Study on the Effects of Microstructural Surfaces on the Attachment of Moving Microbes
Abstract
1. Introduction
2. Research Method
2.1. Governing Equations and Calculation Method
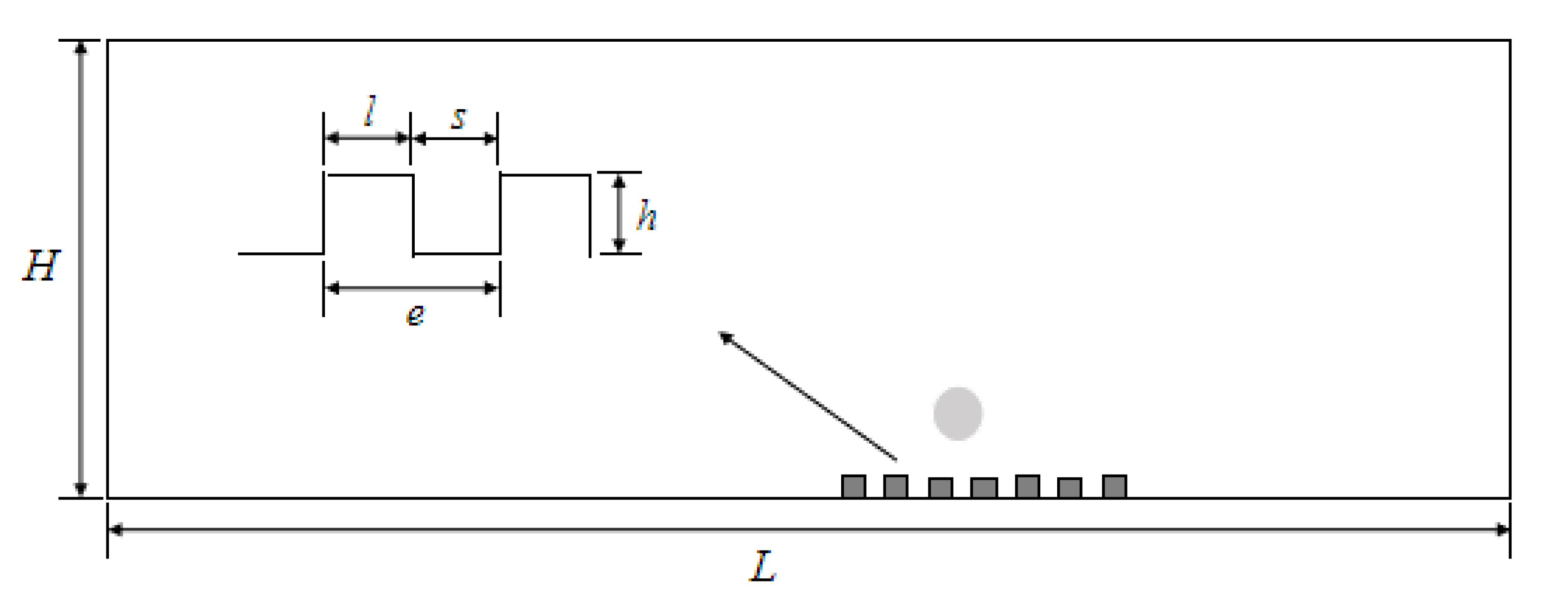
2.2. Model Parameters and Boundary Conditions
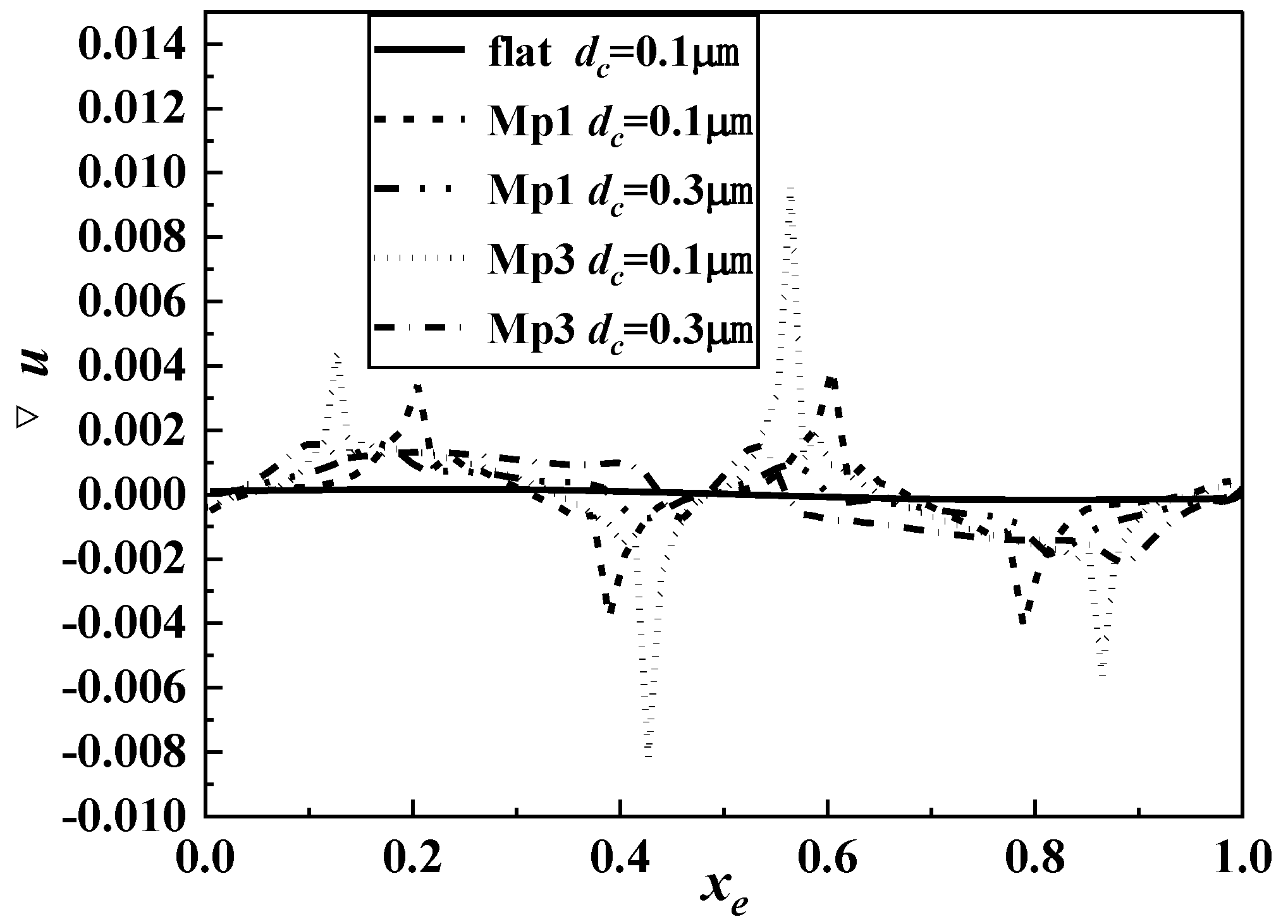
2.3. Mesh Generation and Independence Test
3. Effects of Microstructures on the Attachment of Moving Organisms
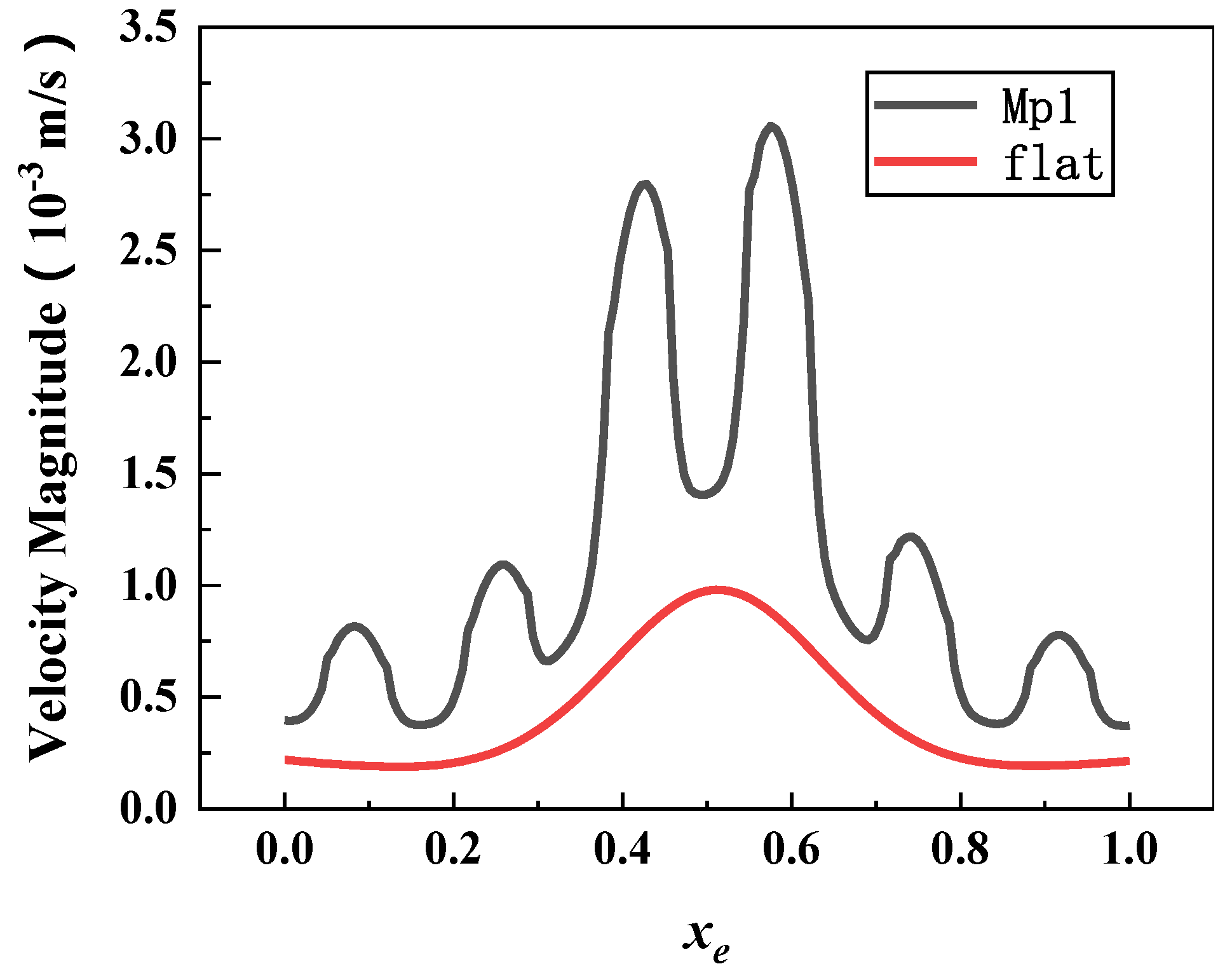
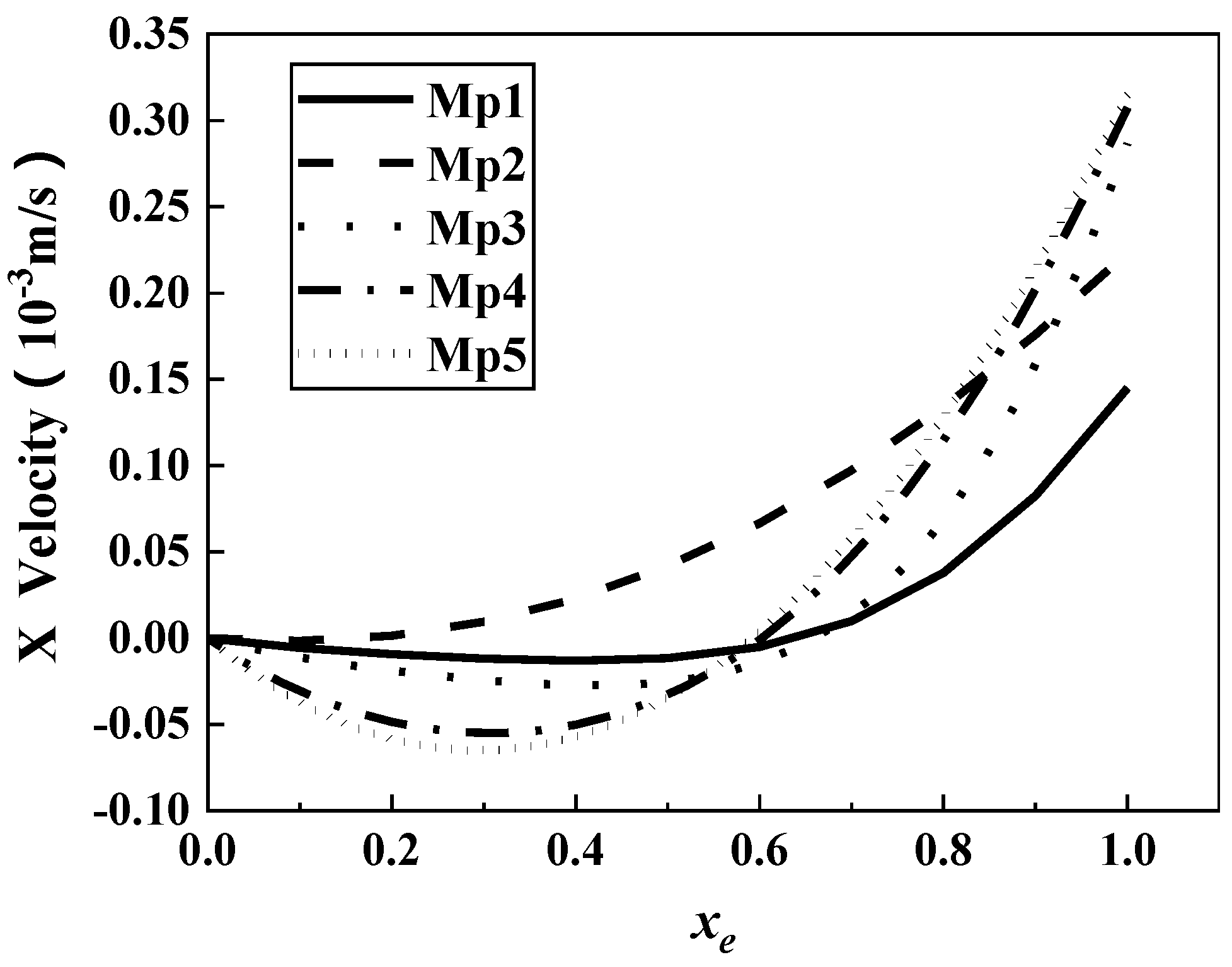
3.1. Characteristics of Fluid Kinematics in Microbial Motion
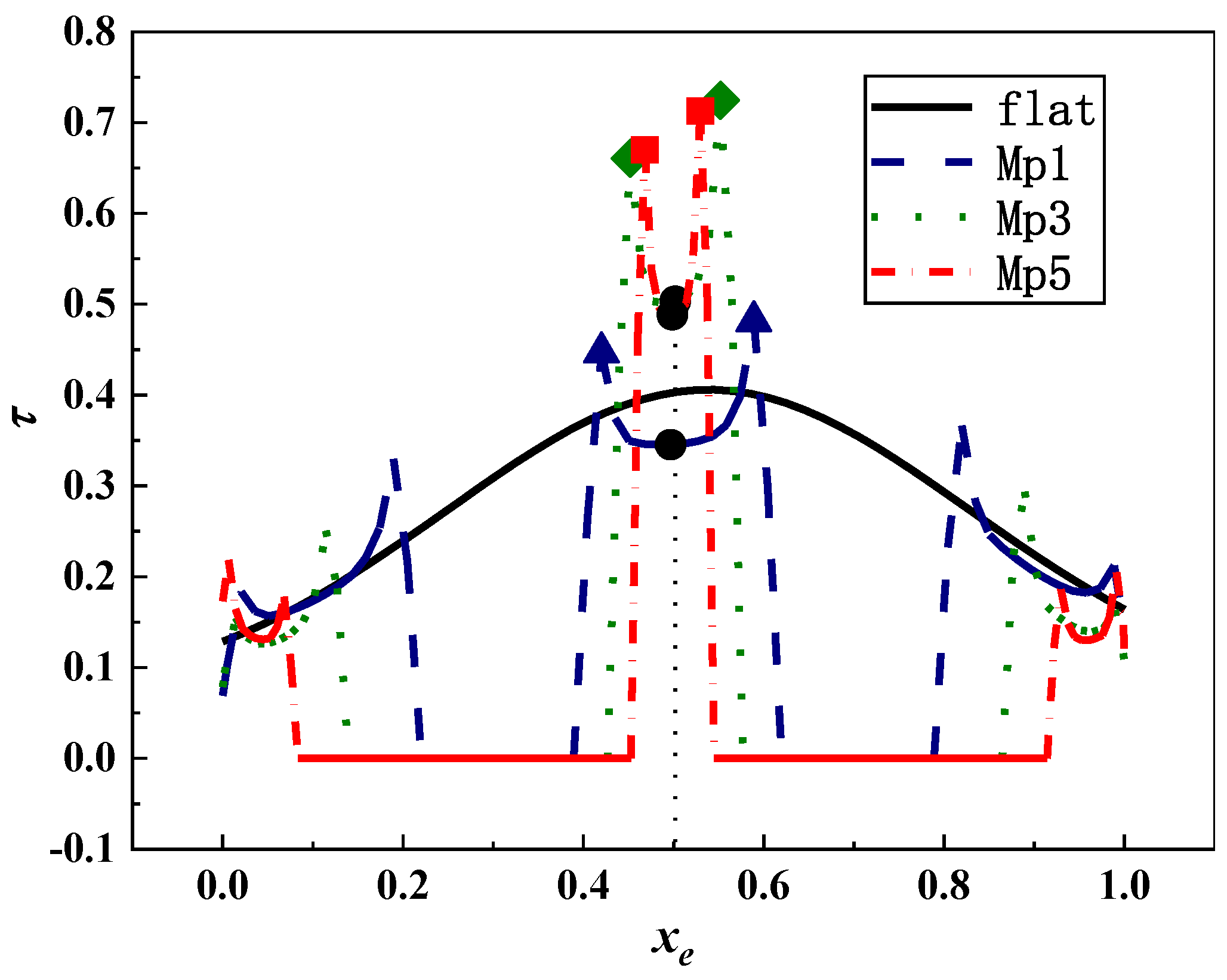
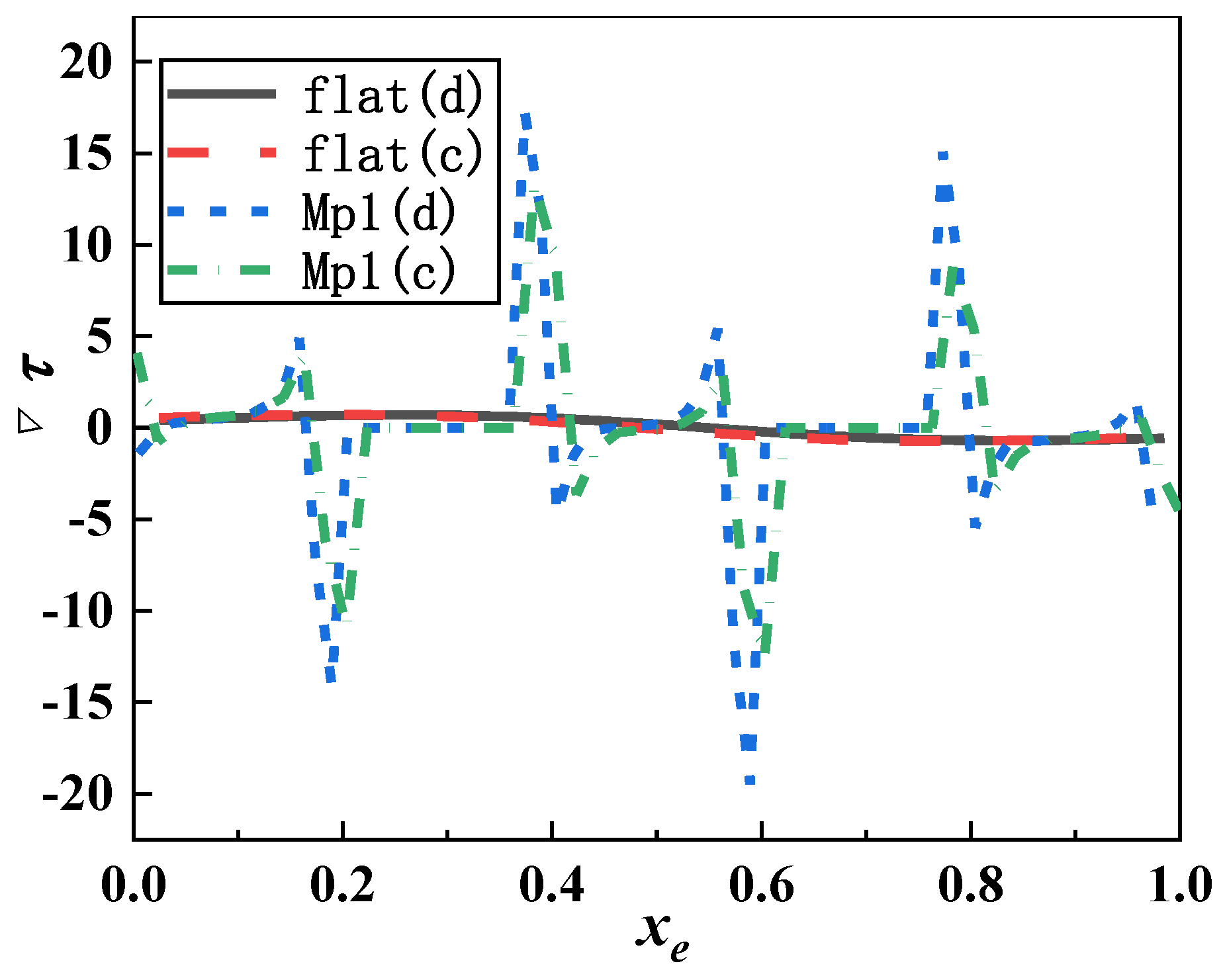
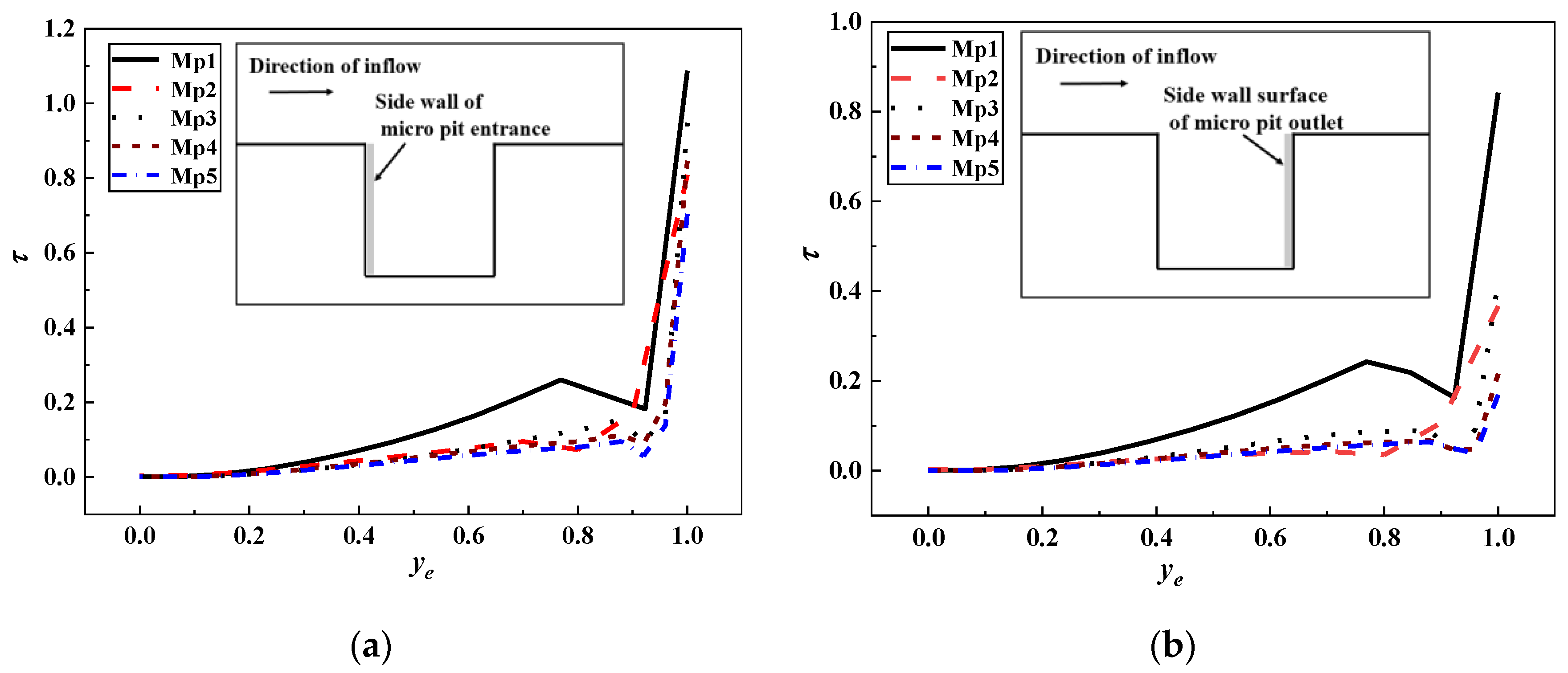
3.2. Shear Stress Distribution of Microorganism Movement
4. Conclusions
- The fluid velocity on the microstructural surface was greater than that on the smooth surface and fluctuated periodically, making the velocity gradient on the microstructural surface change frequently. The smooth surface had zero frequency. Simulation results of five models (0.4 ≤ β ≤ 1) revealed that the smaller the β value, the more difficult to form a vortex in micropits. The larger the β value was, the nearer the low-velocity area of the vortex in micropits to the microstructural surface and the closer the fluid slipping position to microridges. Therefore, the larger the β value, the more difficult it was for microorganisms to attach into micropits.
- The shear stress and its gradient increased with the increase of the depth and width of micropits. When microbes were larger than the microstructure, they could only find the attachment point in microridges. Simulation results expressed that the velocity and shear stress at microridges changed greatly, thus making it hard for microorganisms to attach into micropits. When microorganisms were smaller than the microstructure, they collided with the vortex wall in micropits and then became attached to the torsion node. The shear stress values on the two sides of the micropit with s = 2 μm were significantly higher than those of others, making it difficult for microbes to attach in depth. For other microstructures, the shear stress on the outlet sidewall was significantly lower than that on the inlet sidewall; hence, microbes tended to accumulate on the outlet side of micropits with vortex sediment.
- Microorganisms on five types of microstructures moved through the periodic fluctuation of microridges and micropits. Microridges had vortexes with low velocity and high shear stress, and the opposite phenomenon occurred in micropits. Although the increase of the width of microridges and micropits resulted in greater velocity and shear stress, the distribution of velocity and shear stress in the middle of them was similar to that of the smooth surface, and, thereby, the increase of the width of microridges and micropits was not useful to antifouling.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedrazzi, S.; Allesina, G.; Muscio, A. Are Nano-Composite Coatings the Key for Photovoltaic Panel Self-Maintenance: An Experimental Evaluation. Energies 2018, 11, 3448. [Google Scholar] [CrossRef]
- Lv, Y.K.; Zhao, W.P.; Li, J.G.; Zhang, Y.Z. Simulation of Contamination Deposition on Typical Shed Porcelain Insulators. Energies 2017, 10, 1045. [Google Scholar] [CrossRef]
- Mukherjee, A.; Joshi, M.; Misra, S.C.; Ramesh, U.S. Antifouling paint schemes for green SHIPS. Ocean Eng. 2019, 173, 227–234. [Google Scholar] [CrossRef]
- Lou, T.; Bai, X.Q.; Yuan, C.Q.; Yang, Z.C. Advances in Surface Microstructure Antifouling Technology for Ship Hull. Surf. Technol. 2019, 1, 102–113. [Google Scholar]
- Callow, M.E.; Fletcher, R.L. The influence of low surface energy materials on bioadhesion—A review. Int. Biodeterior. Biodegrad. 1994, 34, 333–348. [Google Scholar] [CrossRef]
- Brennan, A.B.; Baney, R.H.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Schumacher, J.F. Surface Topography for Non-Toxic Bioadhesion Control. U.S. Patent 7143709 B2, 5 December 2006. [Google Scholar]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies–correlating wettability with cell attachment. Biofouling 2006, 22, 11–21. [Google Scholar] [CrossRef]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies-effect of feature size, geometry, and roughness on settlement of zoospores of the green alga ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef]
- Decker, J.T.; Kirschner, C.M.; Long, C.J.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered Antifouling Microtopographies: An Energetic Model That Predicts Cell Attachment. Langmuir 2013, 29, 13023–13030. [Google Scholar] [CrossRef]
- Bai, X.Q.; Xie, G.T.; Fan, H.; Peng, Z.X.; Yuan, C.Q.; Yan, X.P. Study on biomimetic preparation of shell surface microstructure for ship antifouling. Wear 2013, 306, 285–295. [Google Scholar] [CrossRef]
- Fu, Y.F.; Yuan, C.Q.; Bai, X.Q.; Li, T. Study on Drag Reduction Performance of Antifouling Ribbed Surfaces. J. Ship Prod. Des. 2018, 34, 32–41. [Google Scholar]
- Yang, Z.C. Research on Biomimetic Preparation based on Crab Surface Microstructure and Analysis of Antifouling Performance. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2018. (In Chinese). [Google Scholar]
- Wang, X.; Bai, X.Q.; Yuan, C.Q. Analysis of present development on technologies of antifouling and drag reduction based on bionic non-smooth surface. Ship Eng. 2015, 37, 1–5. [Google Scholar]
- Zheng, J.Y.; Lin, C.G.; Zhang, J.W.; Wang, L.; Xu, F.L.; Zhou, J.; Duan, D.X.; Liu, H.H. Antifouling performance of surface microtopographies based on sea star luidia quinaria. Key Eng. Mater. 2013, 562–565, 1290–1295. [Google Scholar] [CrossRef]
- Halder, P.; Nasabi, M.; Jayasuriya, N.; Shimeta, J.; Deighton, M.; Bhattacharya, S.; Mitchell, A.; Bhuiyan, M.A. An assessment of the dynamic stability of microorganisms on patterned surfaces in relation to biofouling control. Biofouling 2014, 30, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Deepti, S.; Mahesh, S.; Sunita, K.; Anil, K.S.; Kulwant, S.; Nidhi, G.; Sucharita, S. Femtosecond laser induced surface modification for prevention of bacterial adhesion on 45S5 bioactive glass. J. Non-Cryst. Solids 2018, 482, 63–72. [Google Scholar] [CrossRef]
- Vucko, M.J.; Poole, A.J.; Carl, C.; Sexton, B.A.; Glenn, F.L.; Whalan, S.; Nys, R. Using textured PDMS to prevent settlement and enhance release of marine fouling organisms. Biofouling 2014, 30, 1–16. [Google Scholar] [CrossRef]
- Won, Y.J.; Jung, S.Y.; Jang, J.H.; Lee, J.W.; Chae, H.R.; Choi, D.C.; Hyun, A.K.; Lee, C.H.; Park, P.K. Correlation of membrane fouling with topography of patterned membranes for water treatment. J. Membr. Sci. 2016, 498, 14–19. [Google Scholar] [CrossRef]
- Jung, S.Y.; Hyun, A.K. Transport and deposition of colloidal particles on a patterned membrane surface: Effect of cross-flow velocity and the size ratio of particle to surface pattern. J. Membr. Sci. 2019, 572, 309–319. [Google Scholar] [CrossRef]
- Fawzan, M.R.B.A.; Myan, F.W.Y. Comparisons of Flow Patterns over a Hierarchical and a Non-hierarchical Surface in Relation to Biofouling Control. MATEC Web Conf. 2018, 152, 02014. [Google Scholar] [CrossRef][Green Version]
- Zhe, Y.; Yan, L. A Comprehensive Study of Dynamic and Heat Transfer Characteristics of Droplet Impact on Micro-Scale Rectangular Grooved Surface. Energies 2018, 11, 1390. [Google Scholar]
- Darnton, N.C.; Turner, L.; Rojevsky, S.; Berg, H.C. Dynamics of bacterial swarming. Biophys. J. 2010, 98, 2082–2090. [Google Scholar] [CrossRef]
- Friedmann, E.; Portl, J.; Richter, T. A Study of Shark Skin and its drag reducing mechanism. Adv. Math. Fluid Mech. 2010, 271–285. [Google Scholar] [CrossRef]
- Rosenhahn, A.; Sendra, G.H. Surface Sensing and Settlement Strategies of Marine Biofouling Organisms. Biointerphases 2012, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Won, Y.J.; Yoo, J.H.; Ahn, K.H.; Lee, C.H. Flow analysis and fouling on the patterned membrane surface. J. Membr. Sci. 2013, 427, 320–325. [Google Scholar] [CrossRef]
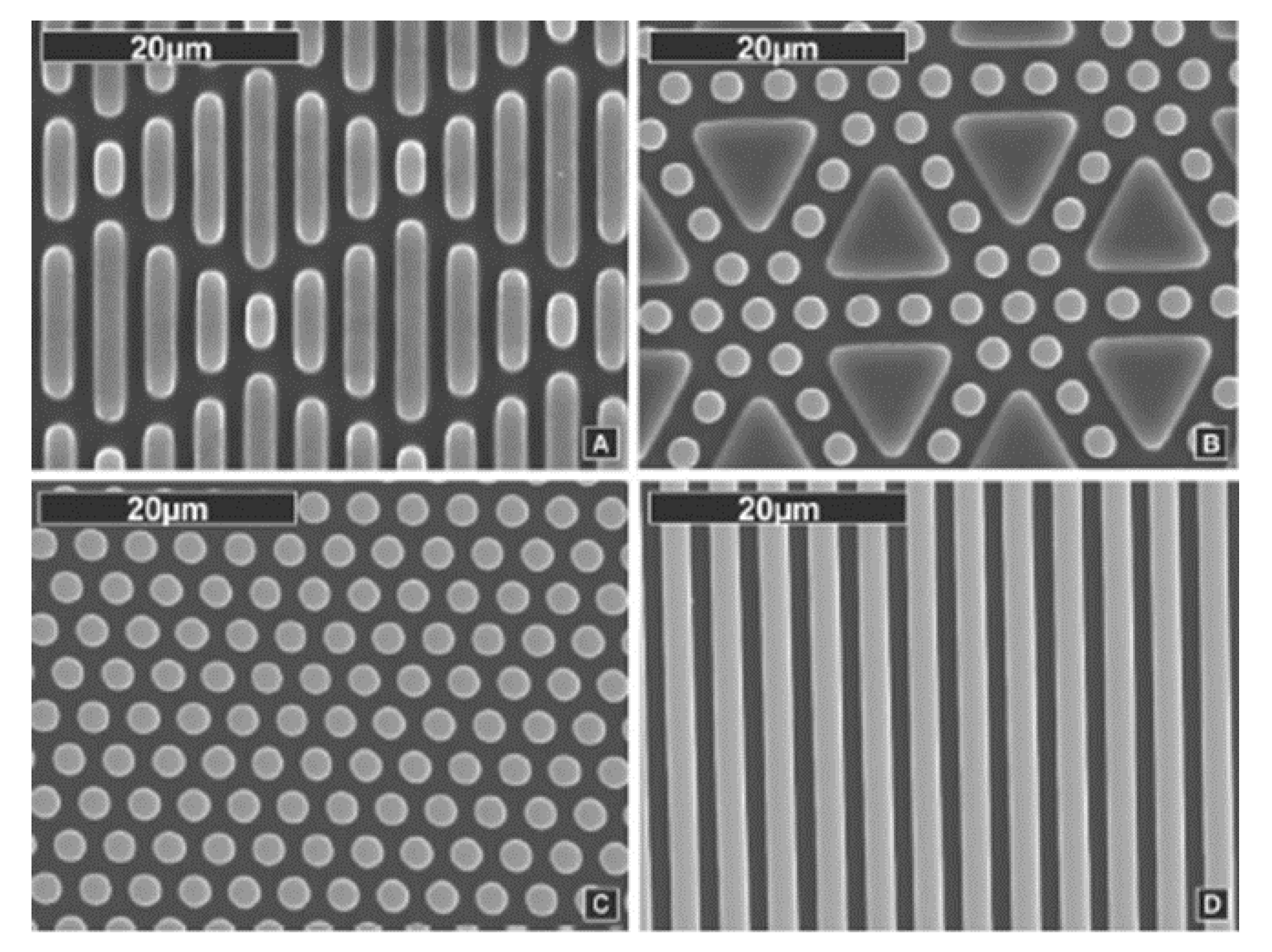



| Structures | Mp1 | Mp2 | Mp3 | Mp4 | Mp5 |
|---|---|---|---|---|---|
| h (μm) | 2 | 2 | 5 | 5 | 5 |
| s (μm) | 2 | 5 | 5 | 8 | 10 |
| β Values and Vortex Centers | Mp1 | Mp2 | Mp3 | Mp4 | Mp5 |
|---|---|---|---|---|---|
| β | 1 | 0.4 | 1 | 0.625 | 0.5 |
| yd (ux = 0) | 0.67 | 0 | 0.645 | 0.534 | 0.523 |
| yc (ux = 0) | 0.64 | 0.132 | 0.66 | 0.6 | 0.591 |
| Shear Stress | Flat | Mp1 | Mp2 | Mp3 | Mp4 | Mp5 |
|---|---|---|---|---|---|---|
| 0.406 | 0.482 | 0.545 | 0.725 | 0.779 | 0.713 | |
| 0.129 | 0 | 0 | 0 | 0 | 0 | |
| 0.287 | 0.254 | 0.210 | 0.283 | 0.286 | 0.274 | |
| 0.414 | 0.482 | 0.547 | 0.683 | 0.778 | 0.737 | |
| 0.130 | 0 | 0 | 0 | 0 | 0 | |
| 0.291 | 0.237 | 0.211 | 0.284 | 0.286 | 0.275 |
| Shear Stress Gradient | Mp1 | Mp2 | Mp3 | Mp4 | Mp5 |
|---|---|---|---|---|---|
| 17.185 | 26.289 | 33.995 | 51.480 | 60.540 | |
| −19.479 | −31.034 | −40.425 | −62.078 | −65.908 | |
| 13.213 | 31.392 | 39.134 | 59.933 | 70.178 | |
| −12.496 | −29.197 | −36.354 | −55.163 | −58.287 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Qian, J.; Yang, M.; Li, C.; Li, H.; Wang, S. Study on the Effects of Microstructural Surfaces on the Attachment of Moving Microbes. Energies 2020, 13, 4421. https://doi.org/10.3390/en13174421
Yang H, Qian J, Yang M, Li C, Li H, Wang S. Study on the Effects of Microstructural Surfaces on the Attachment of Moving Microbes. Energies. 2020; 13(17):4421. https://doi.org/10.3390/en13174421
Chicago/Turabian StyleYang, Hongyue, Ji Qian, Ming Yang, Chunxi Li, Hengfan Li, and Songling Wang. 2020. "Study on the Effects of Microstructural Surfaces on the Attachment of Moving Microbes" Energies 13, no. 17: 4421. https://doi.org/10.3390/en13174421
APA StyleYang, H., Qian, J., Yang, M., Li, C., Li, H., & Wang, S. (2020). Study on the Effects of Microstructural Surfaces on the Attachment of Moving Microbes. Energies, 13(17), 4421. https://doi.org/10.3390/en13174421





