On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glauber, J.R. Pharmacopoea Spagyrica; Johannes Jansson: Amsterdam, The Netherlands, 1654. [Google Scholar]
- Shearon, W.H., Jr.; Dunwoody, W.B. Ammonium nitrate: A staff-industry collaborative report. Ind. Eng. Chem. 1953, 45, 496–504. [Google Scholar] [CrossRef]
- Landucci, G.; Reniers, G.; Cozzani, V.; Salzano, E. Vulnerability of industrial facilities to attacks with imporivised devices aimed at triggering domino scenarios. Reliab. Eng. Syst. Saf. 2015, 143, 53–62. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Kwok, Q.S.M.; Jones, D.E.G. Thermodesorption studies of ammonium nitrate prills by high-resolution thermogravimetry. J. Therm. Anal. Calorim. 2003, 74, 57–63. Available online: https://link.springer.com/article/10.1023/A:1026317517373 (accessed on 1 September 2019). [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Pyra, J.; Twardosz, M. Comparison of structure, morphology and topography of fertilizer-based explosives applied in the mining industry. Microchem. J. 2019, 144, 39–44. Available online: https://www.sciencedirect.com/science/article/pii/S0026265X18305162 (accessed on 5 September 2019). [CrossRef]
- Viktorov, S.D.; Frantov, A.E.; Lapikov, I.N.; Andreev, V.V.; Starshinov, A.V. Effect of microstructure of ammonium nitrate granules on the detonability of composite propellants based on it. Combust. Explos. Shock Waves 2016, 52, 727–731. Available online: https://link.springer.com/article/10.1134/S0010508216060137 (accessed on 1 June 2020). [CrossRef]
- Lotspeich, E.; Petr, V. The characterization of ammonium nitrate mini-prills. In Dynamic Behavior of Materials, Proceedings of the 2014 Annual Conference on Experimental and Applied Mechanics, Greenville, SC, USA, 2–5 June 2014; Song, B., Casem, D., Kimberley, J., Eds.; Springer: Cham, Switzerland, 2015; Volume 1, pp. 319–325. Available online: https://link.springer.com/book/10.1007%2F978-3-319-06995-1 (accessed on 5 September 2019).
- Elzaki, B.I.; Zhang, Y.J. Surface modification of ammonium nitrate by coating with surfactant materials to reduce hygroscopicity. Def Technol. 2019, 15, 615–620. Available online: https://www.sciencedirect.com/science/article/pii/S2214914718305312 (accessed on 1 June 2020). [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł. Research on Morphology and Topology of ANFO Based on Various Types of Oxygen Component; Wydawnictwa AGH: Kraków, Poland, 2018. Available online: https://www.wydawnictwoagh.pl/RESEARCH-ON-MORPHOLOGY-AND-TOPOLOGY-OF-ANFO-BASED-ON-VARIOUS-TYPES-OF-OXYGEN-COMPONENT;s,karta,id,1298 (accessed on 1 June 2020).
- Miyake, A.; Takahara, K.; Ogawa, T.; Ogata, Y.; Wada, Y.; Arai, H. Influence of physical properties of ammonium nitrate on the detonation behavior of ANFO. J. Loss Prev. Process 2001, 14, 533–538. [Google Scholar] [CrossRef]
- Rao, K.V.R.; Hariharan, P.L.; Jagannathan, K.; Yoganarasimhan, S.R. Scanning electron microscopy of ammonium nitrate prills in relations to their application in ammonium nitrate fuel oil systems. Fuel 1989, 68, 1118–1122. Available online: https://www-1sciencedirect-1com-1000027zz0057.wbg2.bg.agh.edu.pl/science/article/pii/0016236189901816 (accessed on 1 May 2020).
- Chaturvedi, S.; Dave, P.N. Review on Thermal Decomposition of Ammonium Nitrate. J. Energetic Mater. 2013, 31, 1–26. Available online: https://www.tandfonline.com/doi/abs/10.1080/07370652.2011.573523?journalCode=uegm20 (accessed on 1 May 2020). [CrossRef]
- Berthelot, M. Explosives and Their Power; John Murray: London, UK, 1892. [Google Scholar]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. FAM Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Oommen, C.; Jain, S.R. Ammonium nitrate: A promising rocket propellant oxidizer. J. Hazard. Mater. 1999, 67, 253–281. [Google Scholar] [CrossRef]
- Gunawan, R.; Freij, S.; Zhang, D.; Beach, F.; Littlefair, M. A mechanistic study into the reactions of ammonium nitrate with pyrite. Chem. Eng. Sci. 2006, 61, 5781–5790. Available online: https://www.sciencedirect.com/science/article/pii/S0009250906002995 (accessed on 1 May 2020). [CrossRef]
- Djerdjev, A.M.; Priyananda, P.; Gore, J.; Beattie, J.K.; Neto, C.; Hawkett, B.S. The mechanism of the spontaneous detonation of ammonium nitrate in reactive grounds. J. Environ. Chem. Eng. 2018, 6, 281–288. Available online: https://www.sciencedirect.com/science/article/pii/S2213343717306425 (accessed on 1 May 2020). [CrossRef]
- Kaniewski, M.; Hoffmann, K.; Hoffmann, J. Influence of selected potassium salts on thermal stability of ammonium nitrate. Thermochim. Acta 2019, 678, 1–10. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0040603119303016 (accessed on 1 May 2020). [CrossRef]
- Han, Z.; Schdeva, S.; Papadaki, M.I.; Mannan, M.S. Ammonium nitrate thermal decomposition with additives. J. Loss Prev. Process 2015, 35, 307–315. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0950423014001703 (accessed on 1 May 2020). [CrossRef]
- Oxley, J.C.; Smith, J.L.; Rogers, E.; Yu, M. Ammonium nitrate: Thermal stability and explosivity modifiers. Thermochim. Acta 2002, 384, 23–45. [Google Scholar] [CrossRef]
- Han, Z.; Schdeva, S.; Papadaki, M.I.; Mannan, M.S. Calorimetry studies of ammonium nitrate—Effect of inhibitors, confinement, and heating rate. J. Loss Prev. Process 2015, 38, 234–242. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0950423015300486 (accessed on 1 May 2020). [CrossRef]
- Biessikirski, A.; Wądrzyk, M.; Janus, R.; Biegańska, J.; Jodłowski, G.; Kuterasiński, Ł. Study on fuel oils used in ammonium nitrate-based explosives. Przem. Chem. 2018, 97, 457–462. Available online: http://www.sigma-not.pl/publikacja-112770-badanie-ciek%C5%82ych-sk%C5%82adnik%C3%B3w-palnych-stosowanych-w-materia%C5%82ach-wybuchowych-opartych-na-azotanie-amonu-przemysl-chemiczny-2018-3.html (accessed on 1 May 2020).
- Zawadzka-Małota, I. Wpływ Struktury i Składu Górniczych Materiałów Wybuchowych na Zawartość Toksycznych Składników w Gazach Postrzałowych. Ph.D. Thesis, GIG, Katowice, Poland, 2009. [Google Scholar]
- Vargeese, A.A.; Joshi, S.S.; Krishnamurthy, V.N. Effect of method of crystallization on the IV-III and IV-II polymorphic transitions of ammonium nitrate. J. Hazard. Mater. 2009, 161, 373–379. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0304389408004949 (accessed on 1 May 2020). [CrossRef]
- Xu, Z.-X.; Fu, X.-Q.; Wang, Q. Phase stability of ammonium nitrate with organic potassium salts. Cent. Eur. J. Energetic Mater. 2016, 13, 736–754. [Google Scholar] [CrossRef]
- Chellappa, R.S.; Dattelbaum, D.M.; Velisavljevic, N.; Sheffield, S. The phase diagram of ammonium nitrate. J. Chem. Phys. 2012, 137, 064504. [Google Scholar] [CrossRef] [PubMed]
- Macy, P.F.; Dudderar, T.D.; Reese, E.F.; Eriksen, L.H. Investigation of Sensitivity of Fertilizer Grade Ammonium Nitrate to Explosion; Technical Report Serial No. 1658; Picatinny Arsenal: Dover, NJ, USA, 1947. [Google Scholar]
- Babrauskas, V. Fire Science Publishers/Society of Fire Protection Engineers; Ignition Handbook; Fire Science Publishers: Issaquah, WA, USA, 2003. [Google Scholar]
- Fedoroff, B.T.; Aaronson, H.A.; Sheffield, O.E.; Reese, E.F.; Clift, G.D.; Dunkle, C.G.; Walter, H.; McLean, D.C. Encyclopedia of Explosives and Related Items; US Department of the Army, Picatinny Arsenal: Dover, NJ, USA, 1960; Volume 1. [Google Scholar]
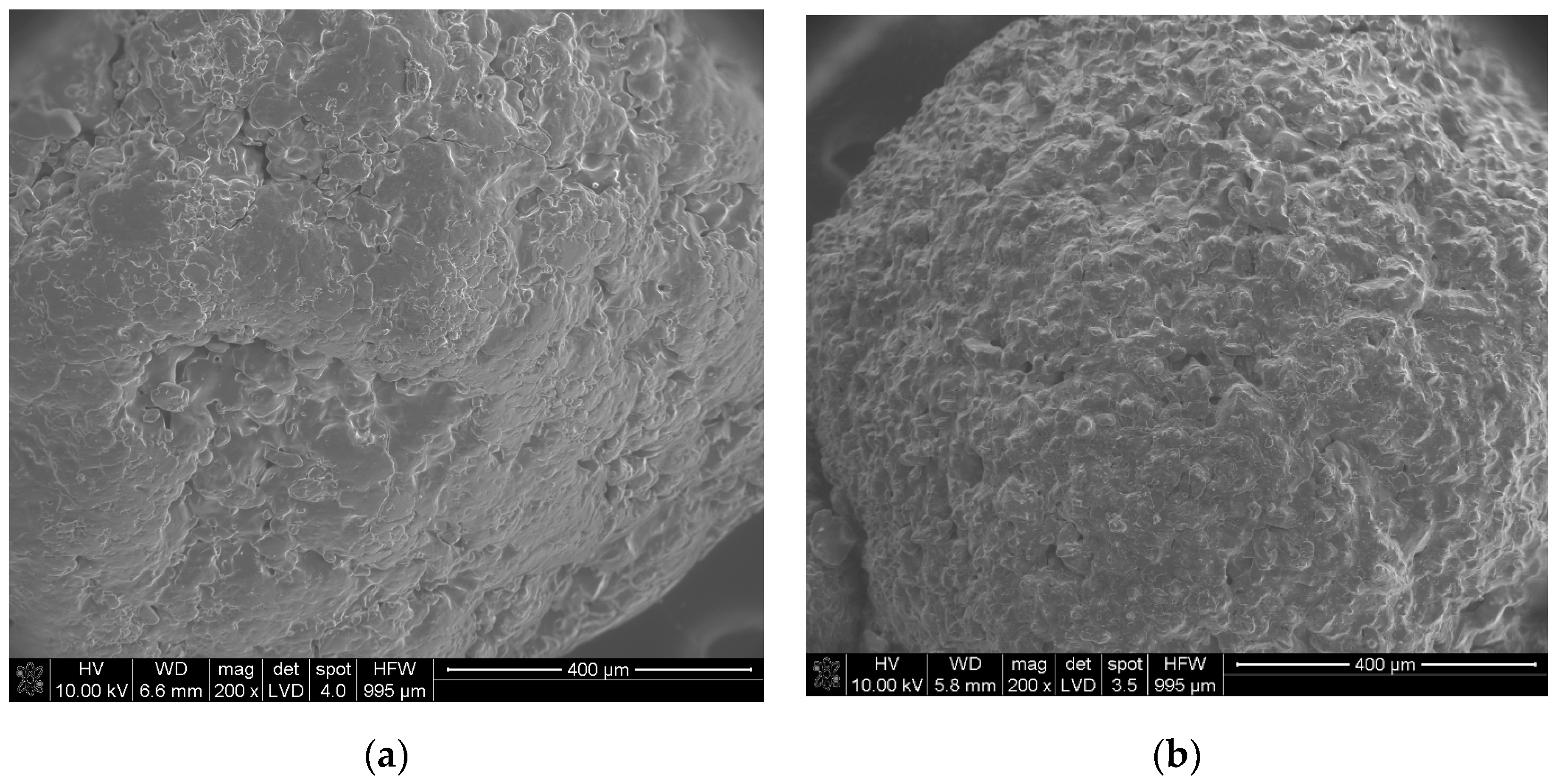

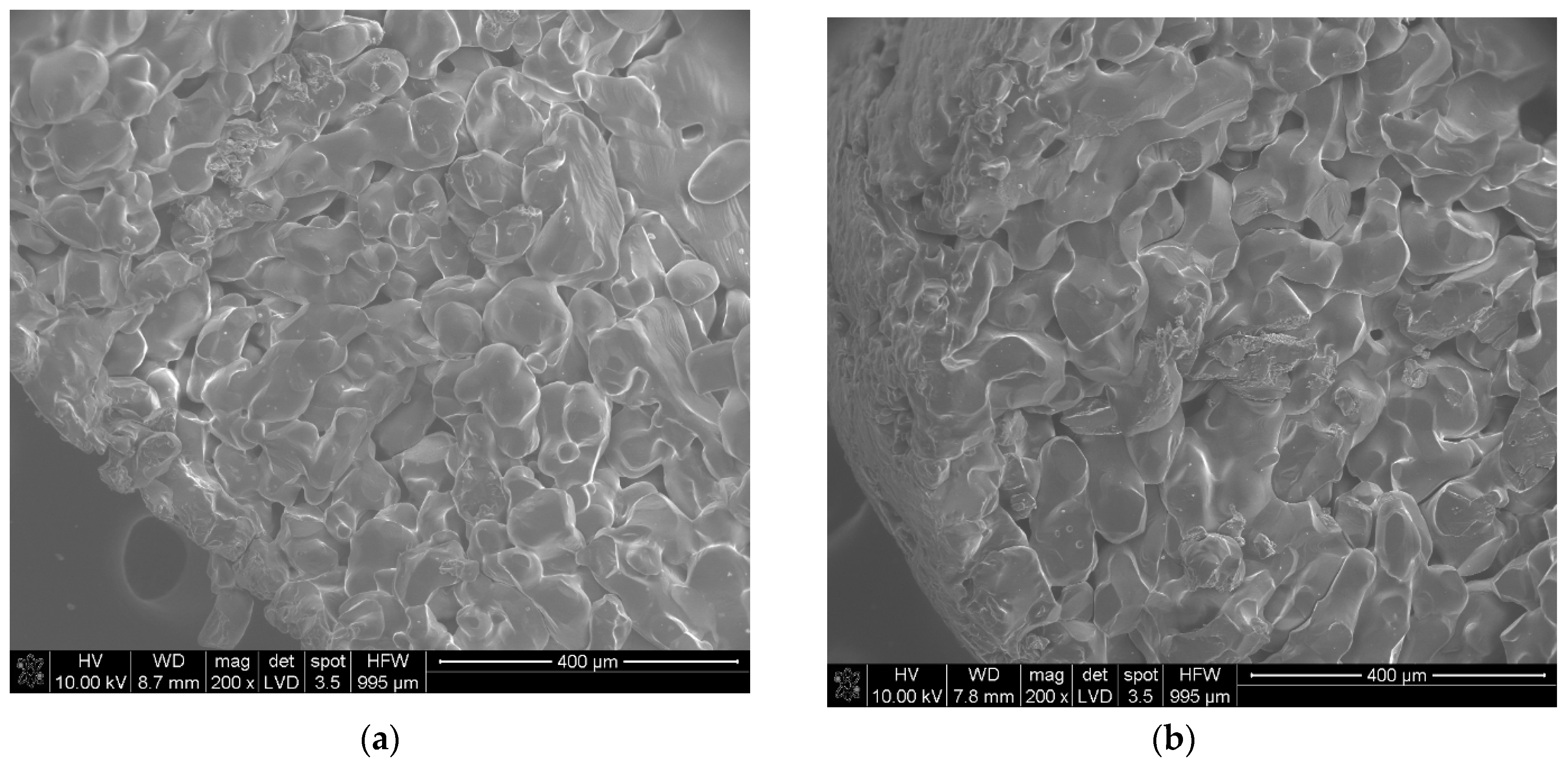
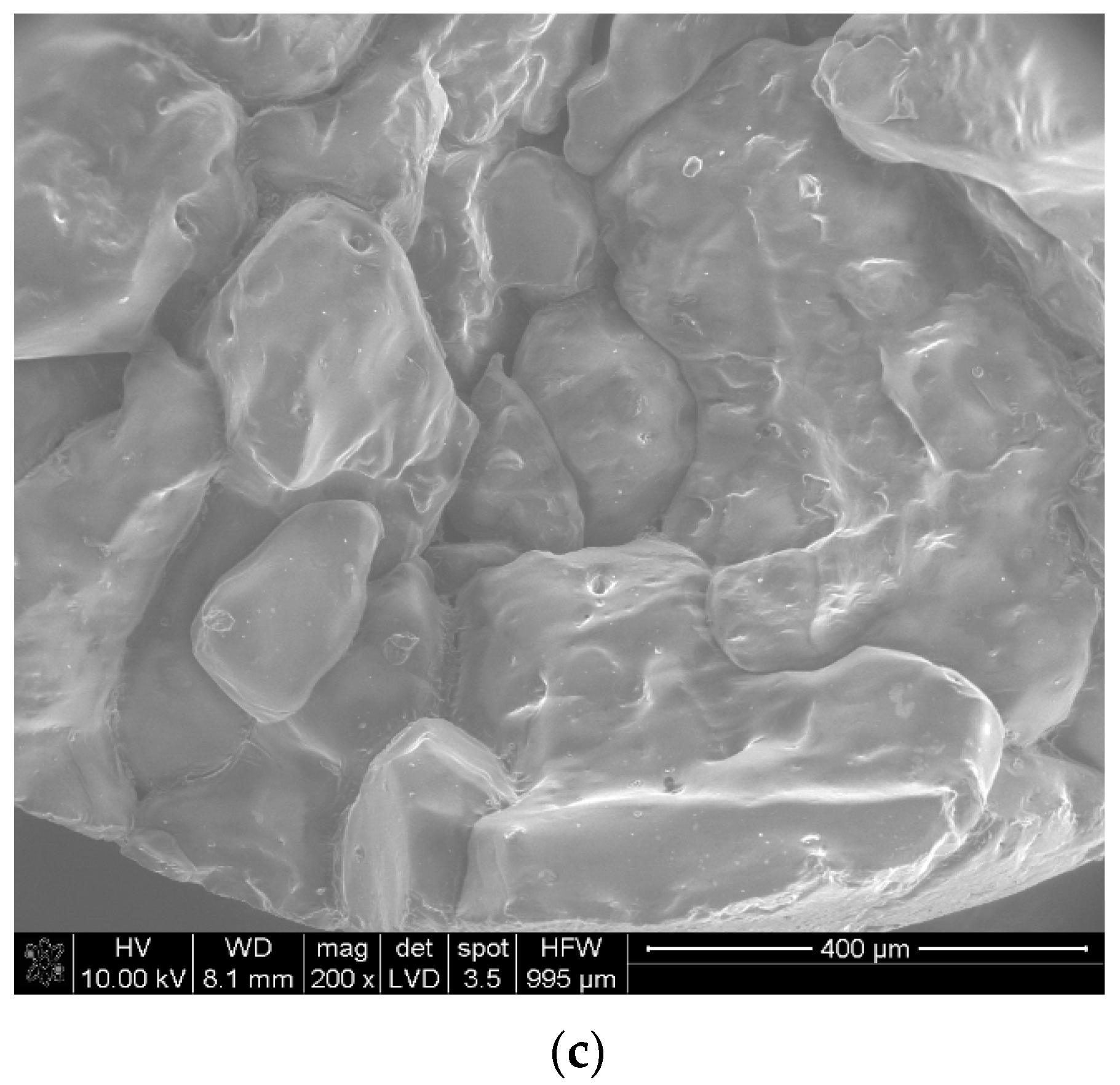
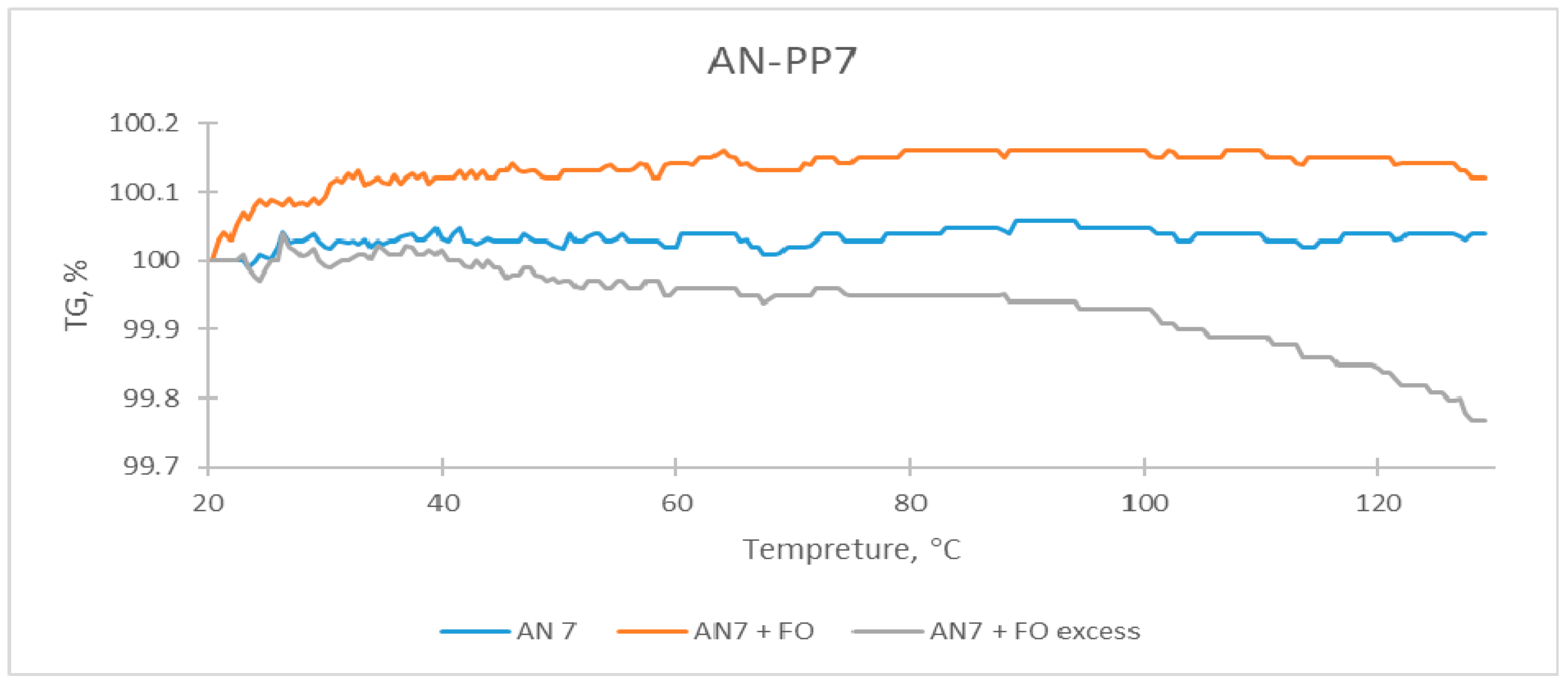
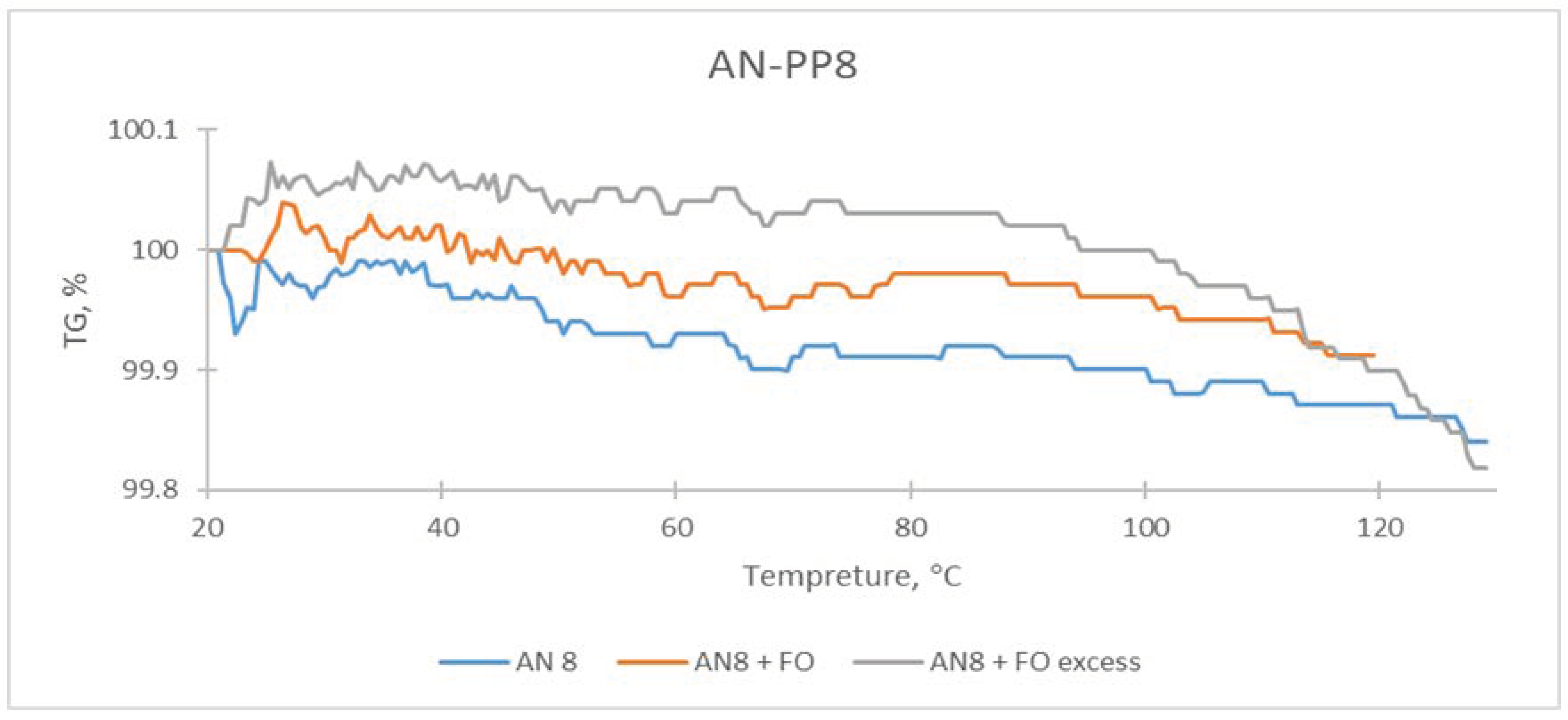
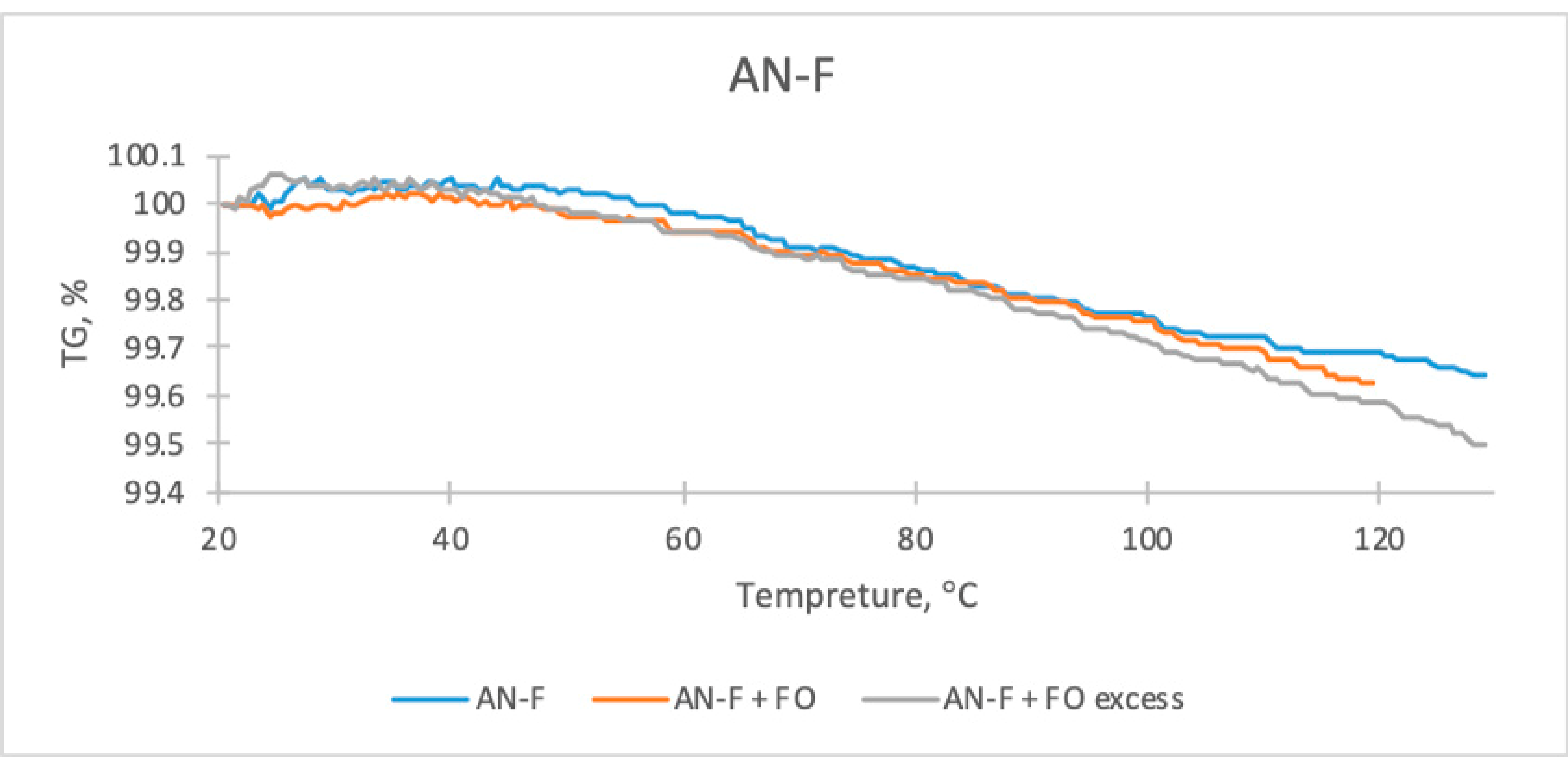
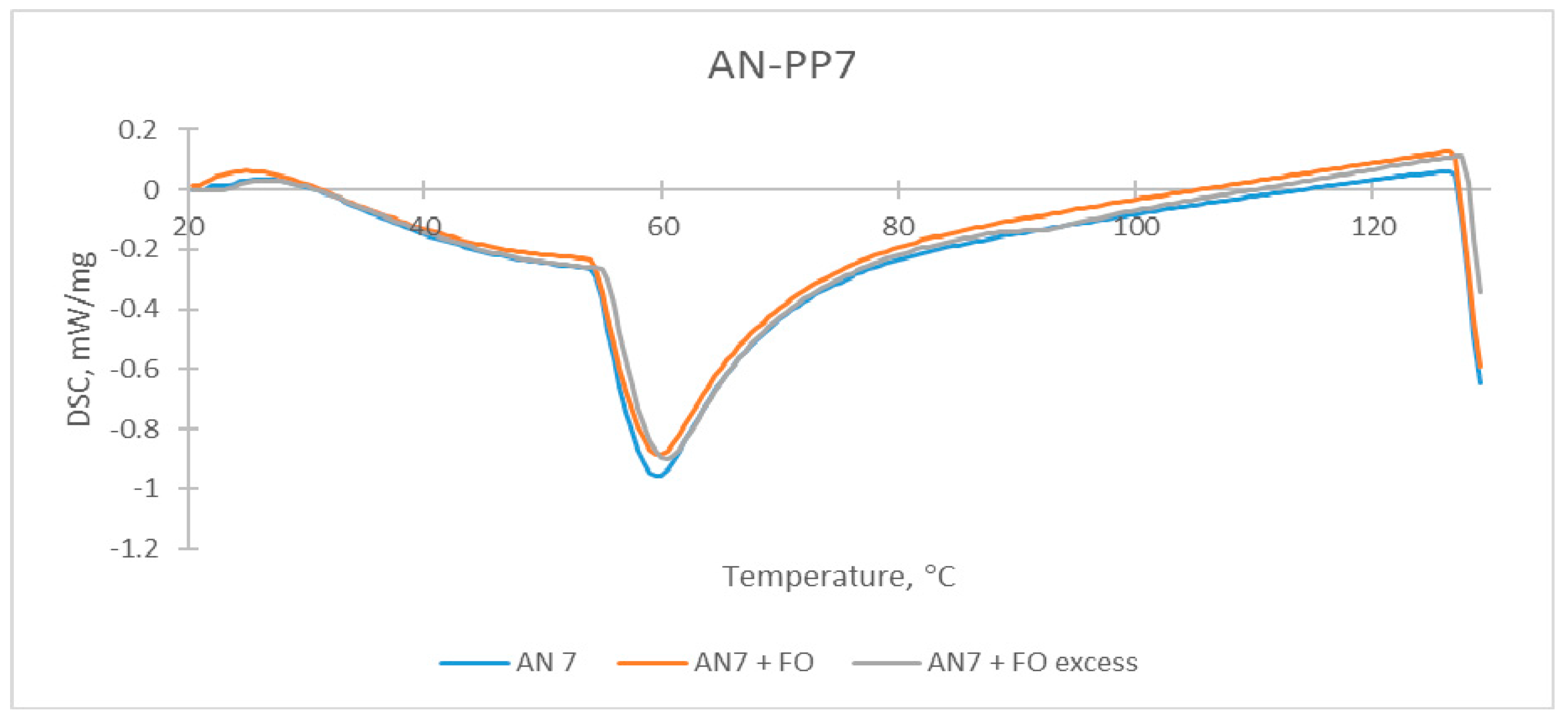
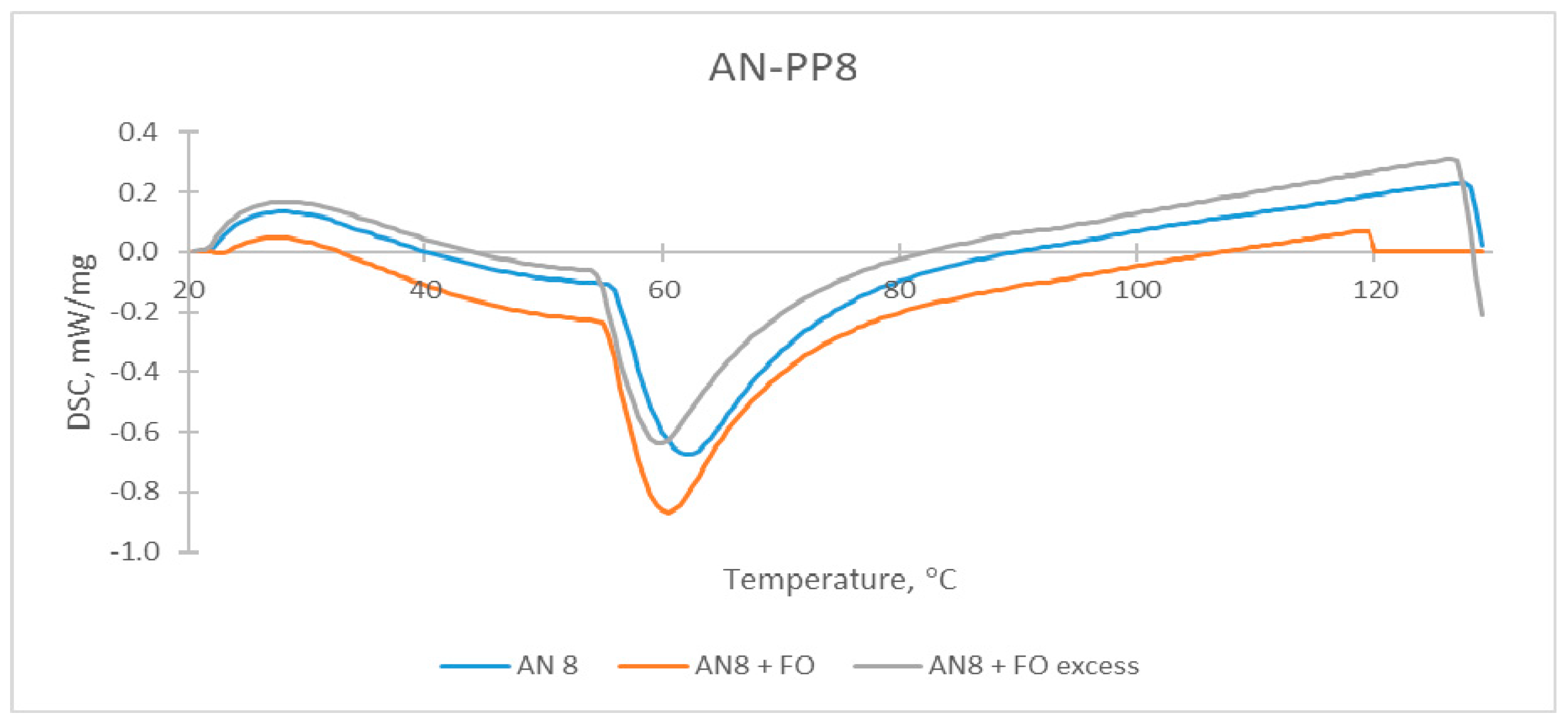
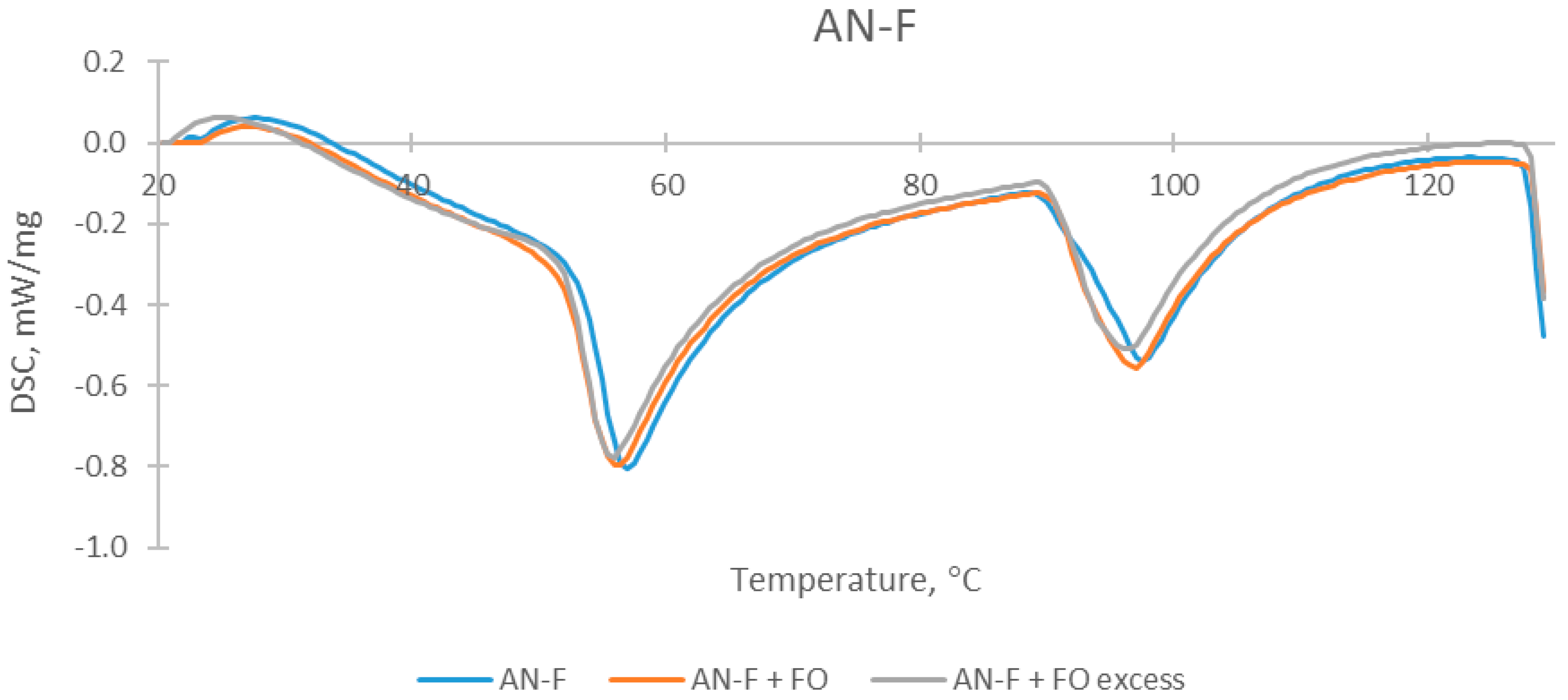
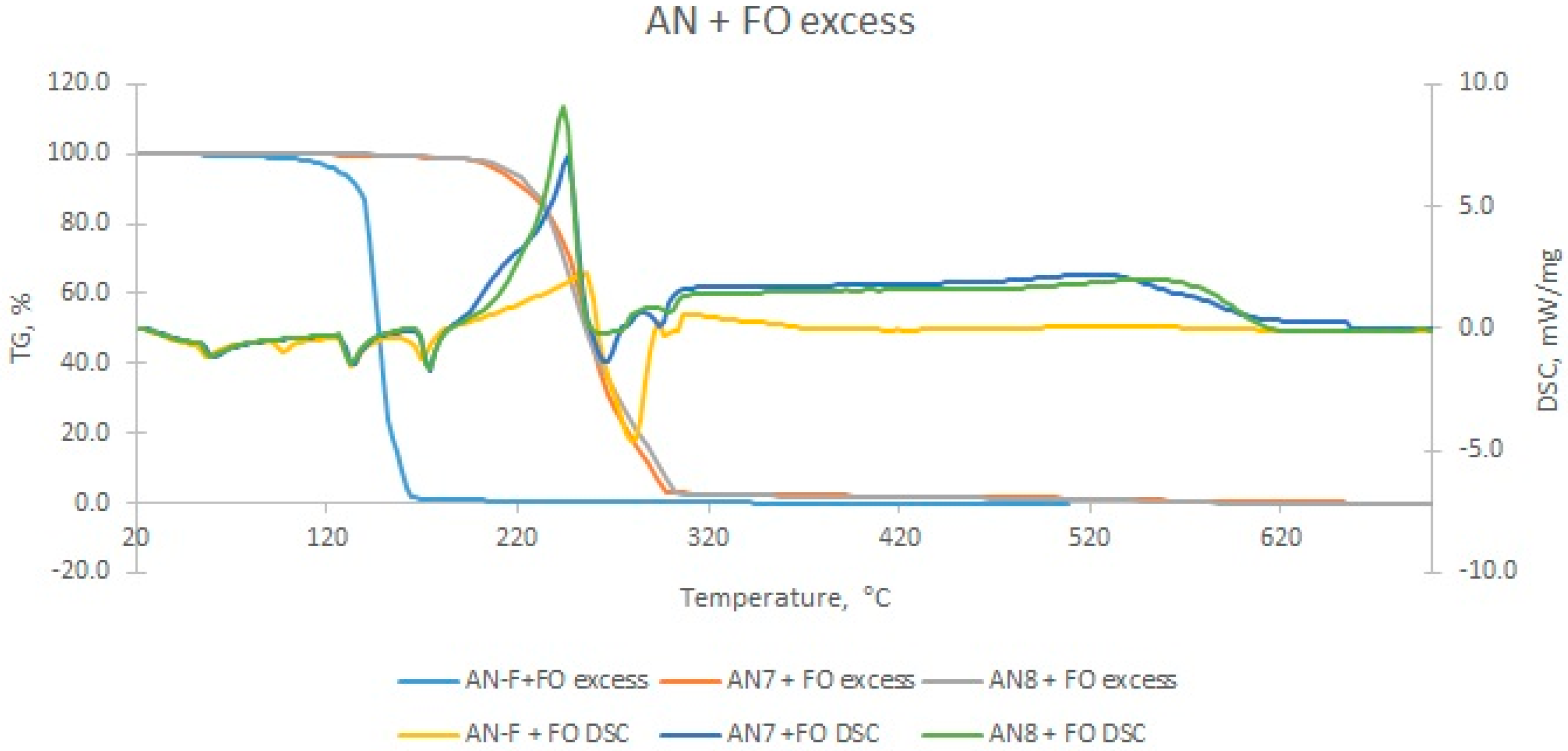
| Sample | The Temperature of the First DSC Peak | The Temperature of the Second DSC Peak | TG Mass Loss |
|---|---|---|---|
| AN-PP7 | 59.9 °C | None | None |
| AN-PP8 | 61.5 °C | None | 0.15% |
| AN-F | 55.0 °C | 95.5 °C | 0.36% |
| AN-PP7 + FO | 60.0 °C | None | None |
| AN-PP8 + FO | 58.5 °C | None | 0.19% |
| AN-F + FO | 56.0 °C | 99.0 °C | 0.42% |
| AN-PP7 + FO excess | 60.5 °C | 88.0–91.5 °C | 0.23% |
| AN-PP8 + FO excess | 60.0 °C | 92.5–94.0 °C | 0.11% |
| AN-F + FO excess | 56.5 °C | 96.5 °C | 0.46% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Tatko, M.; Napruszewska, B.D. On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives. Energies 2020, 13, 4942. https://doi.org/10.3390/en13184942
Biessikirski A, Kuterasiński Ł, Dworzak M, Twardosz M, Tatko M, Napruszewska BD. On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives. Energies. 2020; 13(18):4942. https://doi.org/10.3390/en13184942
Chicago/Turabian StyleBiessikirski, Andrzej, Łukasz Kuterasiński, Michał Dworzak, Michał Twardosz, Maciej Tatko, and Bogna Daria Napruszewska. 2020. "On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives" Energies 13, no. 18: 4942. https://doi.org/10.3390/en13184942
APA StyleBiessikirski, A., Kuterasiński, Ł., Dworzak, M., Twardosz, M., Tatko, M., & Napruszewska, B. D. (2020). On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives. Energies, 13(18), 4942. https://doi.org/10.3390/en13184942





