Advanced Adsorbent Materials for Waste Energy Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Zeolite Synthesis
2.2. Electrospinning SAPO-34/PAN Microfibers
2.3. Samples Characterization
3. Results and Discussion
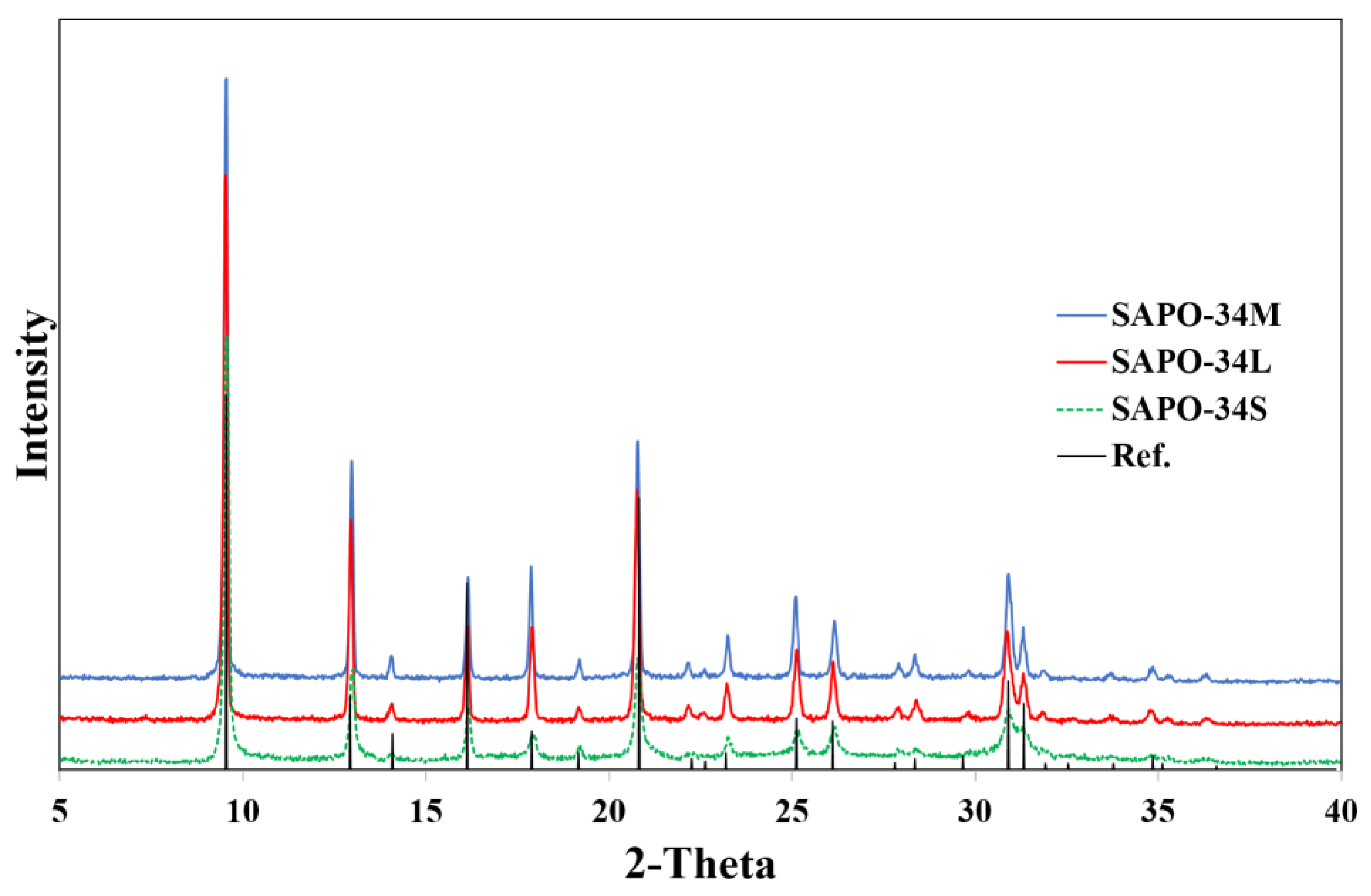
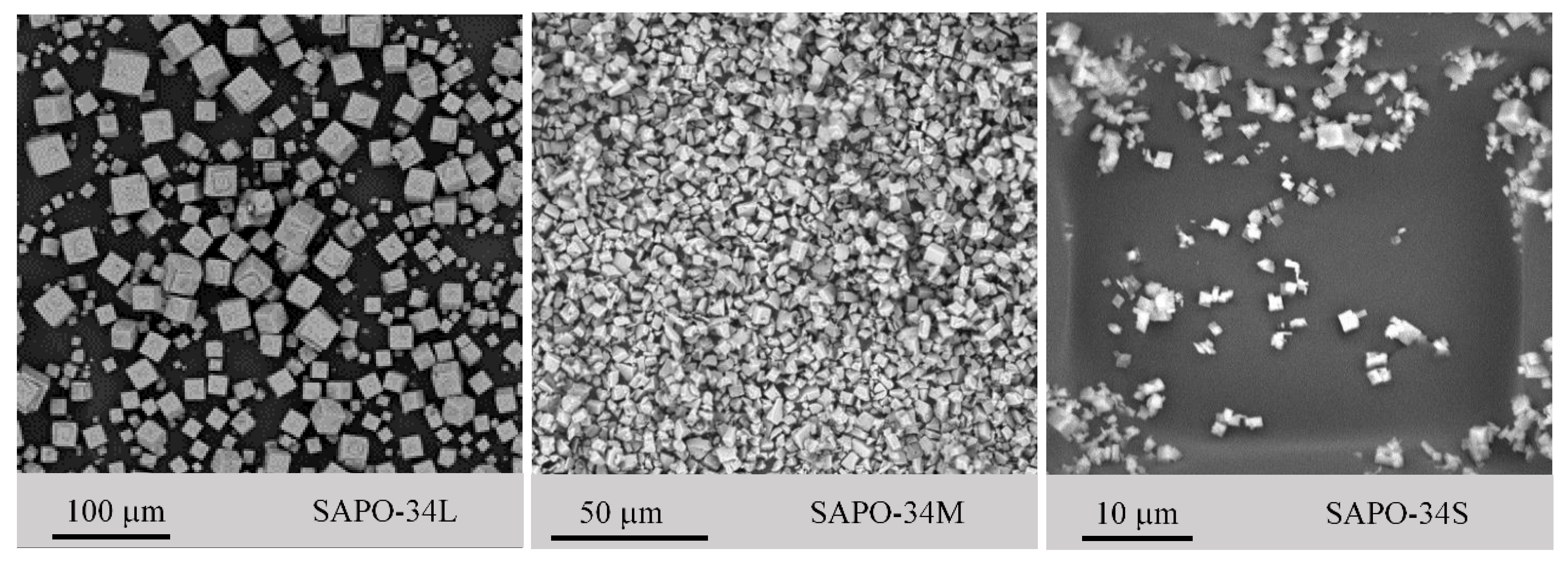
3.1. SAPO-34 Zeolite Characterization
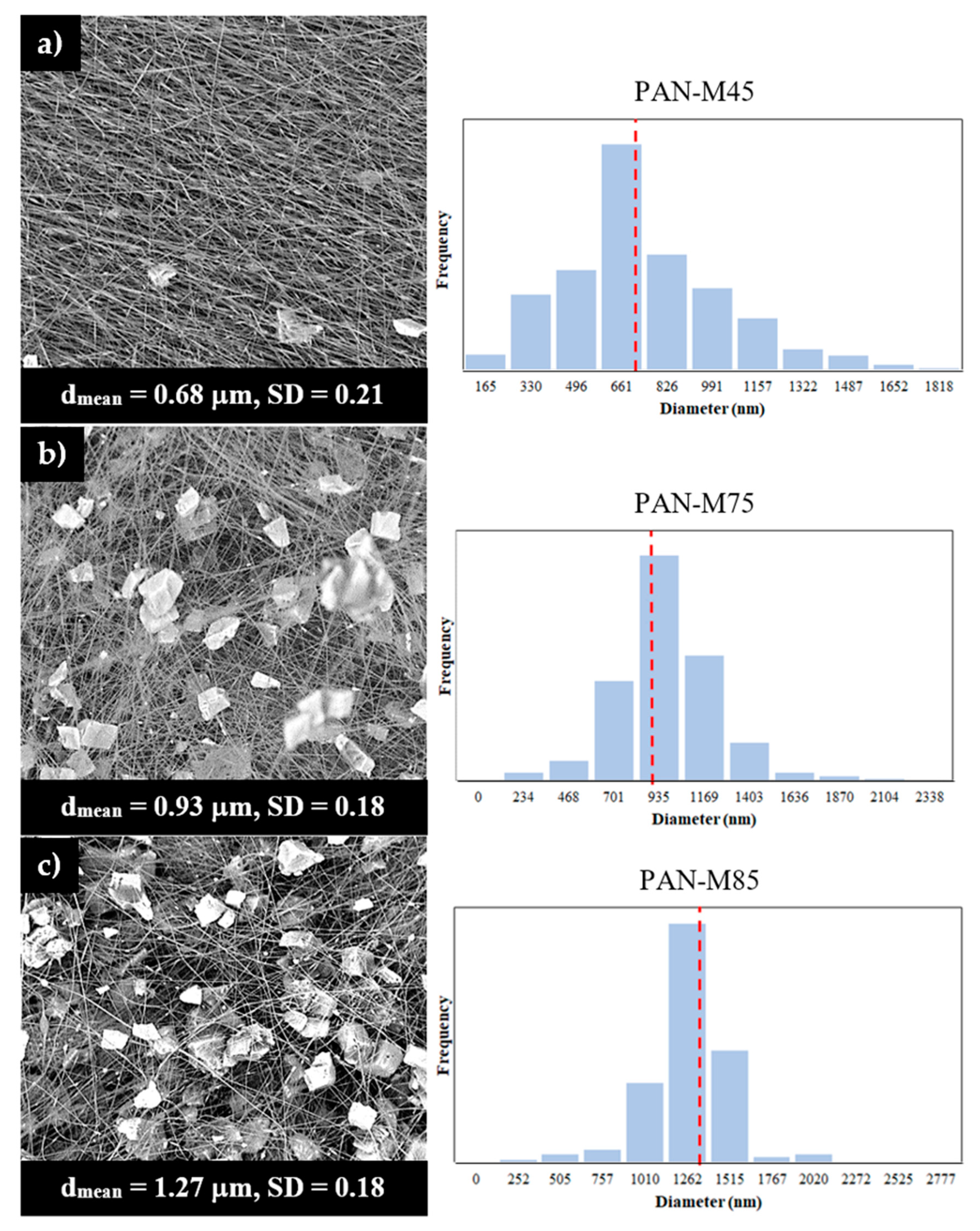
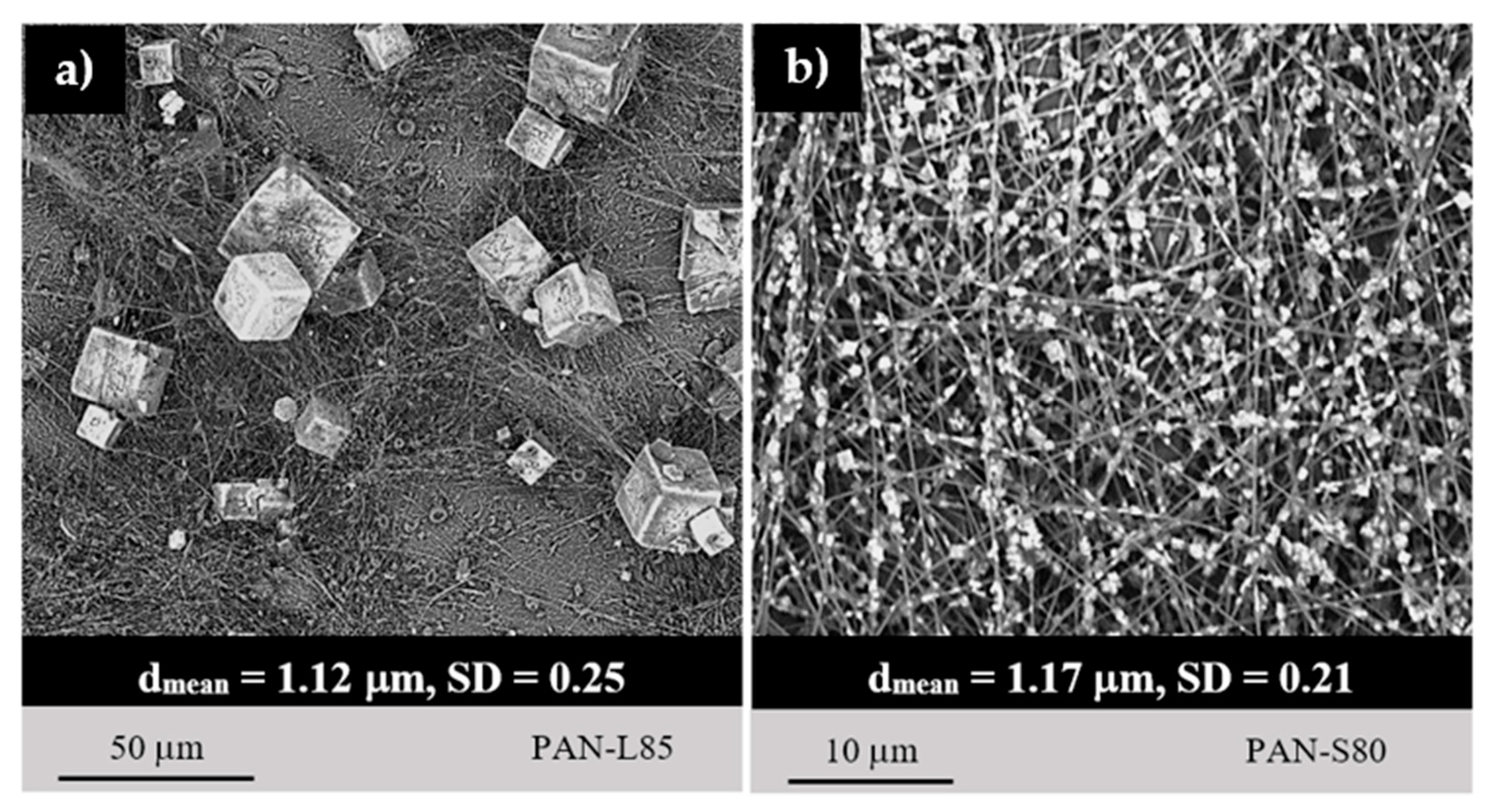
3.2. Morphological Characterization of Hybrid Microfiber Coatings
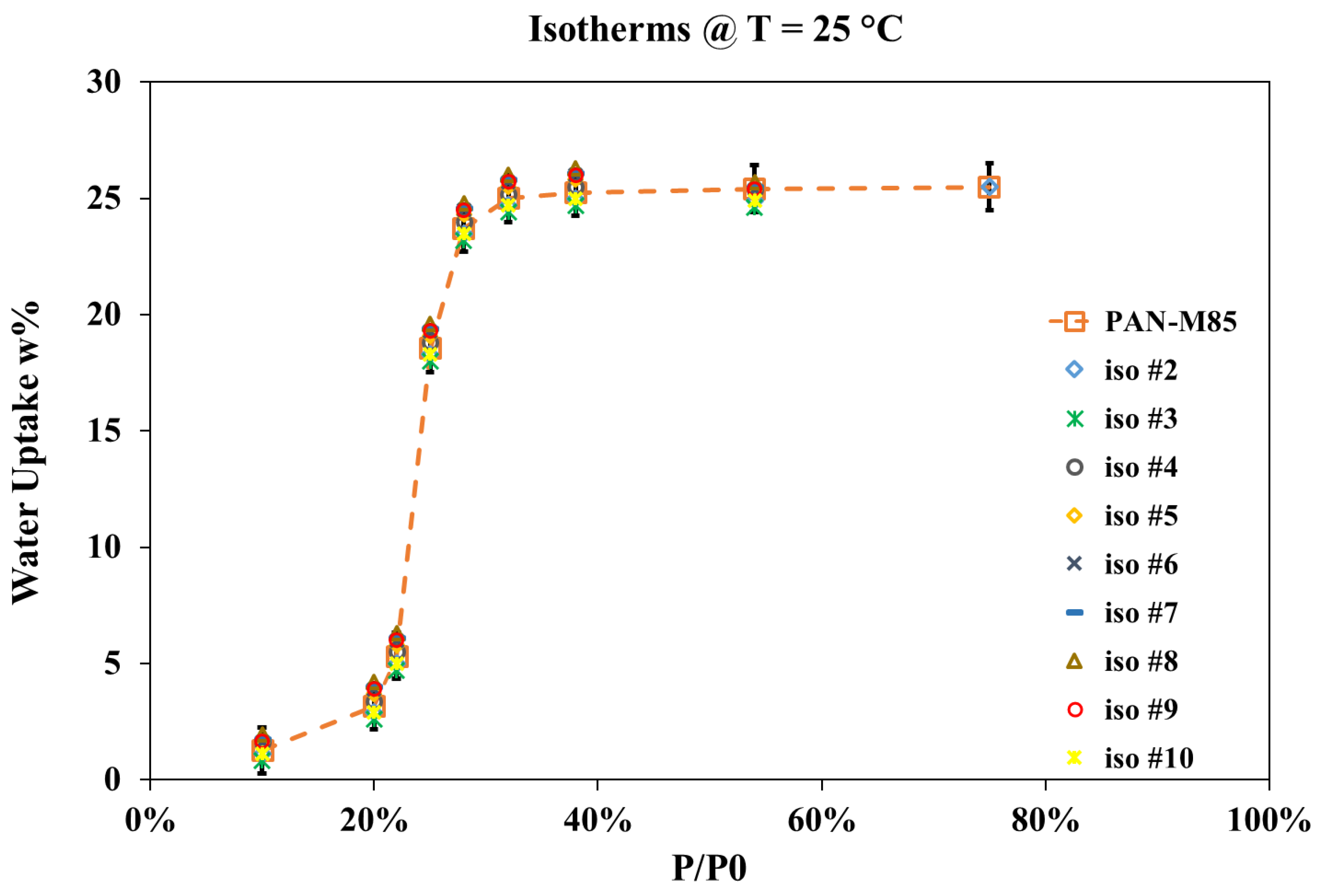
3.3. Thermal and Adsorption Properties of the Microfibers
3.4. Hybrid Microfiber Hydrothermal Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angammana, C.J.; Jayaram, S.H. Fundamentals of electrospinning and processing technologies. Part. Sci. Technol. 2016, 34, 72–82. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Stelitano, S.; Fazio, E.; Neri, F.; Scarpino, L.; Antonucci, P.L.; Santangelo, S. A new approach to the synthesis of titania nano-powders enriched with very high contents of carbon nanotubes by electro-spinning. Mater. Chem. Phys. 2015, 153, 338–345. [Google Scholar] [CrossRef]
- Kara, H.; Xiao, F.; Sarker, M.; Jin, T.Z.; Sousa, A.M.M.; Liu, C.; Tomasula, P.M.; Liu, L. Bio-based packaging. J. Appl. Polym. Sci. 2016, 133, 42475. [Google Scholar] [CrossRef]
- Ke, P.; Jiao, X.N.; Ge, X.H.; Xiao, W.M.; Yu, B. From macro to micro: Structural biomimetic materials by electrospinning. R. Soc. Chem. Adv. 2014, 4, 39704–39724. [Google Scholar] [CrossRef]
- Malara, A.; Frontera, P.; Bonaccorsi, L.; Antonucci, P.L. Hybrid zeolite SAPO-34 fibres made by electrospinning. Materials 2018, 11, 2555. [Google Scholar] [CrossRef]
- Freni, A.; Calabrese, L.; Malara, A.; Frontera, P.; Bonaccorsi, L. Silica gel microfibres by electrospinning for adsorption chillers. Energy 2019, 187, 115971. [Google Scholar] [CrossRef]
- Frontera, P.; Kumita, M.; Malara, A.; Nishizawa, J.; Bonaccorsi, L. Manufacturing and Assessment of Electrospun PVP/TEOS Microfibres for Adsorptive Heat Transformers. Coatings 2019, 9, 443. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Bonaccorsi, L.; Antonucci, P.L. Metodo per la Realizzazione Dello Scambiatore di Calore di una Macchina Termica ad Adsorbimento e Rispettiva Macchina Termica, Nr.102018000003884. EP19164536.5, 22 March 2018. [Google Scholar]
- Ardente, F.; Beccali, M.; Cellura, M.; Mistretta, M. Energy and environmental benefits in public buildings as a result of retrofit actions. Renew. Sustain. Energy Rev. 2011, 15, 460–470. [Google Scholar] [CrossRef]
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Ziemele, J.; Kalnins, R.; Vigants, G.; Vigants, E.; Veidenbergs, I. Evaluation of the industrial waste heat potential for its recovery and integration into a fourth generation district heating system. Energy Procedia 2018, 147, 315–321. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; La Commare, U.; Micale, G. Industrial waste heat: Estimation of the technically available resource in the EU per industrial sector, temperature level and country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Agathokleous, R.; Bianchi, G.; Panayiotou, G.; Arestia, L.; Argyrou, M.C.; Georgiou, G.S.; Tassou, S.A.; Jouhara, H.; Kalogirou, S.A.; Florides, G.A.; et al. Waste heat recovery in the EU industry and proposed new technologies. Energy Procedia 2019, 161, 489–496. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Bloess, A.; Schill, W.P.; Zerrahn, A. Power-to-heat for renewable energy integration: A review of technologies, modeling approaches, and flexibility potentials. Appl. Energy 2018, 212, 1611–1626. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Freni, A.; Maggio, G.; Cipitì, F.; Aristov, Y.I. Simulation of water sorption dynamics in adsorption chillers: One, two and four layers of loose silica grains. Appl. Therm. Eng. 2012, 44, 69–77. [Google Scholar] [CrossRef]
- Girnik, I.S.; Aristov, Y.I. Dynamics of water vapour adsorption by a monolayer of loose AQSOATM-FAM-Z02 grains: Indication of inseparably coupled heat and mass transfer. Energy 2016, 114, 767–773. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Freni, A.; Glaznev, I.S.; Restuccia, G. Kinetics of water adsorption on silica Fuji Davison RD. Microporous Mesoporous Mater. 2006, 96, 65–71. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Restuccia, G.; Schmidt, T.; Py, X. The size of sorbents in low pressure sorption or thermochemical energy storage processes. Energy 2014, 77, 983–998. [Google Scholar] [CrossRef]
- Restuccia, G.; Freni, A.; Maggio, G. A zeolite-coated bed for air conditioning adsorption systems: Parametric study of heat and mass transfer by dynamic simulation. Appl. Therm. Eng. 2002, 22, 619–630. [Google Scholar] [CrossRef]
- Wang, S.G.; Wang, R.Z.; Li, X.R. Research and development of consolidated adsorbent for adsorption systems. Renew. Energy 2005, 30, 1425–1441. [Google Scholar] [CrossRef]
- Freni, A.; Dawoud, B.; Bonaccorsi, L.; Chmielewski, S.; Frazzica, A.; Calabrese, L.; Restuccia, G. Characterization of Zeolite-Based Coatings for Adsorption Heat Pumps; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-09326-0. [Google Scholar]
- Sapienza, A.; Gullì, G.; Calabrese, L.; Palomba, V.; Frazzica, A.; Brancato, V.; La, D.; Vasta, S.; Freni, A.; Bonaccorsi, L.; et al. An innovative adsorptive chiller prototype based on 3 hybrid coated/granular adsorbers. Appl. Energy 2016, 179, 929–938. [Google Scholar] [CrossRef]
- Girnik, I.S.; Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Dynamic optimization of adsorptive chillers: Compact layer vs. bed of loose grains. Appl. Therm. Eng. 2017, 125, 823–829. [Google Scholar] [CrossRef]
- Saha, B.B.; Uddin, K.; Pal, A.; Thu, K. Emerging sorption pairs for heat pump applications: An overview. JMST Adv. 2019, 1, 3. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Freni, A.; Proverbio, E.; Restuccia, G.; Russo, F. Zeolite coated copper foams for heat pumping applications. Microporous Mesoporous Mater. 2006, 91, 7–14. [Google Scholar] [CrossRef]
- Wittstadt, U.; Füldner, G.; Laurenz, E.; Warlo, A.; Große, A.; Herrmann, R.; Schnabel, L.; Mittelbach, W. A novel adsorption module with fiber heat exchangers: Performance analysis based on driving temperature differences. Renew. Energy 2017, 110, 154–161. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone composite foams for adsorption heat pump applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Frazzica, A.; Freni, A.; Proverbio, E. Adsorption performance and thermodynamic analysis of SAPO-34 silicone composite foams for adsorption heat pump applications. Mater. Renew. Sustain. Energy 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Teo, W.E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef]
- Kalashnik, A.T.; Smirnova, T.N.; Chernova, O.P.; Kozlov, V.V. Properties and structure of polyacrylonitrile fibers. Polym. Sci. Ser. A 2010, 52, 1233–1238. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Ismar, E.; Sarac, A.S. Synthesis and characterization of poly (acrylonitrile-co-acrylic acid) as precursor of carbon nanofibers. Polym. Adv. Technol. 2016, 27, 1383–1388. [Google Scholar] [CrossRef]
- Ismar, E.; Sarac, A.S. Oxidation of polyacrylonitrile nanofiber webs as a precursor for carbon nanofiber: Aligned and non-aligned nanofibers. Polym. Bull. 2018, 75, 485–499. [Google Scholar] [CrossRef]
- Van Heyden, H.; Mintova, S.; Bein, T.; Van Heyden, H.; Mintova, S.; Bein, T.; Van Heyden, H.; Mintova, S.; Bein, T. Nanosized SAPO-34 synthesized from colloidal solutions. Chem. Mater. 2008, 20, 2956–2963. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef]
- Gorbach, A.; Stegmaier, M.; Eigenberger, G. Measurement and Modeling of Water Vapor Adsorption on Zeolite 4A—Equilibria and Kinetics. Adsorption 2004, 10, 29–46. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, R.Z.; Ge, T.S.; Hu, L.M. Performance study of SAPO-34 and FAPO-34 desiccants for desiccant coated heat exchanger systems. Energy 2015, 93, 88–94. [Google Scholar] [CrossRef]
- International Zeolite Association. Available online: http://www.iza-online (accessed on 20 May 2020).
- Lenzen, D.; Zhao, J.; Ernst, S.J.; Wahiduzzaman, M.; Ken Inge, A.; Fröhlich, D.; Xu, H.; Bart, H.J.; Janiak, C.; Henninger, S.; et al. A metal–organic framework for efficient water-based ultra-low-temperature-driven cooling. Nat. Commun. 2019, 10, 3025. [Google Scholar] [CrossRef]
- Kohler, T.; Hinze, M.; Müller, K.; Schwieger, W. Temperature independent description of water adsorption on zeotypes showing a type V adsorption isotherm. Energy 2017, 135, 227–236. [Google Scholar] [CrossRef]
- Tański, T.; Matysiak, W.; Hajduk, B. Manufacturing and investigation of physical properties of polyacrylonitrile nanofibre composites with SiO2, TiO2 and Bi2O3 nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, X.; Wu, S. The Microstructure Characterization and the Mechanical Properties of Electrospun Polyacrylonitrile-Based Nanofibers. Nanofibers Prod. Prop. Funct. Appl. 2011. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Li, K.; Wu, Y.; Liu, H. Optimization of stabilization conditions for electrospun polyacrylonitrile nanofibers. Polym. Degrad. Stab. 2012, 97, 1511–1519. [Google Scholar] [CrossRef]
- Dias, J.M.S.; Costa, V.A.F. Adsorption heat pumps for heating applications: A review of current state, literature gaps and development challenges. Renew. Sustain. Energy Rev. 2018, 98, 317–327. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Freni, A.; Proverbio, E. Hydrothermal and microwave synthesis of SAPO (CHA) zeolites on aluminium foams for heat pumping applications. Microporous Mesoporous Mater. 2013, 167, 30–37. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. REPORTING PHYSISORPTION DATA FOR GAS/SOLID SYSTEMS with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Demir, H.; Mobedi, M.; Ulku, S. A review on adsorption heat pump: Problems and solutions. Renew. Sustain. Energy Rev. 2008, 12, 2381–2403. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Proverbio, E.; Freni, A. SAPO-34 based zeolite coatings for adsorption heat pumps. Energy 2019, 187, 115981. [Google Scholar] [CrossRef]




| Sample | PAN/DMF (Polymer/Solvent Ratio, w/w%) | SAPO-34/Solution (Zeolite/Solution Ratio, w/w%) | SAPO-34 |
|---|---|---|---|
| PAN-45 | 5.5/94.5 | 45/55 | M |
| PAN-60 | 5.5/94.5 | 60/40 | M |
| PAN-75 | 5.0/95.0 | 75/25 | M |
| PAN-80 | 5.0/95.0 | 80/20 | S-M-L |
| PAN-85 | 4.5/95.5 | 85/15 | S-M-L |
| Parameters | Sample PAN-M85 | Sample PAN-M85 Aged |
|---|---|---|
| Mean diameter (μm) | 1.27 | 2.06 |
| SD | 0.18 | 0.27 |
| Porosity (%) | 47 | 42 |
| Mean pores area (μm2) | 8.42 | 5.26 |
| N. pores | 447 | 407 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccorsi, L.; Fotia, A.; Malara, A.; Frontera, P. Advanced Adsorbent Materials for Waste Energy Recovery. Energies 2020, 13, 4299. https://doi.org/10.3390/en13174299
Bonaccorsi L, Fotia A, Malara A, Frontera P. Advanced Adsorbent Materials for Waste Energy Recovery. Energies. 2020; 13(17):4299. https://doi.org/10.3390/en13174299
Chicago/Turabian StyleBonaccorsi, Lucio, Antonio Fotia, Angela Malara, and Patrizia Frontera. 2020. "Advanced Adsorbent Materials for Waste Energy Recovery" Energies 13, no. 17: 4299. https://doi.org/10.3390/en13174299
APA StyleBonaccorsi, L., Fotia, A., Malara, A., & Frontera, P. (2020). Advanced Adsorbent Materials for Waste Energy Recovery. Energies, 13(17), 4299. https://doi.org/10.3390/en13174299







