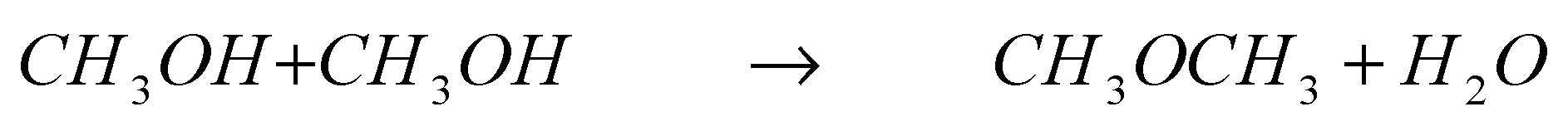1. Introduction
Since the beginning of the industrial age, the burning of fossil fuels has been releasing to the atmosphere the carbon that was slowly sequestered more than 50 million years ago and stored as coal, oil, natural gas and other types of fossil fuels sources such as shale gas and shale oil. The combustion of these fuels produces, besides pollutants, carbon dioxide (CO
2) which has a major effect of trapping the solar heat within the atmosphere (greenhouse effect), is expected to aim to the warming up of the Earth and the severity of the climate [
1].
The general public and most policy makers perceive electric vehicles as a good alternative to fossil fuel-based transportation [
1,
2,
3]. However, regarding emissions linked to vehicle use, electric vehicles are as green as the electricity they consume [
4]. In a country such as Poland [
5] or Australia, where most of the electricity is produced from coal, the running of electric vehicles will increase the contribution to the greenhouse effect comparing to the same car running in Norway or Brazil, where most of the electricity is produced from renewable energy sources [
6]. Additionally, the evaluation of the sustainability of EVs must take into account the whole life cycle of the vehicle, including sensible issues such as the mining of the materials used in batteries and electric motors, as well as battery end-of-life [
1,
4,
7,
8]. Nonetheless, although it is a hot topic with a lot of promising developments [
1], the detailed sustainability comparison between conventional and electric mobility is out of the scope of the present study and is only treated to provide some background.
Now, a large number of countries, regions and cities are proposing the ban on so-called “conventional” vehicles within the next decades [
9,
10] Normally, what policy makers refer to when using this term are cars, buses or lorries propelled by an Internal Combustion Engine (ICE) that burns a fossil fuel. So, if one of these specifications is eliminated from a vehicle, it will no longer be “conventional”. This leaves three types of vehicles: electric, hybrid-electric and alternative-fuel burning.
Nevertheless, some references to the future ruling out of so-called “polluting” vehicles are specified in terms of a ban on Internal Combustion (IC) Engines altogether, or in terms of a ban on vehicles that burn specific fuels such as diesel or gasoline [
4]. This is surprising, as it is accompanied by a growing acceptance of Plug-in Hybrid Electric Vehicles (PHEVs) that are able to run for several tens of kilometres without burning fossil fuel and therefore without producing pollution locally (in city centres, for example) [
11]. But these vehicles rely on ICEs for some of their operation, namely in long trips, once the battery has been depleted. Under these conditions, the ICE usually runs on fossil fuel, therefore producing a non-negligible amount of pollutants and CO
2 during operation [
12].
Therefore, what policy-makers likely aim with the strong limitations to conventional vehicles is the reduction of the emission of fossil CO
2 and the elimination of pollutant emissions within the city limits, which can be jointly achieved by the use of electrified (PHEV) vehicles [
3] This seems to be the direction most OEMs (original equipment manufacturers) are taking. Volvo, for example, had vowed to stop developing “conventional” (non-electrified) vehicles from 2019, only hybrid and battery electric ones [
13]. As of 2020 this has been mostly fulfilled although some of them are only “mild-hybrids” which have a bigger starter generator that displays some braking energy recovery and limited engine assist [
14]. Similar commitments can be seen in other OEMs. Nevertheless, the announcements and proposed timeframes towards electrification or even ICE development abandonment are not always completely fulfilled [
15,
16].
However, GHG (greenhouse gases) reduction in the transport sector can hugely benefit from the use of ICE using CO
2 neutral fuels [
17]. This is particularly important in sectors, such as heavy-duty and aviation, where energy density plays an important role. A recent paper about future trends in transport [
4] calculated that, with the present battery technology, electric passenger airplanes would require between 14 and 31 times its maximum take-off weight in batteries to store the energy that they usually carry as jet fuel. Also, the time for battery charging, using 80 Tesla superchargers would take over one day to fully recharge the battery equivalent of an Airbus 320 fuel tank. In terms of large vessels, the 170 GWh of energy that some these types of container ships carry in their tanks to power the engines would require batteries over five times their dead weight and would take years to recharge [
4]. These estimations seem to be too pessimistic as they do not take into account the difference in efficiencies between electric motors and IC engines, which would cut in half the energy needed, but are high enough to illustrate the impracticality of electrification for large carriers to travel over very long distances unless highly disruptive changes take place in battery technology. But renewable fuels and/or biofuels may be good propositions for these types of transport.
2. Hybrid Vehicles
Electrified vehicles need not specifically be battery electric vehicles (BEV), but there are various levels of hybrid vehicles, from the standard plug-in (parallel) hybrids, in which the engine provides mechanical traction power, to the extended-range (series) hybrids, in which traction is solely made by electric motors and the engine merely generates electricity, to fuel-cell hybrids. All these types of hybrid cars use some type of fuel that is burned in an ICE, with the exception of the fuel-cell hybrids. These latter vehicles use hydrogen (or another hydrogen-rich fuel such as alcohols or ammonia) which does not burn, but goes through a different process normally involving a catalysis through a Proton Exchange Membrane (PEM) producing electricity, water or water and CO
2, when the molecule of the fuel also comprises carbon atoms [
18,
19]. While hybrid systems tend to duplicate systems, which might be a disadvantage in terms of cost and maintenance needs, it might provide a positive trade-off in the short to midterm, as it allows to minimize the main current limitations of electric mobility: energy storage cost, density, reliability and charging time [
8,
20]. While some of these limitations are no longer critical for small urban vehicles, which do not need large storage, they are still critical for driving patterns needing frequent long trips, which would require huge, expensive energy storage systems needing long recharging times or very high power fast charging stations, which also have their own challenges [
21]. For instance, the authors reported that the Plug-in hybrid well-to-wheel CO
2 emissions may become negligible for cases where the long trips are less than 25% of the total mileage [
12]. This was reported for the case where a compact and efficient range extender with two different operating conditions (one for efficiency another one for extra power) was implemented. Moreover, this configuration would allow low fuel consumption, fairly low complexity (comparatively to parallel hybrids) and low system cost [
12].
Of course, the real-world emissions of vehicles are different from the reported emissions of new vehicles. Real driving emissions tests tried to address this issue and are now part of the emissions certification process. They are done under charge depleting (CD—mostly electric mode) and charge sustaining (CS—driving with a stable low state of charge using mostly the engine) modes. The official fuel consumption and emissions is a weighted average between the CD and CS modes, with the weighting factor being the so-called “utility factor” (UF) [
22]. The UF used in Europe is based on the driving statistics described by the SAE J2841 standard [
23]. But these tests are made to new model cars, not to cars being currently driven in roads. There is a loophole in this certification because some users will not use plug-in capability of the vehicle as often as desirable, relying excessively on the charge sustaining mode. Of course, to attain a more realistic measure of the emissions of fleet vehicles, the data mining of this information would be required in these vehicles, but this is still not the case. However, as long as there is an economic incentive in terms of energy cost in order to use electricity, it seems that a hybrid architecture would be well suited for a gradual transition towards full BEV mobility once their challenges have been overcome. Now, the sustainability of hybrid vehicles could be further improved by the use of fuels with lower GHG and pollutant emissions, as discussed in continuation.
3. Fuels
Fuels for IC engines (and fuel cells) are usually a combination of hydrogen (H) and carbon (C) atoms, but occasionally the fuel may also have other elements such as oxygen (O) or nitrogen (N). Fossil fuels (such as petrol or diesel) are, usually, a mixture of different components (hydrocarbons) composed of H and C. Each component has its own physical properties, such as density, boiling temperature and heating value (HV). One of the problems of IC engines is the very high standard for exhaust emissions which requires expensive and bulky after-treatment of the exhaust gases, which also reduces the fuel efficiency of the vehicle.
If a fuel contains oxygen or nitrogen, as these elements do not burn, its HV is lower than others composed just by carbon and hydrogen. In fact, when an alcohol (composed of C, H and O) burns, the oxygen atoms present in the burned gases are in the form of CO
2 or H
2O, mostly the latter [
23,
24].
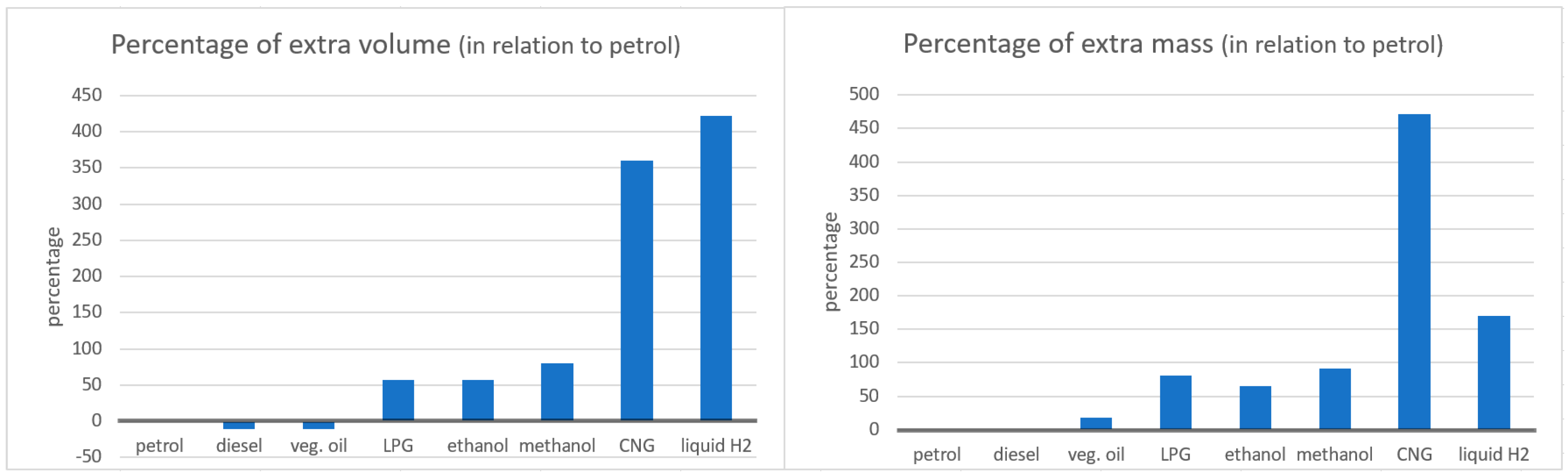
Liquid fuels are more appropriate for vehicle propulsion. They have a very high energy density in terms of mass and volume, enabling the vehicles to have an enormous range. Gaseous fuels require pressurized tanks and have a much lower energy density, resulting in much larger and heavier tanks for the same amount of stored energy (
Figure 1, [
25]).
Battery electric vehicles, for example, require huge volumes for their batteries (
Table 1), adding mass and cost [
8]. Nevertheless, the advances in battery technology have been slow but steady, with emerging technologies gaining traction. Examples are the use of high capacity cathode (e.g., metal oxide) and anode materials, electrolytes with high oxidation potential and metal-air batteries which replace the positive electrode with an air electrode [
26]. Another advantage of liquid fuels is their straightforward and fast refuelling. Gaseous fuels are more complicated and take longer to refuel, while electric vehicles require complex, expensive and time-consuming procedures.
In general, in terms of CO
2 production of fossil fuels, the more carbon the molecule has, the higher is the production of this gas.
Table 2 shows the potential for CO
2 production of various fuels (in terms of LHV) compared to petrol (100). Please note that hydrogen is not a natural fuel, so it has to be produced from other sources, that may be fossil, therefore generating CO
2, not in its burning, but during its production.
4. Biofuels
It is important to know whether a fuel is based on renewable energy, such as crops, as when it burns it does not increase the level of fossil CO
2 in the atmosphere but marginally (if process and process emissions are taken into account [
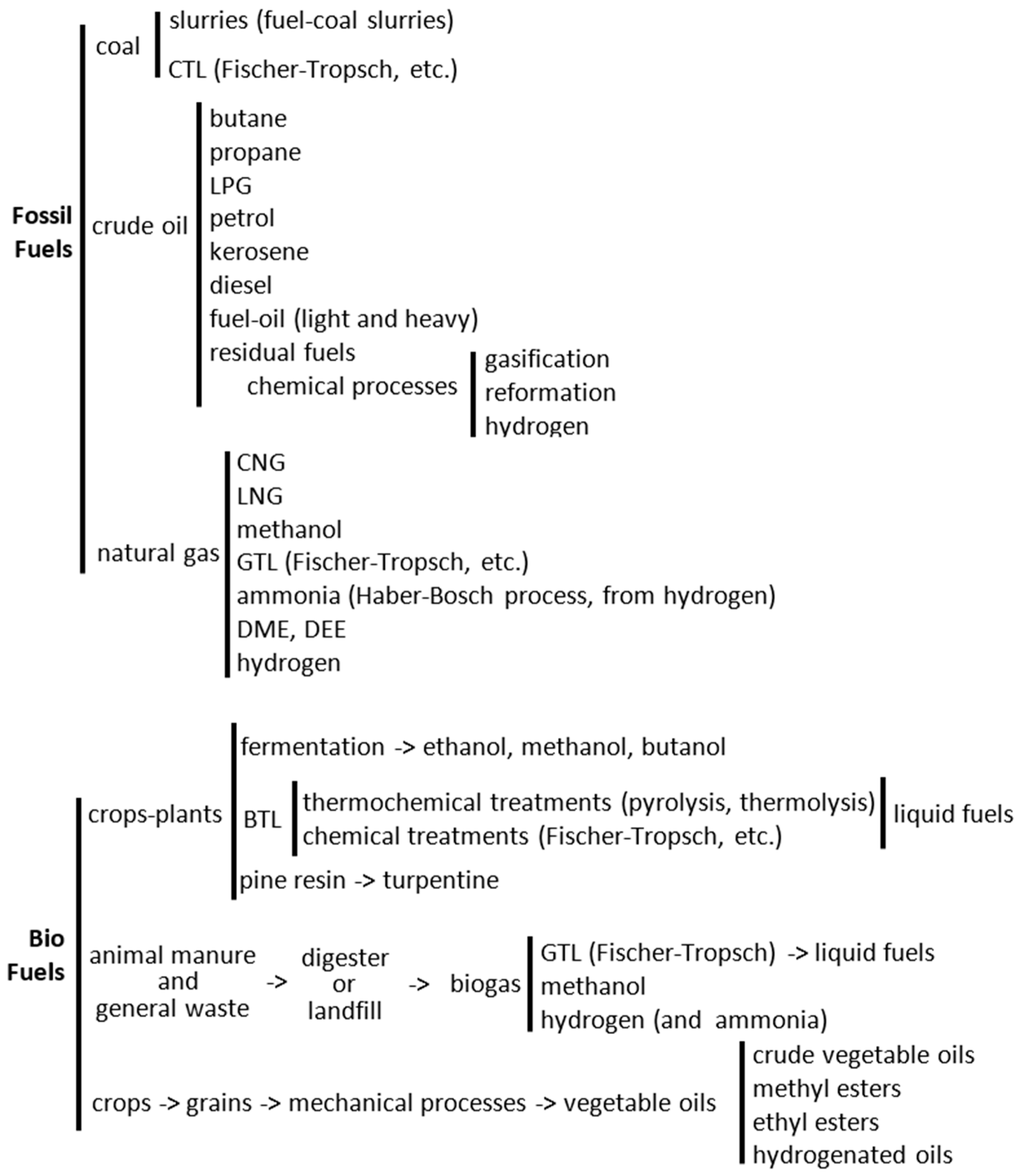
28], so there is an important division between different fuels is if they are generated from fossil or from renewable sources (biofuels) (
Figure 2).
However, as it will be seen later, sometimes it is difficult to assess whether the fuel is “bio” (from renewable energy) or not. For example, biodiesel is seen as a biofuel, but the 10% of methanol required for the transesterification process is usually produced from natural gas, a fossil fuel (
Figure 2). Some fuels (such as hydrogen or ammonia) can be produced from fossil sources (hydrogen from natural gas or oil) but they can also be produced entirely from renewable sources. This is the case for the hydrogen produced from hydrolysis of water using renewable electricity such as solar or wind, as well as photochemical cells. Other so-called solar fuels can also incorporate CO
2 using the same methods [
29]. Also, biomass can be converted to other more convenient fuel forms through the incorporation of solar energy, which aids the pyrolysis process [
30].
This brings another discussion about the fuels, because some of them (such as hydrogen) can be seen as “energy carriers” rather than “energy sources”. Electricity is an energy carrier, as it may be produced from different energies sources (renewables such as solar and wind or fossil such as carbon or nuclear) in a specific location, but then it is transported to the place where the energy is needed, such as houses. The electricity may be transported over large distances by the electrical network or it may be transported by the vehicles within chemical-electric batteries. In this respect, hydrogen, ammonia and other synthetic fuels (such as Fischer-Tropsch petrol or diesel) can also be considered “energy carriers”. If these fuels are produced entirely from renewable sources, then they are considered biofuels. So, these referred fuels (hydrogen, ammonia, synthetic hydrocarbons) may be considered fossil fuels, if they are produced from fossil origins, or they can be partially or entirely considered biofuels. Therefore, the SAME FUEL can be considered a fossil fuel or a biofuel. The methane present in the natural gas is fossil, whereas the same methane contained in the biogas is considered biofuel.
Important Properties
A list of the most common properties for different fuels gathered from several sources [
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38] can be seen in
Table 3. Fuel density and the heating value are important for the determination of the quantity of energy available in the fuel, in terms of volume or mass. The heating value can be specified in terms of higher heating value (HHV) or lower heating value (LHV). The difference between HHV and LHV is the heat related to the condensation of the produced water. Obviously, for carbon, HHV has the same value than LHV, as there is no water produced by its combustion.
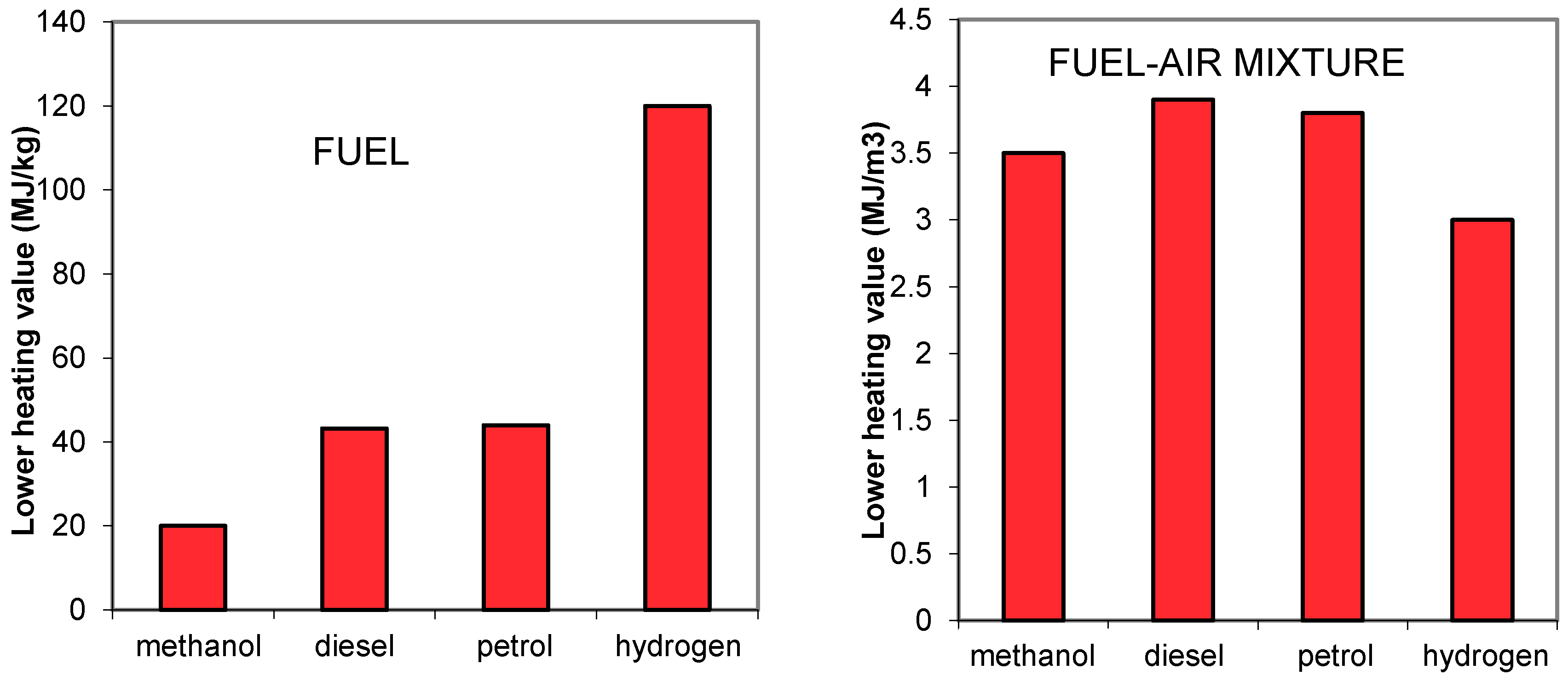
Usually the energy content of liquid and solid fuels is characterized by their LHV, as normally the combustion gases are exhausted at temperatures high enough as not to enable condensation. The extremes, in terms of LHV, are carbon (33 MJ/kg, although a common value for coal is 27 MJ/kg) and hydrogen (120 MJ/kg), and in most hydrocarbons their LHV is a function of the H/C ratio. Gaseous fuels such as methane have a high LHV, as its H/C ratio is one of the highest. The energy density of the fuel on a mass base would be an important parameter for mobile applications (namely, aerospace) but not so relevant for stationary applications.
Another important property is the heating value of a stoichiometric air-fuel mixture in terms of volume at atmospheric pressure. This shows the amount of energy that can be introduced into an IC Engine per cycle, which affects torque and power [
25]. The importance of this parameter can be illustrated with Hydrogen: although it has the highest HV in terms of mass, its value in terms of volume (of its mixture with air) is one of the lowest (
Table 3;
Figure 3). Naturally, this parameter affects storage space, which is also critical for mobile applications.
The latent heat of vaporization is responsible for the cooling effect on the mixture when the fuel is vaporized. Alcohols have a high value, so their mixture with air enters the engine at low temperatures, even when supercharging is used [
25].
The value for the stoichiometric air-fuel ratio (A/F) is an indication of the H/C ratio of the hydrocarbon and/or the amount of oxygen (or nitrogen) in its molecule.
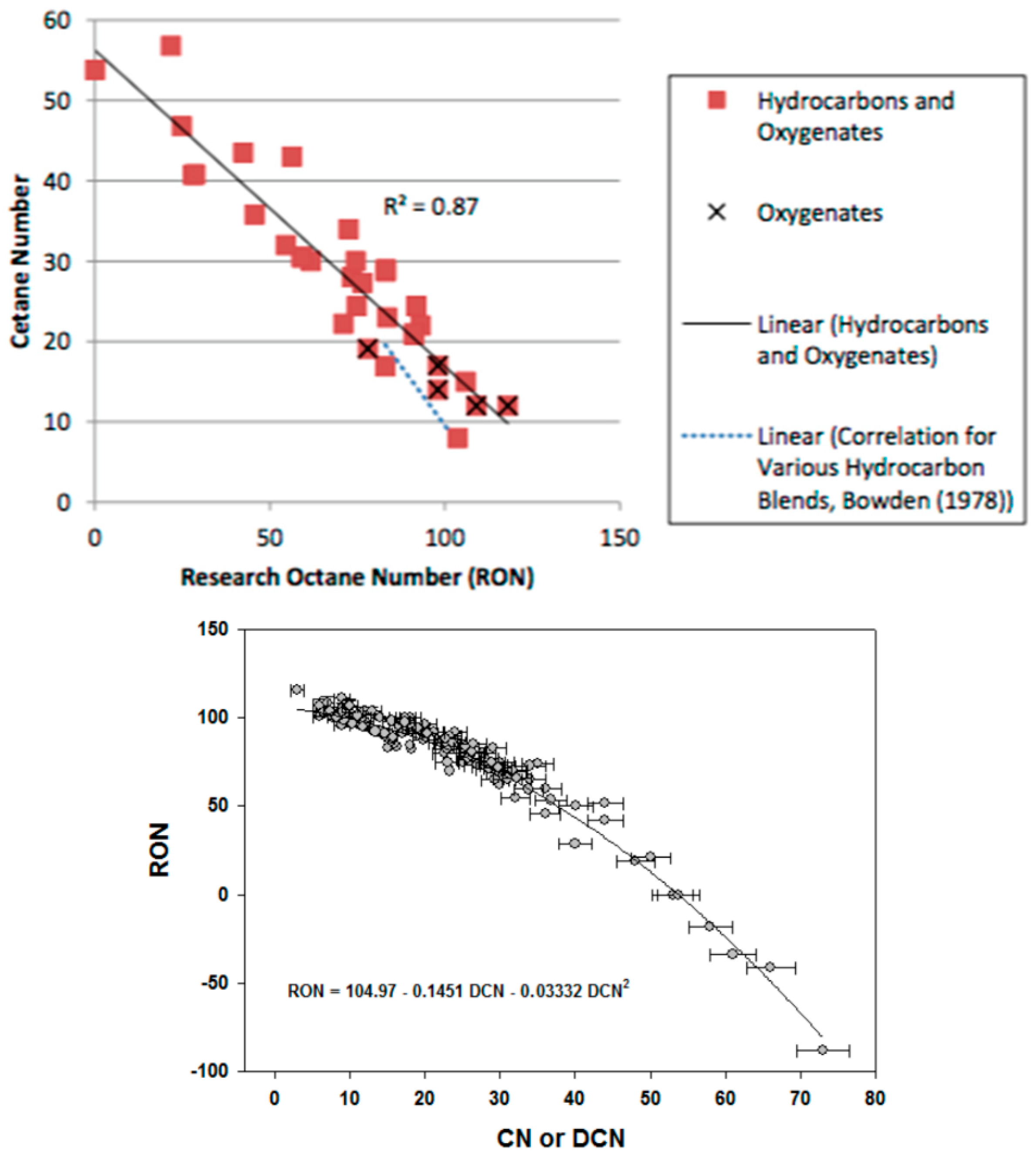
The RON (Research Octane Number) and CN (Cetane Number) used to classify commercial petrol and diesel fuels, respectively, are related to the way the fuel self-ignites. High RON numbers are good for Spark Ignition (SI) engines, whereas high CN numbers are good for diesel engines. In fact, these two numbers are opposed [
39,
40] (
Figure 4).
High values for RON indicate a very difficult auto-ignition behaviour, whereas high values for CN specify fuels that auto-ignite easily. Observing
Figure 4 it may be seen that there is a clear relation between these numbers. The curve fit yields the following formulae:
Other physical properties presented in
Table 3 are viscosity, flash point and Reid vapour pressure. The viscosity values show the potential for the fuel to be injected as a fine spray, very important for diesel engines. For example, diesel fuel has lower viscosity than biodiesel, so the biodiesel spray is coarser than the conventional diesel spray. On the other hand, ethanol and mainly DME and DEE exhibit much less viscosity, so their injection can be made in fine droplet sprays [
41].
Flash point is the temperature at which the liquid releases enough vapour to produce a stoichiometric mixture with air, therefore sustaining a flame, so it is a property related to safety. If the flash point of a fuel (such as diesel) is well above room temperature, a leak of it will not enable combustion. On the other hand, if the fuel has its flash point below 38 °C (100 °F) it is considered flammable [
23].
The Reid vapour pressure is a measure of the volatility of a fuel and is very important for fuels used in SI engines, mainly when they were carburetted fuelled. The flammability limits show the proportions (% in volume) where a spark may ignite the fuel-air mixture. Hydrogen is the fuel with the widest limits for flammability, which is very important to burn very lean mixtures. The burning of very lean mixtures in ICEs has various advantages such as high engine efficiency and low pollutant emissions [
25].
Oxygenated fuels, as the name refers, have oxygen in their molecule, so their energy density is reduced by this fact. Half the mass of methanol is oxygen and its LHV is less than half of that of petrol, but as its stoichiometric A/F is also almost half of the petrol, the HV of the air-methanol mixture is, although lower, at about the same level as for petrol. But, as the high latent heat of methanol greatly reduces the air temperature entering the engine, its density is increased by this fact and much more air enters the engine, effectively increasing engine power output (by around 6%, [
42]). The fuel with the maximum potential for power boost is nitromethane. Although it has a low HV of 12 MJ/kg, the 1.7 stoichiometric A/F enables a huge amount of fuel to be injected at each cycle, increasing the engine power output to more than 2.3 times the value for petrol [
42].
5. Hydrogen
Hydrogen is one of the simplest molecules, with just two atoms joint together, each one with just one proton and an electron. Normally it is in gas form and, unless at very high pressure and/or at very low temperature, its energy density (in terms of volume, or MJ/L–
Figure 3) is extremely low (0.11 MJ/L at atmospheric conditions). And this is one of the disadvantages of this fuel: even at very high pressures (750 bar) or very low temperatures (liquefied at 20 K) its energy density is much lower than that of most other liquid fuels (4.7 and 8.6 MJ/L, respectively [
43]) And it takes a significant amount of energy to pressurize the hydrogen or to liquefy it, a value that is a significant proportion of its own HV. When compared to other liquid hydrocarbons, one litre of liquid hydrogen actually has less hydrogen (atoms) than a litre of a conventional fuel (and the conventional fuel also has, in addition, carbon atoms).
Some recent developments promise the use of materials to store hydrogen at much lower pressures, such as Liquid Organic Hydrogen Carrier (LOHC) systems [
44,
45,
46]. However, these methods are complicated, need pressure and/or temperature control and require time for the storage (hydrogenation) and for the recovery (dehydrogenation), often requiring catalysts when liquids are used [
47,
48]. Yet, these technologies might become viable in the future for specific applications, namely in large-scale stationary cases where the economy of scale eventually compensates for the added complexity.
One other important disadvantage of hydrogen is that its tiny molecule can escape through materials that usually are not permeable to other gases. This requires the use of specific materials for piping and storage, including particular specifications for welding [
49].
Although hydrogen can be used in ICEs, its major advantage is to be used, as energy carrier, in fuel cells, where it produces nothing but electricity and water. Hydrogen production from electricity is usually done by water electrolysis in a process that may have an efficiency between 52% and 67%, so 60% seems a good average value. The hydrogen is then used to produce electricity in fuel cells with efficiencies ranging from 50 to 60% (we will use 55%) [
18]. Furthermore, it is necessary to store the hydrogen as compressed gas at 350 to 700 bar or as liquid at 20K. This requires 15 MJ/kg for the compression up to 700 bar [
50] and 50 MJ/kg for the liquefaction of H
2 [
50]. This leads to overall efficiencies of electricity-to-electricity of 29% (compressed H
2) and 19.5% (liquid H
2) when hydrogen is used as an energy carrier. A novel electrolysis process called high temperature electrolysis or steam electrolysis (at 700 to 1000 °C, much higher than the water critical temperature and at high pressures), shows a potential for much higher efficiencies [
51].
As said before, petrol and diesel also display more hydrogen content on a volume basis than liquid hydrogen, which makes their synthetic versions also good energy carriers, probably better than hydrogen.
But hydrogen has some important properties to be used in ICEs as an additive. It’s very high combustion speed (much higher than petrol) improves the combustion of other fuels even at low fractions (less than 5%). This is beneficial for fuels which burn slowly, such as ammonia [
52].
When the percentage of hydrogen is high or when it is burned on its own (pure), the higher adiabatic flame temperature generates a high quantity of NOx, so it is common to inject water as a means to reduce the maximum temperature, especially when supercharging is used. One of the problems of hydrogen is its potential for auto-ignition, as the activation energy (of a spark) required for ignition is very low (0.01 mJ). This fact interferes with the measurement of its knock behaviour and the value for its octane number. The measurement of RON requires an intake temperature of 149 °C, too high for the use of hydrogen. Usually the RON for hydrogen is reported as higher than 100 (
Table 3), but some researchers [
31] report values as low as 60. Others report RON of over 130 when lean mixtures are used. When the intake is at atmospheric temperature hydrogen shows a very high RON, enabling compression ratios (CR) higher than 14.5:1 without knock, probably helped by its very high combustion velocities [
53].
But, as discussed earlier, the major problem with hydrogen is its energy density. 50 L of petrol can be stored in a 72 L tank (weighing 84 kg, including the fuel), whereas the same amount of energy in hydrogen (19 kg) requires a cylindrical tank with 272 L and 129 kg ([
54]; see
Figure 1). So, the main interests of hydrogen seem to be its potential for use in PEM fuel cells, the absence of CO
2 emissions and its use as an energy carrier and energy storage in stationary applications (although at very high pressures or volumes). If other types of high performance fuel cells capable of consuming liquid fuels are developed, other biofuels (with the potential for not producing fossil CO
2) are used and other synthetic fuels may be used as energy carriers, what will be the benefit of hydrogen? It is our opinion that, if that is the case, the justification for the “hydrogen economy” will lose a lot of its appeal and other high energy density (liquid) synthetic fuels and/or biofuels will likely replace it. So, it seems that the major advantage of hydrogen in transportation is its higher energy density compared to batteries, which makes fuel cell hybrid electric cars a better proposition than full electric vehicles, with higher range and much lower refueling times.
6. Alcohols
The most common alcohol used in propulsion is ethanol, with vast amounts being deployed in Brazil and USA in the so called “flex-fuel” engines. These SI engines can burn petrol, straight ethanol, or any mixture of these two fuels. Although the stoichiometry of both fuels is very different (AFR of 14.5 for petrol and 9.0 for ethanol—see
Table 3), the injection system uses the lambda sensor in the exhaust to assess the richness of the mixture and to adjust it. If, for example, the engine is running on straight petrol and the driver fills the tank with ethanol, when the new fuel reaches the injectors, they produce a lean mixture (less fuel than required) but, within one or two seconds the lambda sensor reads the strength of the mixture, sends the information to the ECU (electronic control unit) and the right amount of fuel is then injected to the cylinders and from that point onwards the ECU (electronic control unit) of the engine assumes that fuel. Therefore, only during this very short period the driver may feel some swift glitch in the engine, but then it works seamlessly afterwards.
Methanol is another alcohol occasionally used, mainly in the USA and mainly for racing engines. Ethanol and mostly methanol are exceptional racing fuels for various reasons. They have a high value for RON and a high latent heat of vaporization (see
Table 3) leading to cold and dense mixtures entering the engine (more mass) and allowing the use of high compression ratio (CR), which brings higher efficiencies and power [
25].
Ethanol is mainly produced from the enzymatic breakdown of starch (grains) leading to sugar and then to ethanol. In the USA the base is corn but in Brazil, where the sugar cane is used, the first transformation is avoided, therefore greatly enhancing the overall efficiency of production.
Methanol is mainly produced from natural gas from the steam reforming equation:
followed by a catalytic reaction between CO and hydrogen:
One of the less known advantages of alcohol combustion is the so-called “alcohol bonus”. When methanol, is burned, the equation is:
which in terms of moles (which translate into volume) gives:
When petrol is used (considering CH
2):
which in terms of volumes is:
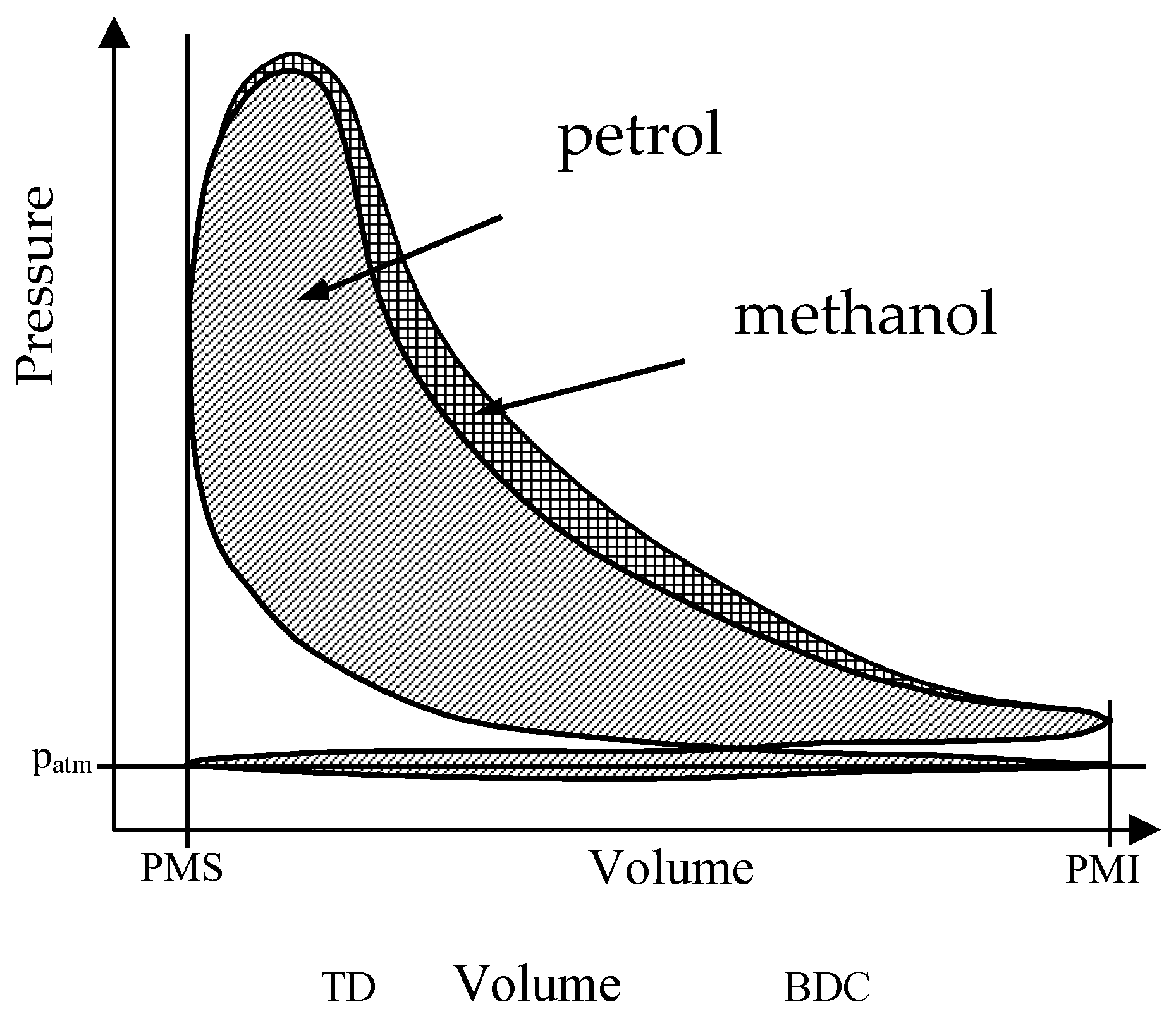
Thus, for the same volume of reactants (2.5) only two volumes are produced when petrol is used, but three are produced when methanol is used. This means there is a substantial higher volumetric expansion when alcohol is used. This can be seen on the indicated diagram, where the methanol shows higher pressure during expansion (
Figure 5). This is considering that both petrol and methanol are entirely vaporized when entering the engine, although it is much more difficult to vaporize methanol than petrol, as the latent heat of vaporization of the former (1100 kJ/kg) is much higher than that of the latter (350 kJ/kg), so the relation is even higher.
As methanol (and ethanol) have much higher latent heat of vaporization than petrol and, for the same power, the amount of injected mass is also considerable higher, the total heat required for the total vaporization of the fuel is much higher when alcohols are used. This creates a cooling effect on the intake mixture, even when supercharging is used. This is beneficial for motorsports, as the thermal loads of engine internals are very high. With the use of alcohols, a supercharged engine can work without intercooling and face no thermal problems or knock.
As the RON values for alcohols are higher than those for petrol (see
Table 3), the compression ratio of the engines can be increased without knock, therefore improving power and efficiency. As the adiabatic flame temperature of alcohols is lower than that of petrol, the thermal losses to the combustion chamber are reduced, further improving the overall efficiency.
Other advantages of the alcohols are the fact that, unlike petrol, they mix very well with water, enabling firefighting by the use of water. Throwing water into a petrol fire usually aggravates the problem, as the water is denser than petrol, so the fuel floats over it and spreads easily.
But alcohols also have some problems. The methanol flame has no colour, so it is very difficult to assess whether a fire is taking place. Also, as the heat required for full vaporization of the alcohols is high, mixture preparation may be a problem [
55], and a large proportion of liquid may enter the cylinders and “wash” away the oil from the cylinder surfaces, enabling piston-cylinder contact. Also, in cold countries (even in south Brazil) a small tank of petrol is required to start the engine, as ethanol or methanol does not have enough vapour pressure to produce an ignitable mixture. Also, ethanol and especially methanol, tend to induce heavy corrosion on various metals and also other materials such as rubber.
As the flammability limits of alcohols are much wider than those of petrol, the engines may work with much leaner mixtures, improving the efficiency of the engine and reducing all the pollutants.
Despite being a clean and efficient fuel, the major contribution from methanol to transportation is its use to the biodiesel production in the transesterification process [
56].
7. Ethers
Ethers are molecules with an atom of oxygen connecting two radicals that usually are similar. For example, dimethyl ether (DME) is composed by two identical methyl radicals connected by the oxygen atom. They are highly flammable liquids or gases which, therefore, may be used in IC engines.
7.1. DME
Dimethyl ether (DME) is the simplest ether and is a gas at atmospheric pressure, but it is easily condensed by applying pressure (<10 bar), so it may be stored in similar containers as propane. Its cetane number (CN > 55) is higher than that of diesel (see
Table 3), has a low ignition temperature (320 °C), its viscosity is very low and, as it is composed of 35% oxygen and it has no C-C bonds, its combustion is smoke free [
57]. As it is highly volatile, its mixture preparation with air is much easier than diesel, which makes it a perfect compression ignition fuel. Also, it burns fast and without knock (silent combustion—[
41]), it has a potential for higher efficiency but it produces more NOx than diesel [
56].
DME (and other ethers) can be produced by the dehydration of two alcohol molecules, also producing water (
Figure 6). It can also be produced from the “black liquor”, a by-product of pulp and paper production, or from lignite-cellulose biomass, which makes it a second-generation biofuel.
The compressibility of DME is much higher than diesel, which increases the required energy for fuel compression, although it does not require the huge injection pressures required for fine diesel spray formation. However, it cannot be used directly on diesel injection systems, as it has poor lubrication properties, but a small amount of biodiesel may be added to enable the lubricity. Also, its low density and low heating value require higher injection mass flowrates than diesel but in overall it has the potential for producing more power from the same engine using diesel [
56].
7.2. DEE
Diethyl ether (DEE) is a volatile ether conventionally produced as a by-product of ethylene hydration during the production of ethanol, but it can also be produced from the reaction of sulphuric acid with ethanol or by catalytic dehydration of ethanol. As its cetane number is very high (ignition temperature of 160 °C, one of the lowest,
Table 3), DEE is used as an IC Engine starting agent, both in SI and diesel engines.
With such a high cetane number (up to 158 [
41]) and being able to be stored as a liquid at atmospheric conditions (T
boiling = 34 °C,
Table 3), this fuel seems to be a good candidate for use in Compression Ignition (CI) engines. It can be added to diesel and, during WWII in Japan, it was used as an additive (up to 5%) in aeroplane engines [
41]. Its use as a straight CI engine fuel may have problems as it does not have lubricity and it has tendency to oxidation, producing peroxides. Some organic peroxides are dangerously reactive because they combine both fuel (carbon) and oxygen in the same compound. As it has oxygen in its molecule and has no C-C bonds, its burning does not produce smoke.
8. Esters (Biodiesel)
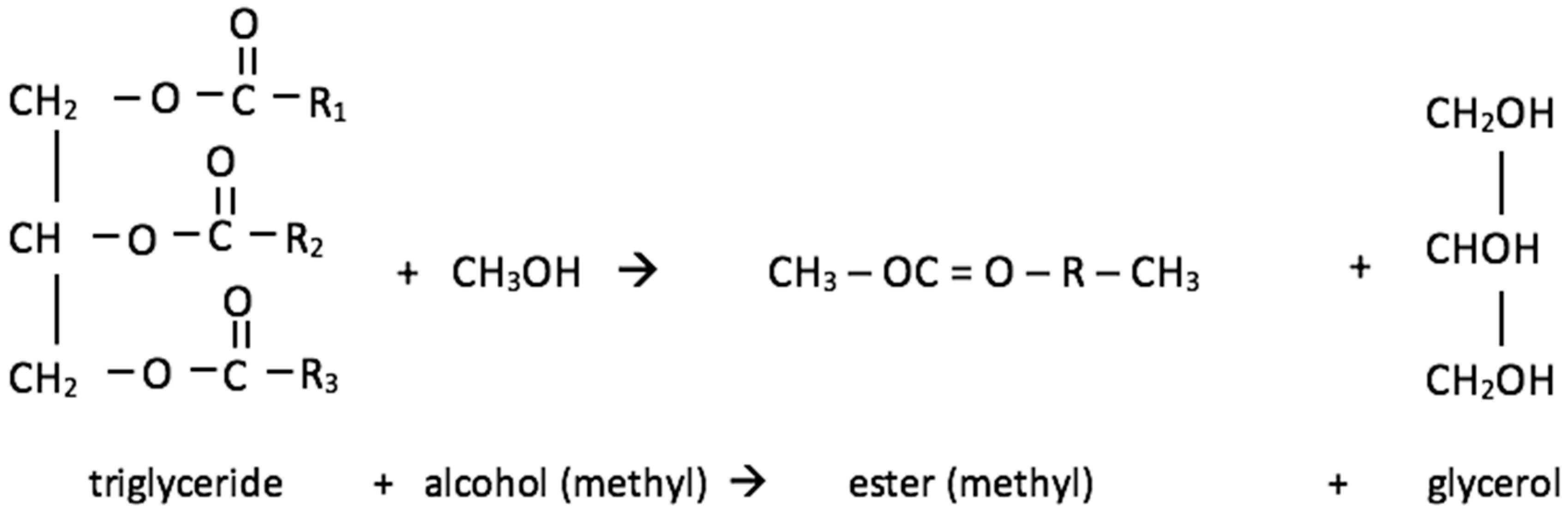
Common esters are better known as biodiesel and they are good substitutes for fossil diesel fuel in CI engines. They are produced from vegetable oils (and other fats) in esterification or, more commonly, transesterification processes (
Figure 7). In the latter process a triglyceride reacts with an alcohol, in the presence of a catalyst, producing the ester and glycerol. Methods for glycerol usage will be discussed in a following chapter.
The esters of different vegetable oils (rape seed, soy, peanut, sunflower, etc.) are known as biodiesel or FAME (fatty acid methyl ester), if they are produced from methanol. They are usually produced using methanol, but it is possible to produce biodiesel using ethanol. In this case the process is slower and has lower efficiency, but the final product may be considered 100% biofuel, if bioethanol is used and if the vegetable oil is 100% bioproduced. While methanol can also be produced from renewable sources, usually it is derived from natural gas. One of the advantageous properties of biodiesel is its lubricity. When sulphur was removed from diesel, its lubricity was reduced drastically, and the solution was the addition of 2% biodiesel to restore it.
Biodiesel, although with slightly different properties according to the original oil it was made from, is a fuel with higher cetane number than diesel, has no sulphur, the CO and HC emissions are lower [
58] and is a biodegradable liquid. In terms of disadvantages, it has higher viscosity than diesel, produces higher values of NOx and is not stable (oxidises) during prolonged storage. Also, the production process is inefficient (is intensive in energy), its heating value is lower than diesel and it may attack elastomers.
For the same amount of injected fuel as diesel, the power reduction using biodiesel should be about 10%, but engine data shows only a reduction of 5% [
37], showing a higher efficiency. For the same vehicle energy consumption, the author [
58] measured an increase of only 3.5% (in volume) and a reduction of 6% in terms of energy (in fuel) used, when compared to fossil fuel in a long (12,350 km) trip in South America. This also shows a better engine efficiency on the use of biodiesel when compared to diesel [
58].
It is important to state that the referred comparative tests were performed in common-rail engines. When using traditional pump-pipe-injector systems the lower compressibility and higher cetane number of the biodiesel generates an earlier and faster combustion which further improves efficiency (and increases NOx) if the engine is developed to minimize NOx emissions. But the lower compressibility of the biodiesel does not interfere with the injection in common-rail systems, so the higher efficiency in these type of engines is only explained by the better combustion potential of the biodiesel [
58].
Biodiesel has some disadvantages in relation to diesel. It solidifies at higher temperature (~0 °C) which may be problematic in cold countries. Also, the cold weather additives for diesel do not work for biodiesel, so other additives have to be developed. Biodiesel produced from animal fats (a significant proportion of Brazil biodiesel, 25%) has a much higher solidification temperature (~15 °C) [
59].
In prolonged storage it may oxidize and, as it is a biofuel, it may be a source of bacteriologic contamination [
59].
Acrolein, which is a toxic substance, is seen as a problem for the biodiesel burning. However, acrolein is a by-product of glycerol burning and biodiesel should have almost no glycerol. In fact, a study [
60] showed that biodiesel exhaust emissions may present a lower risk to human health than diesel emissions in IC engines.
There are other processes for the production of biodiesel other than esterification and transesterification. One of the routes involves a mixture of biomass and water (to keep it moisten) undergoing a high temperature (300–350 °C) and high pressure (120–180 bar) process (hydro thermal upgrading—HTU) to remove part (85%) of its oxygen [
61]. The resulting oil can be physically or chemically refined into biodiesel. This is a second-generation process.
Iodine Value (IV)
The iodine value (IV) is a measure of the level of unsaturation of the biodiesel or oils. This is an easy test to perform, basically it consists on measuring the amount of iodine that can be added to saturate 100 g of the fuel. The degree of unsaturation relates to the number of double bonds (
Figure 8a) between carbon atoms and shows its stability to oxidation and/or to polymerization. A biodiesel from an unsaturated oil has various double bonds. When iodine is added, two atoms are connected to the carbon atoms that were previously connected by the double bond (
Figure 8b). The higher the iodine value of the biodiesel (oil or fat), the lower the melting temperature. So, biodiesels produced from animal fat (saturated) have melting points usually above 15 °C [
59].
The hydrogenation of oil occurs when the double bonds of the unsaturated oil are transformed into single bonds and hydrogen atoms are included where the iodine atoms were placed, as seen in
Figure 8, right.
A fuel with a higher degree of unsaturation has a higher IV, usually produces higher values of NOx [
62,
63,
64] and has lower stability to oxidation. With that in mind, in Europe the IV of the biodiesel is limited to 120, restricting biodiesel produced from unsaturated oils such as sunflower or soy [
64] and allowing rapeseed oil based biodiesel. This restriction imposes limitations to the biodiesel production of southern European countries and imports from Brazil and the USA (usually from soy) and there is a large debate about it. Biodiesel specifications in the USA, Brazil and Australia do not restrict IV.
Biodiesel produced from animal fats, a highly saturated fat, induces a reduction in NOx [
63] and its IV is also low [
65]. Brazil uses a mixture of biodiesel produced from soy oil (75%) and tallow fat (25%), enabling the reduction of the high IV from the soy oil biodiesel [
65]. However, biodiesel produced from anchovies, an unsaturation fat, has an IV of 185 and tends to reduce the emission of NOx (in 11%), when compared to diesel fuel for similar conditions [
66], proving that, at least in some cases, there is no direct link between IV and NOx emissions.
9. Vegetable Oils
The first diesel engines developed by Rudolf Diesel were fuelled by vegetable oils and only later was mineral diesel oil used. One of the problems of using raw vegetable oils is their very high viscosity. It is possible to reduce the viscosity by increasing the temperature of the vegetable oil to values similar to those of diesel prior to injection. But oils and fats have various levels of saturation (double bonds) indicated by their iodine value (IV). The higher the IV, the higher is the probability of the oil or fat to polymerize at high temperature, leading to the formation of heavy and sticky deposits (gums) at the injector tips and piston rings, resulting in a damaged engine.
10. Other Oxygenate Fuels
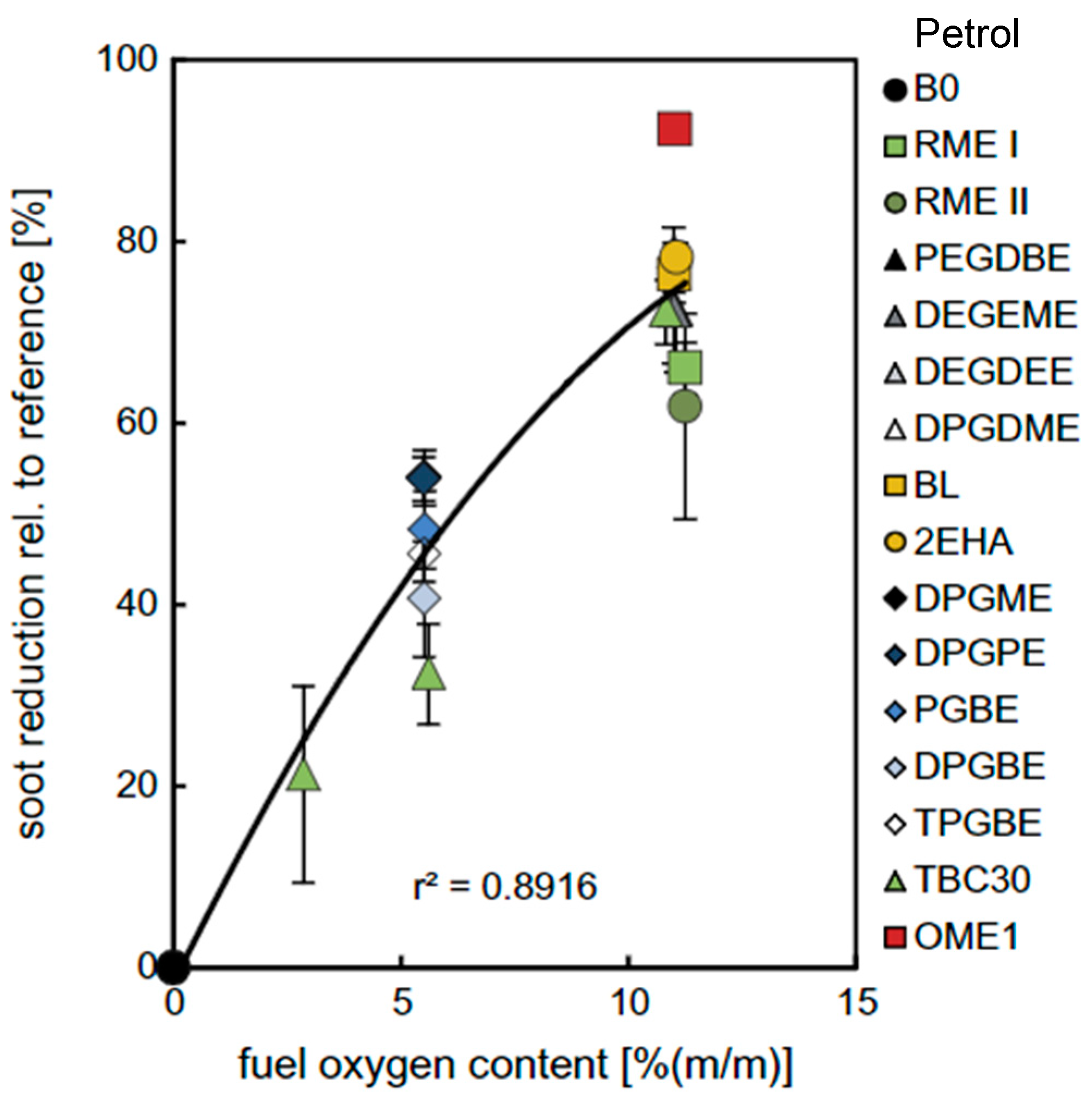
The previous fuels (alcohols, ethers and esters) have oxygen in their molecule, for which they are called oxygenate fuels. These oxygen atoms significantly improve the fuel combustion and they reduce the potential for particulate matter (PM) production. A work by Harlt [
67] showed (
Figure 9) that there is a strong correlation between oxygen mass content of the fuel and relative soot (PM) reduction. Furthermore, these researchers proved that oxygenate fuels with higher hydrogen content (higher H/C ratio) tend to further reduce soot formation. Therefore, light oxygenate fuels such as DME or DMM (dimethoxy methane) are better suited as far as soot emissions are concerned. While DME has a high cetane number (~60), DMM, has a relatively low CN of 30 (see
Table 3), which reduces the CN number of the mixture diesel-DMM, increasing its ignition delay [
68]. But its addition to diesel fuel greatly reduces PM production. DMM has low lubricity so it cannot be used as straight fuel on a diesel engine, without additives for lubricity and cetane number enhancement [
67].
Diesel engines and recent Spark-Ignition direct injection engines suffer from PM production, as the time for fuel preparation is scarce. In these types of engines, the fuel injection takes place very late in the cycle, reducing the time required for proper fuel-air mixture, leading to PM production. Oxygenate fuels, such as alcohols and ethers, are known for PM production reduction as they lack C-C bonds [
69].
Dimethyl carbonate (DMC) and methyl formate (MeFo) are knock resistant (with high RON) esters which are suitable for direct-injection (DI) high-compression Spark-Ignition (SI) engines [
70,
71]. These fuels also offer the potential for significantly PM production reduction of SI-DI engines, which is a beneficial surplus. They are both used in the chemical industry as solvents and other applications. Both DMC (C
3H
6O
3) and MeFo (C
2H
4O
2) have equal elemental ratios, so they have the same heating value (
Table 3) and similar knocking resistance. Tests comparing to petrol showed a higher fuel consumption for these oxygenates, but the engine power output was significantly increased (by 13%) as a result of a higher mixture heating value when compared to a petrol-air mixture. But the better improvement was in terms of PM reduction, where the particulate number (PN) was reduced by one (DMC) and two (MeFo) orders of magnitude [
69] compared to diesel.
In terms of novel oxygenate fuels for compression ignition engines, the oxymethylene ethers OMEs (CH
3O(CH
2O)nCH
3) seem very promising [
72]. These fuels are also known by the name polyoxymethylene dimethyl ethers (PODEn). This is a class of different fuels, with n varying from 1 (dimethoxy methane or DMM) to more than 5. However, for n = 1 we have the DMM which is very volatile (almost like the DME, which is n = 0) and for n = 2 the fuel has a low flash point [
73] so the more usable fuels have the n ranging from 3 to 4 (OME3-4 or PODE3-4). Higher values of n have very high melting points and may precipitate when mixed with diesel fuel. Diesel engine tests of this fuel (PODE3-4 mixed with diesel) showed the potential for a faster combustion, lower PM production and slightly higher NO
x emissions. The engine efficiency improved for all conditions, when compared to straight diesel fuel and the CO and HC emissions were lowered [
73]. These fuels have the added benefit of being able to be produced from biomass feedstock [
74] and do not have C-C bonds, therefore burning easily and cleanly.
11. Synthetic Fuels—Fischer-Tropsch Process
It is possible to produce synthetic liquid fuels from more traditional fuels such as coal, natural gas or hydrogen. The processes are initiated by the production of syngas (a mixture of hydrogen and carbon monoxide) which then passes through catalytic reactions, such as the Fischer-Tropsch (F-T) process, leading to the production of liquid hydrocarbons [
75]. The ratio between H
2 and CO on the syngas and the type of catalyst determines the types of hydrocarbons produced, which can be similar to petrol, diesel or lubricating oil. The relevant equations are the following:
Diesel F-T has a higher compressibility than fossil diesel (which is not an issue for common-rail engines), has a higher cetane number (
Table 3) and the potential for NOx and PM (particulate matter) production is lower [
36]. As these synthetic fuels are sulphur free, their combustion is very clean with low PM emitting potential. However, it seems to be sensitive to EGR (exhaust gas recirculation) levels, producing high levels of smoke above a certain value of EGR. But diesel F-T may have different formulations with different distillation curves, which changes some of its properties [
76]. These synthetic fuels are hydrocarbons, which do not have oxygen in their molecule, so the reduction of PM production cannot be attributed to that element, as is for oxygenate fuels.
These fuels are seldom known as GTL (gas to liquid). If the base fuel to produce the syngas is biomass, the name changes to BTL (biomass to liquid) and are considered second generation biofuels. During WWII the axis countries had huge shortages of oil, so most of the required fuels and lubricants were produced with these techniques (synthetic fuels) from coal (called CTL—coal to liquid, [
77]), like some decades later did South Africa to overcome the oil embargo they were subjected to [
78].
11.1. CTL
CTL (coal to liquid) fuels burn cleaner than fossil petrol or fossil diesel, as they are specifically produced to be burned in a particular type of engine. There are two methods to produce them. The indirect coal liquefaction (ICL) requires the crushing of the coal, which then is exposed to high temperature and high pressure together with water (steam) and oxygen to produce the syngas:
and also, with limited air:
Then, the syngas is transformed into liquid fuels by the F-T process. According to the required fuel (petrol, kerosene, diesel or lubricant), the F-T process has to be fed with the right proportions of H
2 and CO, so it is possible to change these proportions, for example by:
In the direct coal liquefaction (DCL) the pulverized coal is exposed to hydrogen (hydrogenation) also at high temperatures and pressures (pyrolysis), resulting in a syncrude liquid, which is then refined. The Belgius process involves the mixing of coal with heavy oil recovered from the process and hydrogen at high pressures and temperatures, resulting in a liquid hydrocarbon:
The major differences between ICL and DCL are:
- -
DCL uses just one step and is more energy efficient;
- -
ICL is easier to control (“design”) the type of produced fuel.
The production of huge quantities of CO
2 (almost twice the overall emission of fossil fuels, in a WTW basis, [
79]) is one of the major drawback of these processes. Other problems are the large amounts of necessary thermal energy and water. The required water consumption is in the region of 1m
3 (1 ton) per each barrel of fuel production [
77] and the coal (bituminous) consumption is between 0.73 to 1.04 ton per barrel, according to Sasol experience [
77]. Suitable catalysts are essential for each process.
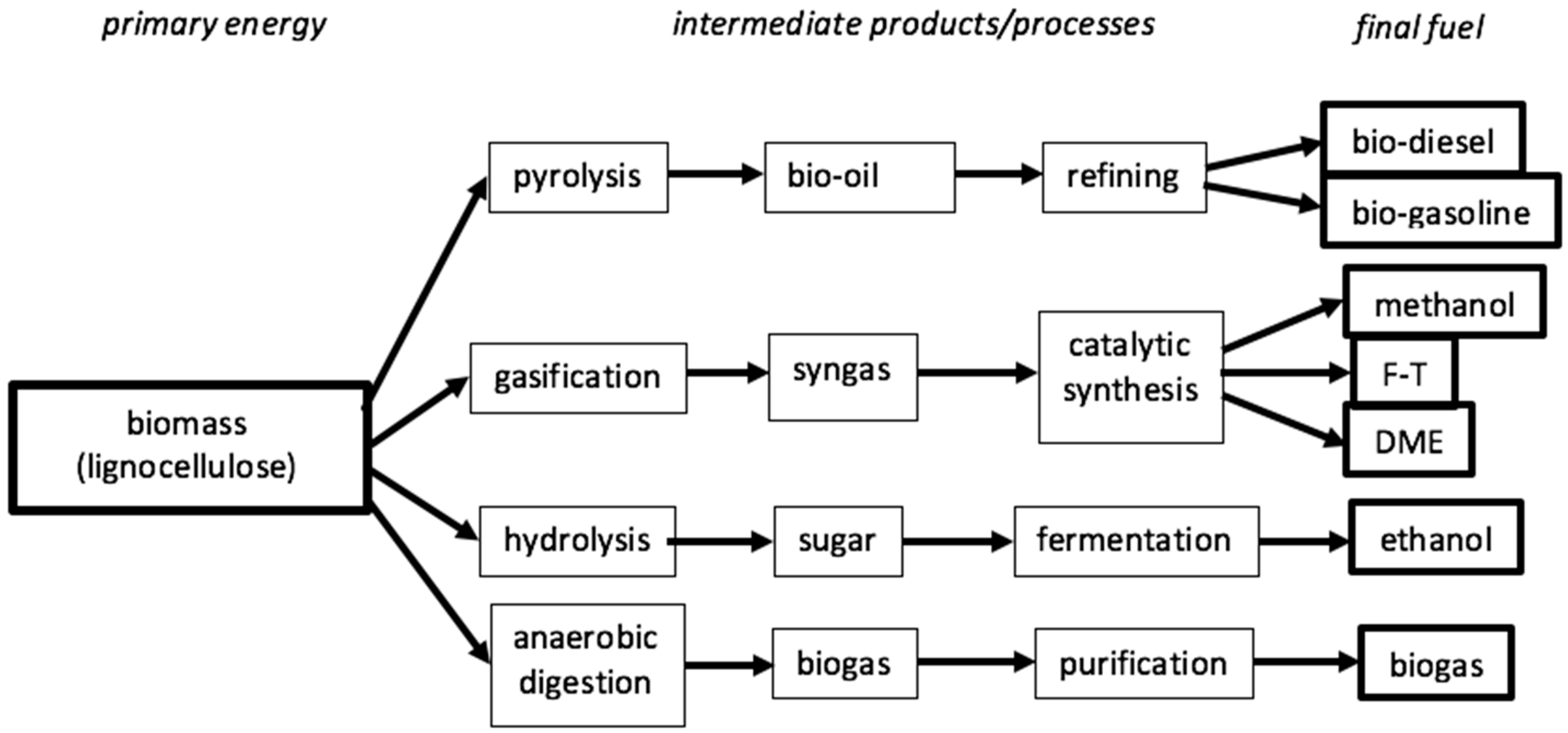
11.2. BTL
Some of the processes for transforming solid biomass (plants, wood, crops, straw –lignocellulosic) into liquid fuels (
Figure 10) are similar to the above referred processes. The solid biomass is burned under a low oxygen environment (gasification) or is reacted with steam (high pressure and temperature, although lower than CTL) in the presence of adequate catalysts to produce syngas, which is then transformed into liquid fuels using the F-T process. Or, the biomass goes through a pyrolysis process (
Figure 10), producing a pyrolysis oil, that is processed and distilled into the required liquid fuels. However, these processes are very biomass intensive (6 ton of biomass to produce 1 ton of BTL, [
80]).
There are other processes for the transformation of lignocellulosic biomass into fuels such as the second-generation hydrolysis process (
Figure 10) followed by the fermentation of the sugar into ethanol and the old process of anaerobic digestion. However, this process requires novel ways of increasing the bioconversion efficiency, such as performing the pre-treatment leading to cell wall degradation [
81].
11.3. GTL
The easier way of using the F-T process is using a mixture of natural gas (methane) and steam in a catalyst bed, where it produces the syngas:
These are called gas-to-liquid or GTL. One way to produce BTL fuels is using GTL plants and “hybridizing” them to accept syngas produced from biomass, what is sometimes called hybrid BGTL plants. These plants have a maximum efficiency of around 22% of production fuel derived from biomass [
82].
11.4. HVO
Vegetable oils can undergo processes of cracking and/or hydrogenation similar to those in oil refineries, leading to oxygen free linear paraffinic hydrocarbons usually called HVO (hydrogenated vegetable oils) and propane. HVO are not biodiesels, as they have no oxygen in their molecule and have properties similar to fossil diesel.
The hydrogen brakes the links between the glycerol and the fatty acids and deoxidize the hydroxyl and carboxyl groups, leading to a hydrocarbon without oxygen [
83]. Part of the carbon is used to “brake” the glycerol and generate propane. So, this process consumes fat and hydrogen and produces long chain hydrocarbons (diesel fuel) using catalysts and high temperature (300 °C) and pressure (50 to 180 bar). CO and CO
2 are also formed as by-products of the process.
The removal of the oxygen alters some of the properties. It reduces the lubricity and its PM (smoke) production and heating value are between those of biodiesel and diesel. The cetane number of HVO is very high (CN = 82:
Table 3) which will require a remapping of the engines, namely of the injection advance. Although its heating value is somehow higher than diesel, its lower density results in a lower heating value per unit volume.
11.5. Gasification Fuels—VGO
Gasification fuels can be obtained from biomass using forest residues (small branches and leaves) and black liquor (a paper and pulp production by-product), which produce syngas at high temperature and pressure, followed by the F-T process.
Different plastics (namely those non-recyclable) can undergo a gasification process where, after decontamination, the gases are condensed in a high-quality oil (VGO—vacuum gas oil) that may be distilled into petrol and diesel. Such a processing plant could be a floating platform used to eliminate and treat the enormous amounts of plastic that litter vast parts of the oceans. The process to produce VGO is already used for the recovery of fossil heavy oils, where the heating at low pressure allows the heavy oil to boil at much lower temperatures than at atmospheric pressure. This prevents the production of cokes and therefore increase the liquid fuel production.
11.6. Pyrolysis Fuels (PL)
Pyrolysis is a high temperature reaction without air contact. It produces gases, liquids and solids, being the liquid fractions (PL) the important ones for IC engines. Various substances, such as plastics, biomass and used tires can be used as raw material. Using the latter material (tires), the resulting liquid has a high heating value (over 40 MJ/kg [
84]) and some other properties are similar to fossil diesel, but the cetane number is very low (17.6—
Table 3), which allows it to be used mixed with fossil diesel (or biodiesel) only in small percentages.
11.7. Electrofuels
The denomination electrofuels has been gaining importance in the last decade [
28]. It does mean renewable energy-based fuels which are of non-biologic origin, so they are not based on crops. The EC [
85] introduced the term Indirect Land Use Change (ILUC) to account for the consequences (sustainability) of the production of biofuels on the land use. As a sustainability measure, the biofuels produced through ILUC will not be included in terms of renewable targets after 2030. The electro-fuels, such as hydrogen and its derivatives, are basically produced from the (renewable) electricity and water by electrolysis or other physical/chemical processes. Then the hydrogen is combined with the carbon in the CO
2 (through carbon monoxide) to produce the syngas required for the synthesis (Fischer-Tropsch) process [
86].
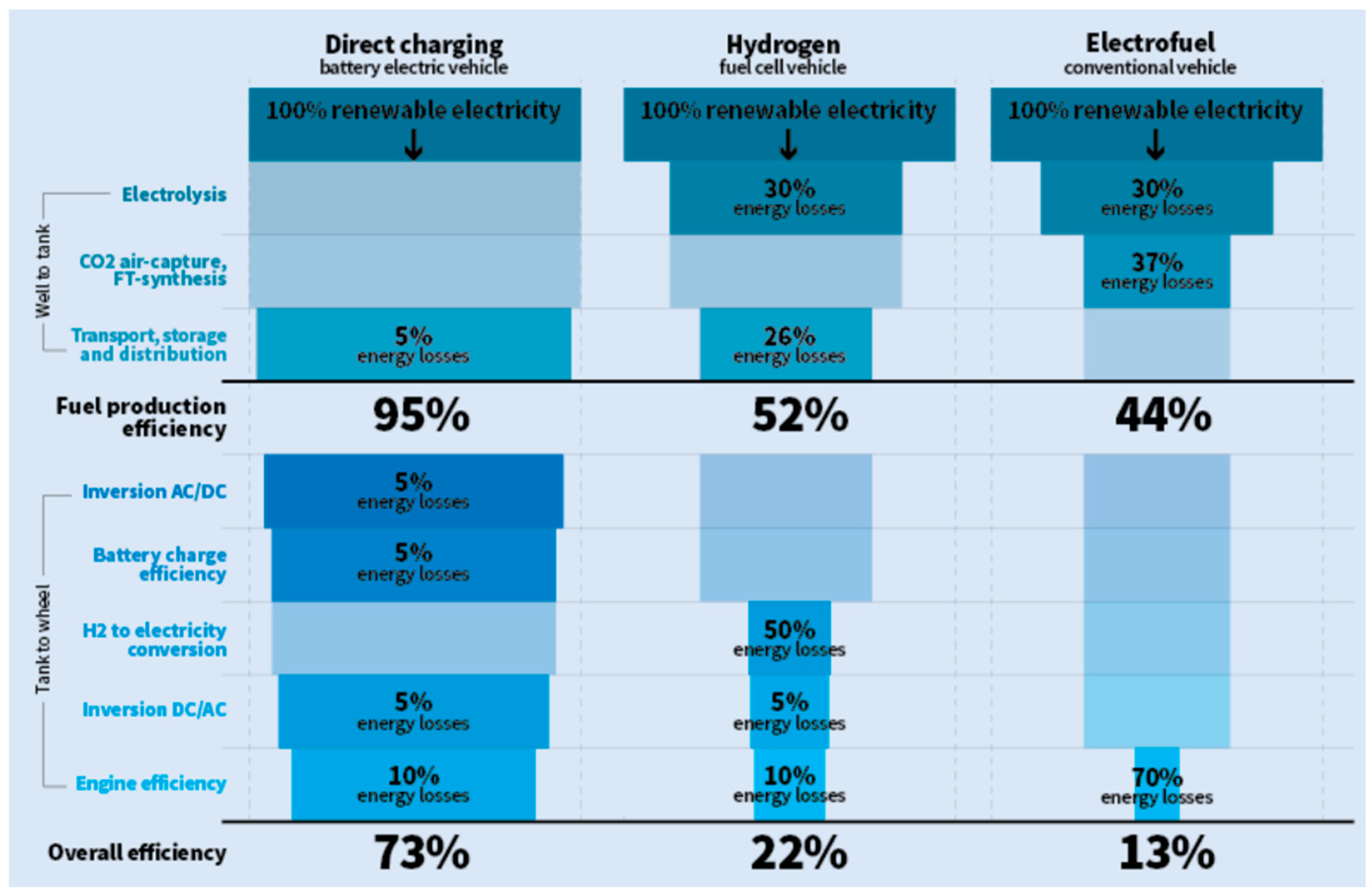
Electro-fuels are very expensive to produce as the energy efficiency of its production is very low, requiring much more energy in the production process than the available in the fuel. Bannon [
87] refers overall efficiency values of 73%, 22% and 13% for vehicles running on batteries, on hydrogen fuel-cell and on IC engines burning electrofuel, respectively (
Figure 11). So, it is more logical to use the electricity directly stored in batteries in electric vehicles. However, this is not possible in aviation, where the heavy batteries prevent its use. Therefore, airplanes need liquid fuels to fly, so electro-fuels seem to have a future in the huge market for the renewable energy use in the air transport [
51,
87]. And bear in mind the proposed 50% of EU aviation renewable fuel (electrofuel) by 2050 [
85].
When comparing to biofuels, electrofuels from zero-carbon renewable sources (solar, wind, etc.) have much less sustainability risks and use one order of magnitude less land [
51] Additionally, the requirement for water is far inferior and there is no risk for groundwater pollution (through chemical fertilization, such as nitrogen). However, if the electricity production is based on any amount of carbon intensity, its inherent low WTW efficiency causes it to produce high levels of CO
2. For example, electrofuels produced from the European energy mix average grid [
6] production would have a CO
2 intensity three times higher than current fossil fuels [
51]. In terms of cost, the values are extremely high, more than five times the cost of fossil fuels and, therefore, much more than the price for biofuels [
88]. One of the important factor on the production of electrofuels is the production of hydrogen by electrolysis, which conventionally has a low efficiency [
51]. However, high temperature electrolysis, or steam electrolysis, is a novel process where the hydrogen and oxygen are generated at temperatures between 700 and 1000 °C [
89] with much higher efficiencies. The higher efficiencies are, in part, a result of the high temperature of the steam and because the heat required for these high temperatures comes from the subsequent F-T process itself [
51]
Another possibility of producing electrofuels is through the high-temperature co-electrolysis of CO
2 and H
2O [
90] using solid oxide electrolysis cells (SOECs) [
91]. These promising advanced electrochemical energy storage and conversion devices have high conversion efficiencies and convert directly CO
2 and water into syngas, leading directly to the production of F-T fuels, but are still in an early stage of development [
92].
11.8. Solar Fuels
Solar fuels are new concepts for renewable liquid fuel production. These fuels are produced from solar energy through direct or indirect techniques. They can be electrofuels, where the required electricity is generated from photovoltaic sources, or fuels generated from processes involving photochemical, thermochemical or biochemical (photosynthesis) involving solar energy [
29]. The solar energy in these processes are used for breaking the water and/or the CO
2 producing the H
2 and CO (syngas) required for the subsequent Fischer-Tropsch process. So, some of the earlier mentioned synthetic fuels produced using electricity or thermal energy can also be created using solar energy, through photovoltaic and/or thermal concentration. Enzymatic conversion of CO
2 can also be accomplished with the use of solar energy, leading to chemicals such as methane and CO [
93].
One process involves the reaction of CO
2 and H
2O at high temperatures (~1400 °C) in the presence of a cerium oxide catalyst, followed by hydrolysis at 800 °C, producing H
2 and CO [
94]. This process of producing liquid hydrocarbons through the processing of the syngas is already being explored commercially, although requires further optimisation involving the use of metal oxides in powerful solar concentrators [
29].
Photochemical and photoelectrochemical systems have the active light-absorbing materials directly integrated into the cathode and/or anode electrodes which are placed in contact with the electrolyte. The photosensitized electrodes convert light into an electric current that is then used to split the water into hydrogen and oxygen. The combination of photovoltaic and electrochemical processes is also a promising technology as it allows the separate optimization of both processes [
95].
Thermochemical processes have a lot of potential when using very high values of solar concentration [
29]. However, the integration of the process into the solar reactor originates significant thermal losses and various other difficulties for upscaling installations have hindered the viability of this approach, with efficiencies lower than 10% [
29], prior to considering its use in IC engines, where the efficiency barely reaches the 40% mark or in fuel cells (~60% efficiency).
12. Dimethylfuran (DMF)
Dimethylfuran (DMF—CH
3-C
4H
2-O-CH
3—
Table 3) is a biofuel with the potential to substitute petrol, as it has properties between petrol and ethanol. Its RON is even higher than for ethanol, but its knocking behaviour is slightly lower than ethanol, probably because its latent heat of vaporization is much lower (similar to gasoline), preventing the effective mixture cooling of the ethanol [
96]. Also, its laminar flame speed is lower than for ethanol, being even slightly lower than for petrol [
97]. Its boiling temperature is high (93 °C,
Table 3), which makes it a less volatile fuel and more practical for transport and storage, although it may be difficult to start a cold engine. Unlike ethanol and methanol, it is insoluble in water, reducing some of the alcohol’s storage problems.
DMF can be obtained from fructose, so it may be a biofuel produced through a chemical or biochemical route using a direct process using catalysts and its production consumes about one third of the energy required for the production of ethanol [
98], where its low latent heat of vaporization helps.
13. Nitromethane
Nitromethane (CH
3NO
2—
Table 3) is a fuel with oxygen and nitrogen beyond the usual carbon and hydrogen, known for its explosive behaviour and by the huge power improvement it can offer to powerful engines. As it has a high proportion of O and N (52.5% of oxygen and 75.4% of N + O), its heating value is low but, as it has a very low stoichiometric A/F (1.7,
Table 3), its mixture with air, in a volume base, carries much more energy than any other fuel, a massive 2.3 times the mixture of air-petrol (see
Table 3). The very low A/F requires large amounts of injected fuel (8.5 times more mass, compared to petrol) which, allied to the high latent heat of vaporization (almost twice of the petrol, see
Table 3), requires huge amounts of heat to vaporize.
Nitromethane is used mostly on the “top fuel” category of drag racing, where the consumption can be 25 L for a race of 300 m ran in 3.6 s and where the finishing speeds are in excess of 530 km/h. Strangely these 10,000 cv plus engines have no cooling other than the latent heat of the fuel. Some other applications are its use as an additive (~5%), usually of methanol-based fuels, such as those for aero models.
14. Acetylene
This fuel (C
2H
2) was occasionally used in IC engines, mostly during the world wars, as there were no oil-based fuels for the general public. It is produced from the reaction of calcium carbide and water:
This process was commonly used in the pit gasometers and on the front lamps of old cars and was occasionally produced in-board (in the trunk of the car) and supplied to the engine.
Acetylene RON is 40 (
Table 3), so it is unsuitable for today’s high compression ratio (CR) engines, but it has a fast flame propagation speed, which may reduce knock occurrence. One of the best properties of acetylene is its adiabatic temperature, one of the highest, that can exceed the 3000 °C when burned with straight oxygen, but this is not an advantage for an IC engine.
15. Ammonia
Ammonia (NH3) is an important substance used as a fertilizer throughout the world with a yearly production of over 150 million tons. More than half the world population rely on the enhanced crop production boosted by the nitrogen in the ammonia, but its production is very high energy intensive (uses about 2% of the total energy consumed in the world) and it produces approximately 1% of the CO2 emissions worldwide. In general, the production of one molecule of NH3 results in the emission of a molecule of CO2. In nature, there is the production of ammonia by the decomposition (rotting) of vegetable and animal wastes by bacteria. It can also be produced during the pyrolysis of coal, as a by-product of coke and coal gas production. In these cases, the ammonia appears as ammonium hydroxide, a liquid normally used as a cleaning agent usually known as “ammonia”. Ammonia is also used in absorption cycles refrigeration systems.
Anhydrous ammonia (without added water) may be a substitute for petrol in SI engines or even diesel engines and the main interest is to use it as an “energy carrier”, to substitute the electricity (or the hydrogen) as a means to transport energy from the place where is produced (wind farm or nuclear power station) to the location where will be used, such as to power vehicles. As there is no carbon in its molecule, it does not produce any CO2, CO nor HC. Comparing it to hydrogen (another energy carrier), one litre of liquid ammonia (@10 bar and 25 °C) has 30% more hydrogen than 1 L of liquid hydrogen (@-253 °C). Therefore, it makes much more sense to use ammonia as an energy carrier than hydrogen.
In terms of NOx production, the burning of ammonia will produce some thermally (Zeldovich mechanism) but its combustion also produces
NOx because its molecule has nitrogen atoms. However, ammonia has a relatively low adiabatic temperature, lower than usual hydrocarbons and much lower than hydrogen (
Table 3) reducing the potential for Zeldovich NOx production.
In terms of heating value, ammonia has a low value (18.6 MJ/kg,
Table 3), less than half of petrol and the comparison gets worse when done in volume, where it has slightly more than 1/3 of the energy of petrol. Also, as ammonia is a gas at atmospheric conditions, it requires a pressure tank, cylindrical or toroidal, similar to those for LPG, where only 80% of the volume can be filled, which reduces the vehicle range.
Other crucial properties of ammonia are the very high auto-ignition temperature (651 °C,
Table 3), a very high energy required for ignition (much higher than the required for petrol) and the very high latent heat of vaporization (2450 kJ/kg, compared to 380 for petrol), which introduces further difficulties on its use as an IC engine fuel. Other problems are the narrow limits for ignition (flammability, see
Table 3) and the reduced speed for flame propagation, five times slower than petrol [
99] and 30 times slower than hydrogen [
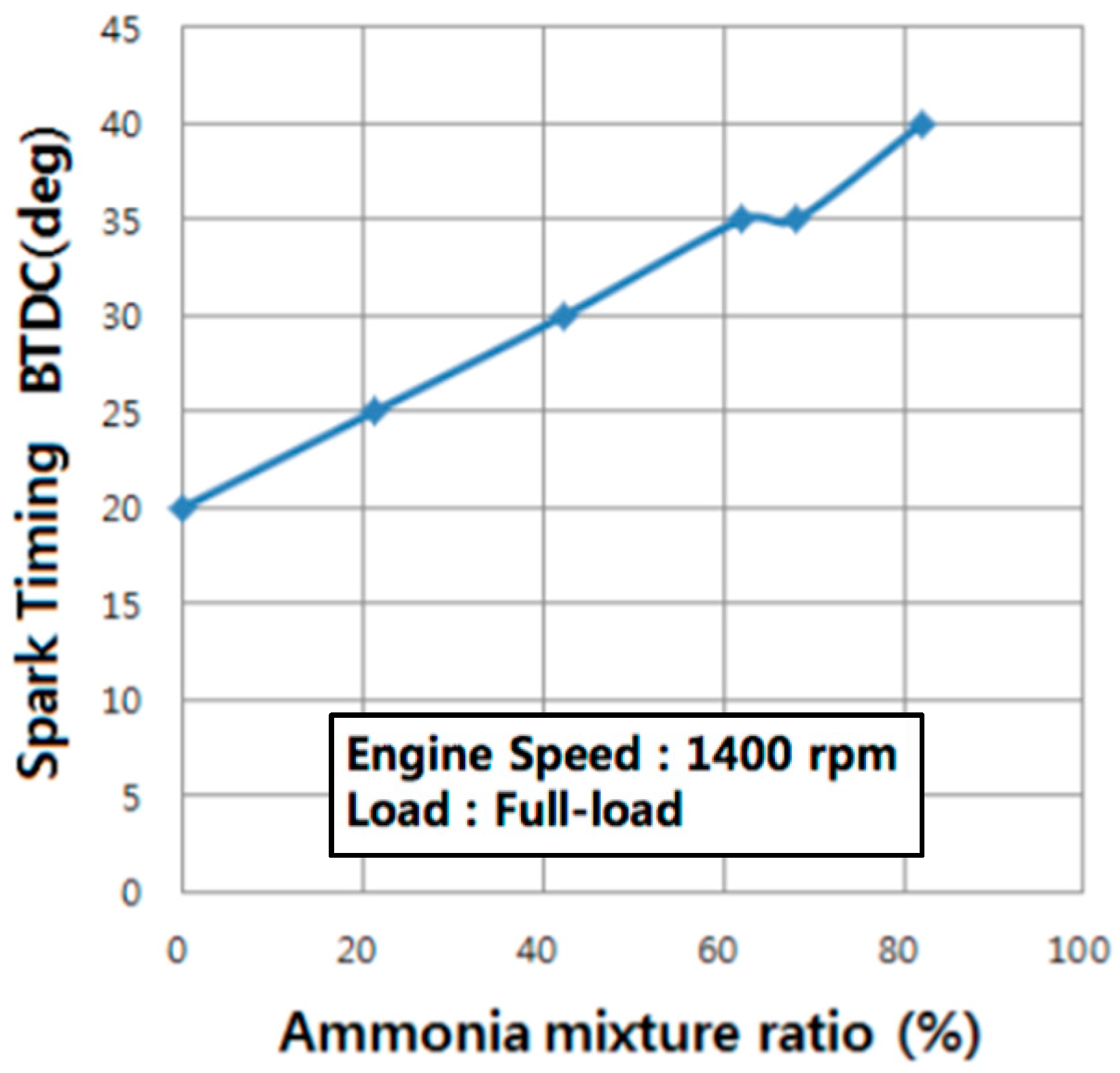
52]. Adding 4% of ammonia to petrol reduced its burning speed in 15% [
99]. As a result, the spark timing required for the burning of the petrol-ammonia mixture increases with the percentage of ammonia (
Figure 12, [
100]).
Thus, the combustion of straight ammonia in a SI engine is not easy but it is possible if measures are taken to ensure that the referred combustion deficiencies (very high ignition energy, flammability limits and slow combustion) are overcome, for example using multiple spark location and very high energy ignition and compact combustion chambers [
101]. Supercharging seems to be a good option [
102] and some researchers used plasma ignition with good results [
103]. As one of the major problems is its slow combustion, engine conditions of low charge and high speed can only be achieved using a combustion “promotor” such as hydrogen [
53], petrol or even diesel fuel, enabling a better ignibility and increased combustion velocity.
It can be burned in CI engines when mixed with diesel, but it would be advantageous to be mixed with biodiesel or DME as these fuels have higher cetane numbers (CN) [
104]. Ammonia causes irritation in small amounts and may be lethal in higher concentrations. However, its distinct and strong smell and the fact that is lighter than air, reduces its risks. Ammonia is largely used in the word, having specific production, storage and delivery installations and procedures, so it has been extensively tested throughout the world. Also, unlike petrol it is not carcinogenic, its combustion does not produce smoke and it is much less prone to explosions [
104].
At the moment, ammonia is produced from natural gas (70%) and from coal (30%) by the Haber-Bosch process [
105] where hydrogen and nitrogen react (3H
2 + N
2 → 2NH
3) in an iron oxide catalyst at temperatures ranging from 380 to 500 °C. Ammonia was produced from renewable energy (hydro) by water hydrolyses in the 40s, but high production costs and the aging of the facilities originate production to stop on the 80s [
106].
Synthetic gasoline (by the Fisher-Tropsch process) production requires 95.3 MJ/kg and its heating value is 42.5 MJ/kg, which means that it requires 2.25 times its energy content to be produced. In the case of ammonia (Haber-Bosch process plus H
2 production and N
2 separation), the production of 1 kg requires 43.2 MJ [
107] and its heating value is 18.6 MJ/kg. Therefore, it requires 2.3 times its energy content to be produced, value similar to the F-T petrol production.
In terms of bio-production, it is necessary to use 2.72 kg of corn to produce 1 kg of ethanol, whereas it is necessary to use 3 kg of the same cereal to produce 1 kg of ammonia, using processes involving gasification and synthesis [
108]. As ethanol has an energy density of 25 MJ/kg and ammonia only has 18.6 MJ/kg, in terms of energy, ammonia use is 1.5 worse than ethanol. And the production of ethanol from corn is a bad energetic efficiency when compared to the Brazilian production from sugar cane.
For ammonia to be used as an energy carrier, we know that its production through the Haber-Bosch process (3H
2 + N
2 → 2NH
3 @ 500 °C and 300 bar) requires 43 MJ/kg [
107], already including the production of hydrogen and separation of nitrogen from the air. If ammonia if burned in an IC engine with an efficiency of 40%, then the overall electricity-to-electricity efficiency will be 16%. The same study for liquid hydrogen (see in the hydrogen section) showed this efficiency to be 19.5%.
These examples show that the production and use of ammonia is not, as yet, energetically nor economically viable and it will require the development of more efficient processes. An off-shore wind project for the production of ammonia for use in vessels (Zero Emission Energy Distribution at Sea—ZEEDS [
109]) plans water hydrolysis and nitrogen separation from air using the generated wind electricity. It is estimated that this ammonia would be three times more expensive than 3.5% sulphur heavy oil. Another interesting use of ammonia is the direct use in fuel cells [
110]), that generally require pure hydrogen.
16. Turpentine
Spirit of turpentine or just turpentine is the resulting fluid from the distillation of pine resin. Traditionally it was used as a solvent (as in dry cleaners) and different trees produce slightly different compositions of the turpentine. Turpentine can also be produced straight from the wood through what is called destructive distillation, a kind of pyrolysis.
Although world annual pine resin production is low at less than a thousand tons [
111], there is the development of genetically improved trees to produce high yields of resin [
112,
113]. However, only the small fraction of 20% of the resin may be transformed into turpentine [
114]. Another source of turpentine is the black liquor, a by-product of the pulp and paper industry.
Turpentine (C
10H
16) has been used in IC engines from 1824, when Samuel Morey [
115] patented an atmospheric engine using it. But the better-known use of turpentine has been in the post-WWII by Soichiro Honda (founder of the Honda Motor Co), who produced motorcycles with small army-surplus engines (
Figure 13) which run on turpentine, that he would sell himself after 1946, because of the lack of petrol in post-war Japan.
Turpentine (see
Table 3) has a heating value higher than petrol or diesel and, as its density is also higher, its energy density (in volume or mass) is higher than the conventional fuels. Also, as its stoichiometric A/F (14.2) is lower than petrol, in fact the mixture air-turpentine has more energy than air-petrol and theoretically should produce higher torque and power from the same engine [
116]. But its RON is lower than petrol, so the engines require less ignition advance when turpentine is added to petrol.
Turpentine can also be added to diesel fuel, but its low cetane number (20 to 25) tends to reduce the engine efficiency [
117], although some authors [
118] report a 1–2% efficiency improvement when the mixtures are below 40%. It can be used in dual-fuel mode by fumigation, enabling the substitution of up to 75% of the diesel fuel, with noticeable reductions of smoke production [
119].
17. Glycerine (or Glycerol)
Before the intense production of biodiesel, the glycerin was a valuable substance for skin creams, lip sticks and as a food additive. However, the huge quantities of biodiesel produced worldwide generated large surpluses of glycerin, with its value plummeting, as there is no market for it. For each part of generated biodiesel, the transesterification process produces 10% of glycerin.
Although glycerin (C
3H
8O
3) may be burned, its atmospheric combustion (at temperatures lower than 300 °C) may produce toxic compounds such as the aldehyde acrolein. Acrolein is produced by the dehydration of glycerol and it is the black and sticky substance produced during the exposure at high temperature of vegetable oils, such as the deposits on the frying pans and responsible for their acrid smell. It has been associated to lung cancer [
120], so its emission should be avoided.
As a fuel, glycerin is a very difficult substance to burn in an engine. It solidifies at 18 °C, so it has a high viscosity and has to be injected hot (~100 °C) to enable sufficient atomization. Its auto-ignition temperature is 390 °C, so it is too high for straight use in compression ignition engines. Some researchers mixed it with diesel up to 20% [
121], but the intake air needed to be heated up to 100 °C to sustain stable combustion. The power was slightly reduced, and the efficiency was slightly increased, whereas NOx and PM production were reduced, mostly at high power. One of the reported problems was the difficulty to produce and maintain stable mixtures of hydrocarbons and glycerol.
However, at least one company has achieved the combustion of straight glycerin in a diesel engine (Aquafuel Research Ltd., Smarden, Kent, UK). The idea is to increase the intake temperature [
122] up to a level that almost any fuel (even petrol) would burn in the diesel engine. So, the intake air should be heated (~200 °C) as well as the fuel (~100 °C), so the glycerin burns cleanly and efficiently. Formula E racing uses electric cars which batteries that have to be charged in the circuit garages. This company developed the electric generators to be used by the different teams to charge the car batteries. These generators are modified diesel engines (Cummins KTA50, 50 L, V16, turbocharged, capable of over 1 MW) that work on glycerol, because it is a clean biofuel. Each generator can produce 850 kWe, enough for charging 40 car batteries in 50 min. These generators are also used by biodiesel producers, enabling them to use the by-product glycerin to produce electricity in their plants. The engines have a drop in power because the intake air density is reduced by the increased temperature. It seems that the high intake temperature is attained by reducing the heat removed in the inter-cooler after the turbo-charger. The emissions NOx and PM were reported as “virtually eliminated” [
122], but these statements were reported in the company’s site.
A paper reporting on the same company [
123] refers 90 °C as the minimum intake temperature to enable stable combustion in a CI engine with glycerin, and 100 °C for petrol RON98. The diesel engine needs to be started on diesel and only can be run on glycerin after warm-up and needs to run again on diesel before being switched-off, to purge the injection system. In terms of laminar flame speed, glycerol is similar to petrol [
124].
18. Fage
There are different processes to transform glycerin into usable fuels such as its reaction with dimethyl sulphate and or methanol (producing glycerol dimethoxy ether—[
125]), etherification acetylification or anaerobic fermentation [
126]. However, these processes are slow and economic unviable. But it is possible to produce FAGE (fatty acid glycerol formal ester) from a reaction between glycerine and other fats (vegetable or animal). FAGE has an LHV lower than biodiesel but is very dense (
Table 3), so its energy density (by volume) is similar to biodiesel. But its boiling temperature is almost 300 °C and solidifies at 14 °C, which makes it very viscous.
There is another way of transforming fats without the production of glycerol [
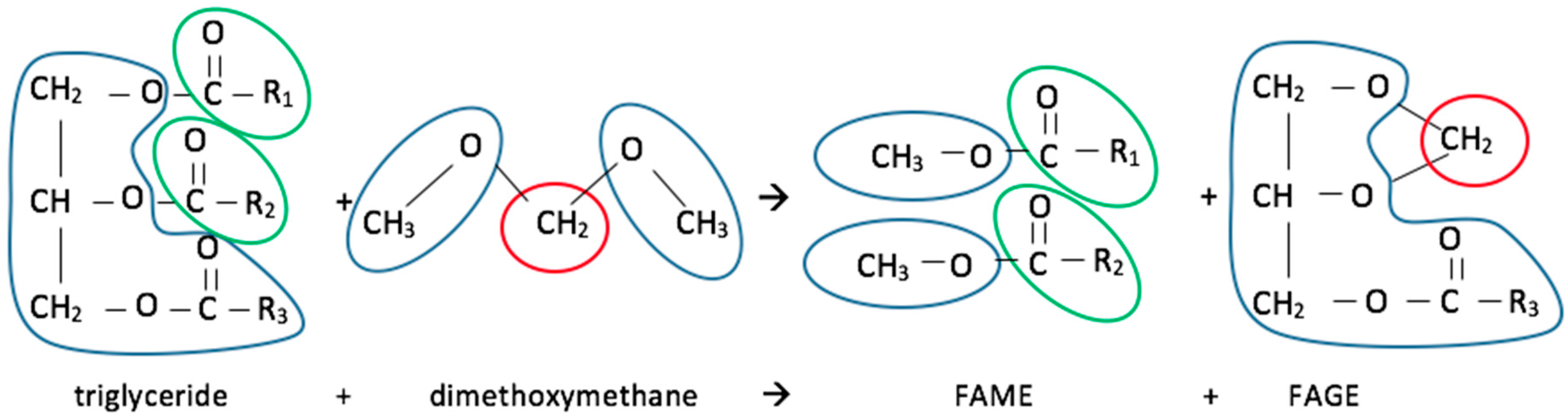
126]. Instead of methanol, dimethoxymethane (DMM) is mixed with fat in a combined transesterification-trans-ketalization process producing fatty acid methyl ester (FAME) common biodiesel and FAGE. In overall, the process can be shown as
Figure 14).
19. Conclusions
In a time where the future use of internal combustion engines and/or fossil fuels for road transport is being put into question by many policy makers all over the world, this paper presents an overview of various solutions of alternative fuels that may be used to fuel car engines of the future in a sustainable way.
Some alternatives to the combination internal combustion engine—fossil fuels to propel vehicles exist, such as battery electric cars, fuel cell hybrid vehicles or just conventional vehicles fuelled with renewable and/or biofuels. The latter alternative seems to be particularly attractive, as liquid fuels have very high energy density and they are used in devices (IC engines) that have been developed for over a century. With this in mind, the authors discussed the various propositions for alternatives to fossil fuels. It seems that future liquid fuels will still be burned in IC engines, but the vehicles will be electrically assisted (hybrids) and the exhaust emissions, CO2 emissions and fuel consumption will be lower than today’s fossil fuels.
The various properties, applications and production processes of the various alternative fuels were presented and discussed, from the more conventional alcohols and biodiesel to the more unusual ammonia or turpentine. New concepts such as electrofuels and solar fuels were introduced and discussed. These will be very important in the future, as the land used for the production of biofuels will be limited and restrained. These renewable fuels use significantly less resources in terms of land and water than conventional and second-generation biofuels, but they still have a huge problem of energy efficiency, requiring much more energy for its production than their energy content. These fuels may also be known as “energy carriers” a concept firstly used for hydrogen, whereas they “transport” the energy from where it is produced to where it is used. However, for the most part this is still done with extremely low energy efficiencies.
Some of these fuels are readily available and usable in IC engines with little or no modifications, but others require significant engine modifications and/or adaptations. However, it is possible to burn (in IC engines) unexpected fuels such as ammonia or glycerin, which are currently used in large diesel generators to charge the batteries of Formula E cars. Oxygenated fuels, for instance, are able to retain low particulate matter emissions due to the lack of carbon-carbon bonds.
While synthetic fuels may more easily improve pollutant emissions to the degree that they can be custom manufactured, their greenhouse gas footprint will mostly depend on the sustainability level of its raw materials (fossil/renewable), the energy source and from which they are developed and the energy efficiency of the process.