Process Modelling and Simulation of Waste Gasification-Based Flexible Polygeneration Facilities for Power, Heat and Biofuels Production
Abstract
1. Introduction
2. Materials and Methods
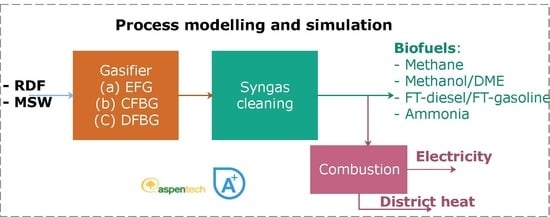
2.1. Polygeneration Concepts
2.2. Ultimate and Proximate Analyses of MSW and RDF
2.3. Modelling and Simulation
2.3.1. Air Separation Unit Modelling
2.3.2. Pretreatment (Grinding and Drying) Modelling
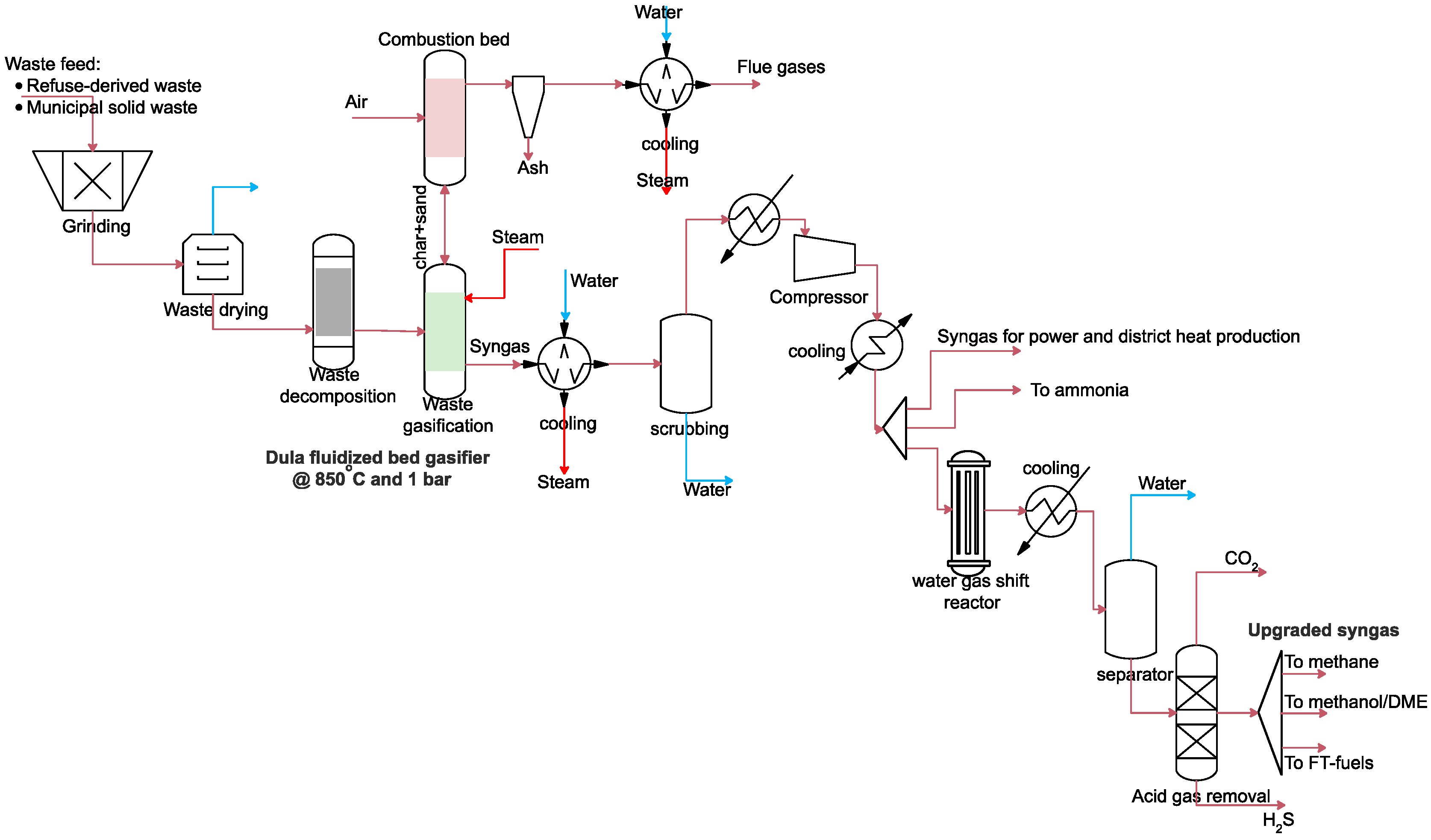
2.3.3. Modelling of Gasifiers, Syngas Cooling, Acid Gas Removal and Water–Gas Shift Reactor
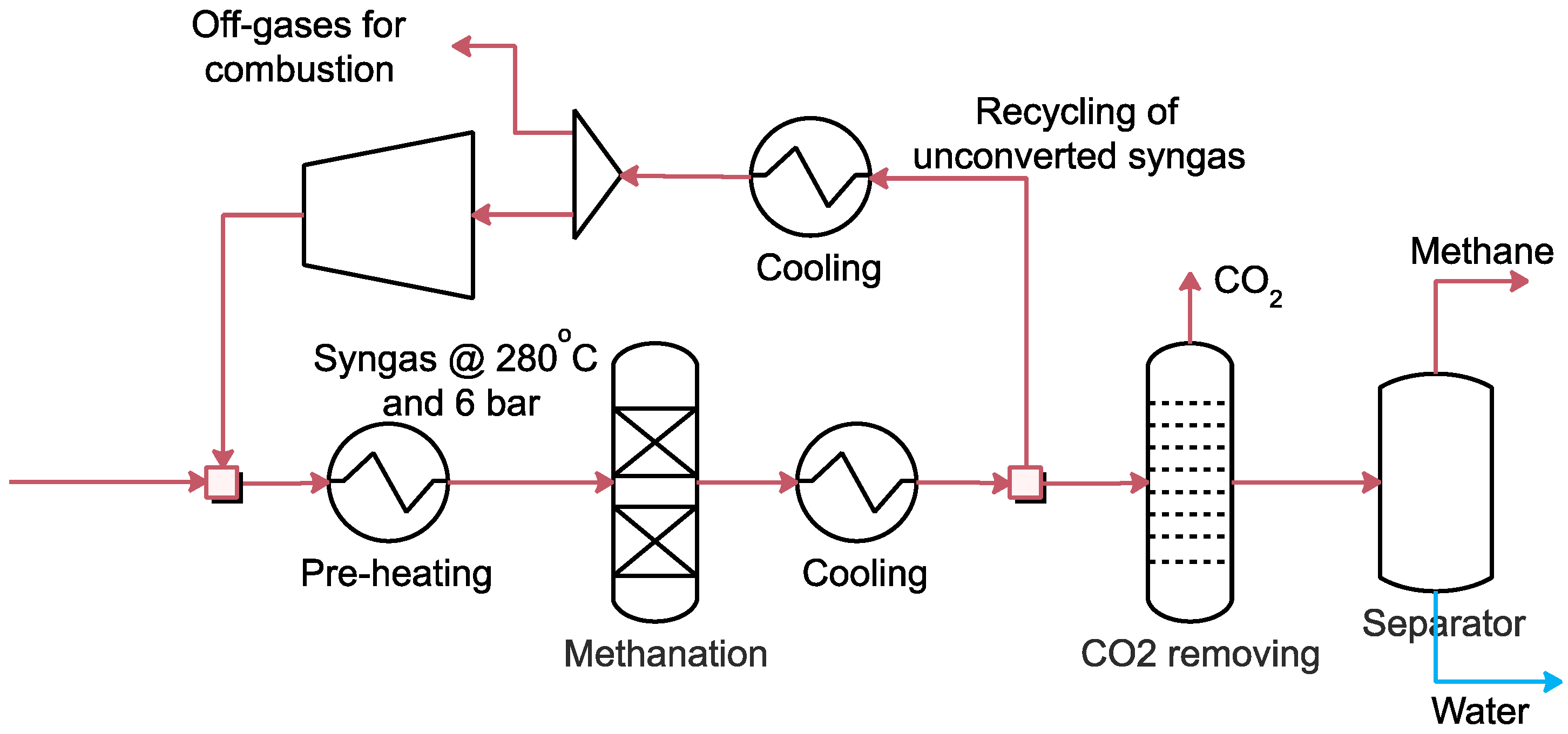
2.3.4. Methane Synthesis Modelling
2.3.5. Methanol/DME Synthesis Modelling
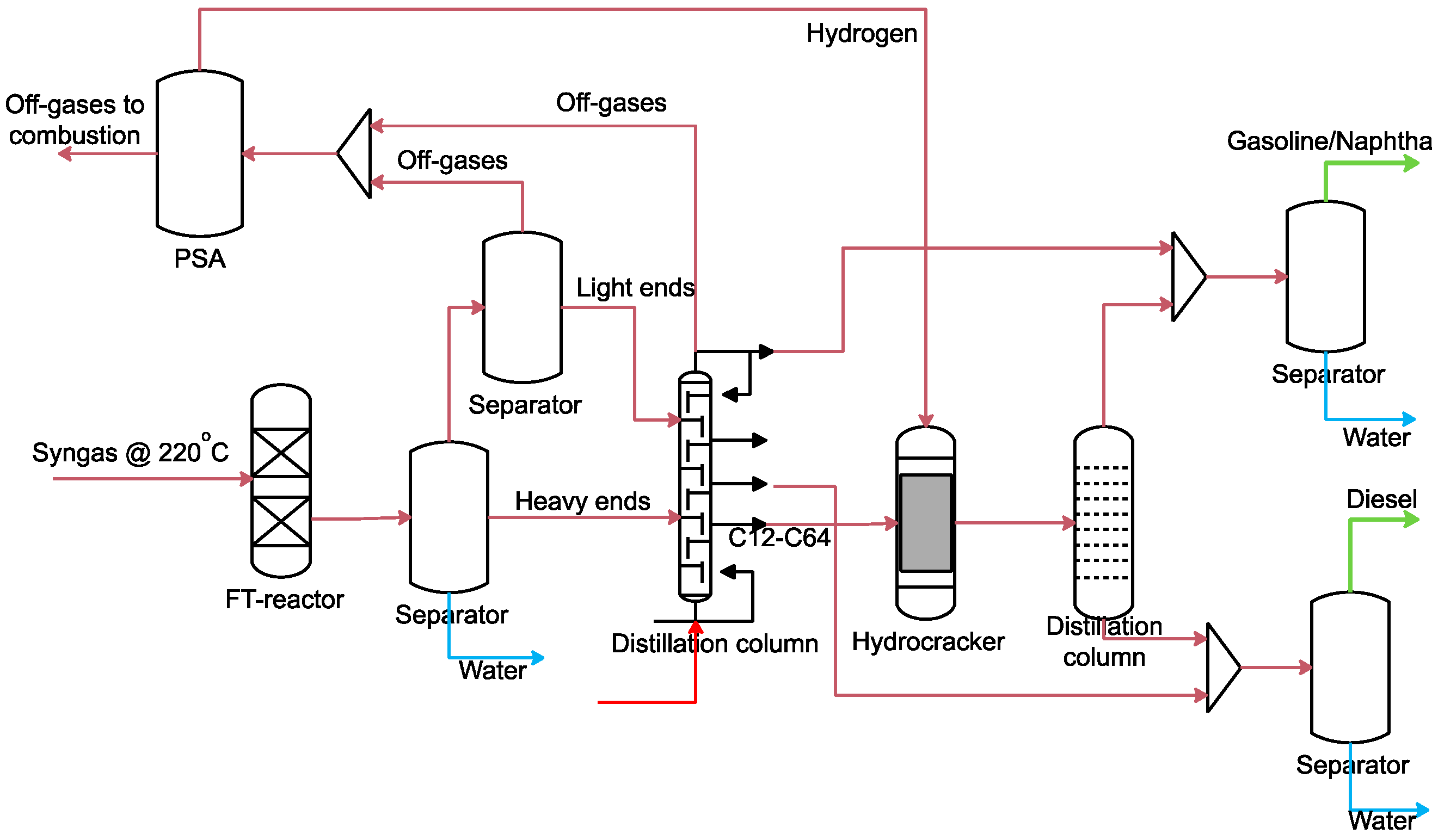
2.3.6. FT Fuels Synthesis Modelling
2.3.7. Ammonia Synthesis Modelling
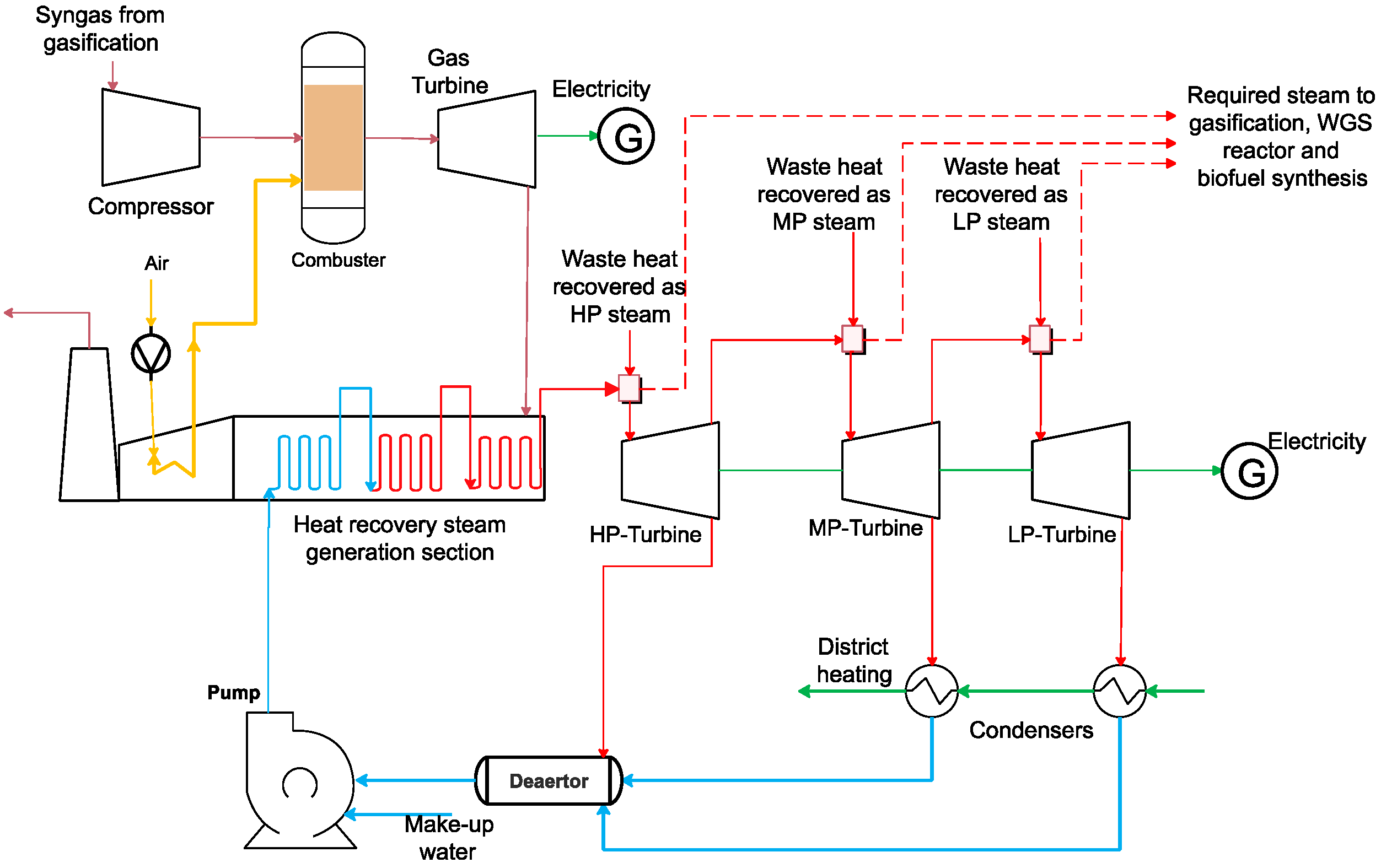
2.3.8. Power and DH Production Modelling
2.4. Performance Indicators and Case Studies
3. Results
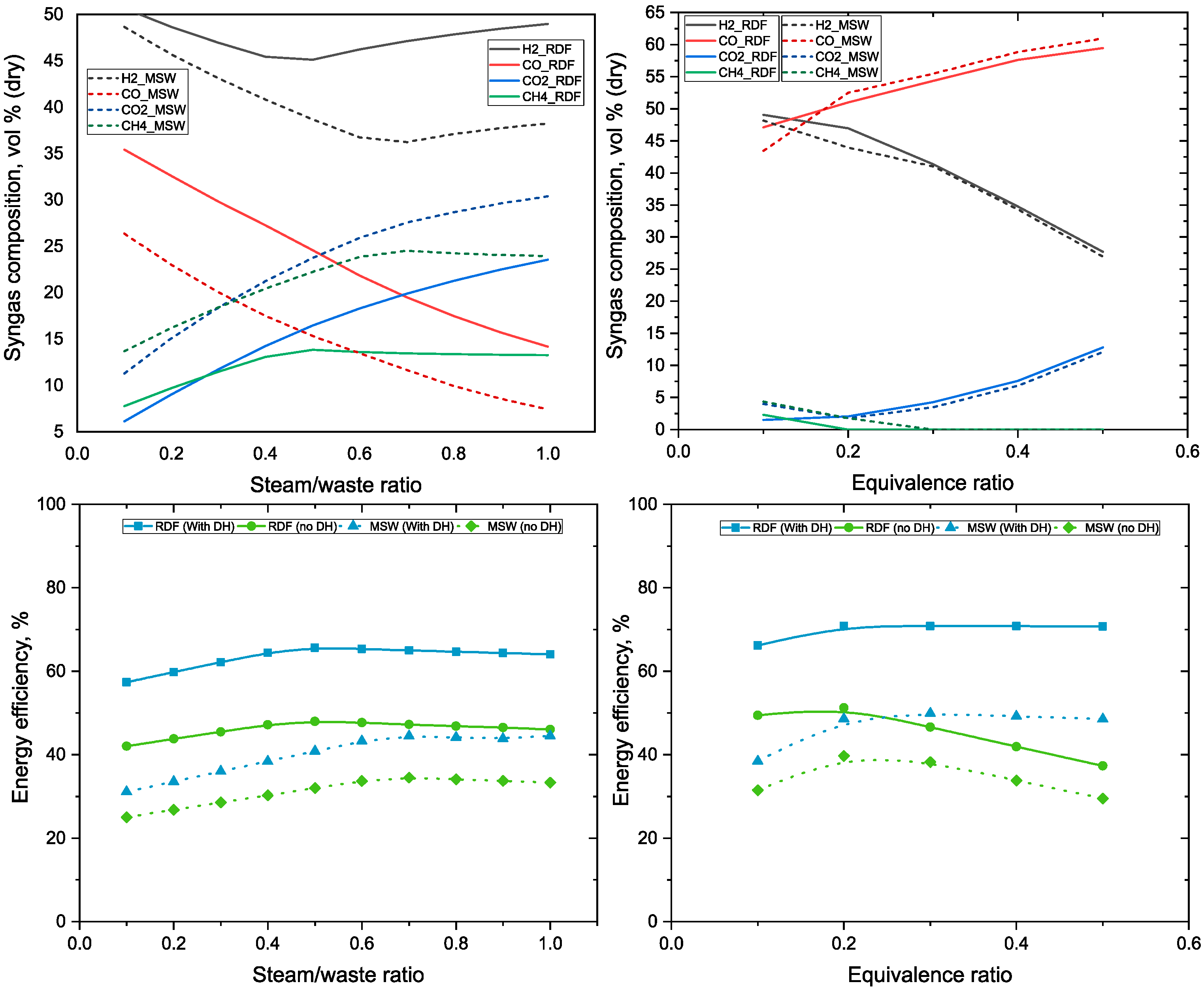
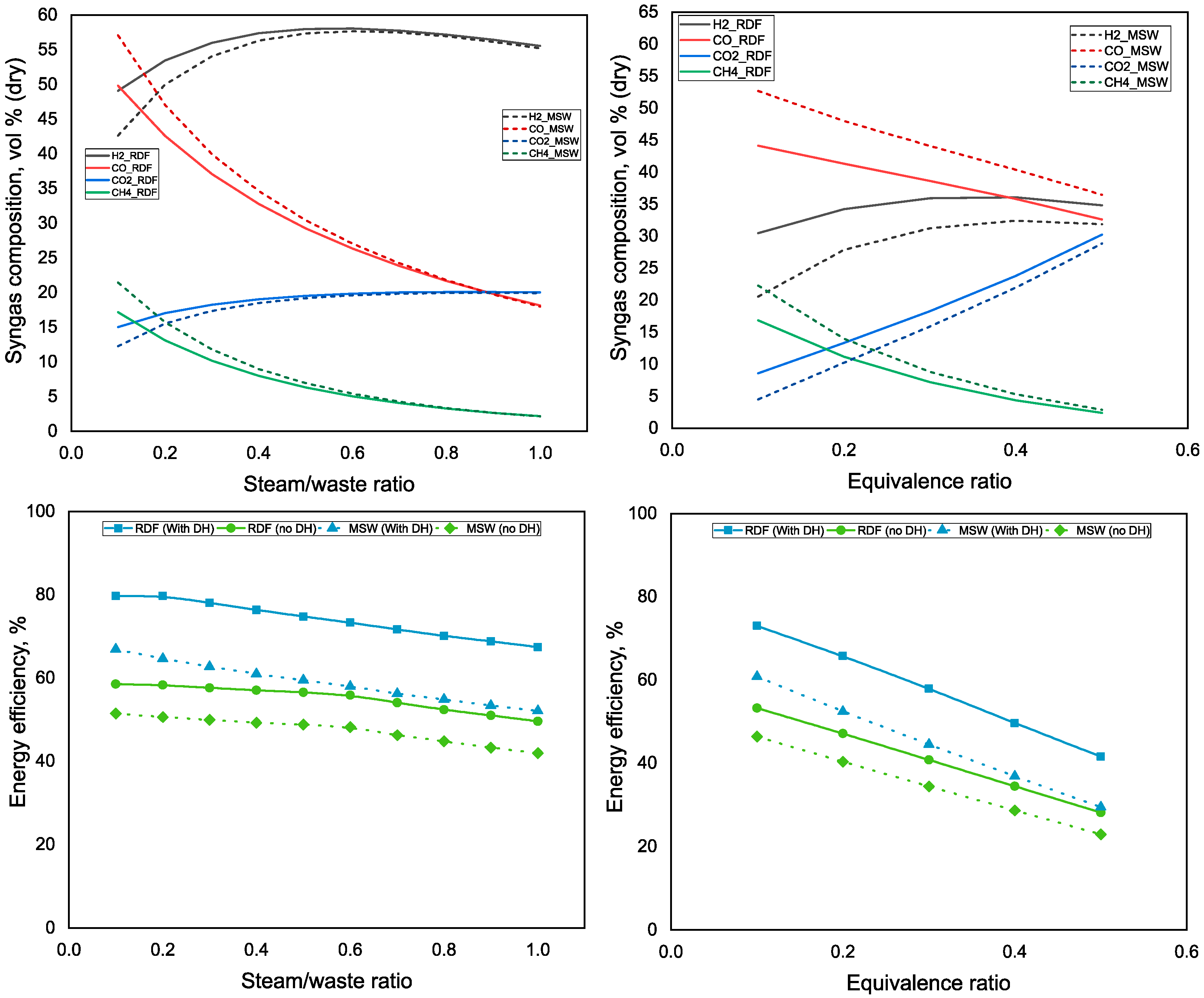
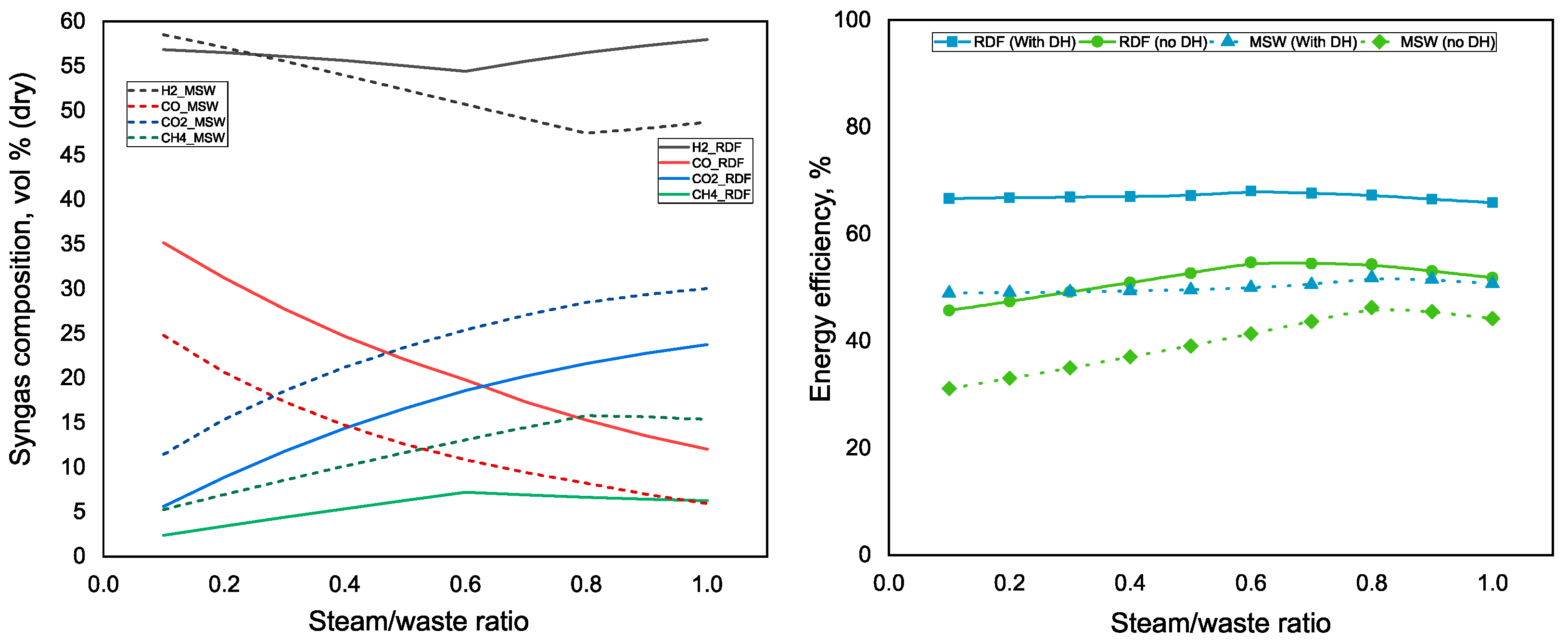
3.1. Influence of the Steam/Waste Ratio and ER on Syngas Composition and Energy Efficiency of the Polygeneration Plant
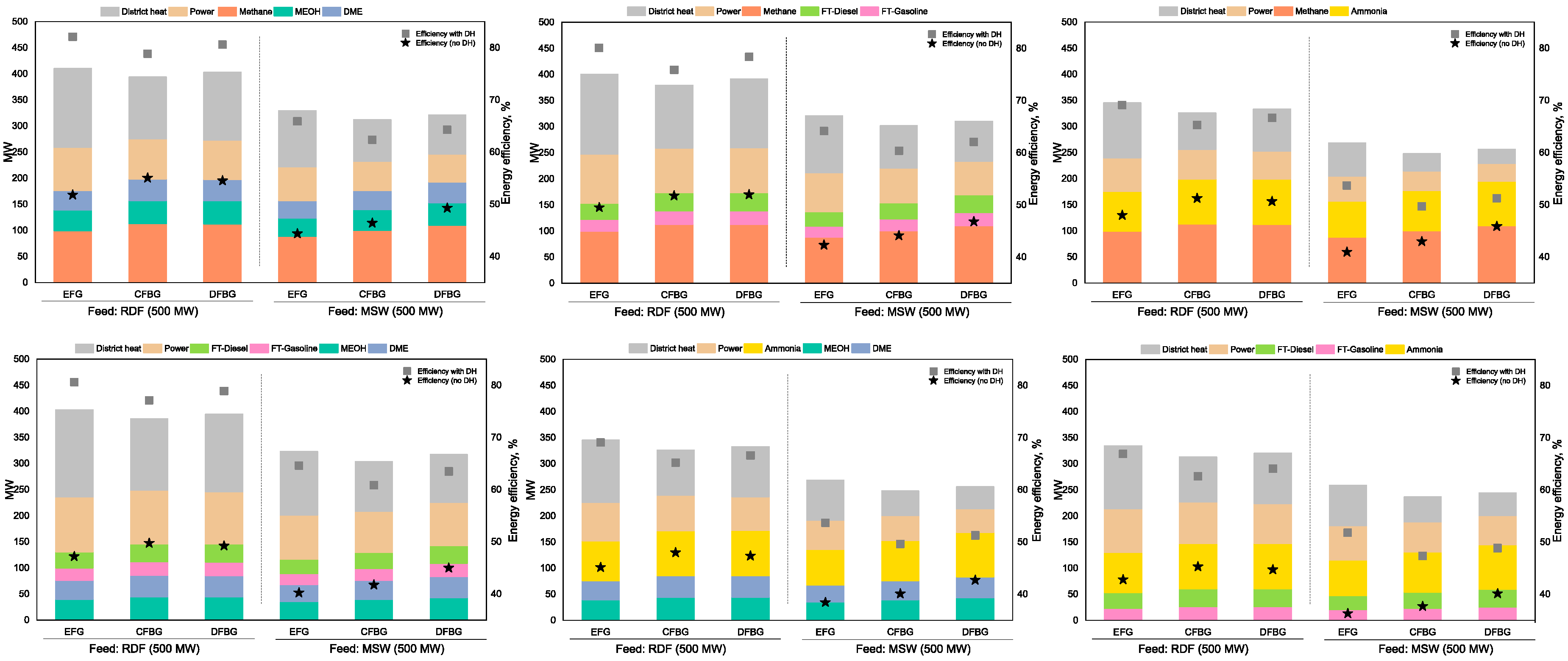
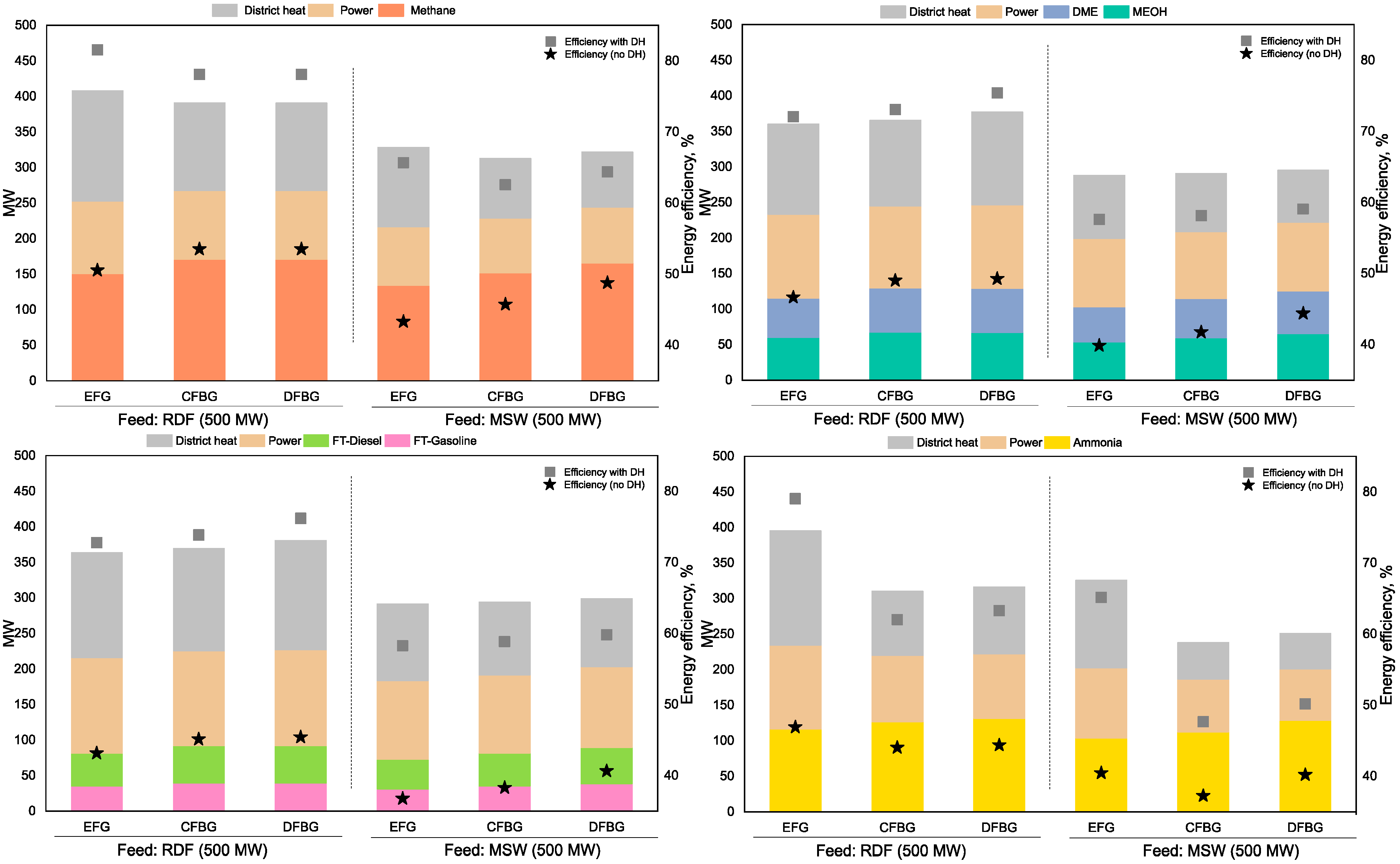
3.2. Case 1 Results
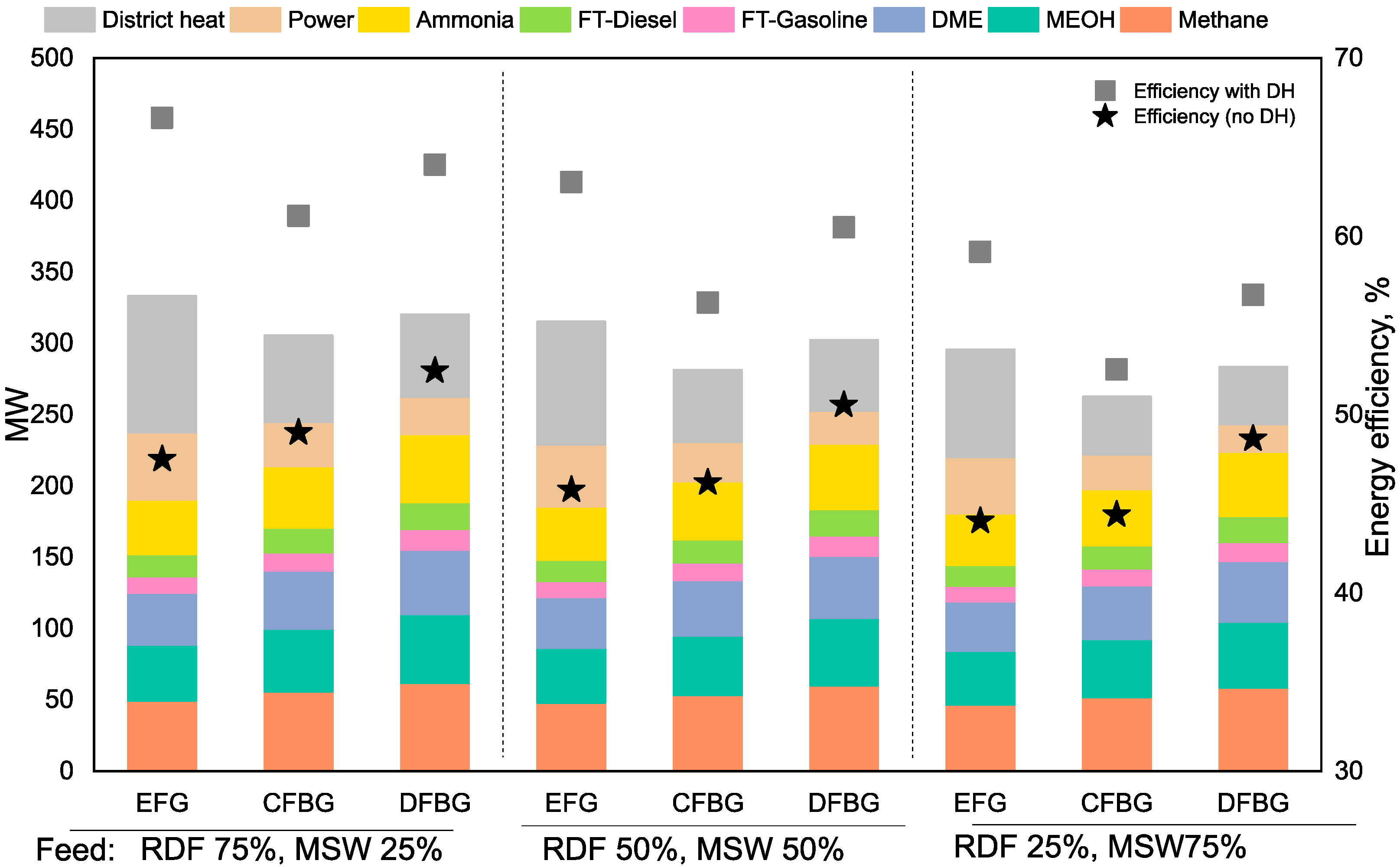
3.3. Case 2 Results
3.4. Case 3 Results
3.5. Case 4 Results
3.6. Carbon Dioxide Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AGR | Acid gas removal |
| ASF | Anderson–Schultz–Flory |
| ASU | Air separation unit |
| CFBG | Circulating fluidized bed gasifier |
| CGE | Cold gas efficiency |
| DFBG | Dual fluidized bed gasifier |
| DH | District heating |
| DME | Dimethyl ether |
| DSTWU | Shortcut distillation column block in Aspen Plus |
| EFG | Entrained flow gasifier |
| ER | Equivalence ratio |
| FT | Fischer Tropsch |
| HHV | Higher heating value |
| HP | High pressure |
| HRSG | Heat recovery steam generation |
| LHV | Lower heating value |
| LP | Low pressure |
| MDEA | N-methyl diethanolamine |
| MP | Medium pressure |
| MSW | Municipal solid waste |
| PSA | Pressure swing adsorption |
| RDF | Refuse derived fuel |
| REQUIL | Aspen Plus reactor block based on equilibrium reactions |
| RGIBBS | Aspen Plus reactor block based on minimizing Gibbs energy |
| RSTOICH | Aspen Plus reactor block based on known stoichiometric reactions |
| RYIELD | Aspen Plus reactor block based on component yields |
| WGS | Water–gas shift |
References
- Bhaskar, T.; Pandey, A.; Rene, E.; Tsang, D. Waste Biorefinery Integrating Biorefineries for Waste Valorisation; Elsevier: Amsterdam, The Netherlands, 2020; Volume 53. [Google Scholar] [CrossRef]
- Istrate, I.-R.; Iribarren, D.; Galvez-Martos, J.; Dufour, J. Review of life-cycle environmental consequences of waste-to-energy solutions on the municipal solid waste management system. Resour. Conserv. Recycl. 2020, 157, 104778. [Google Scholar] [CrossRef]
- Moharir, R.V.; Gautam, P.; Kumar, S. Waste Treatment Processes/Technologies for Energy Recovery. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–77. [Google Scholar] [CrossRef]
- Giugliano, M.; Ranzi, E. Thermal Treatments of Waste. In Waste to Energy (WtE); Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, W.; Yang, Y.; Yang, Y.; Man, X. Global trends of municipal solid waste research from 1997 to 2014 using bibliometric analysis. J. Air Waste Manag. Assoc. 2015, 65, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Bioenergy IEA. Gasification of Waste for Energy Carriers; IEA: Paris, France, 2018. [Google Scholar]
- Whiting, K.; Wood, S.; Fanning, M. Waste Technologies: Waste to Energy Facilities—A Report for the Strategic Waste Infrastructure Planning (SWIP) Working Group. Available online: http://large.stanford.edu/courses/2017/ph240/kim-d2/docs/wsp-may13.pdf (accessed on 5 July 2020).
- Segurado, R.; Pereira, S.; Correia, D.; Costa, M. Techno-economic analysis of a trigeneration system based on biomass gasification. Renew. Sustain. Energy Rev. 2019, 103, 501–514. [Google Scholar] [CrossRef]
- Jana, K.; Ray, A.; Majoumerd, M.M.; Assadi, M.; De, S. Polygeneration as a future sustainable energy solution—A comprehensive review. Appl. Energy 2017, 202, 88–111. [Google Scholar] [CrossRef]
- Ciuta, S.; Tsiamis, D.; Castaldi, M.J. Gasif Waste Mater Technol Gener Energy, Gas, Chem from Munic Solid Waste, Biomass, Nonrecycled Plast Sludges, Wet Solid Wastes. In Critical Development Needs; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–141. [Google Scholar] [CrossRef]
- Gootz, M.; Forman, C.; Wolfersdorf, C.; Meyer, B. Polygeneration-Annex: Combining lignite-based power generation and syngas-based chemicals production. Fuel 2017, 203, 989–996. [Google Scholar] [CrossRef]
- Buttler, A.; Kunze, C.; Spliethoff, H. IGCC–EPI: Decentralized concept of a highly load-flexible IGCC power plant for excess power integration. Appl. Energy 2013, 104, 869–879. [Google Scholar] [CrossRef]
- Yu, G.-W.; Xu, Y.-Y.; Hao, X.; Li, Y.-W.; Liu, G.-Q. Process analysis for polygeneration of Fischer–Tropsch liquids and power with CO2 capture based on coal gasification. Fuel 2010, 89, 1070–1076. [Google Scholar] [CrossRef]
- Narvaez, A.; Chadwick, D.; Kershenbaum, L. Small-medium scale polygeneration systems: Methanol and power production. Appl. Energy 2014, 113, 1109–1117. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Yang, Y.; Zhai, D.; Zhang, K.; Xu, G. Thermodynamic analysis of a coal-based polygeneration system with partial gasification. Energy 2014, 72, 201–214. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Q.; Fang, M.; Luo, Z.; Cen, K. Thermodynamic and economic analysis of polygeneration system integrating atmospheric pressure coal pyrolysis technology with circulating fluidized bed power plant. Appl. Energy 2014, 113, 1301–1314. [Google Scholar] [CrossRef]
- Li, M.; Zhuang, Y.; Zhang, L.; Liu, L.; Du, J.; Shen, S. Conceptual design and techno-economic analysis for a coal-to-SNG/methanol polygeneration process in series and parallel reactors with integration of waste heat recovery. Energy Convers. Manag. 2020, 214, 112890. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Cui, P. Design concept for coal-based polygeneration processes of chemicals and power with the lowest energy consumption for CO2 capture. Energy Convers. Manag. 2018, 157, 186–194. [Google Scholar] [CrossRef]
- Heinze, C.; May, J.; Peters, J.; Ströhle, J.; Epple, B. Techno-economic assessment of polygeneration based on fluidized bed gasification. Fuel 2019, 250, 285–291. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kim, Y.T.; Kwak, G.; Kim, J. Techno-economic evaluation of the integrated polygeneration system of methanol, power and heat production from coke oven gas. Energy Convers. Manag. 2019, 182, 240–250. [Google Scholar] [CrossRef]
- Fan, J.; Hong, H.; Jin, H. Biomass and coal co-feed power and SNG polygeneration with chemical looping combustion to reduce carbon footprint for sustainable energy development: Process simulation and thermodynamic assessment. Renew. Energy 2018, 125, 260–269. [Google Scholar] [CrossRef]
- Meerman, H.; Ramírez, A.; Turkenburg, W.; Faaij, A. Performance of simulated flexible integrated gasification polygeneration facilities. Part A: A technical-energetic assessment. Renew. Sustain. Energy Rev. 2011, 15, 2563–2587. [Google Scholar] [CrossRef]
- Sahoo, U.; Kumar, R.; Pant, P.; Chaudhary, R. Development of an innovative polygeneration process in hybrid solar-biomass system for combined power, cooling and desalination. Appl. Therm. Eng. 2017, 120, 560–567. [Google Scholar] [CrossRef]
- Jana, K.; De, S. Polygeneration using agricultural waste: Thermodynamic and economic feasibility study. Renew. Energy 2015, 74, 648–660. [Google Scholar] [CrossRef]
- Jana, K.; De, S. Sustainable polygeneration design and assessment through combined thermodynamic, economic and environmental analysis. Energy 2015, 91, 540–555. [Google Scholar] [CrossRef]
- Gładysz, P.; Saari, J.; Czarnowska, L. Thermo-ecological cost analysis of cogeneration and polygeneration energy systems—Case study for thermal conversion of biomass. Renew. Energy 2020, 145, 1748–1760. [Google Scholar] [CrossRef]
- Villarroel-Schneider, J.; Mainali, B.; Martí-Herrero, J.; Malmquist, A.; Martin, A.; Alejo, L. Biogas based polygeneration plant options utilizing dairy farms waste: A Bolivian case. Sustain. Energy Technol. Assess. 2020, 37, 100571. [Google Scholar] [CrossRef]
- Jana, K.; De, S. Environmental impact of an agro-waste based polygeneration without and with CO2 storage: Life cycle assessment approach. Bioresour. Technol. 2016, 216, 931–940. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, E.; Martín, M. Optimal CSP-Waste Based Polygeneration Coupling for Constant Power Production; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 46, pp. 1645–1650. [Google Scholar]
- McKendry, P. Energy production from biomass (Part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Heyne, S.; Thunman, H.; Harvey, S. Exergy-based comparison of indirect and direct biomass gasification technologies within the framework of bio-SNG production. Biomass Convers. Biorefin. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Van Der Meijden, C.M.; Veringa, H.J.; Rabou, L.P. The production of synthetic natural gas (SNG): A comparison of three wood gasification systems for energy balance and overall efficiency. Biomass Bioenergy 2010, 34, 302–311. [Google Scholar] [CrossRef]
- Gassner, M.; Wang, L. Thermo-economic process model for thermochemical production of Synthetic Natural Gas (SNG) from lignocellulosic biomass. Biomass Bioenergy 2009, 33, 1587–1604. [Google Scholar] [CrossRef]
- Tungalag, A.; Lee, B.; Yadav, M.; Akande, O. Yield prediction of MSW gasification including minor species through ASPEN plus simulation. Energy 2020, 198, 117296. [Google Scholar] [CrossRef]
- Buckley, T.J. Calculation of higher heating values of biomass materials and waste components from elemental analyses. Resour. Conserv. Recycl. 1991, 5, 329–341. [Google Scholar] [CrossRef]
- Özyuğuran, A.; Yaman, S.; Küçükbayrak, S. Prediction of calorific value of biomass based on elemental analysis. Int. Adv. Res. Eng. J. 2018, 2, 254–260. [Google Scholar]
- Aspen Technology, Inc. Aspen Plus. USA. 2020. Available online: https://www.aspentech.com/ (accessed on 25 March 2020).
- Aspentech. Getting Started Modeling Processes with Solids; Aspen Technology: Burlington, VT, USA, 2013. [Google Scholar]
- Salman, C.A. Techno Economic Analysis of Wood Pyrolysis in Sweden; KTH Stockholm: Stockholm, Sweden, 2014. [Google Scholar]
- Harris, D.; Roberts, D. Coal Gasification and Conversion; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 2, pp. 427–454. [Google Scholar]
- Li, K.; Cousins, A.; Yu, H.; Feron, P.H.; Tade, M.; Luo, W.; Chen, J. Systematic study of aqueous monoethanolamine-based CO2 capture process: Model development and process improvement. Energy Sci. Eng. 2015, 4, 23–39. [Google Scholar] [CrossRef]
- Heyne, S.; Thunman, H.; Harvey, S. Extending existing combined heat and power plants for synthetic natural gas production. Int. J. Energy Res. 2011, 36, 670–681. [Google Scholar] [CrossRef]
- Clausen, L.R.; Elmegaard, B.; Ahrenfeldt, J.; Henriksen, U.B. Thermodynamic analysis of small-scale dimethyl ether (DME) and methanol plants based on the efficient two-stage gasifier. Energy 2011, 36, 5805–5814. [Google Scholar] [CrossRef]
- Pondini, M.; Ebert, M. Process Synthesis and Design of Low Temperature Fischer-Tropsch Crude Production from Biomass Derived Syngas. Master’s Thesis, Chalmers University of Technology, Göteborg, Sweden, 2013. [Google Scholar]
- Song, H.-S.; Ramkrishna, R.; Trinh, S.; Wright, H. Operating strategies for Fischer-Tropsch reactors: A model-directed study. Korean J. Chem. Eng. 2004, 21, 308–317. [Google Scholar] [CrossRef]







| RDF | MSW | |
|---|---|---|
| Carbon | 52.11 | 58.45 |
| Hydrogen | 7.40 | 6.87 |
| Nitrogen | 0.85 | 1.36 |
| Sulfur | 0.46 | 2.49 |
| Chlorine | 0.07 | 0.30 |
| Oxygen | 39.11 | 30.53 |
| Ash | 17.47 | 30.81 |
| Moisture | 13.12 | 51.87 |
| Feedstock | EFG | CFBG | DFBG | |||
|---|---|---|---|---|---|---|
| RDF | MSW | RDF | MSW | RDF | MSW | |
| Steam/waste ratio | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 |
| ER | 0.2 | 0.2 | 0.1 | 0.1 | - | - |
| H2, % | 54.3 | 49.7 | 42.4 | 40.5 | 55.6 | 54 |
| CO, % | 40.0 | 41.4 | 33.8 | 35.0 | 24.7 | 14.7 |
| CO2, % | 5.7 | 8.9 | 13.5 | 16.2 | 14.4 | 21.2 |
| CH4, % | negligible | negligible | 10.3 | 8.3 | 5.3 | 10.1 |
| LHV, MJ/kg | 16.4 | 14.2 | 16.2 | 14.1 | 16.5 | 15.2 |
| CGE, % | 84.4 | 80.2 | 83.3 | 79.4 | 84.9 | 78.4 |
| RDF, % | MSW, % | Mass Flow of Waste Equivalent to 500 MW, kg/s |
|---|---|---|
| 100 | 0 | 23.25 |
| 0 | 100 | 40.88 |
| 75 | 25 | 26 |
| 50 | 50 | 29.6 |
| 25 | 75 | 34.4 |
| Feed | CO2 Emissions, Tonne/MJ Feed | ||
|---|---|---|---|
| EFG | CFBG | DFBG | |
| RDF | 37.38 | 35.52 | 37.06 |
| MSW | 31.6 | 31.2 | 31.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, C.A.; Omer, C.B. Process Modelling and Simulation of Waste Gasification-Based Flexible Polygeneration Facilities for Power, Heat and Biofuels Production. Energies 2020, 13, 4264. https://doi.org/10.3390/en13164264
Salman CA, Omer CB. Process Modelling and Simulation of Waste Gasification-Based Flexible Polygeneration Facilities for Power, Heat and Biofuels Production. Energies. 2020; 13(16):4264. https://doi.org/10.3390/en13164264
Chicago/Turabian StyleSalman, Chaudhary Awais, and Ch Bilal Omer. 2020. "Process Modelling and Simulation of Waste Gasification-Based Flexible Polygeneration Facilities for Power, Heat and Biofuels Production" Energies 13, no. 16: 4264. https://doi.org/10.3390/en13164264
APA StyleSalman, C. A., & Omer, C. B. (2020). Process Modelling and Simulation of Waste Gasification-Based Flexible Polygeneration Facilities for Power, Heat and Biofuels Production. Energies, 13(16), 4264. https://doi.org/10.3390/en13164264