Systematic Analysis of Materials for Coated Adsorbers for Application in Adsorption Heat Pumps or Refrigeration Systems
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Material Compatibility
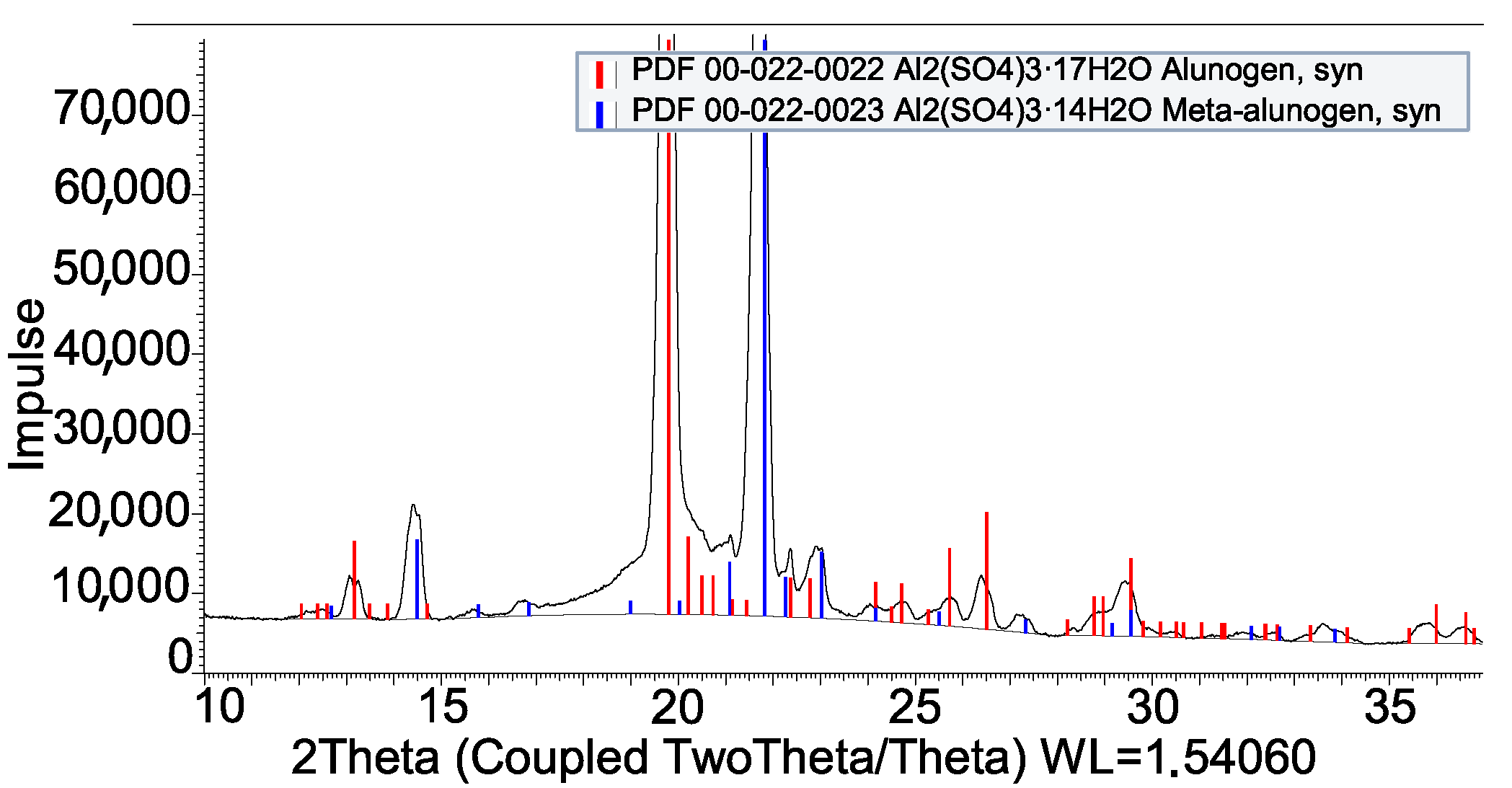
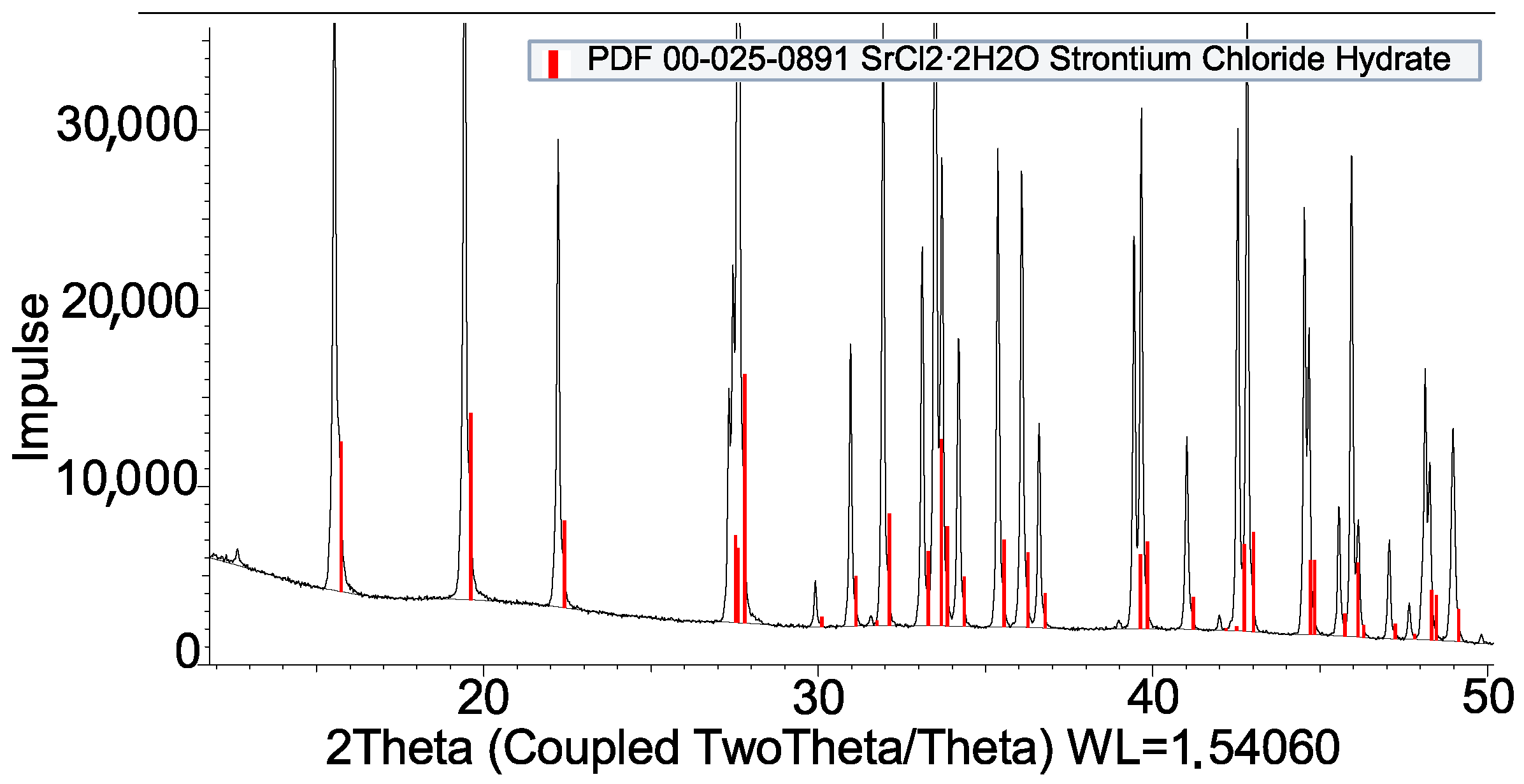
3.2. Coating by Reaction
3.3. Composites with Binding Material
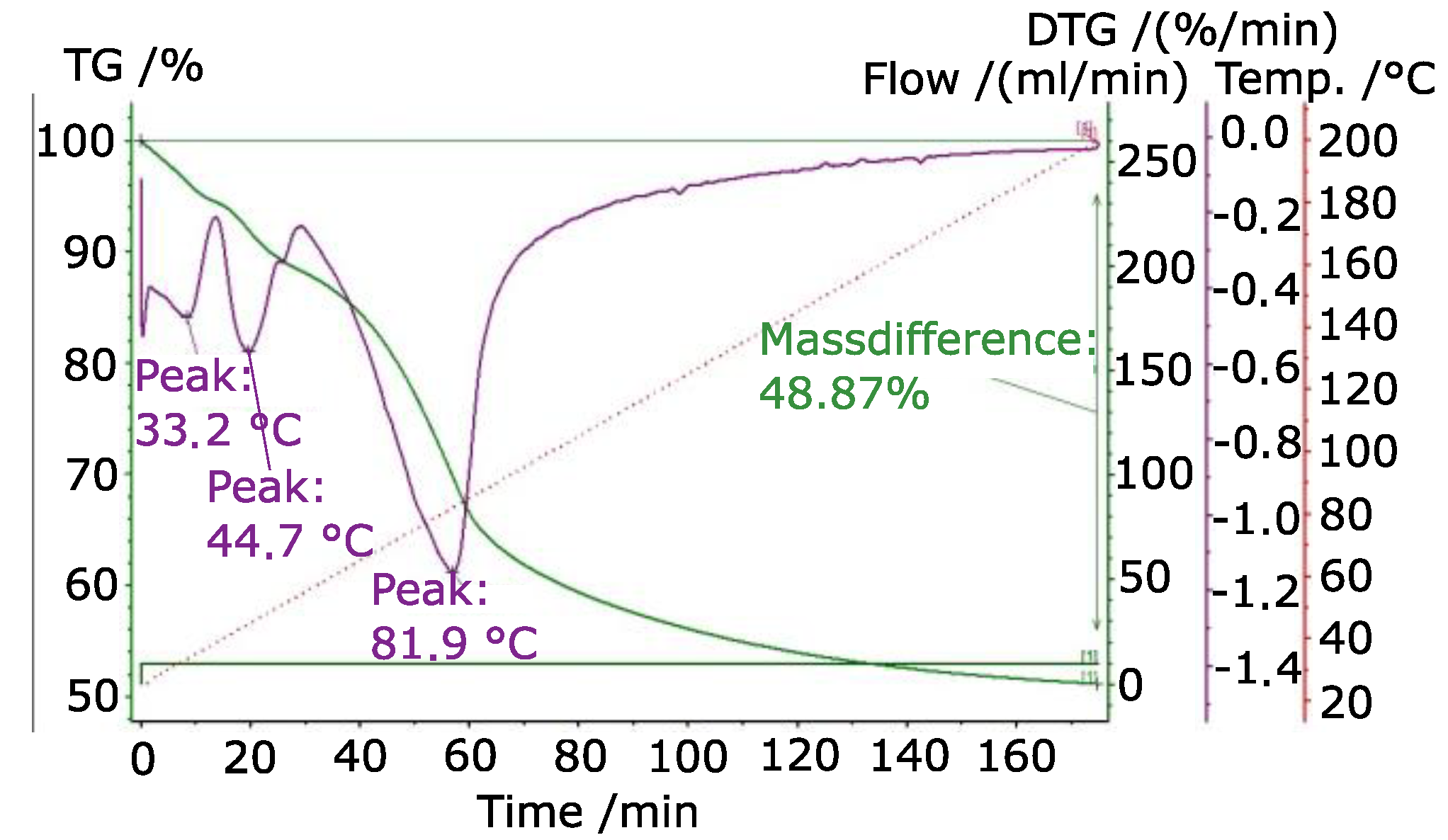
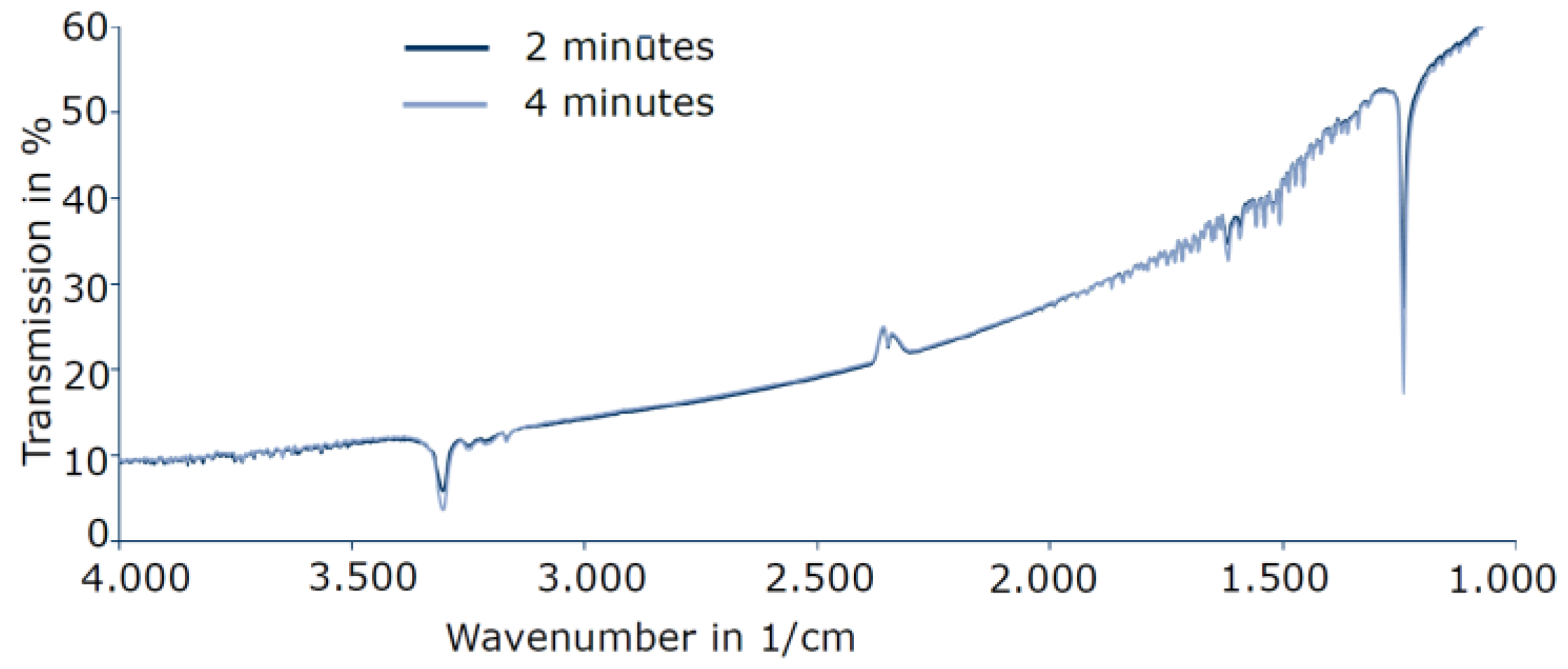
3.4. TPD
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, R.; Oliveira, R.G. Adsorption refrigeration—An efficient way to make good use of waste heat and solar energy. Prog. Energy Combust. Sci. 2006, 32, 424–458. [Google Scholar] [CrossRef]
- Wang, R.Z.; Wang, L.W.; Wu, J.Y. Adsorption Refrigeration Theory and Applications; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Dias, J.M.; Costa, V.A.F. Adsorption heat pumps for heating applications: A review of current state, literature gaps and development challenges. Renew. Sustain. Energy Rev. 2018, 98, 317–327. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Luo, W.; Wang, R. Experimental study on working pairs for two-stage chemisorption freezing cycle. Renew. Energy 2015, 74, 287–297. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Sole, A.; Cabeza, L.F. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schossig, P.; Henning, H.-M. Novel Sorption Materials for Solar Heating and Cooling. Energy Procedia 2012, 30, 279–288. [Google Scholar] [CrossRef]
- Aristov, Y. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Capri, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Osterland, T.; Priehs, B.; Opel, O.; Ruck, W.K. Thermochemical heat storage materials—Performance of mixed salt hydrates. Sol. Energy 2016, 136, 571–589. [Google Scholar] [CrossRef]
- Lee, J.S.; Yoon, J.W.; Mileo, P.G.M.; Cho, K.H.; Park, J.; Kim, K.; Kim, H.; De Lange, M.F.; Kapteijn, F.; Maurin, G.; et al. Porous Metal–Organic Framework CUK-1 for Adsorption Heat Allocation toward Green Applications of Natural Refrigerant Water. ACS Appl. Mater. Interfaces 2019, 11, 25778–25789. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.; Wang, L. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Guilleminot, J.J.; Chalfen, J.B.; Poyelle, F. Transfers de masse et de chaleur dans les composites adsorbent consolides. Influence sur les performances d’une pompe a chaleur a adsorption solide. In Proceedings of the 19th International Conference of Refrigeration, The Hague, The Netherlands, 20–25 August 1995. [Google Scholar]
- Yuan, Y.; Bao, H.S.; Ma, Z.; Lu, Y.; Roskilly, A.P. Investigation of equilibrium and dynamic performance of SrCl2-expanded graphite composite in chemisorption refrigeration system. Appl. Therm. Eng. 2019, 147, 52–60. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Freni, A.; Proverbio, E.; Restuccia, G. Synthesis of SAPO-34 on graphite foams for adsorber heat exchangers. Appl. Therm. Eng. 2013, 61, 848–852. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Proverbio, E.; Freni, A.; Restuccia, G. In situ Growth of Zeolites on Metal Foamed Supports for Adsorption Heat Pumps. J. Chem. Eng. Jpn. 2007, 40, 1307–1312. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Proverbio, E.; Maggio, G.; Restuccia, G. Zeolite synthesised on copper foam for adsorption chillers: A mathematical model. Microporous Mesoporous Mater. 2009, 120, 402–409. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Z. Heat transfer enhancement of high temperature thermal energy storage using metal foams and expanded graphite. Sol. Energy Mater. Sol. Cells 2011, 95, 636–643. [Google Scholar] [CrossRef]
- Wittstadt, U.; Füldner, G.; Andersen, O.; Herrmann, R.; Schmidt, F.P. A New Adsorbent Composite Material Based on Metal Fiber Technology and Its Application in Adsorption Heat Exchangers. Energies 2015, 8, 8431–8446. [Google Scholar] [CrossRef]
- Fortes, A.D.; Wood, I.G.; Vočadlo, L.; Brand, H.E.A.; Knight, K.S. Crystal structures and thermal expansion of α-MgSO4 and β-MgSO4 from 4.2 to 300 K by neutron powder diffraction. J. Appl. Crystallogr. 2007, 40, 761–770. [Google Scholar] [CrossRef]
- Freni, A.; Frazzica, A.; Dawoud, B.; Chmielewski, S.; Calabrese, L.; Bonaccorsi, L. Adsorbent coatings for heat pumping applications: Verification of hydrothermal and mechanical stabilities. Appl. Therm. Eng. 2013, 50, 1658–1663. [Google Scholar] [CrossRef]
- Schnabel, L.; Tatlıer, M.; Schmidt, F.; Erdem-Şenatalar, A.; Tatlier, M. Adsorption kinetics of zeolite coatings directly crystallized on metal supports for heat pump applications (adsorption kinetics of zeolite coatings). Appl. Therm. Eng. 2010, 30, 1409–1416. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Proverbio, E.; Freni, A. SAPO-34 based zeolite coatings for adsorption heat pumps. Energy 2019, 187, 115981. [Google Scholar] [CrossRef]
- Saeed, A.; Al-Alili, A. A review on desiccant coated heat exchangers. Sci. Technol. Built Environ. 2016, 23, 136–150. [Google Scholar] [CrossRef]
- Van Heyden, H.; Munz, G.; Schnabel, L.; Schmidt, F.; Mintova, S.; Bein, T. Kinetics of water adsorption in microporous aluminophosphate layers for regenerative heat exchangers. Appl. Therm. Eng. 2009, 29, 1514–1522. [Google Scholar] [CrossRef]
- Donkers, P.; Sögütoglu, L.; Huinink, H.; Fischer, H.; Adan, O. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Kahlenberg, V.; Braun, D.E.; Krüger, H.; Schmidmair, D.; Orlova, A. Temperature- and moisture-dependent studies on alunogen and the crystal structure of meta-alunogen determined from laboratory powder diffraction data. Phys. Chem. Miner. 2016, 44, 95–107. [Google Scholar] [CrossRef]
- Çılgı, G.K.; Cetisli, H. Thermal decomposition kinetics of aluminum sulfate hydrate. J. Therm. Anal. Calorim. 2009, 98, 855–861. [Google Scholar] [CrossRef]
- Van Essen, V.M.; Gores, J.C.; Bleijendaal, L.P.J.; Zondag, H.; Schuitema, R.; Bakker, M.; Van Helden, W.G.J. Characterization of Salt Hydrates for Compact Seasonal Thermochemical Storage, Proceedings of the ASME 2009 3rd International Conference on Energy Sustainability, San Francisco, CA, USA, 19–23 July 2009; ASME International: New York, NY, USA, 2009; Volume 2, pp. 825–830. [Google Scholar]
- Fortes, A.D.; Wood, I.G.; Alfredsson, M.; Vocadlo, L.; Knight, K.S. The thermoelastic properties of MgSO47D2O (epsomite) from powder neutron diffraction and ab initio calculation. Eur. J. Miner. 2006, 18, 449–462. [Google Scholar] [CrossRef]
- Grevel, K.-D.; Majzlan, J. Internally consistent thermodynamic data for magnesium sulfate hydrates. Geochim. Cosmochim. Acta 2009, 73, 6805–6815. [Google Scholar] [CrossRef]
- Schuitema, R.; van Helden, W.G.J.; Zondag, H.A.; van Essen, V.M.; Bleijendaal, L.P.J.; Cot Gores, J.; Planje, W.; Epema, T.; Oversloot, H. Comparison of reactor concepts for thermochemical storage of solar heat. In Proceedings of the 3rd International Renewable Energy Storage Conference (IRES 2008), Berlin, Germany, 24–25 November 2008. [Google Scholar]
- Ferchaud, C.; Zondag, H.A.; de Boer, R.; Rindt CC, M. Characterization of the sorption process in thermochemical materials for seasonal solar heat storage application. In Proceedings of the 12th international conference on energy storage (Innostock 2012), Lleida, Spain, 16–18 May 2012. [Google Scholar]
- Donkers, P.; Pel, L.; Adan, O. Experimental studies for the cyclability of salt hydrates for thermochemical heat storage. J. Energy Storage 2016, 5, 25–32. [Google Scholar] [CrossRef]
- Wang, T.; Debelak, K.A.; Roth, J.A. Dehydration of iron(II) sulfate heptahydrate. Thermochim. Acta 2007, 462, 89–93. [Google Scholar] [CrossRef]
- Kanari, N.; Menad, N.-E.; Ostrosi, E.; Shallari, S.; Diot, F.; Allain, E.; Yvon, J. Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres. Metals 2018, 8, 1084. [Google Scholar] [CrossRef]
- Fortier, H.; Westreich, P.; Selig, S.; Zelenietz, C.; Dahn, J. Ammonia, cyclohexane, nitrogen and water adsorption capacities of an activated carbon impregnated with increasing amounts of ZnCl2, and designed to chemisorb gaseous NH3 from an air stream. J. Colloid Interface Sci. 2008, 320, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Rusch, A.W. Identifying Hydrated Salts Using Simultaneous Thermogravimetric Analysis and Differential Scanning Calorimetry. J. Chem. Educ. 2012, 90, 235–238. [Google Scholar] [CrossRef]
- Kuwata, K.; Masuda, S.; Kobayashi, N.; Fuse, T.; Okamura, T. Thermochemical Heat Storage Performance in the Gas/Liquid-Solid Reactions of SrCl2 with NH3. Nat. Resour. 2016, 7, 655–665. [Google Scholar]
- Gaeini, M.; Shaik, S.A.; Rindt, C.C.M. Characterization of potassium carbonate salt hydrate for thermochemical energy storage in buildings. Energy Build. 2019, 196, 178–193. [Google Scholar] [CrossRef]
- Sögütoglu, L.; Donkers, P.; Fischer, H.; Huinink, H.; Adan, O. In-depth investigation of thermochemical performance in a heat battery: Cyclic analysis of K2CO3, MgCl2 and Na2S. Appl. Energy 2018, 215, 159–173. [Google Scholar] [CrossRef]
- D’Ans, P.; Courbon, E.; Frère, M.; Descy, G.; Segato, T.; Degrez, M. Severe corrosion of steel and copper by strontium bromide in thermochemical heat storage reactors. Corros. Sci. 2018, 138, 275–283. [Google Scholar] [CrossRef]
- Farrell, A.J.; Norton, B.; Kennedy, D.M. Corrosive effects of salt hydrate phase change materials used with aluminium and copper. J. Mater. Process. Technol. 2006, 175, 198–205. [Google Scholar] [CrossRef]
- Moreno, P.; Miró, L.; Sole, A.; Cabeza, L.F.; Solé, C.; Martorell, I.; Cabeza, L.F. Corrosion of metal and metal alloy containers in contact with phase change materials (PCM) for potential heating and cooling applications. Appl. Energy 2014, 125, 238–245. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Wang, Z.; Shi, Z.; Gao, B.; Hu, X.; Tao, W.; Liu, F.; Yu, J. Study on the thermal decomposition of aluminium chloride hexahydrate. Can. Met. Q. 2017, 57, 235–244. [Google Scholar] [CrossRef]
- Glasser, L. Thermodynamics of Inorganic Hydration and of Humidity Control, with an Extensive Database of Salt Hydrate Pairs. J. Chem. Eng. Data 2014, 59, 526–530. [Google Scholar] [CrossRef]
- Van Essen, V.M.; Zondag, H.A.; Gores, J.C.; Bleijendaal, L.P.J.; Bakker, M.; Schuitema, R.; Van Helden, W.G.J.; He, Z.; Rindt, C.C.M. Characterization of MgSO4 Hydrate for Thermochemical Seasonal Heat Storage. J. Sol. Energy Eng. 2009, 131. [Google Scholar] [CrossRef]
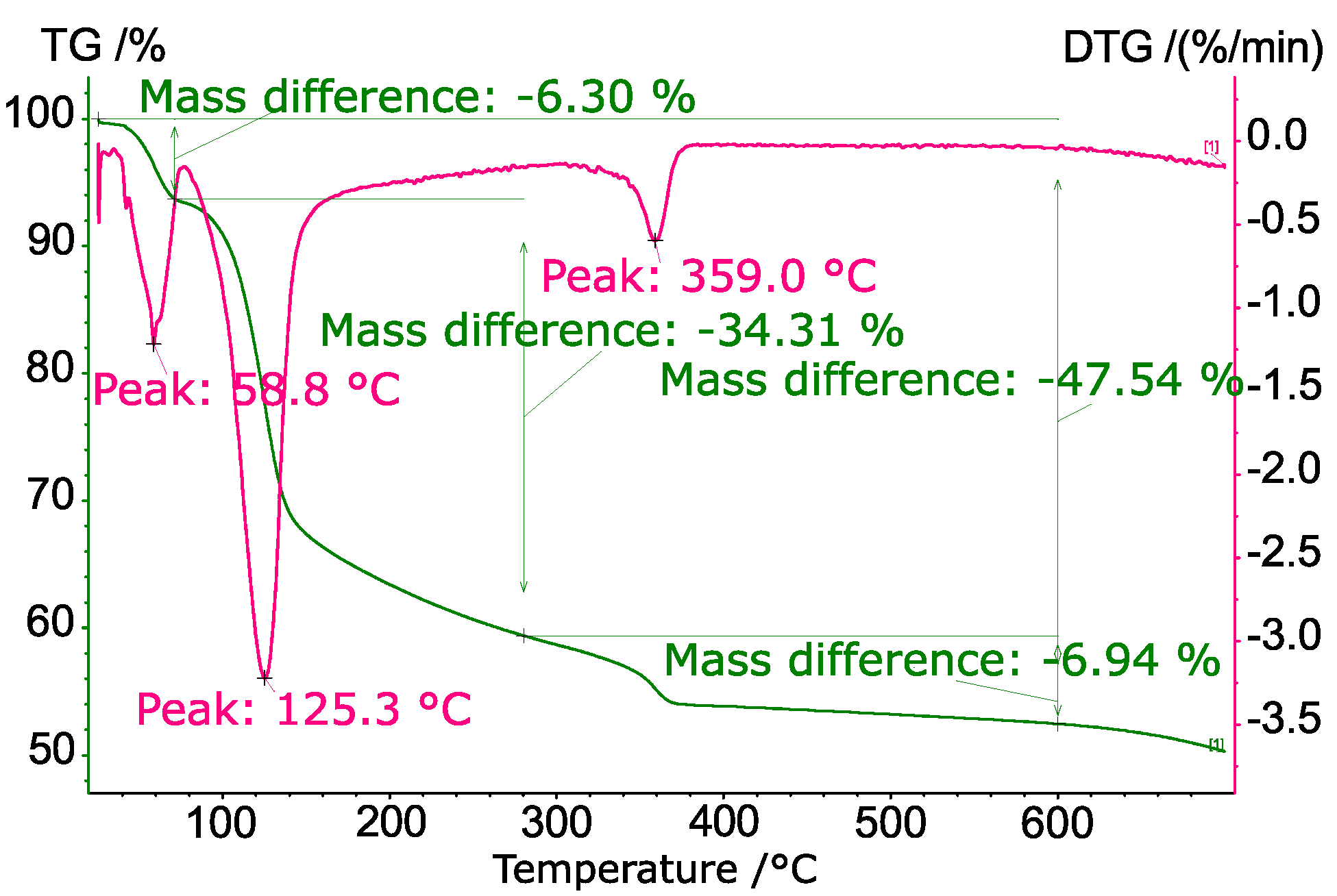

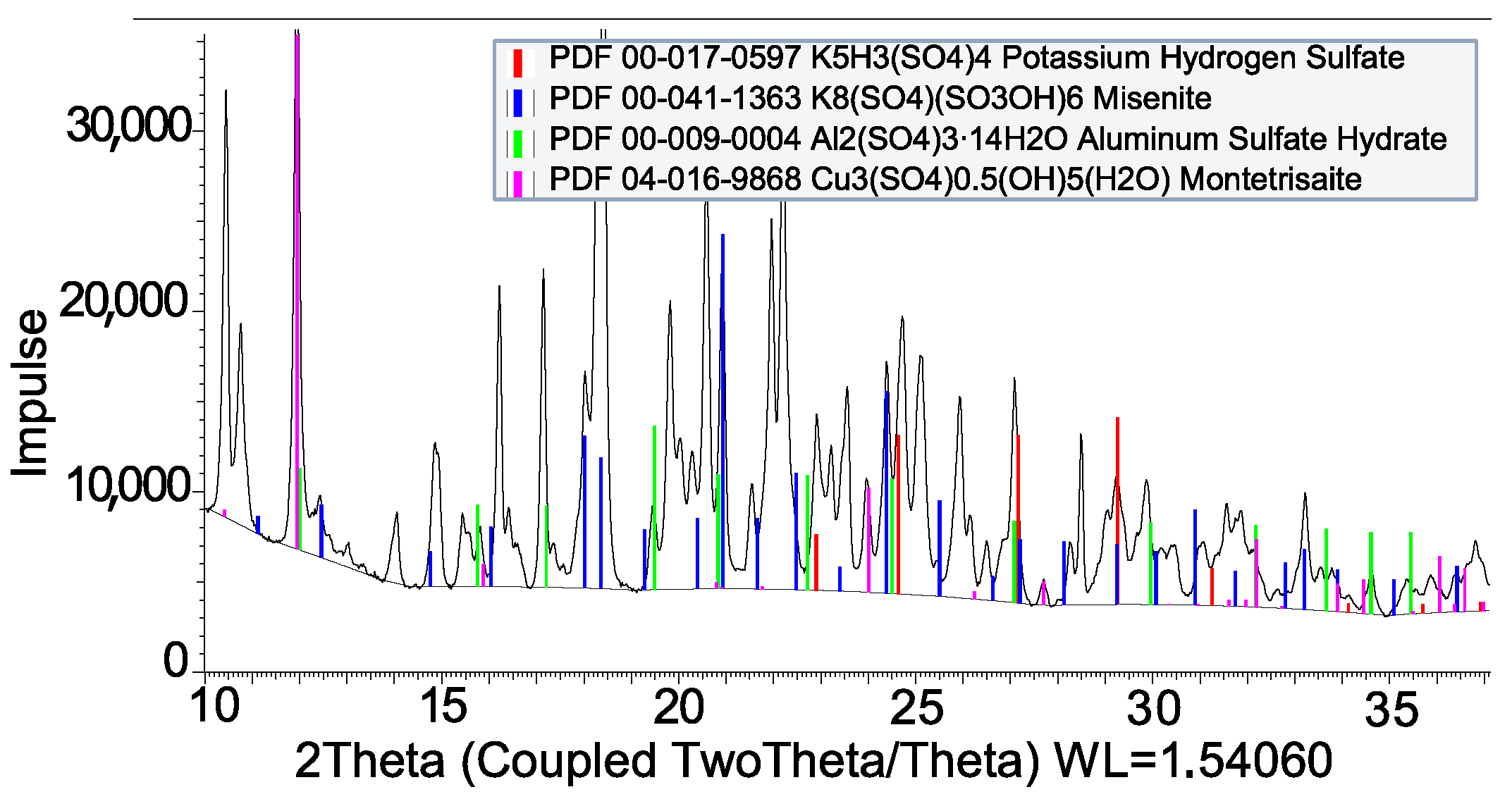
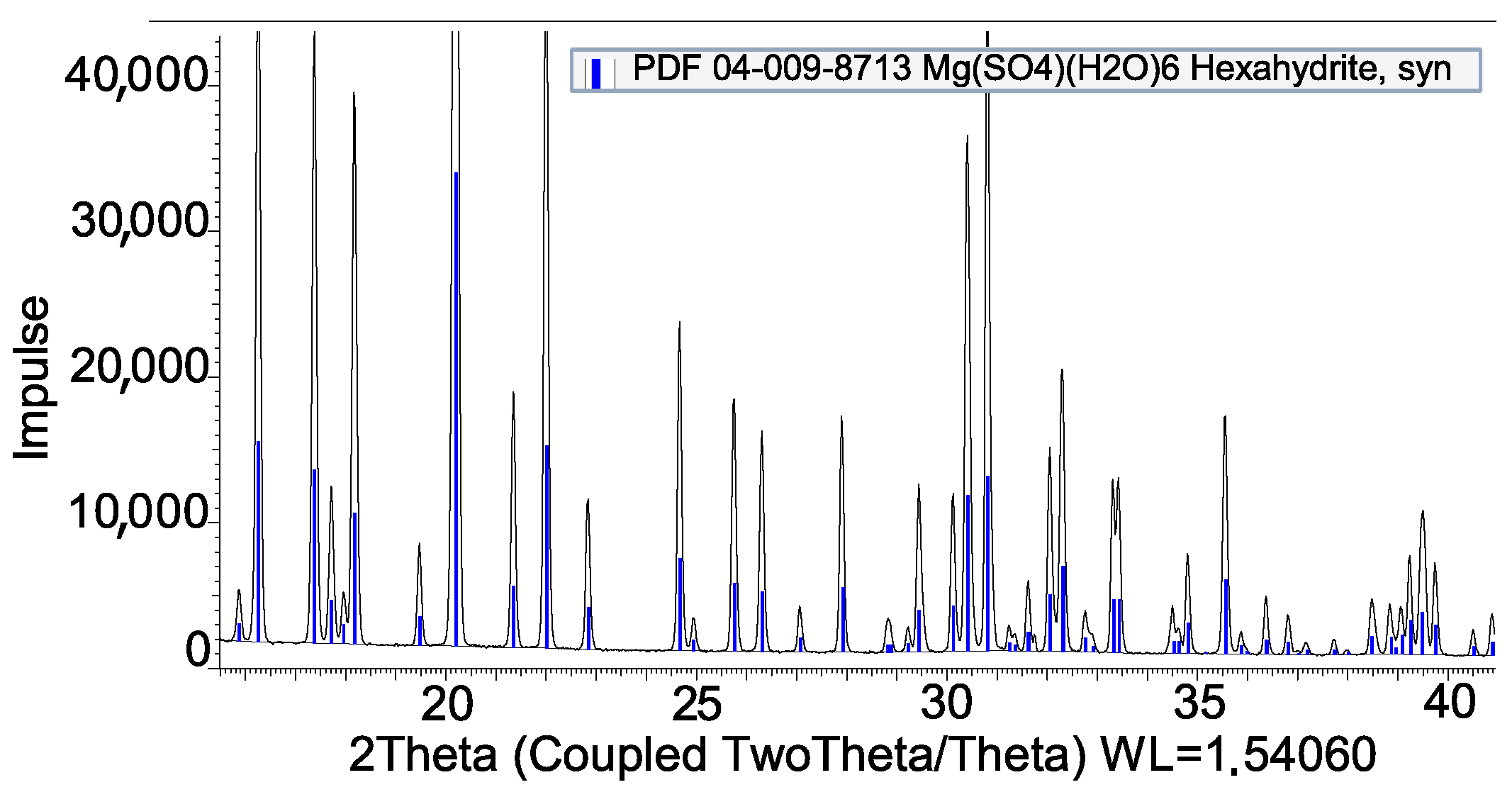
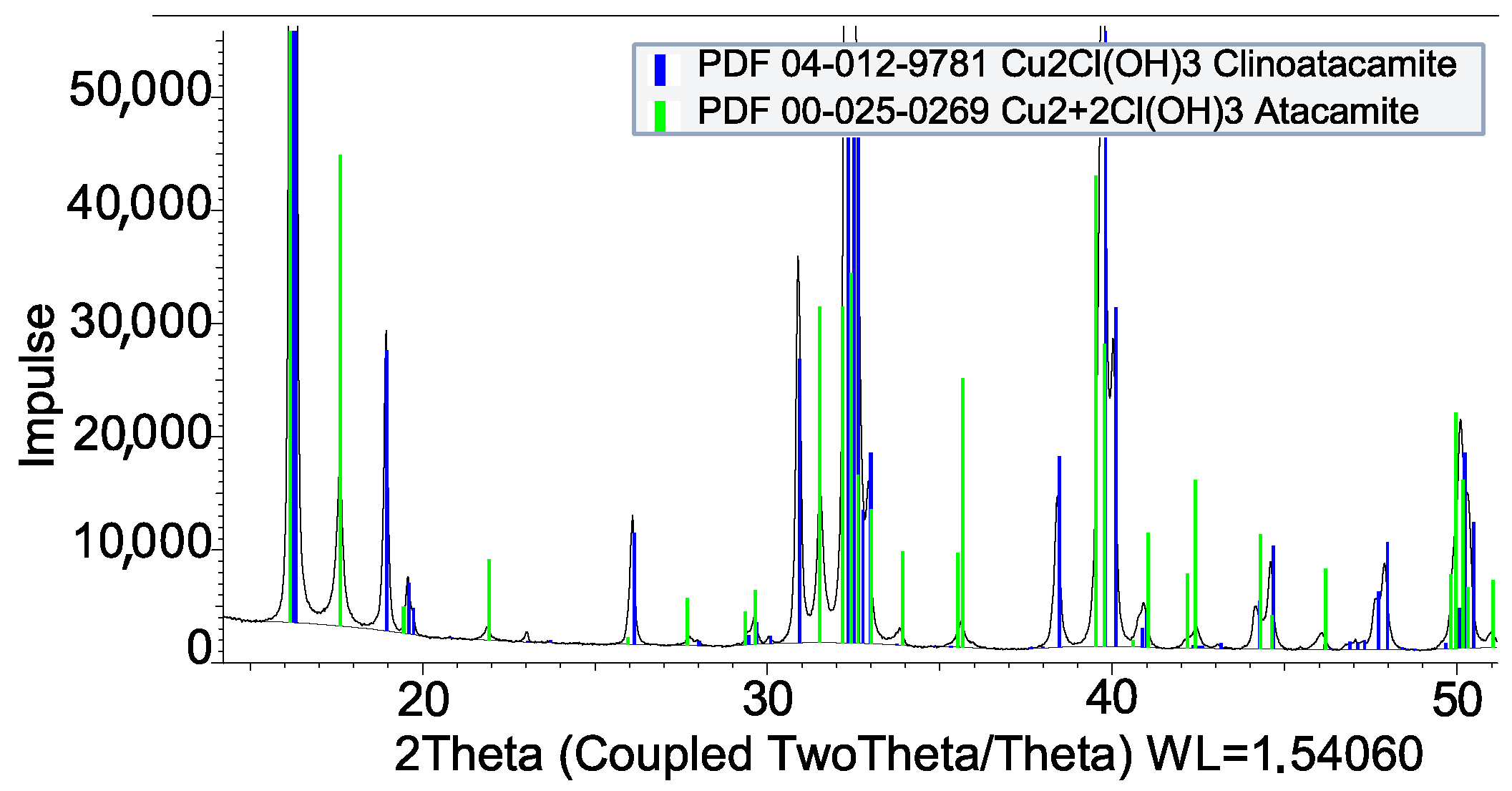
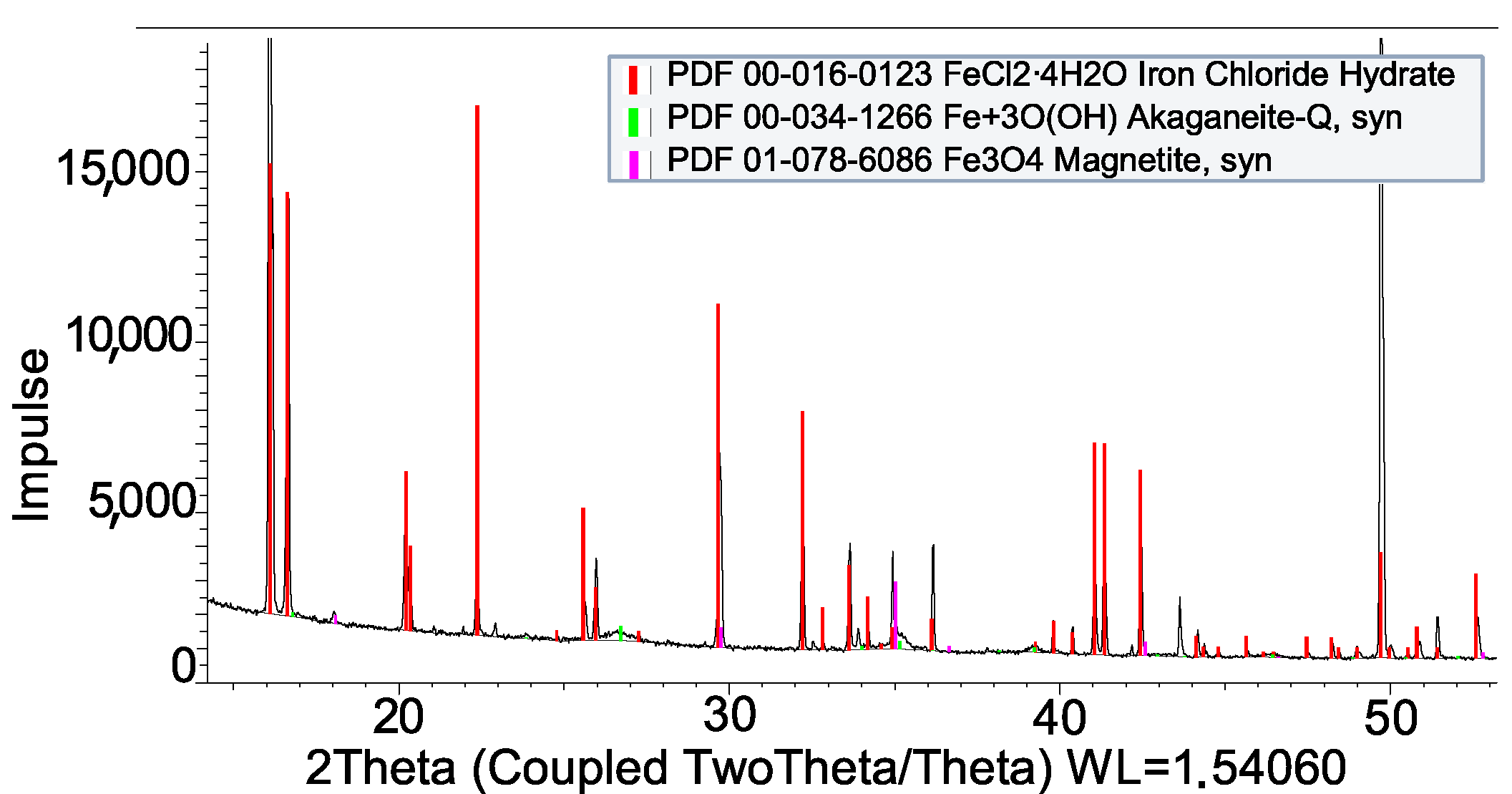
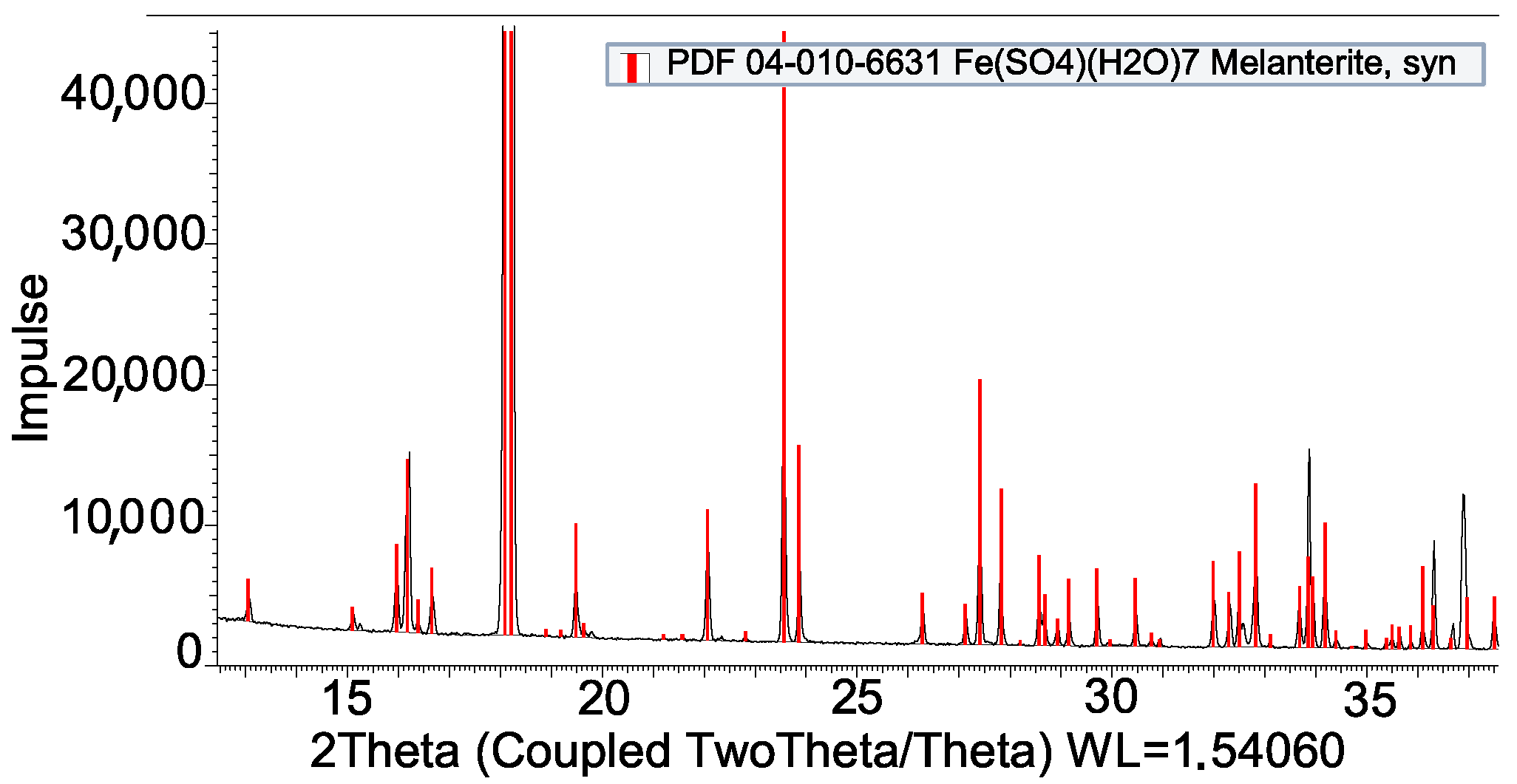
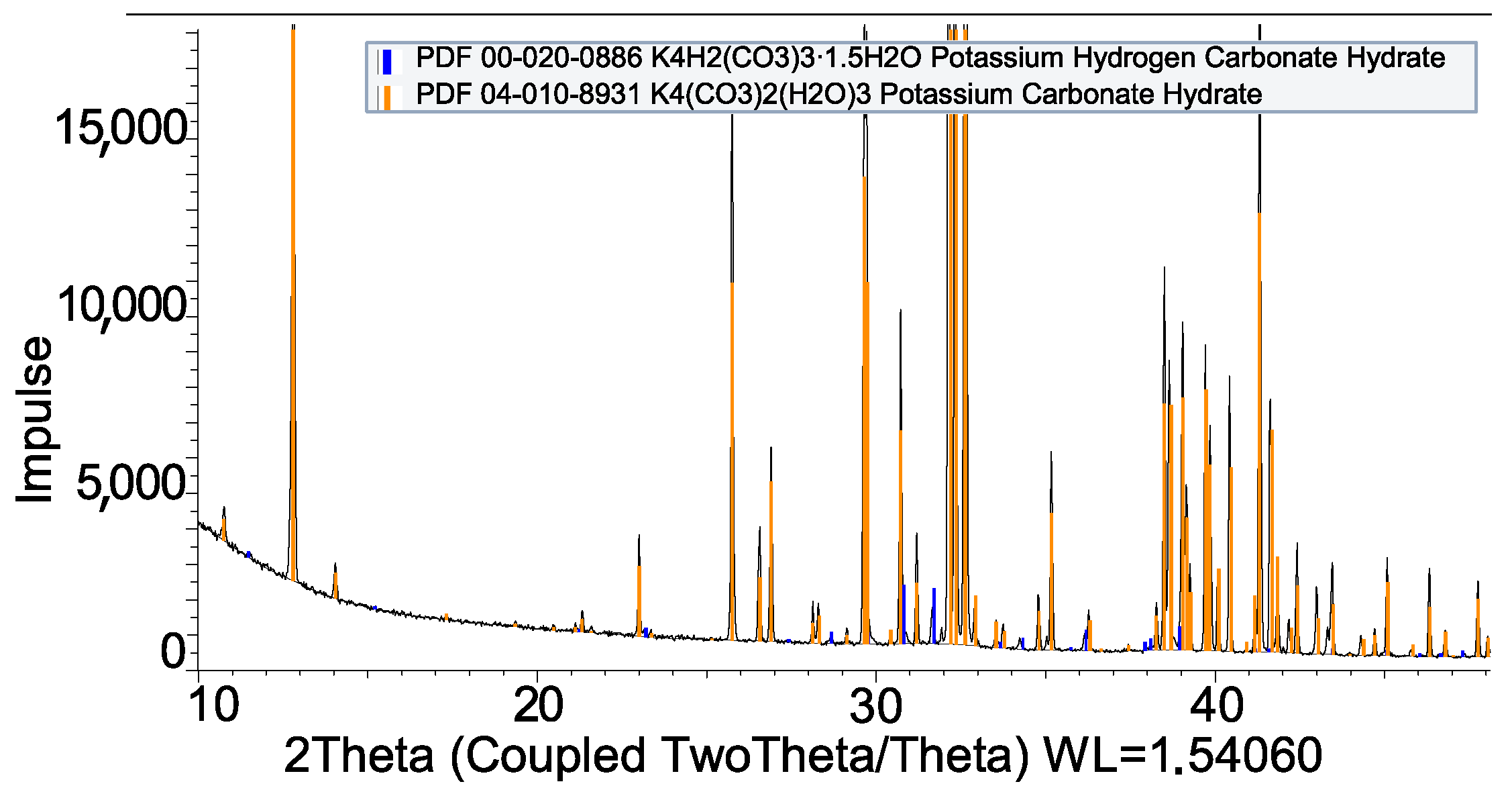

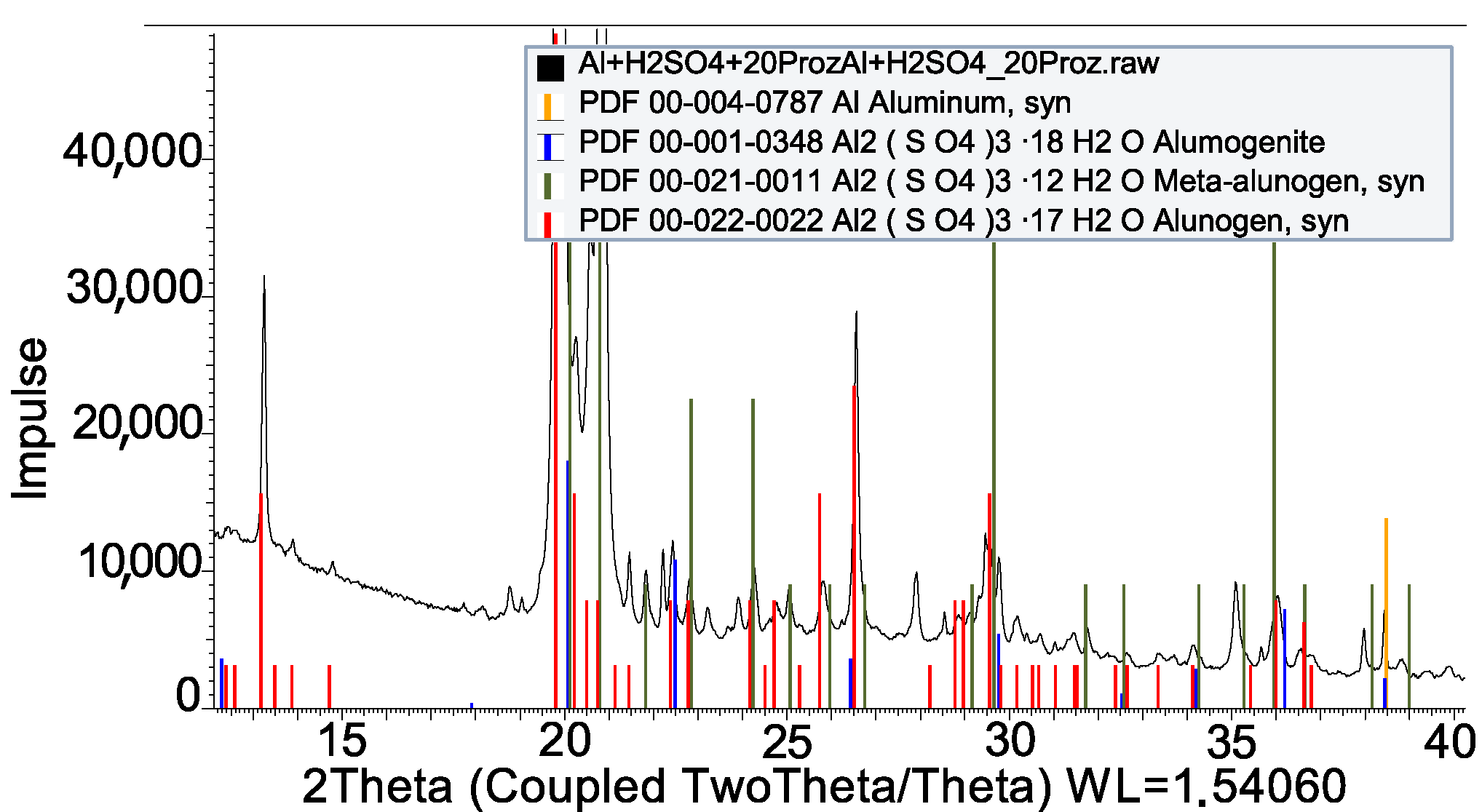

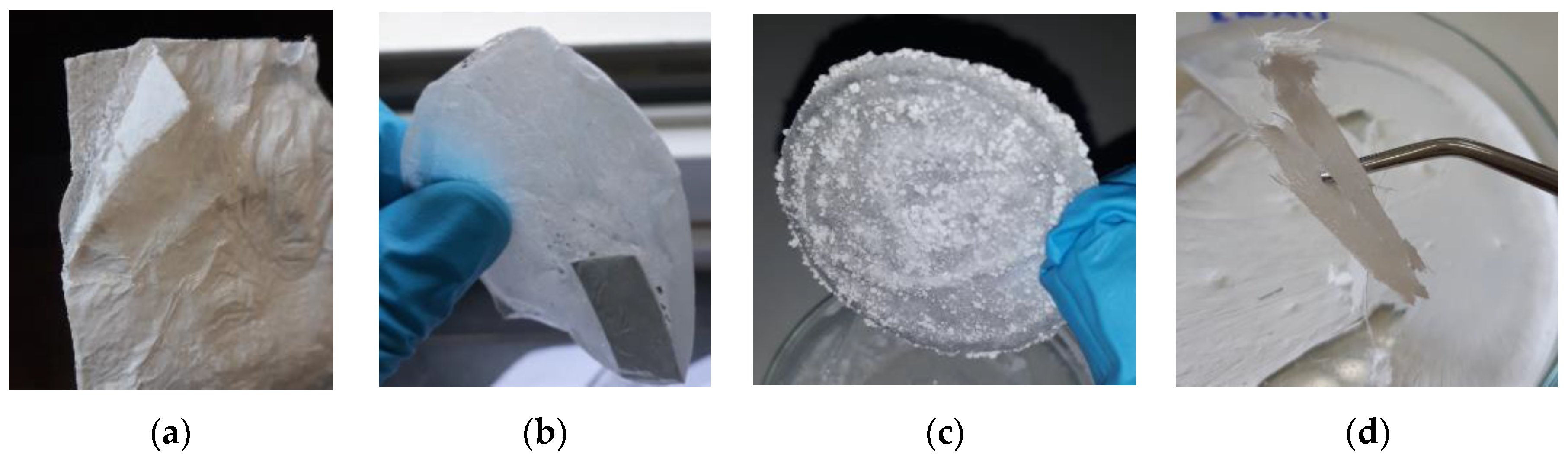

| Impregnation | |
| Salt + Metal | Compatibility |
| FeCl2 + Al | Not compatible |
| ZnCl2 + Al | Not compatible |
| FeSO4 + Al | Not compatible |
| K2CO3 + Al | Not compatible |
| CuSO4 + Al | Not compatible |
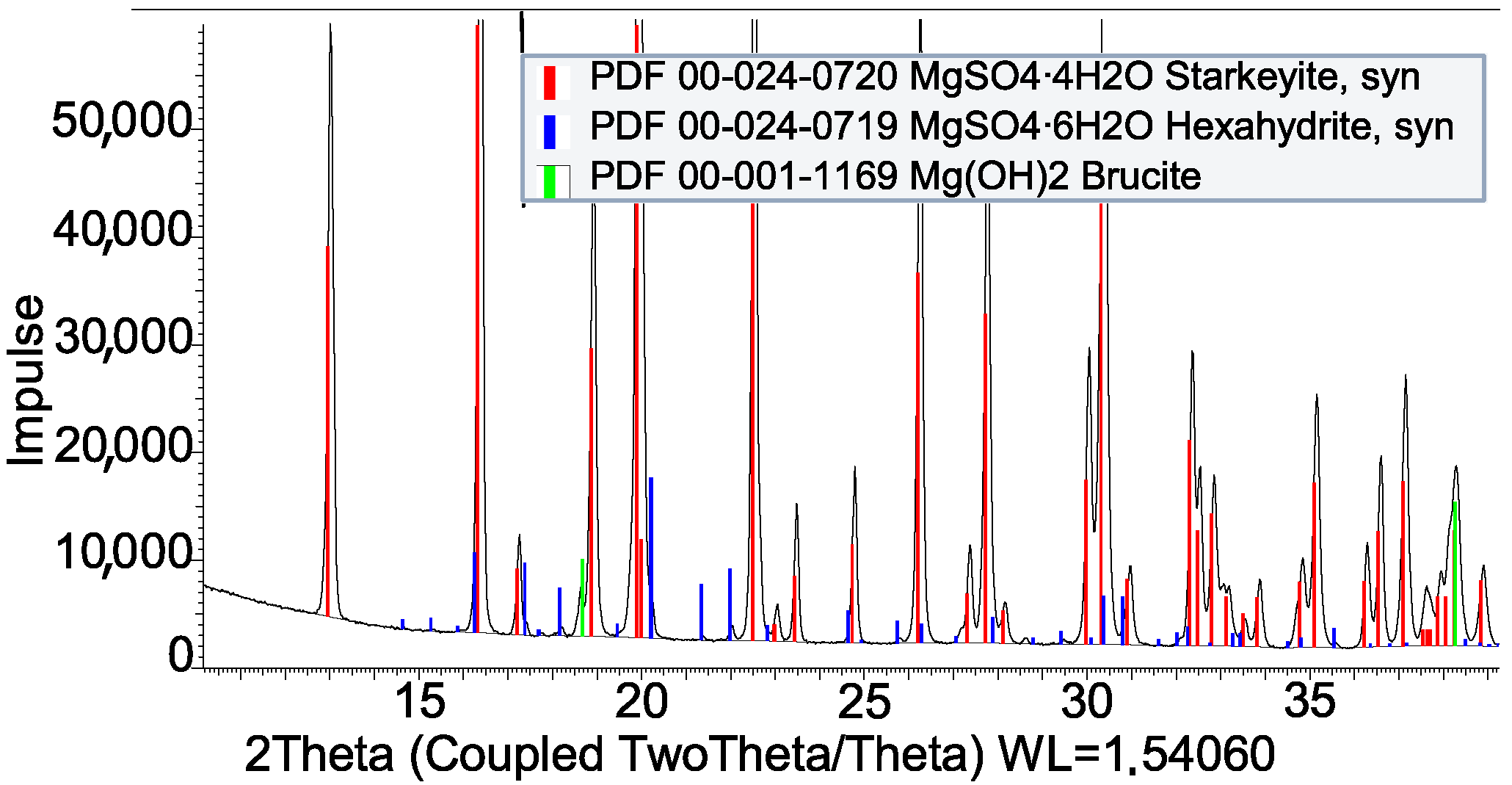
| MgSO4 + Al | Compatible |
| CuCl2 + Cu | Not compatible |
| FeCl2 + SS-316L | Compatible |
| Fe(SO4) + SS-316L | Compatible |
| K2CO3 + SS-316L | Compatible |
| Direct reactions | |
| Reactants | Products |
| H2SO4 + Al | Al2(SO4)3 · 16H2O |
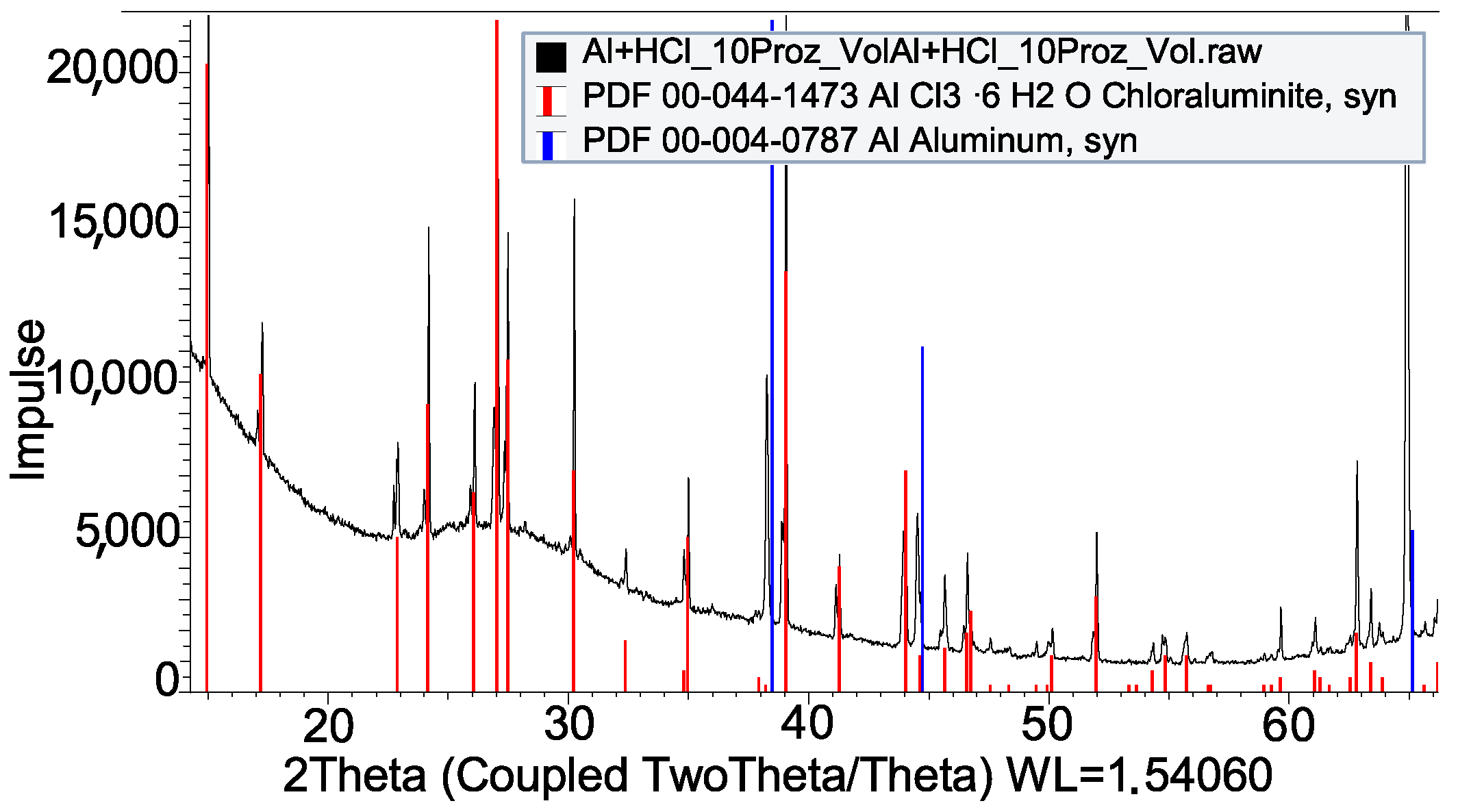
| HCl + Al | AlCl3 · 6H2O |
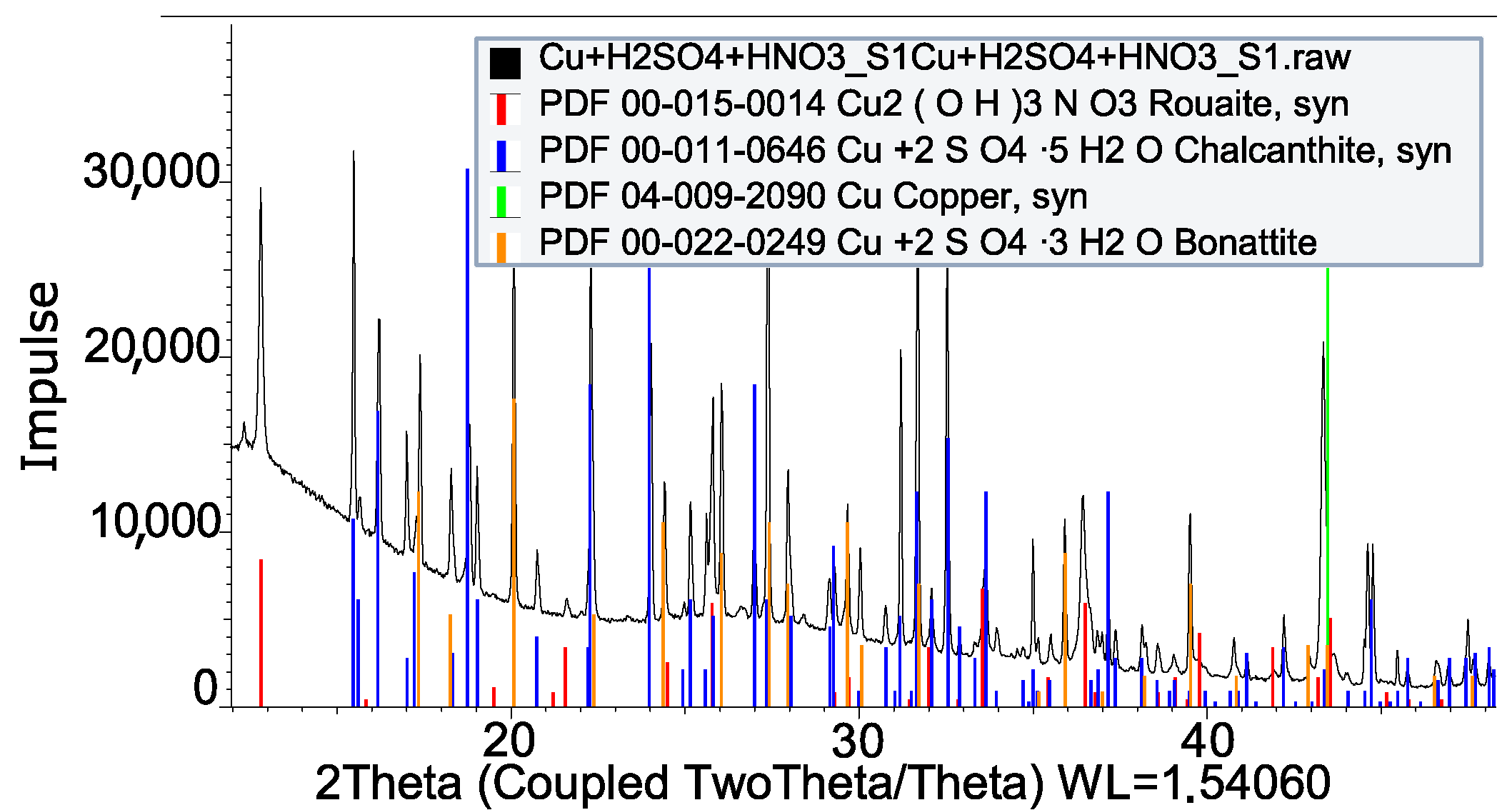
| HNO3 + H2SO3 + Cu | Not valid |
| HCl + Cu | CuCl2 · 2H2O |
| Composites | |
| Salt + Composite | Compatibility |
| MgSO4 + PVOH | Compatible |
| Al2(SO4)3 + PVOH | Compatible |
| SrCl2 + PVOH | Compatible |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banos, O.; Ohmann, S.; Alscher, F.; Breitkopf, C.; Pacheco, V.; Glorius, M.; Veit, M. Systematic Analysis of Materials for Coated Adsorbers for Application in Adsorption Heat Pumps or Refrigeration Systems. Energies 2020, 13, 4962. https://doi.org/10.3390/en13184962
Banos O, Ohmann S, Alscher F, Breitkopf C, Pacheco V, Glorius M, Veit M. Systematic Analysis of Materials for Coated Adsorbers for Application in Adsorption Heat Pumps or Refrigeration Systems. Energies. 2020; 13(18):4962. https://doi.org/10.3390/en13184962
Chicago/Turabian StyleBanos, Oscar, Sven Ohmann, Felix Alscher, Cornelia Breitkopf, Vicente Pacheco, Maja Glorius, and Matthias Veit. 2020. "Systematic Analysis of Materials for Coated Adsorbers for Application in Adsorption Heat Pumps or Refrigeration Systems" Energies 13, no. 18: 4962. https://doi.org/10.3390/en13184962
APA StyleBanos, O., Ohmann, S., Alscher, F., Breitkopf, C., Pacheco, V., Glorius, M., & Veit, M. (2020). Systematic Analysis of Materials for Coated Adsorbers for Application in Adsorption Heat Pumps or Refrigeration Systems. Energies, 13(18), 4962. https://doi.org/10.3390/en13184962







