Performance Evaluation of a Direct Absorption Collector for Solar Thermal Energy Conversion
Abstract
1. Introduction
2. Material and Methods
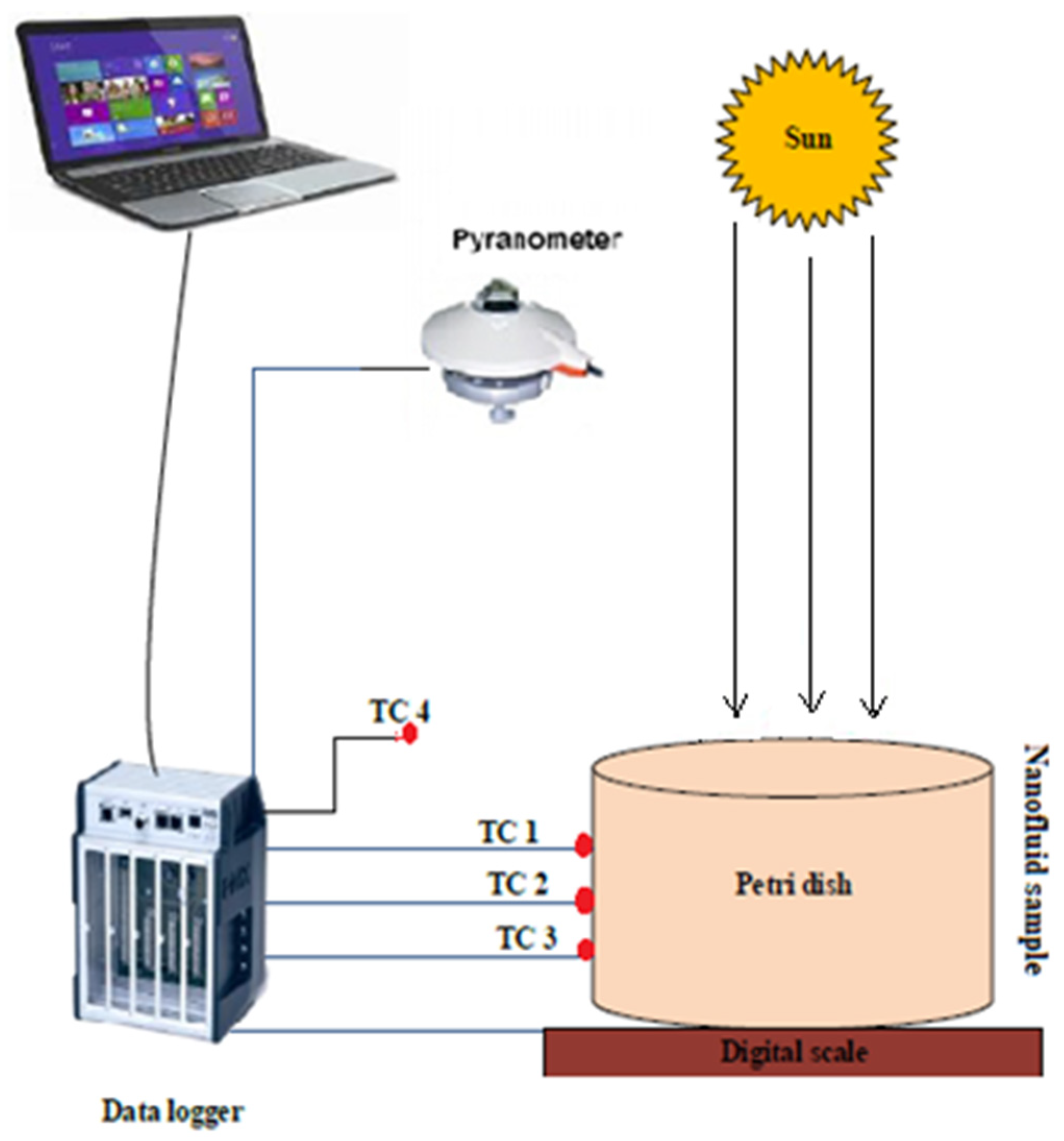
2.1. Experimental Setup
2.2. Nanofluids’ Preparation
3. Results and Discussion
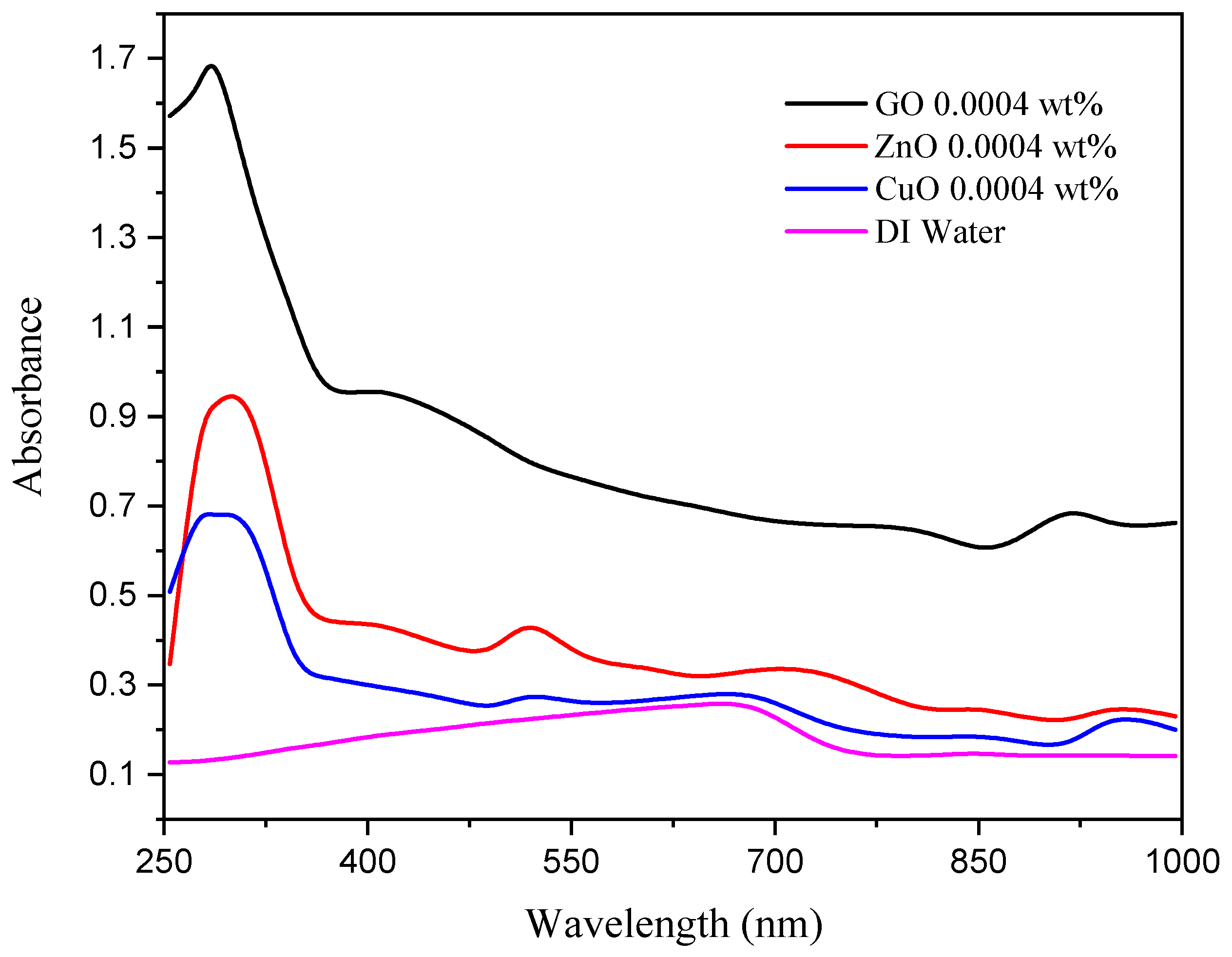
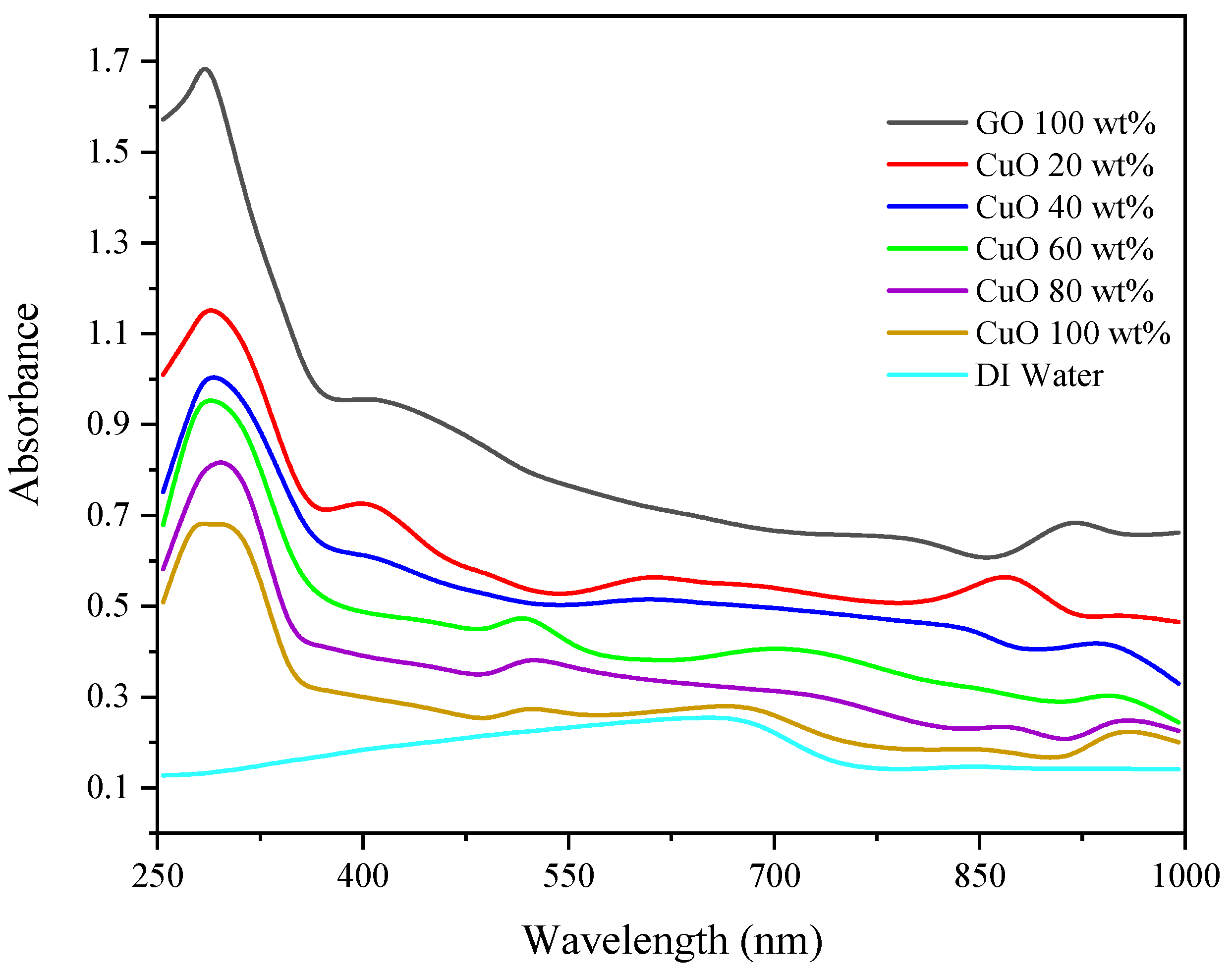
3.1. Optical Absorbance
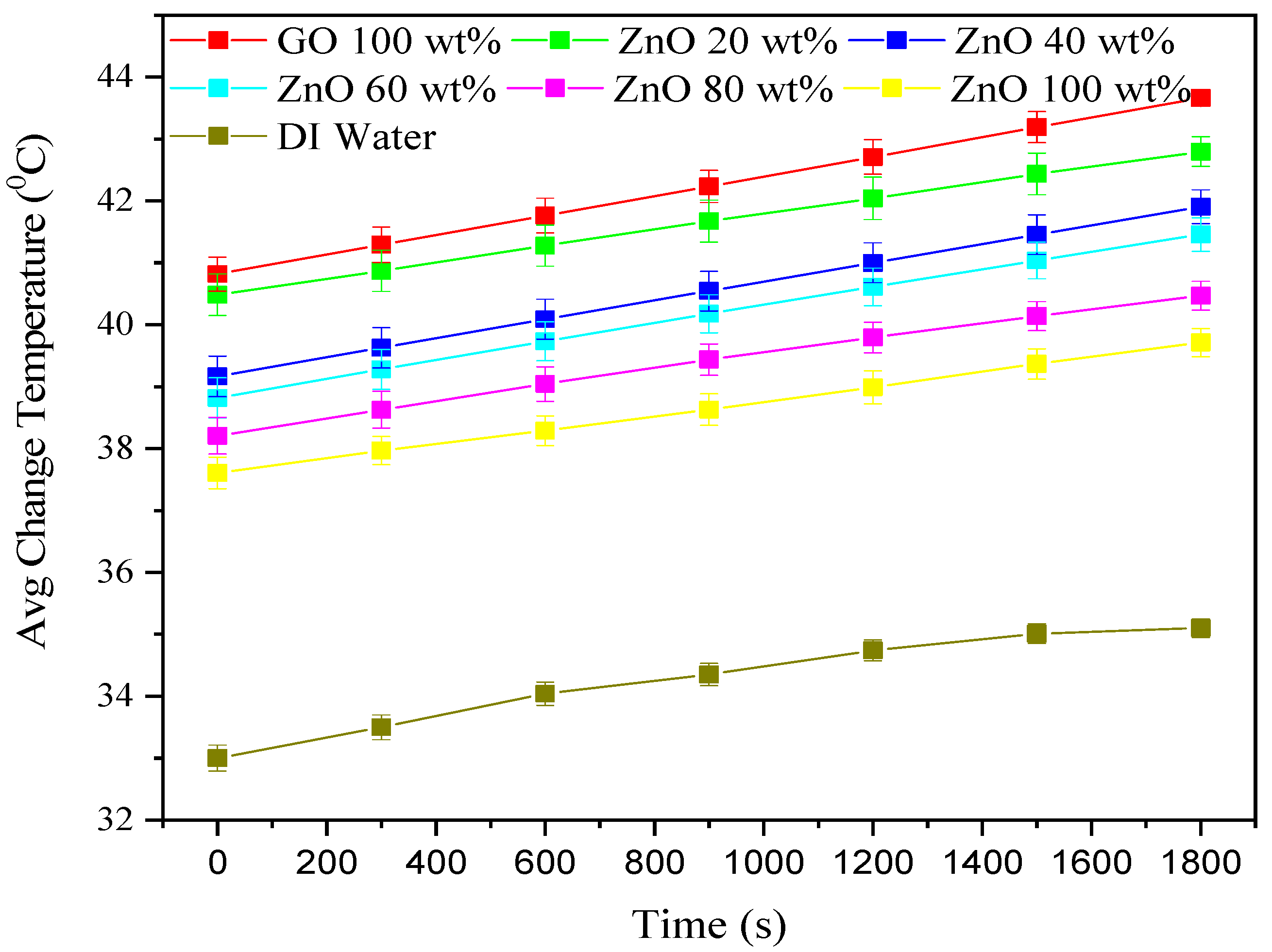
3.2. Change in Fluid Volume Temperature
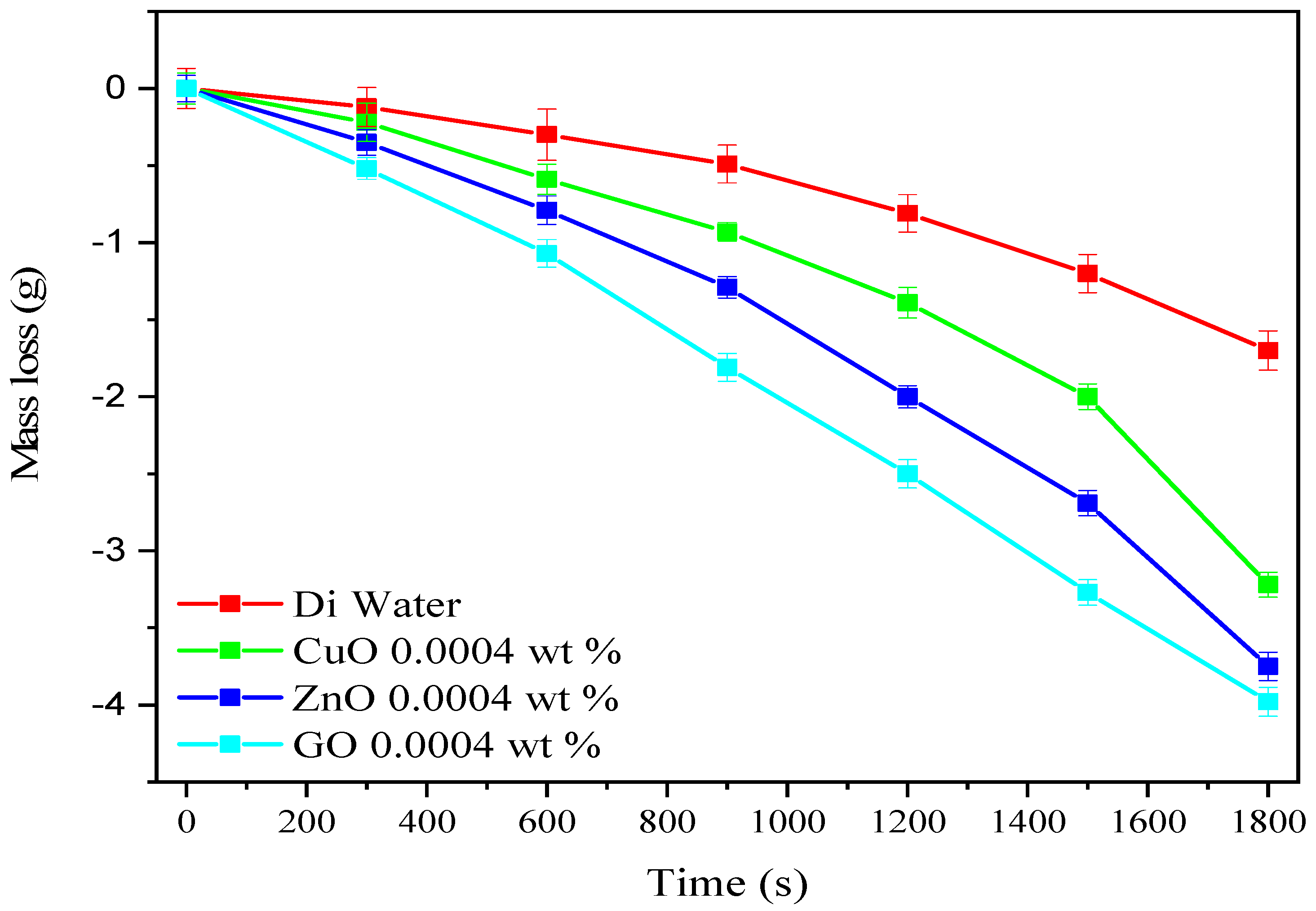
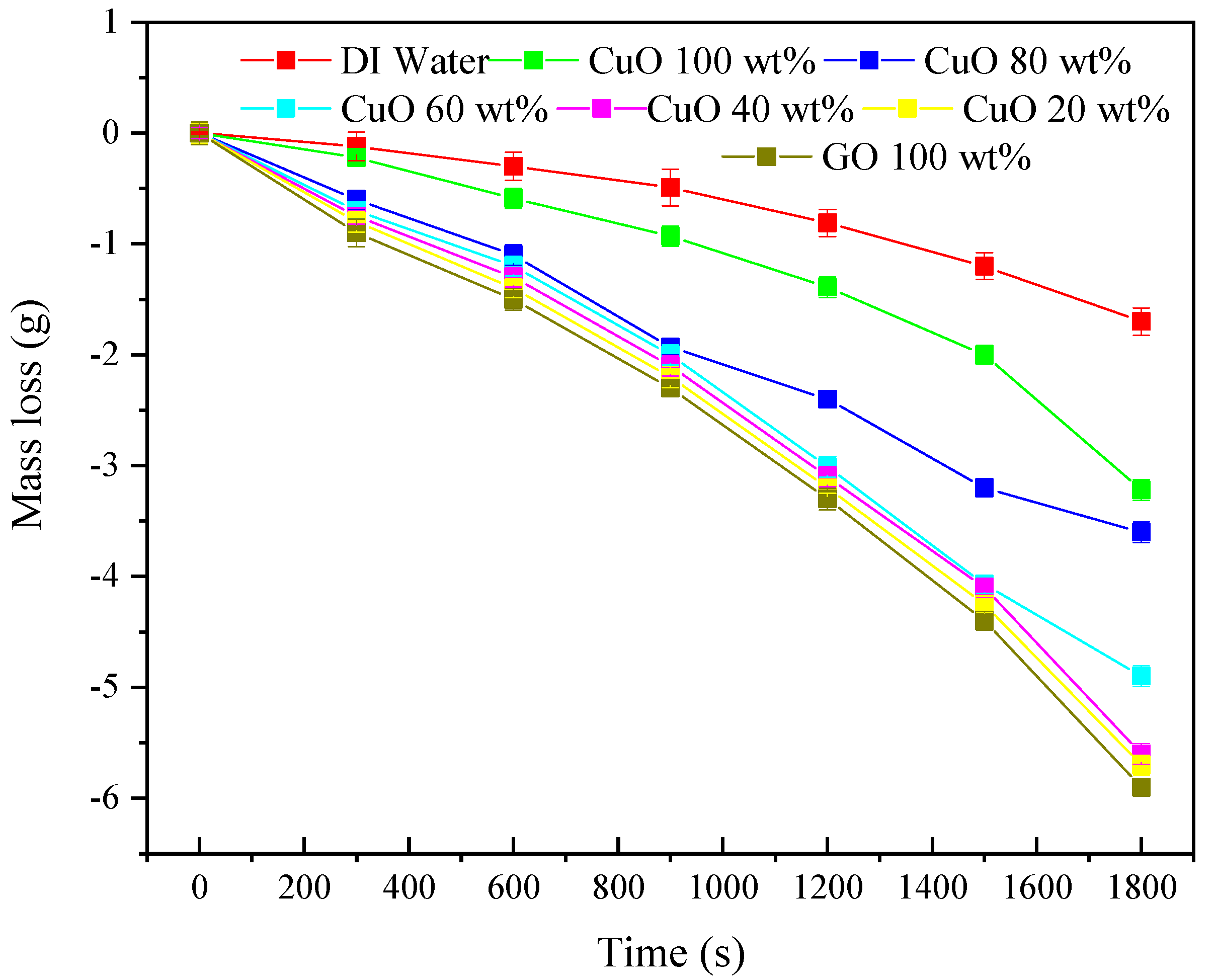
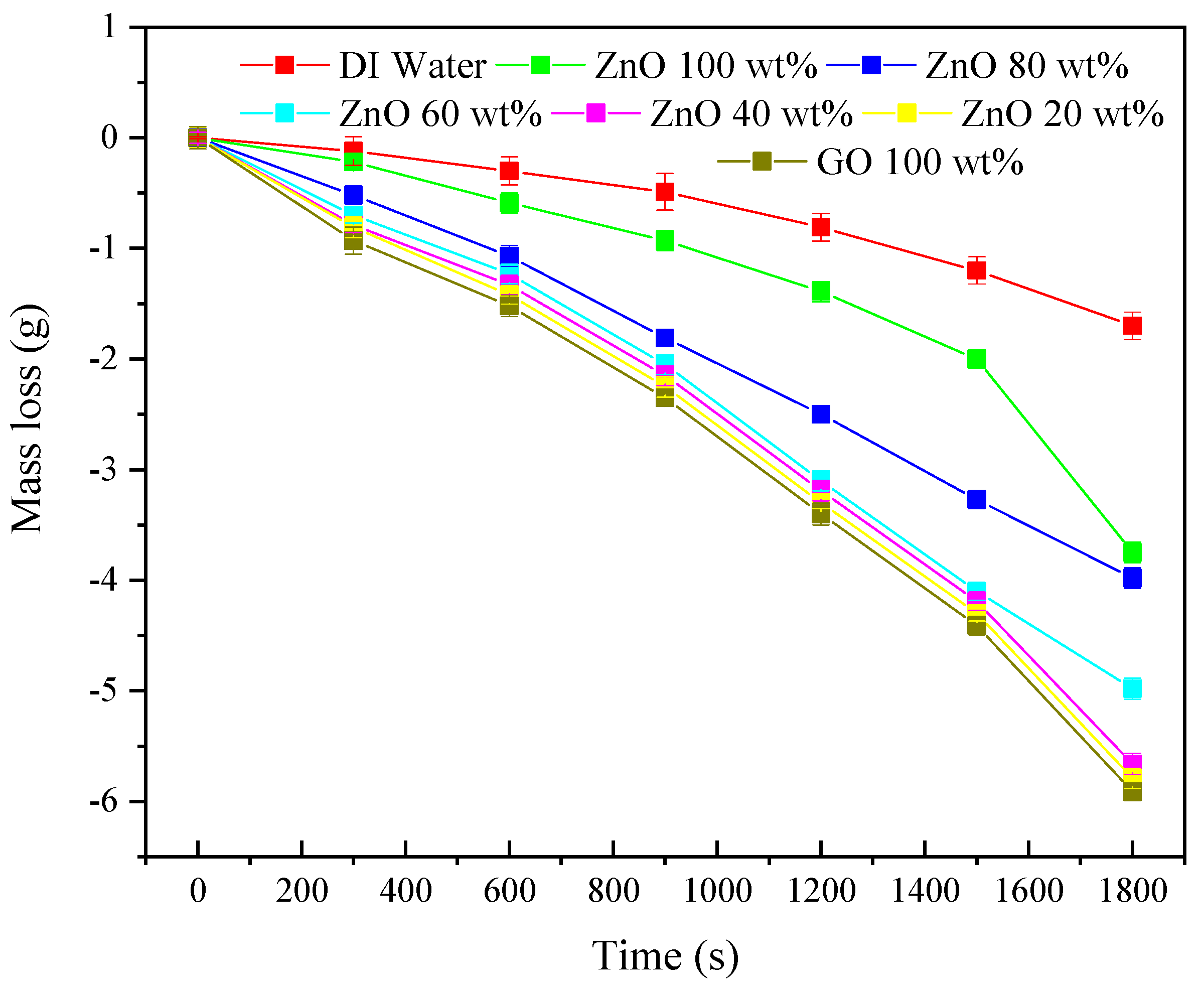
3.3. Mass Loss of Nanofluids
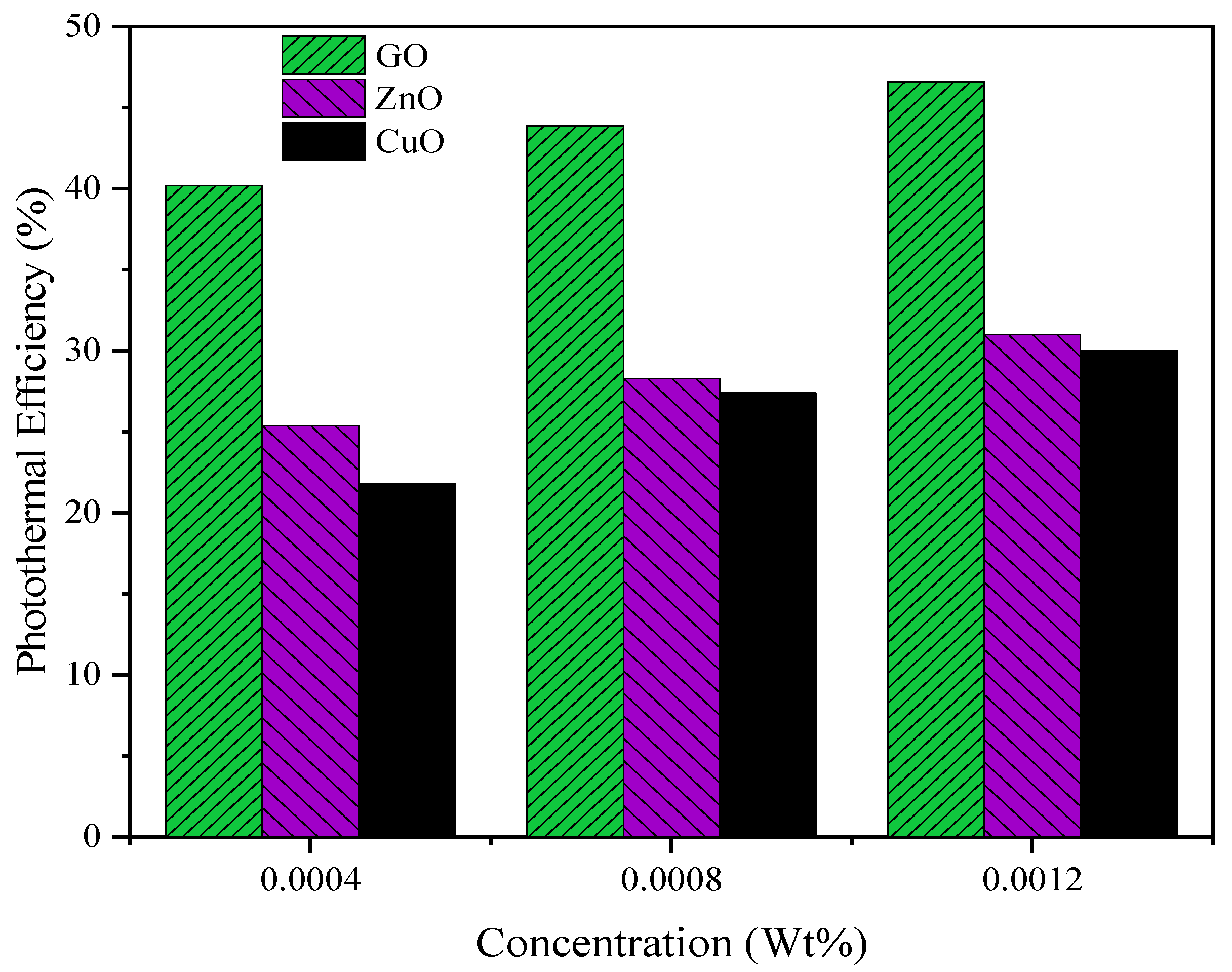
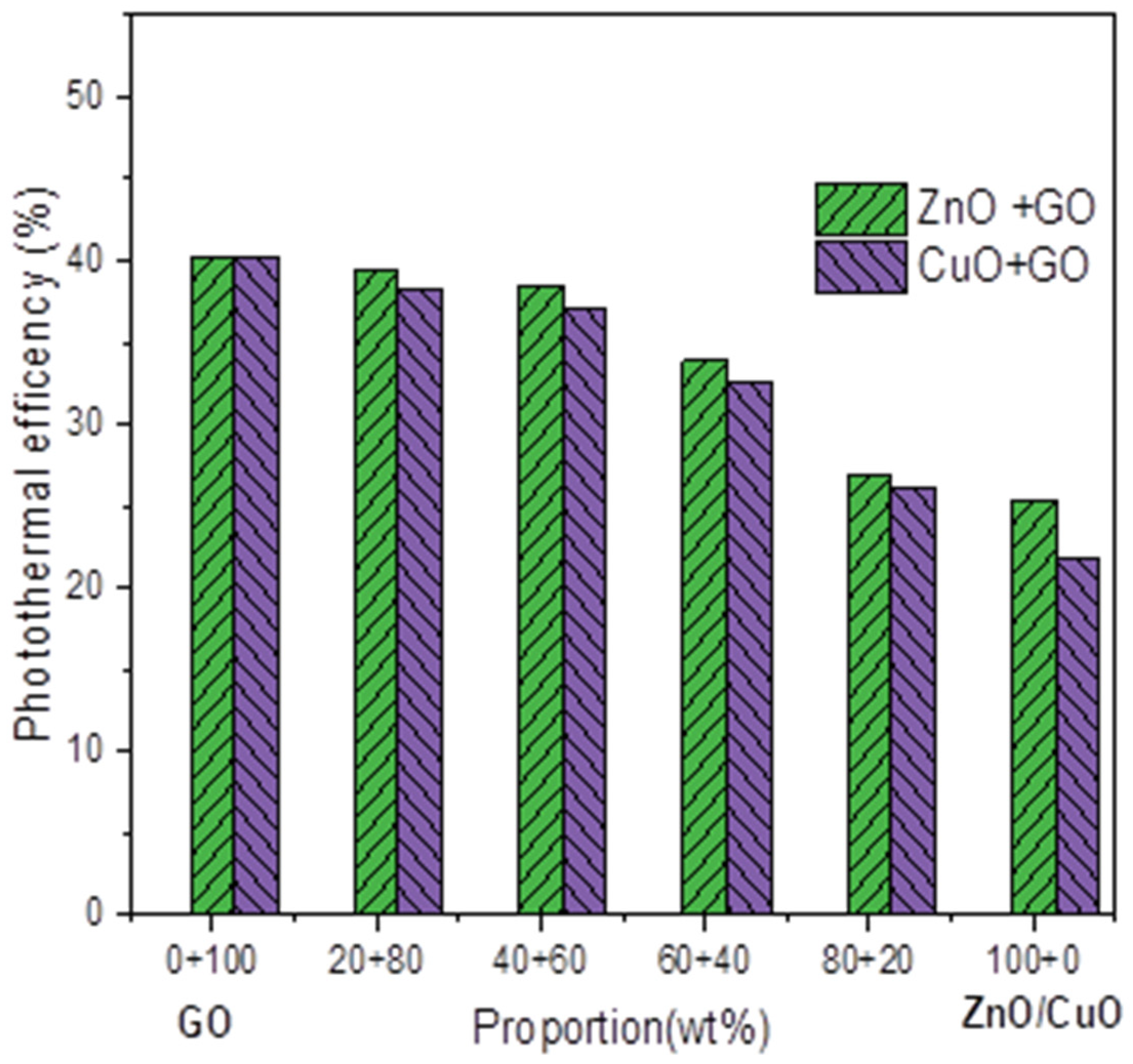
3.4. Photothermal Efficiency
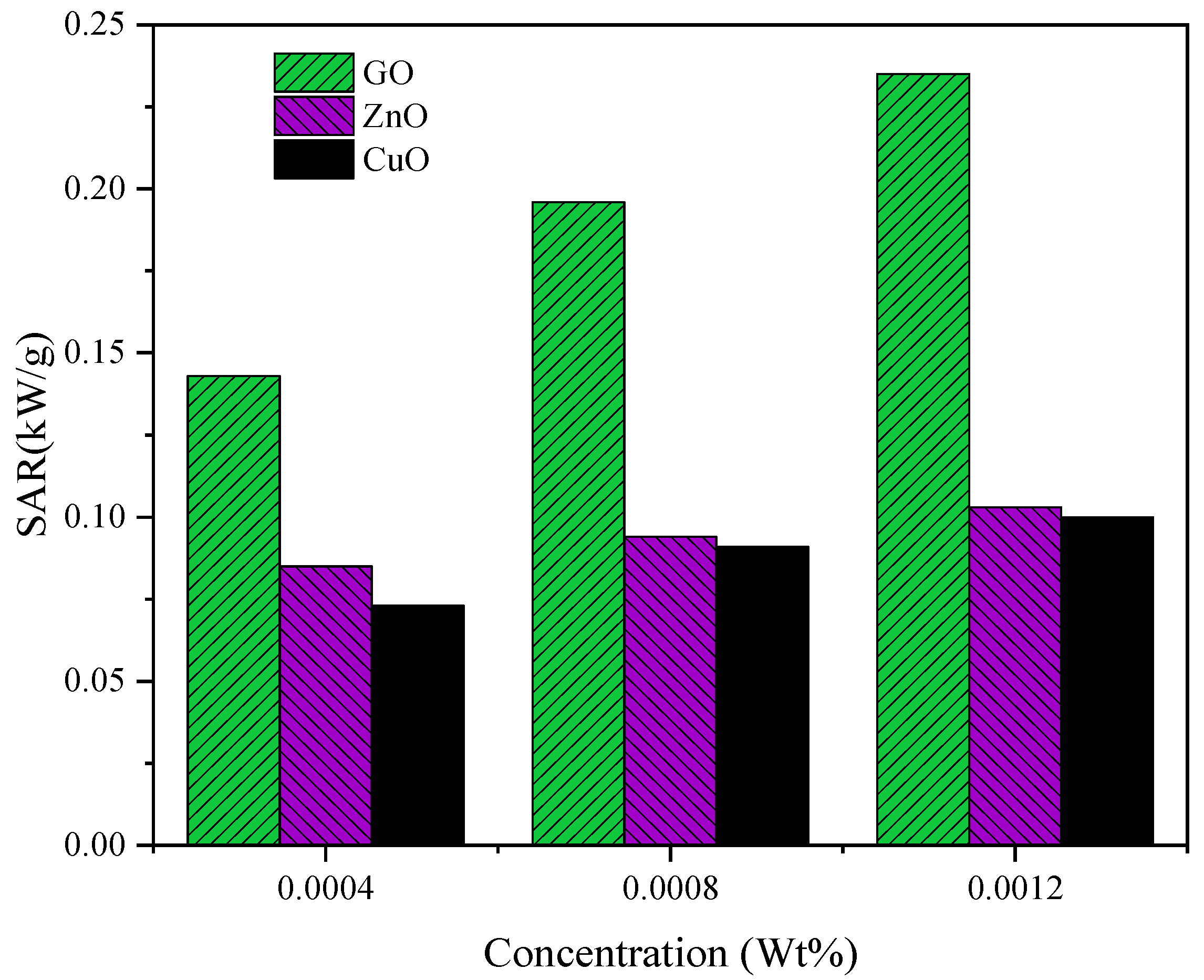
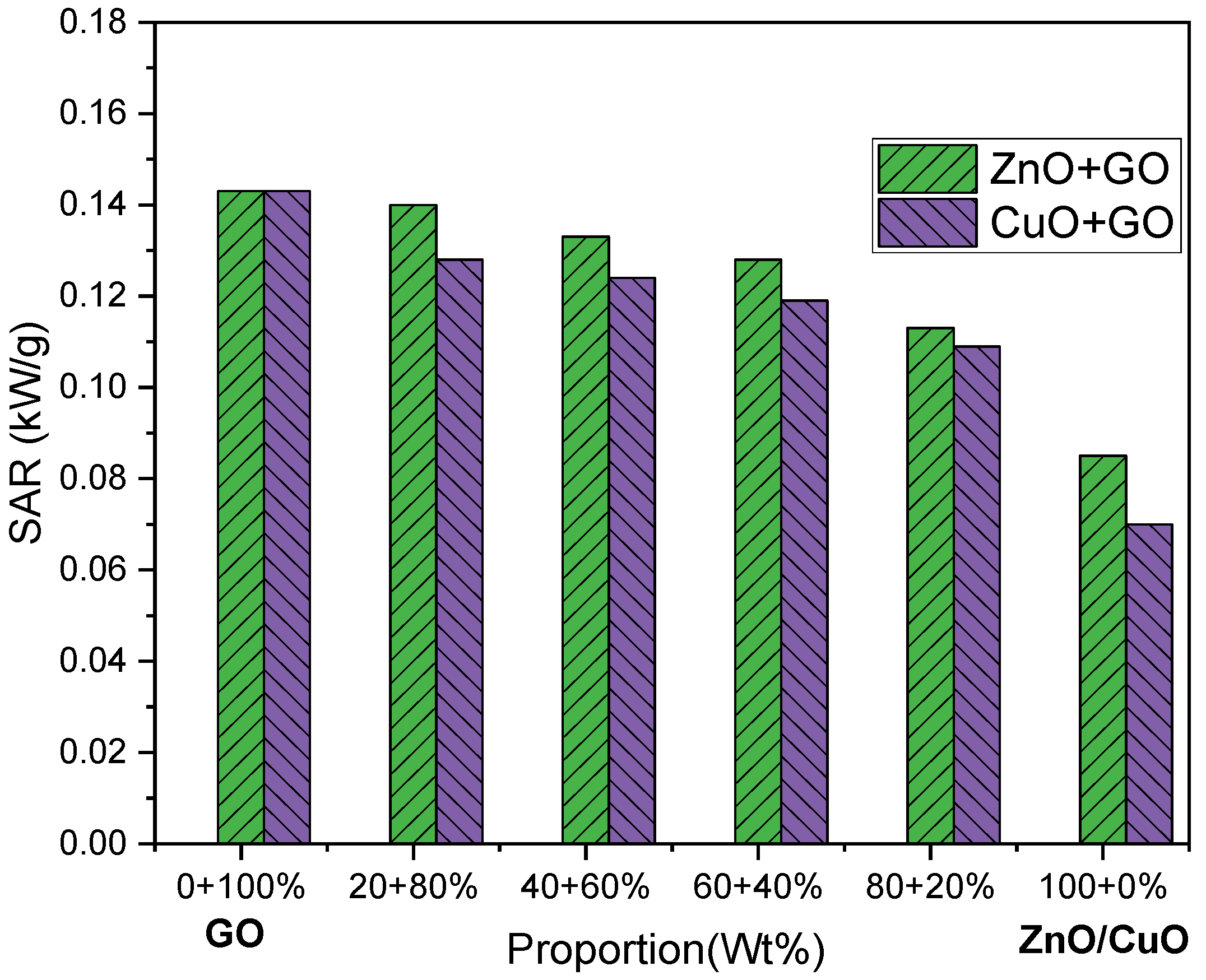
3.5. Specific Absorption Rate (SAR)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saçık, S.Y.; Yokus, N.; Alagöz, M.; Yokuş, T. Optimum Renewable Energy Investment Planning in Terms of Current Deficit: Turkey Model. Energies 2020, 13, 1509. [Google Scholar] [CrossRef]
- Bhalla, V.; Khullar, V.; Tyagi, H. Experimental investigation of photo-thermal analysis of blended nanoparticles (Al2O3/Co3O4) for direct absorption solar thermal collector. Renew. Energy 2018, 123, 616–626. [Google Scholar] [CrossRef]
- Zeren, F.; Akkuş, H.T. The relationship between renewable energy consumption and trade openness: New evidence from emerging economies. Renew. Energy 2020, 147, 322–329. [Google Scholar] [CrossRef]
- Lu, Y.; Alghassab, M.; Alvarez-Alvarado, M.; Gunduz, H.; Khan, Z.A.; Imran, M. Optimal Distribution of Renewable Energy Systems Considering Aging and Long-Term Weather Effect in Net-Zero Energy Building Design. Sustainability 2020, 12, 5570. [Google Scholar] [CrossRef]
- Hussain, M.I.; Ménézo, C.; Kim, J.-T. Advances in solar thermal harvesting technology based on surface solar absorption collectors: A review. Sol. Energy Mater. Sol. Cells 2018, 187, 123–139. [Google Scholar] [CrossRef]
- Kasaeian, A.; Daneshazarian, R.; Pourfayaz, F. Comparative study of different nanofluids applied in a trough collector with glass-glass absorber tube. J. Mol. Liq. 2017, 234, 315–323. [Google Scholar] [CrossRef]
- Khullar, V.; Bhalla, V.; Tyagi, H. Potential Heat Transfer Fluids (Nanofluids) for Direct Volumetric Absorption-Based Solar Thermal Systems. J. Therm. Sci. Eng. Appl. 2017, 10, 011009. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, G.; Wang, M.; Yu, W.; Zeng, J.; Yu, X.; Xie, H.; Li, Q. Dual plasmonic Au/TiN nanofluids for efficient solar photothermal conversion. Sol. Energy 2019, 184, 240–248. [Google Scholar] [CrossRef]
- Moon, G.-H.; Ko, R.; Joo, S.-K. Integration of Smart Grid Resources into Generation and Transmission Planning Using an Interval-Stochastic Model. Energies 2020, 13, 1843. [Google Scholar] [CrossRef]
- Cui, W.; Shen, Z.; Yang, J.; Wu, S.; Bai, M. Influence of nanoparticle properties on the thermal conductivity of nanofluids by molecular dynamics simulation. RSC Adv. 2014, 4, 55580–555894. [Google Scholar] [CrossRef]
- Amjad, M.; Gardy, J.; Hassanpour, A.; Wen, D. Novel draw solution for forward osmosis based solar desalination. Appl. Energy 2018, 230, 220–231. [Google Scholar] [CrossRef]
- Abdul-Ganiyu, S.; Quansah, D.A.; Ramde, E.W.; Seidu, R.; Adaramola, M.S. Investigation of Solar Photovoltaic-Thermal (PVT) and Solar Photovoltaic (PV) Performance: A Case Study in Ghana. Energies 2020, 13, 2701. [Google Scholar] [CrossRef]
- Vall, S.; Medrano, M.; Solé, C.; Castell, A. Combined Radiative Cooling and Solar Thermal Collection: Experimental Proof of Concept. Energies 2020, 13, 893. [Google Scholar] [CrossRef]
- Suh, J.; Yoon, M.; Jung, S. Practical Application Study for Precision Improvement Plan for Energy Storage Devices Based on Iterative Methods. Energies 2020, 13, 656. [Google Scholar] [CrossRef]
- Sang, L.; Ai, W.; Wu, Y.; Ma, C. Enhanced specific heat and thermal conductivity of ternary carbonate nanofluids with carbon nanotubes for solar power applications. Int. J. Energy Res. 2019, 44, 334–343. [Google Scholar] [CrossRef]
- Garg, K.; Khullar, V.; Das, S.K.; Tyagi, H. Application of Nanofluid-Based Direct Absorption Solar Collector in Once-Through Multistage Flash Desalination System. In Advances in Solar Energy Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 519–535. [Google Scholar]
- Jin, H.; Lin, G.; Bai, L.; Amjad, M.; Filho, E.P.B.; Wen, D. Photothermal conversion efficiency of nanofluids: An experimental and numerical study. Sol. Energy 2016, 139, 278–289. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Mishra, P.C.; Nayak, S.K.; Mukherjee, S. Thermal conductivity of nanofluids-an extensive literature review. Int. J. Eng. Res. Technol. 2013, 2, 734–745. [Google Scholar]
- Alade, I.O.; Rahman, M.A.A.; Saleh, T.A. Predicting the specific heat capacity of alumina/ethylene glycol nanofluids using support vector regression model optimized with Bayesian algorithm. Sol. Energy 2019, 183, 74–82. [Google Scholar] [CrossRef]
- Al Shdaifat, M.Y.; Zulkifli, R.; Sopian, K.; Salih, A.A. Thermal and Hydraulic Performance of CuO/Water Nanofluids: A Review. Micromachines 2020, 11, 416. [Google Scholar] [CrossRef]
- Wang, X.; Ou, G.; Wang, N.; Wu, H. Graphene-Based Recyclable Photo-Absorbers for High-Efficiency Seawater Desalination. ACS Appl. Mater. Interfaces 2016, 8, 9194–9199. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Solar powered desalination—Technology, energy and future outlook. Desalination 2019, 453, 54–76. [Google Scholar] [CrossRef]
- Palenzuela, P.; Alarcón-Padilla, D.C. Concentrating Solar Power and Desalination Plants. In Solar Resources Mapping; Springer: Berlin/Heidelberg, Germany, 2019; pp. 327–340. [Google Scholar]
- Amin, I.; Ali, M.E.; Bayoumi, S.; Oterkus, S.; Shawky, H.; Oterkus, E. Conceptual Design and Numerical Analysis of a Novel Floating Desalination Plant Powered by Marine Renewable Energy for Egypt. J. Mar. Sci. Eng. 2020, 8, 95. [Google Scholar] [CrossRef]
- Menbari, A.; Alemrajabi, A.A.; Ghayeb, Y. Investigation on the stability, viscosity and extinction coefficient of CuO–Al2O3/Water binary mixture nanofluid. Exp. Therm. Fluid Sci. 2016, 74, 122–129. [Google Scholar] [CrossRef]
- Męczyńska-Wielgosz, S.; Wojewódzka, M.; Matysiak-Kucharek, M.; Czajka, M.; Jodłowska-Jędrych, B.; Kruszewski, M.; Kapka-Skrzypczak, L. Susceptibility of HepG2 Cells to Silver Nanoparticles in Combination with other Metal/Metal Oxide Nanoparticles. Materials 2020, 13, 2221. [Google Scholar] [CrossRef]
- Soysal, F.; Çıplak, Z.; Getiren, B.; Gökalp, C.; Yıldız, N. Synthesis and characterization of reduced graphene oxide-iron oxide-polyaniline ternary nanocomposite and determination of its photothermal properties. Mater. Res. Bull. 2020, 124, 110763. [Google Scholar] [CrossRef]
- Gimeno-Furio, A.; Hernandez, L.; Navarrete, N.; Mondragón, R. Characterisation study of a thermal oil-based carbon black solar nanofluid. Renew. Energy 2019, 140, 493–500. [Google Scholar] [CrossRef]
- Amjad, M.; Raza, G.; Xin, Y.; Pervaiz, S.; Xu, J.; Du, X.; Wen, D. Volumetric solar heating and steam generation via gold nanofluids. Appl. Energy 2017, 206, 393–400. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, X.; Shi, L.; Zhu, J. Investigation of photothermal heating enabled by plasmonic nanofluids for direct solar steam generation. Sol. Energy 2017, 157, 35–46. [Google Scholar] [CrossRef]
- Filho, E.P.B.; Mendoza, O.S.H.; Beicker, C.L.L.; Menezes, A.; Wen, D. Experimental investigation of a silver nanoparticle-based direct absorption solar thermal system. Energy Convers. Manag. 2014, 84, 261–267. [Google Scholar] [CrossRef]
- Jeon, J.; Park, S.; Lee, B.J. Analysis on the performance of a flat-plate volumetric solar collector using blended plasmonic nanofluid. Sol. Energy 2016, 132, 247–256. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Hamze, S.; Fal, J.; Marcos, M.A.; Estellé, P.; Żyła, G. Thermal and Physical Characterization of PEG Phase Change Materials Enhanced by Carbon-Based Nanoparticles. Nanomaterials 2020, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, R.; Wang, Z.; Wang, Q. Photo-thermal conversion properties of hybrid CuO-MWCNT/H2O nanofluids for direct solar thermal energy harvest. Appl. Therm. Eng. 2019, 147, 390–398. [Google Scholar] [CrossRef]
- Xiao, X.; Jia, H.; Wen, D.; Zhao, X. Thermal performance analysis of a solar energy storage unit encapsulated with HITEC salt/copper foam/nanoparticles composite. Energy 2020, 192, 116593. [Google Scholar] [CrossRef]
- Khullar, V.; Tyagi, H.; Hordy, N.; Otanicar, T.; Hewakuruppu, Y.; Modi, P.; Taylor, R.A. Harvesting solar thermal energy through nanofluid-based volumetric absorption systems. Int. J. Heat Mass Transf. 2014, 77, 377–384. [Google Scholar] [CrossRef]
- Hordy, N.; Rabilloud, D.; Meunier, J.-L.; Coulombe, S. High temperature and long-term stability of carbon nanotube nanofluids for direct absorption solar thermal collectors. Sol. Energy 2014, 105, 82–90. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Pei, G.; Yang, H.; Cao, J.; Hu, M. Assessment of Performance Enhancement Potential of a High-Temperature Parabolic Trough Collector System Combining the Optimized IR-Reflectors. Appl. Sci. 2020, 10, 3744. [Google Scholar] [CrossRef]
- Nagarajan, P.; Subramani, J.; Suyambazhahan, S.; Sathyamurthy, R. Nanofluids for Solar Collector Applications: A Review. Energy Procedia 2014, 61, 2416–2434. [Google Scholar] [CrossRef]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Campos, C.; Vasco, D.A.; Angulo, C.; Burdiles, P.A.; Cardemil, J.M.; Palza, H. About the relevance of particle shape and graphene oxide on the behavior of direct absorption solar collectors using metal based nanofluids under different radiation intensities. Energy Convers. Manag. 2019, 181, 247–257. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Ye, Q.; Wang, X.; Hu, Y. Shape-dependent solar thermal conversion properties of plasmonic Au nanoparticles under different light filter conditions. Sol. Energy 2019, 182, 340–347. [Google Scholar] [CrossRef]
- Chavan, S.; Babrekar, M.; More, S.; Jadhav, K. Structural and optical properties of nanocrystalline Ni–Zn ferrite thin films. J. Alloy. Compd. 2010, 507, 21–25. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.A.; Mahur, A.K.; Virk, H.S.; Singh, F.; Khan, S.; Avasthi, D.K.; Prasad, R. Study of optical band gap and carbonaceous clusters in swift heavy ion irradiated polymers with UV–Vis spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 1788–1792. [Google Scholar] [CrossRef]
- Amjad, M.; Jin, H.; Du, X.; Wen, D. Experimental photothermal performance of nanofluids under concentrated solar flux. Sol. Energy Mater. Sol. Cells 2018, 182, 255–262. [Google Scholar] [CrossRef]



| Sr. No. | Nanofluid (%) | Amount of GO (g) | Nanofluid Concentration (wt %) |
|---|---|---|---|
| 1 | 100% GO | 0.0001 | 0.0004 |
| 2 | 80%GO + 20%CuO | 0.00008 | 0.0004 |
| 3 | 60%GO + 40%CuO | 0.000060 | 0.0004 |
| 4 | 40%GO + 60%CuO | 0.000040 | 0.0004 |
| 5 | 20%GO + 80%CuO | 0.000020 | 0.0004 |
| 6 | 100%CuO | 0.0000 | 0.0004 |
| 7 | 80%GO + 20%ZnO | 0.000080 | 0.0004 |
| 8 | 60%GO + 40%ZnO | 0.000060 | 0.0004 |
| 9 | 40%GO + 60%ZnO | 0.000040 | 0.0004 |
| 10 | 20%GO + 80%ZnO | 0.000020 | 0.0004 |
| 11 | 100%ZnO | 0.0000 | 0.0004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattar, A.; Farooq, M.; Amjad, M.; Saeed, M.A.; Nawaz, S.; Mujtaba, M.A.; Anwar, S.; El-Sherbeeny, A.M.; Soudagar, M.E.M.; Bandarra Filho, E.P.; et al. Performance Evaluation of a Direct Absorption Collector for Solar Thermal Energy Conversion. Energies 2020, 13, 4956. https://doi.org/10.3390/en13184956
Sattar A, Farooq M, Amjad M, Saeed MA, Nawaz S, Mujtaba MA, Anwar S, El-Sherbeeny AM, Soudagar MEM, Bandarra Filho EP, et al. Performance Evaluation of a Direct Absorption Collector for Solar Thermal Energy Conversion. Energies. 2020; 13(18):4956. https://doi.org/10.3390/en13184956
Chicago/Turabian StyleSattar, Abdul, Muhammad Farooq, Muhammad Amjad, Muhammad A. Saeed, Saad Nawaz, M.A. Mujtaba, Saqib Anwar, Ahmed M. El-Sherbeeny, Manzoore Elahi M. Soudagar, Enio P. Bandarra Filho, and et al. 2020. "Performance Evaluation of a Direct Absorption Collector for Solar Thermal Energy Conversion" Energies 13, no. 18: 4956. https://doi.org/10.3390/en13184956
APA StyleSattar, A., Farooq, M., Amjad, M., Saeed, M. A., Nawaz, S., Mujtaba, M. A., Anwar, S., El-Sherbeeny, A. M., Soudagar, M. E. M., Bandarra Filho, E. P., Ali, Q., Imran, M., & Pettinau, A. (2020). Performance Evaluation of a Direct Absorption Collector for Solar Thermal Energy Conversion. Energies, 13(18), 4956. https://doi.org/10.3390/en13184956












