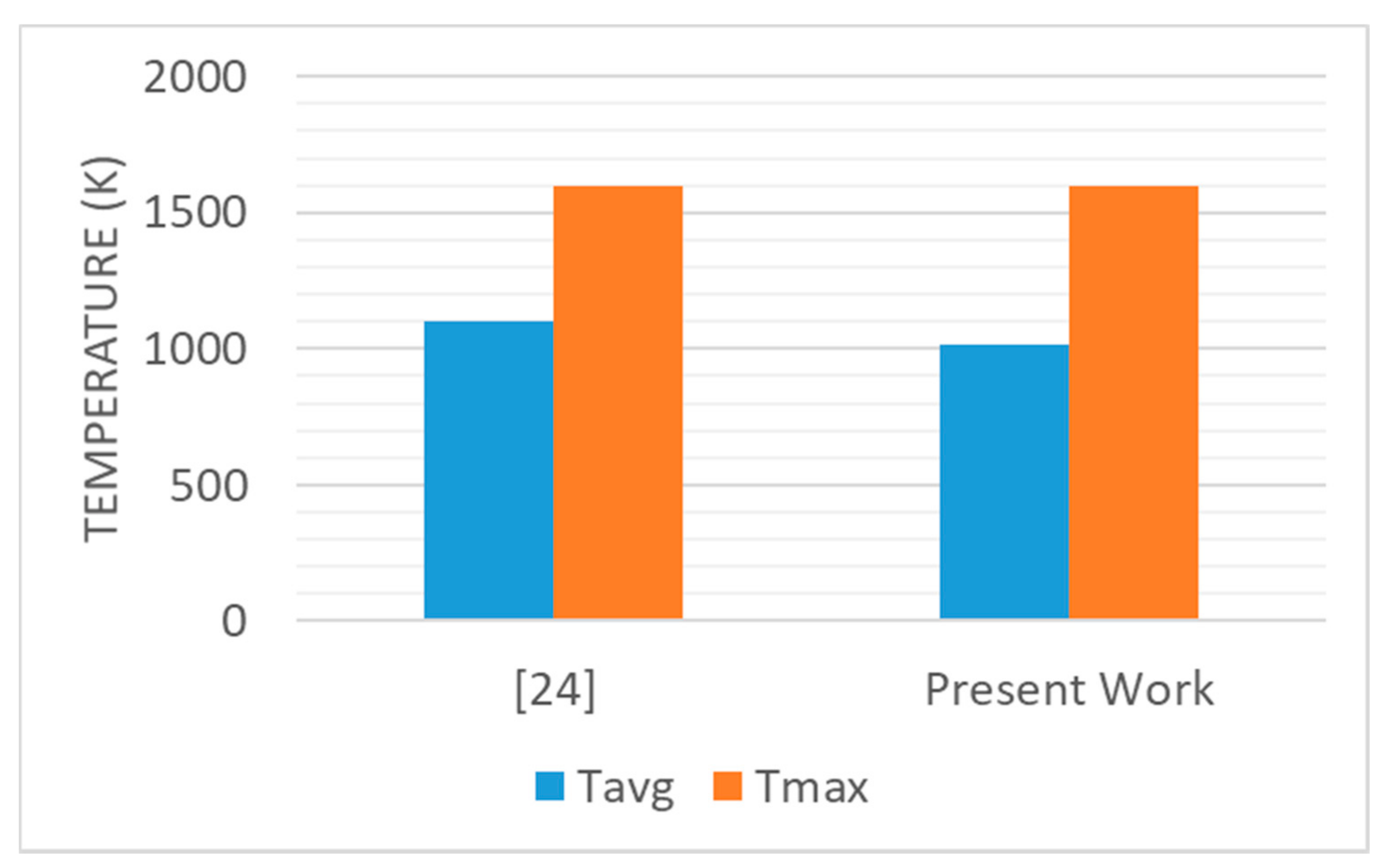
- Simulation results are compared with published data and are found to be in good agreement.
Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Symbols
| xi, xj | direction vectors, [-] |
| ui, uj | velocity vector, [m/s] |
| P | pressure, [Pa] |
| gi | gravitational force, [m/s] |
| Fi | mass force, [N] |
| H | enthalpy, [J/kg] |
| kt | thermal conductivity, [W/m·K] |
| cp | average specific heat, [J/kg.K] |
| Sh | heat source term, [W/m] |
| ft | mass fraction |
| Si | mass source term, [kg/m2·s] |
| density, [kg/m3] | |
| K | turbulence kinetic energy, [m2/s2] |
| Gk | generation of turbulence kinetic energy, [m2/s2] |
| Tf | reforming temperature, [K] |
| Uf | stack fuel utilization factor, [-] |
| hf | Heat transfer co-efficient, [W/m2.K] |
| , | constant, [-] |
| Greek symbols | |
| turbulence Prandtl no. for , [-] | |
| σk | turbulent Prandtl no. for k,[-] |
| µ | viscosity, [Pa·s] |
| µt | turbulent velocity, [Pa·s] |
| stress tensor, [Pa] | |
| Ε | dissipation turbulence kinetic energy, [m2 s−3] |
| Abbreviations | |
| CCS | Carbon Capture and Storage |
| PEMFC | Polymer Electrolyte Membrane Fuel Cell |
| SOFC | Solid Oxide Fuel Cell |
| GHG | Green House Gases |
| HT-PEMFC | High Temperature Polymer Electrolyte Fuel Cell |
| LTS | Low Temperature Water Gas Shift |
| PL | Power Law |
| LH Model | Langmuir Hinshelwood Model |
| MSR | Multistage Reforming |
| CHP | Combined Heating and Power |
| MS-EGC | Multistage Exhaust Gas Combustion |
| AOGR | Anode Off Gas Recycling |
| AOGC | Anode Off Gas Combustion |
| CFD | Computational Fluid Dynamics |
| O/C | Oxygen to Carbon Ratio |
| NG | Natural Gas |
| A/F | Air to Fuel Ratio |
| AOG | Anode Off Gas |
| k−ε | Turbulence model |
| MCFC | Molten Carbonate Fuel Cell |
| AFC | Alkaline Fuel Cell |
| PAFC | Phosphoric Acid Fuel Cell |
| H/C | Hydrogen to Carbon ratio |
| SMR | Steam Methane Reforming |
| PEM | Proton Exchange Membrane |
References
- Fossil Fuels, Environmental and Energy Study Institute (EESI), Washington, DC, USA. Available online: https://www.eesi.org/topics/fossil-fuels/description (accessed on 23 June 2020).
- Denchak, M. Fossil Fuels: The Dirty Facts; Natural Resources Defense Council (NRDC): New York, NY, USA, 2018; Available online: https://www.nrdc.org/stories/fossil-fuels-dirty-facts (accessed on 30 June 2020).
- Renewable Energy, Environmental and Energy Study Institute (EESI), Washington, DC, USA. Available online: https://www.eesi.org/topics/renewable-energy/description (accessed on 25 June 2020).
- Hydrogen Fuel Cells, Renewable Energy, Environmental and Energy Study Institute (EESI), Washington, DC, USA. Available online: https://www.eesi.org/topics/hydrogen-fuel-cells/description (accessed on 25 July 2020).
- Zhang, L.; Xing, Y.; Xu, H.; Wang, H.; Zhong, J.; Xuan, J. Comparative study of solid oxide fuel cell combined heat and power system with Multi-Stage Exhaust Chemical Energy Recycling: Modeling, experiment and optimization. Energy Convers. Manag. 2017, 139, 79–88. [Google Scholar] [CrossRef]
- Ozcan, H.; Dincer, I. Performance evaluation of an SOFC based trigeneration system using various gaseous fuels from biomass gasification. Int. J. Hydrog. Energy 2015, 40, 7798–7807. [Google Scholar] [CrossRef]
- Obara, S. Dynamic-characteristics analysis of an independent microgrid consisting of a SOFC triple combined cycle power generation system and large-scale photovoltaics. Appl. Energy 2015, 141, 19–31. [Google Scholar] [CrossRef]
- Kupecki, J. Off-design analysis of a micro-CHP unit with solid oxide fuel cells fed by DME. Int. J. Hydrog. Energy 2015, 40, 12009–12022. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Jiang, J.; Li, S.; Yang, J.; Li, J. Dynamic modeling and analysis of a 5-kW solid oxide fuel cell system from the perspectives of cooperative control of thermal safety and high efficiency. Int. J. Hydrog. Energy 2015, 40, 456–476. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, J.; Cheng, H.; Deng, Z.; Li, X. Control strategy for power management, efficiency-optimization and operating-safety of a 5-kW solid oxide fuel cell system. Electrochim. Acta 2015, 177, 237–249. [Google Scholar] [CrossRef]
- Gholamian, E.; Zare, V. A comparative thermodynamic investigation with environmental analysis of SOFC waste heat to power conversion employing Kalina and Organic Rankine Cycles. Energy Convers. Manag. 2016, 117, 150–161. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Moradpoor, I. Combined solid oxide fuel cell, micro-gas turbine and organic Rankine cycle for power generation (SOFC–MGT–ORC). Energy Convers. Manag. 2016, 116, 120–133. [Google Scholar] [CrossRef]
- Lee, K.; Yun, J.; Ahn, K.; Lee, S.; Kang, S.; Yu, S. Operational characteristics of a planar steam reformer thermally coupled with a catalytic burner. Int. J. Hydrog. Energy 2013, 38, 4767–4775. [Google Scholar] [CrossRef]
- Powell, M.; Meinhardt, K.; Sprenkle, V.; Chick, L.; McVay, G. Demonstration of a highly efficient solid oxide fuel cell power system using adiabatic steam reforming and anode gas recirculation. J. Power Sources 2012, 205, 377–384. [Google Scholar] [CrossRef]
- Peters, R.; Deja, R.; Engelbracht, M.; Frank, M.; Nguyen, V.N.; Blum, L.; Stolten, D. Efficiency analysis of a hydrogen-fueled solid oxide fuel cell system with anode off-gas recirculation. J. Power Sources 2016, 328, 105–113. [Google Scholar] [CrossRef]
- Halinen, M.; Thomann, O.; Kiviaho, J. Effect of Anode off-gas Recycling on Reforming of Natural Gas for Solid Oxide Fuel Cell Systems. Fuel Cells 2012, 12, 754–760. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, P.H.; Park, C.S.; Park, B.I.; Hwang, S.S. Combustion characteristics of mixture of anode off gas and LNG in reformer. Int. J. Hydrog. Energy 2011, 36, 5181–5188. [Google Scholar] [CrossRef]
- Ilbas, M.; Yılmaz, İ.; Kaplan, Y. Investigations of hydrogen and hydrogen-hydrocarbon composite fuel combustion and NOx emission characteristics in a model combustor. Int. J. Hydrog. Energy 2015, 30, 1139–1147. [Google Scholar] [CrossRef]
- Sarioglan, A.; Korkmaz, Ö.; Kaytaz, A.; Akar, E.; Akgun, F. A 5 kWt catalytic burner for PEM fuel cells: Effect of fuel type, fuel content and fuel loads on the capacity of the catalytic burner. Int. J. Hydrog. Energy 2010, 35, 11855–11860. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Minh, N. Solid oxide fuel cell technology? Features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Gardemann, U.; Roes, J.; Heinzel, A. A Pollutant emissions of burners for steam reformers for residential power supply. Int. J. Hydrog. Energy 2011, 36, 5189–5199. [Google Scholar] [CrossRef]
- Dwook, K.; Taehyun, J.; Bonchan, K.; Hyunkyoo, S. Combustion characteristics of anoe off-gas on the steam reforming performance. Int. J. Hydrog. Energy 2019, 44, 4688–4697. [Google Scholar]
- Pianko-Oprych, P.; Jaworski, Z. Numerical investigation of a novel burner to combust anode exhaust gases of SOFC stacks. Pol. J. Chem. Technol. 2017, 19, 20–26. [Google Scholar] [CrossRef]
- Stylianidis, N.; Azimov, U.; Birkett, M. Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism. Energies 2019, 12, 2442. [Google Scholar] [CrossRef]
- Lingstädt, T.; Grimm, F.; Krummrein, T.; Bücheler, S.; Aigner, M. Experimental investigation of an sofc off-gas combustor for hybrid power plant usage with low heating values realised by natural gas addition. In Proceedings of the GPPS Forum 18, Global Power and Propulsion Society, Montreal, QC, Canada, 7–9 May 2018. [Google Scholar]









| Type | Governing Equations | No. | Source |
|---|---|---|---|
| Mass Balance | (1) | [24] | |
| Momentum Balance | (2) | [24] | |
| Energy Balance | (3) | [24] | |
| Species Balance | (4) | [24] | |
| Turbulent K.E. | (5) | [24] | |
| Dissipation K.E. | + | (6) | [24] |
| Reactions | Pre-Exponential Factor (ko) (kmol∙m−3∙s−1) | Activation Energy (E) (J∙kmol−1) | Reaction Enthalpy (ΔHr) (kJ∙mol−1) | No. | Source |
|---|---|---|---|---|---|
| 4.4 × 1011 | 1.255 × 108 | −36 * | (7) | [24] | |
| 2.24 × 1012 | 1.703 × 108 | −283 * | (8) | [24] | |
| 5.68 × 1011 | 1.46 × 108 | −241.8 * | (9) | [24] | |
| 6.186 × 109 | 1.256 × 108 | −1427.7 * | (10) | [24] | |
| 6.186 × 109 | 1.256 × 108 | −2043 * | (11) | [24] |
| Case | Inlet Velocity (m/s) | Inlet Temperature [K] | ||
|---|---|---|---|---|
| AOG | NG+ Air | AOG | NG + Air | |
| Case I | - | 0.5, 0.7 | - | 300 |
| Case II | 0.5, 0.7 | 0.5, 0.7 | 623 | 573 |
| Case III | 0.5, 0.7 | 0.5, 0.7 | 623 | 573 |
| Species | Mass Fraction (kg/kg) | |||||
|---|---|---|---|---|---|---|
| Case I | Case II | Case III | ||||
| AOG | NG + Air | AOG | NG + Air | AOG | NG + Air | |
| CH4 | - | 0.085 | - | 0.085 | - | 0.085 |
| C2H6 | - | 0.01 | - | 0.01 | - | 0.01 |
| C3H8 | - | 0.005 | - | 0.005 | - | 0.005 |
| O2 | - | 0.207 | - | 0.207 | - | 0.207 |
| H2 | - | - | 0.35 | - | 0.35 | - |
| H2O | - | - | - | - | 0.25 | - |
| Case | Inlet Velocity = 0.5 m/s | Inlet Velocity = 0.7 m/s | ||||||
|---|---|---|---|---|---|---|---|---|
| Tmax, K | Tavg, K | Tout, K | CO2, % | Tmax, K | Tavg, K | Tout, K | CO2, % | |
| Case I | 1050 | 884 | 826 | 8.4 | 1060 | 908 | 850 | 8.0 |
| Case II | 1601 | 1014 | 861 | 11.3 | 1636 | 1072 | 301 | 10.9 |
| Case III | 1613 | 1018 | 860 | 11.6 | 1647 | 1078 | 901 | 10.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashmiri, S.A.; Tahir, M.W.; Afzal, U. Combustion Modeling and Simulation of Recycled Anode-off-Gas from Solid Oxide Fuel Cell. Energies 2020, 13, 5186. https://doi.org/10.3390/en13195186
Kashmiri SA, Tahir MW, Afzal U. Combustion Modeling and Simulation of Recycled Anode-off-Gas from Solid Oxide Fuel Cell. Energies. 2020; 13(19):5186. https://doi.org/10.3390/en13195186
Chicago/Turabian StyleKashmiri, Sataish Asghar, Muhammad Wasim Tahir, and Umer Afzal. 2020. "Combustion Modeling and Simulation of Recycled Anode-off-Gas from Solid Oxide Fuel Cell" Energies 13, no. 19: 5186. https://doi.org/10.3390/en13195186
APA StyleKashmiri, S. A., Tahir, M. W., & Afzal, U. (2020). Combustion Modeling and Simulation of Recycled Anode-off-Gas from Solid Oxide Fuel Cell. Energies, 13(19), 5186. https://doi.org/10.3390/en13195186






