3.1. Characteristics of Methane Reforming
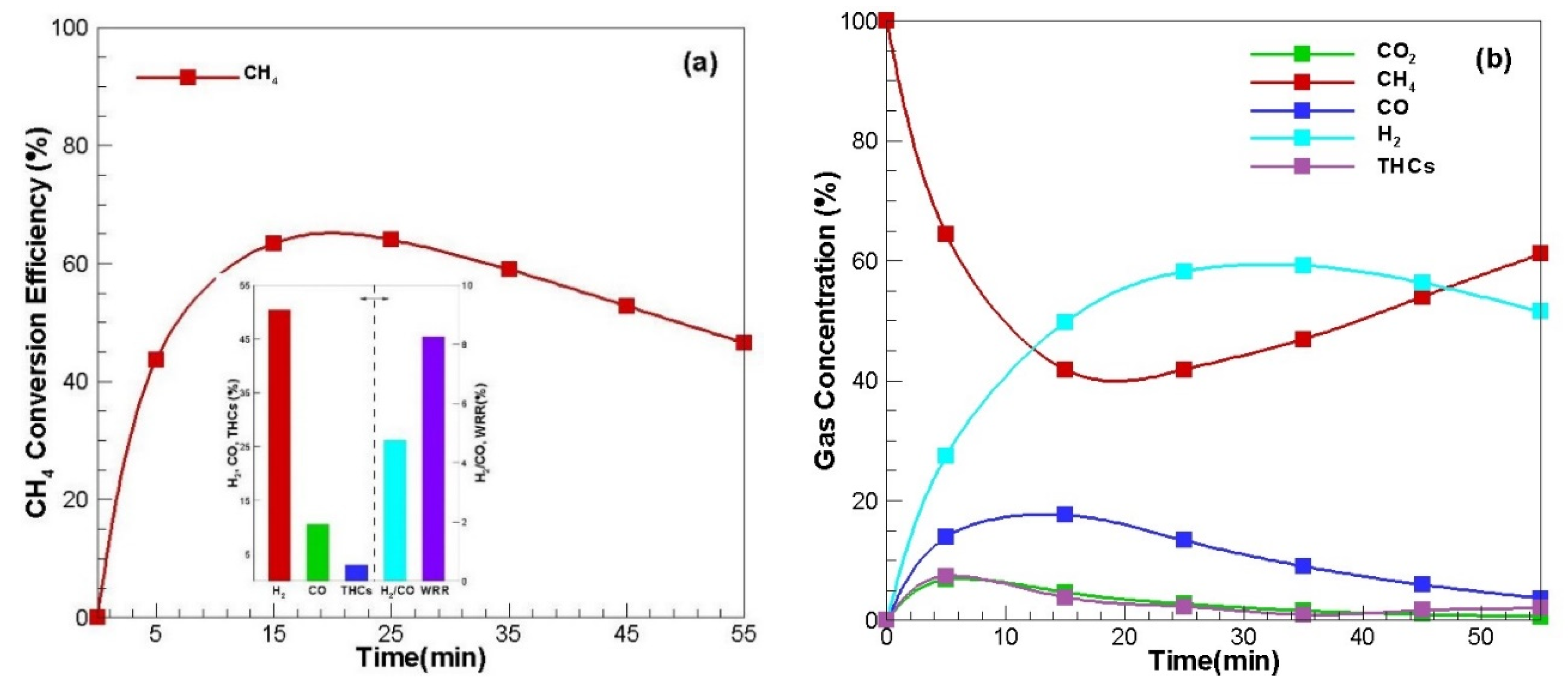
The reforming conversion characteristics of natural gas, city gas and carbon dioxide purified biogas, which are mainly composed of methane, were identified, and the results are shown in
Figure 2.
The CH
4 conversion efficiency in
Figure 2a increased rapidly as the reformation started, and then decreased again after showing the maximum value. CH
4 conversion efficiency increased in the first half of reforming because methane was converted to hydrogen and carbon by the decomposition mechanism of the thermal decomposition (Equation (4)). In addition, a partial oxidation reaction by residual oxygen in the reformer was performed as shown in Equation (5), converting it to hydrogen and carbon monoxide. This can also be found in the similar pattern of the concentration of product gas in
Figure 2b. That is, as CH
4 conversion efficiency increased, the concentration of methane in the product gas decreased, and the concentration of hydrogen and carbon monoxide increased.
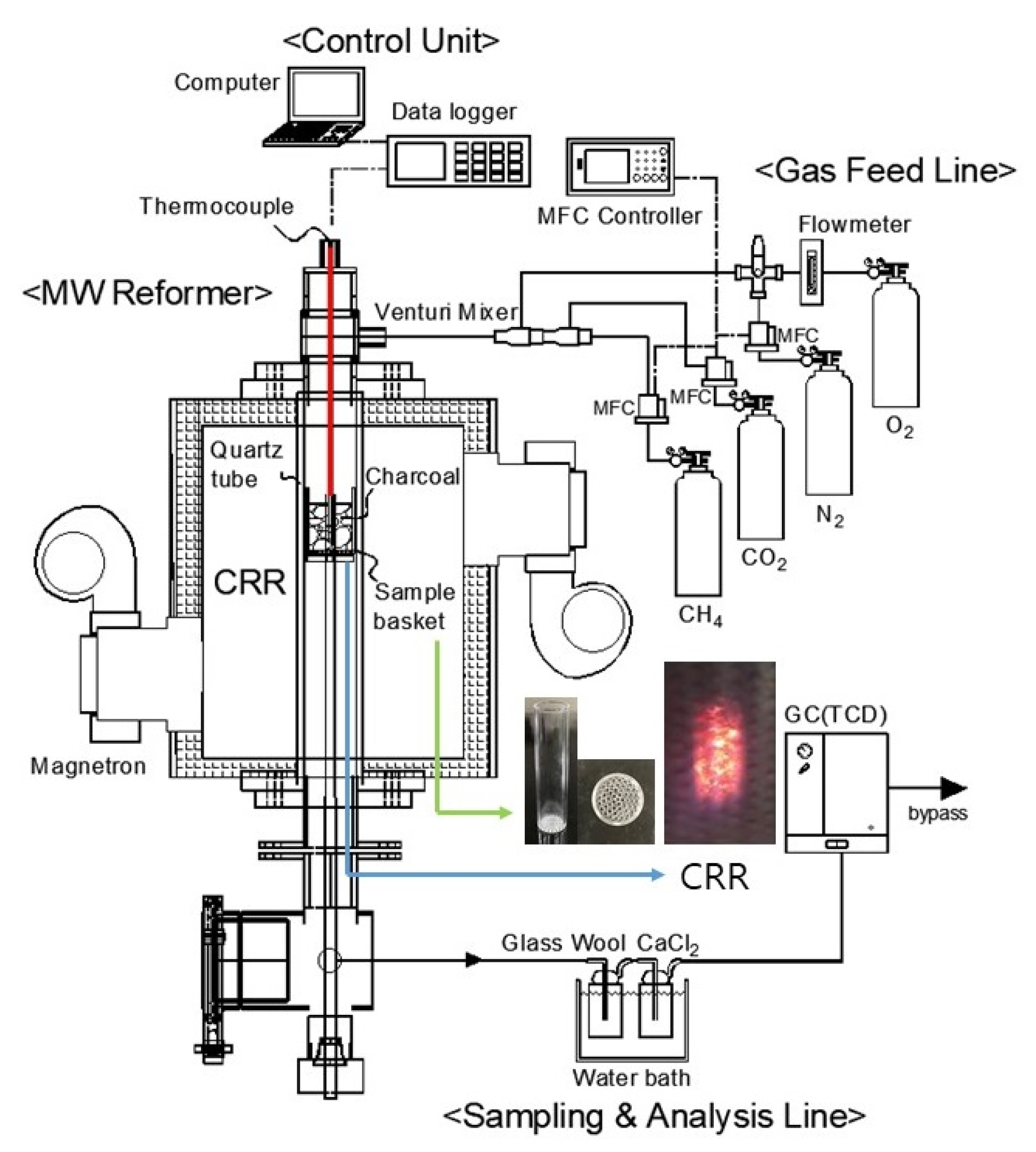
Unlike the conventional heating method in which the heat source is transferred from the outside of the receptor, microwave heating is a method in which microwave energy is transferred into a receptor and kinetic energy by object vibration is converted into thermal energy and heated. Therefore, microplasma is generated inside the carbon receptor, which is a dielectric solid, showing the pattern that the high temperature is maintained at a specific location than the normal temperature of the carbon receptor bed. The microplasma phenomenon can be seen in the photo in
Figure 1 [
14]. The thermal decomposition reaction Equation (4) and partial oxidation reaction Equation (5) mentioned above are gaseous homogeneous reactions in which the catalytic reaction of the carbon receptor containing the catalyst components such as K, Ca, Fe, etc., are activated (see
Table 1). Additionally, as already described, microplasma is formed on the carbon receptor bed, which is a microwave absorbing dielectric and the high temperature is maintained, so the reactivity of the surrounding gas is improved due to the high temperature, especially in the thermal decomposition reaction of Equation (4).
However, CH
4 conversion efficiency decreased again after showing the maximum value. This is because carbon (C) generated in Equation (4), which is a thermal decomposition reaction, is adsorbed on the surface of the receptor and prevents methane, which is a reforming target gas, from penetrating into catalytic activity pores of the carbon receptor, hindering the catalytic activity that helps the thermal decomposition reaction. As a result, the problem of microwave heating methane reforming using a catalyst-containing receptor is that it is adsorbed on the surface of the catalyst receptor of the carbon generated in the methane pyrolysis reaction to inhibit the catalyst activity of the receptor. Similar results have been found in the results of other researchers’ studies on several types of carbonaceous-based catalysts, such as char [
15], activated carbon [
16] and carbon black [
17]. The gas concentration of the bar graph in
Figure 2a is the arithmetic average of the measured values over time: H
2 50%, CO 11% and THCs (total hydrocarbons) 3%. Additionally, H
2/CO ratio is 4.8 and weight reduction rate (WRR) is 8.3%.
3.2. Characteristic of Biogas Reforming
In order to identify the reforming characteristics of biogas generated during anaerobic digestion of organic waste, a microwave reforming conversion experiment was conducted with regard to simulated gas mixed with CH
4 and CO
2, and the results are shown in
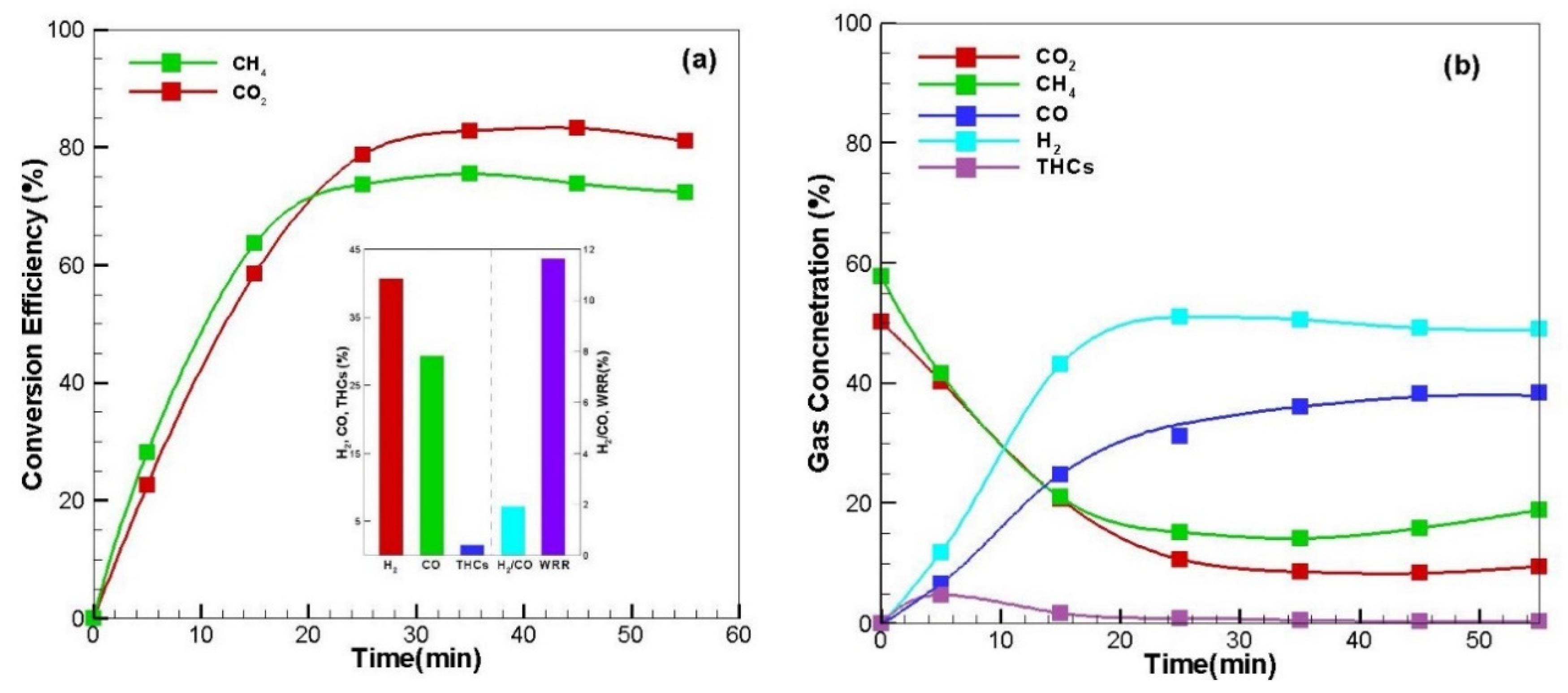
Figure 3.
After reforming, CH
4 conversion efficiency and CO
2 conversion efficiency were significantly increased, and these values continued (see
Figure 3a). In the case of biogas, methane is first thermally decomposed (Equation (4)) to produce hydrogen with attached carbon (C
CH4), which will be removed by Equation (7), and then fixed carbon (C
fc) is gasified with carbon dioxide (Equation (6)) to produce carbon monoxide.
Additionally, unlike the case where only methane in
Figure 2 is supplied, it can be seen that CH
4 conversion efficiency is not reduced over time in the case of biogas. This is because attached carbon (C
CH4) generated when methane is reformed (see Equation (4)), is reduced by carbon gasification as shown in Equation (7). As described above, it has been reported that the catalytic active center can be cleaned by steam gasification. In addition, it is known that the microwave method in which microplasma is generated in the carbon receptor bed is more effective for this cleaning than the conventional hot air heating method [
1].
In addition to this heterogeneous solid–gas reaction, carbon monoxide and hydrogen are generated by the dry reforming reaction of the following Equation (8), which is the homogeneous reaction of methane and carbon dioxide gas. Therefore, it was found that the conversion efficiency of mixed gas of methane and carbon dioxide was higher than that of carbon dioxide reforming, and that the conversion efficiency was somewhat lower than that of methane reforming, but it remained constant.
In the case of biogas, carbon dioxide is converted more easily than methane because carbon (CCH4) generated by the methane thermal decomposition reaction (Equation (4)) is adsorbed to the receptor pores to promote the generation of microplasma formed inside the receptor, facilitating the carbon gasification reaction (Equation (6)).
Conversion of the above-mentioned mixed gas is also found in that the concentrations of methane and carbon dioxide decrease and the concentrations of hydrogen and carbon monoxide increase as microwave reforming progresses (see
Figure 3b).
Bar graphs in
Figure 3a shows the average concentration of product gas: H
2 41%, CO 29%, THCs 1.5%, H
2/CO ratio of 1.9 and WRR (weight reduction rate) of 11.6. In the case of biogas reforming, unlike methane reforming (
Figure 2a), the carbon gasification reaction (Equation (6)) was carried out, so a larger amount of carbon, which is the main component of the carbon receptor, was exhausted, and the weight reduction rate (WRR) increased. As a result, the CO concentration increased from 11% to 29%, while the hydrogen concentration decreased from 50% to 41%. As a result, it was found that the H
2/CO ratio decreased from 4.8 to 1.9.
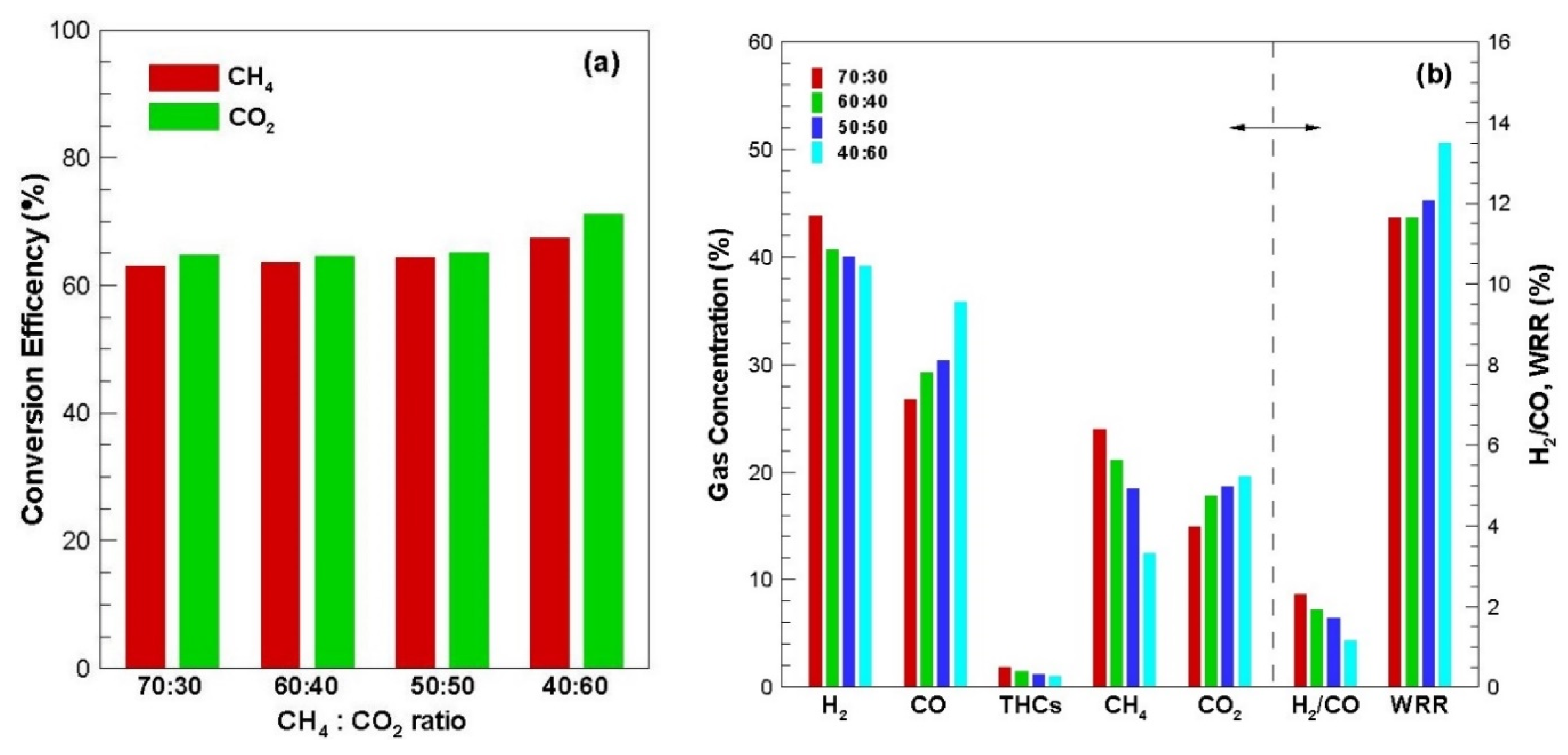
Figure 4 shows the conversion efficiency, average product gas concentration, H
2/CO ratio and weight reduction rate according to the CH
4:CO
2 ratio change of biogas.
As shown in
Figure 4a, CO
2 and CH
4 conversion efficiencies increased as the amount of CO
2 in the biogas simulated mixed gas increased. In particular, the increase was large when the ratio of CH
4:CO
2 was 40:60, which had a larger amount of CO
2 than CH
4. As already mentioned, the conversion efficiency was increased because attached carbon (C
CH4) generated when methane was reformed (see Equation (4)) was reduced by the gasification reaction Equation (7), and CO
2 was converted by the carbon gasification reaction (Equation (6)) in which the carbon receptor that fixed carbon (C
fc) itself was exhausted. In the case of methane decomposition reforming, it is known that the carbon receptor is affected by catalysts such as Ca, Fe and Cu contained and methane conversion efficiency was also reduced because the attached carbon (C
CH4), which impairs the receptor catalytic active center, is reduced by the gasification reaction Equation (7) [
18]. The previous study using activated carbon as a microwave receptor reported that the increase in CH
4 conversion efficiency was greater than the increase in CO
2 conversion efficiency, while the results of this study showed that the increase in CO
2 conversion efficiency was greater [
9].
As shown in
Figure 4b, as the ratio of CO
2 in the product gas concentration biogas increased, the CO concentration in the product gas increased, H
2 and THCs decreased and the H
2/CO ratio decreased as well. Additionally, weight reduction rate was increased by the fixed carbon gasification reaction.
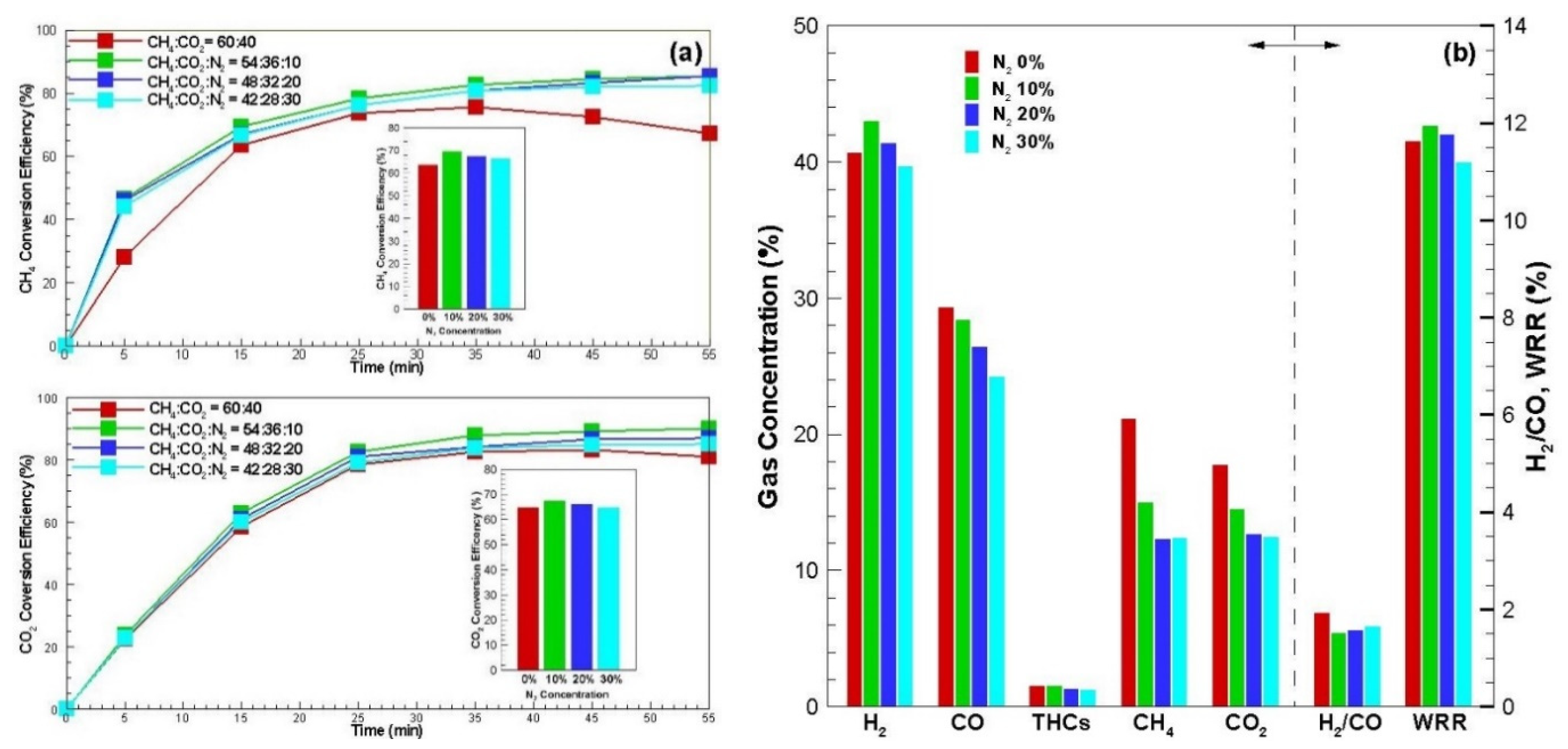
3.3. Characteristic of Nitrogen Effect Reforming
Figure 5 shows the studies on four cases of CH
4:CO
2:N
2 ratios of 60:40:0, 54:36:10, 48:32:20 and 42:28:30 respectively in order to determine how nitrogen concentration in biogas affects reforming properties.
Figure 5a shows CH
4 conversion efficiency and CO
2 conversion efficiency over time. Although somewhat different, both conversion efficiencies showed a pattern similar to that of biogas dry reforming (see
Figure 3a) in a state not containing nitrogen even if containing nitrogen. As the amount of nitrogen in the gas increased, both CH
4 conversion efficiency and CO
2 conversion efficiency increased, but the effect was great in the case of CH
4 conversion. It is known that the formation of microplasma in the catalytic active center occurs more vigorously when nitrogen is included in the feed gas during microwave heating [
19]. This increased both conversion efficiencies when nitrogen was included as 10% in the feed gas, rather than when not included. As a result, it was found that the effect of nitrogen concentration in biogas is more effective in methane reforming, which is influenced by the catalytic activity by the acceleration of microplasma generation.
However, although the nitrogen concentration increased from 20% to 30%, there was a slight decrease. This is because CO2 was diluted with nitrogen during carbon gasification and penetrated into the active pores inside the carbon receptor, the chance for the heterogeneous gasification reaction (Equation (6)) was not relatively greater than the case without nitrogen. Additionally, dry reforming (Equation (8)) did not have enough time due to an increase of retention time at the carbon receptor.
Figure 5b shows the product gas concentration, H
2/CO ratio and weight reduction rate. When the concentration of nitrogen was 10%, the concentration of hydrogen in the carbon product gas showed the maximum value because CH
4 conversion efficiency was the highest. Additionally, CO decreased as nitrogen concentration increased because the carbon gasification reaction increased slightly. As a result, when the nitrogen concentration was 10%, the H
2/CO ratio showed the maximum value and the weight reduction rate also showed the maximum value.
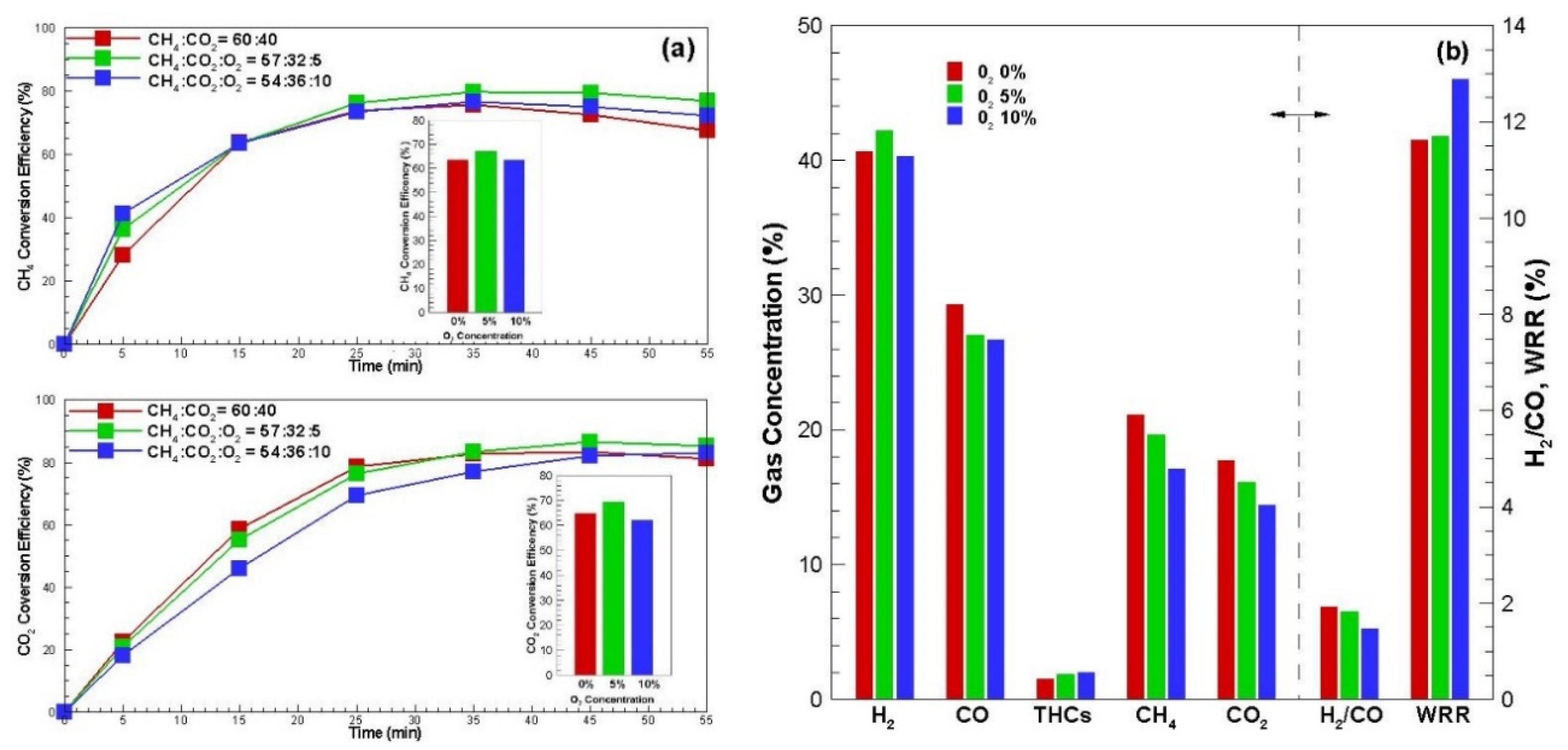
3.4. Characteristic of Oxygen Effect Reforming
Figure 6 shows the studies on three cases of CH
4:CO
2:O
2 ratio of 60:40:0, 57:38:5 and 54:36:10 to understand how the concentration of oxygen in biogas affects the reforming properties.
Figure 6a shows the conversion efficiency over biogas reforming time like line graphs, and the conversion efficiency of the arithmetic average of these values as bar graphs. CH
4 conversion efficiency over reforming time increased slightly in the first half, and the second half of reforming as the oxygen concentration increased. This is because methane is converted to the methane oxidation reaction Equation (4) in which methane is partially oxidized and reformed at the beginning of reforming, and the increase in the latter part is that attached carbon generated by the methane thermal decomposition reaction (Equation (5)) was treated in the carbon oxidation reaction Equation (9), so the carbon gasification (Equation (6)) reforming proceeded well. CO
2 conversion efficiency increased slightly in the second half when the oxygen concentration was 5% because attached carbon is removed by the carbon oxidation reaction (Equation (9)), promoting the role of catalytic activity pores (Equation (9)).
However, 10% showed the lowest overall value. This is because carbon was exhausted by the carbon oxidation reaction (Equation (9)) due to the excessive oxygen concentration, resulting in relatively few opportunities for reforming. When the oxygen concentration in the biogas was 5%, it was better than when the oxygen was not present, while it tended to decrease when 10%. It can be seen that in the case of oxygen concentration in biogas, energy efficiency is increased by autothermal reforming of methane and stable reforming can be achieved, but when the amount is more than 10%, the fuel value of the product gas decreases by the oxidation reaction (Equations (11) and (12)) or methane oxidation reaction (Equation (10)) [
20].
Figure 6b shows the product gas concentration, H
2/CO ratio and weight reduction rate. As already mentioned in the conversion efficiency, hydrogen showed the maximum value because the methane reforming effect was excellent when the oxygen concentration was 5%. However, carbon monoxide was somewhat reduced because some of the reformed amount was oxidized (Equation (12)). As the oxygen concentration increased, the weight reduction rate increased, which is due to carbon gasification (Equation (6)), but it was exhausted by carbon oxidation (Equation (10)), so the value was highest when the oxygen concentration was 10%.