Thermogravimetric Kinetics of Selected Energy Crops Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experiment Procedure
2.3. Analytical Methods
3. Results and Discussion
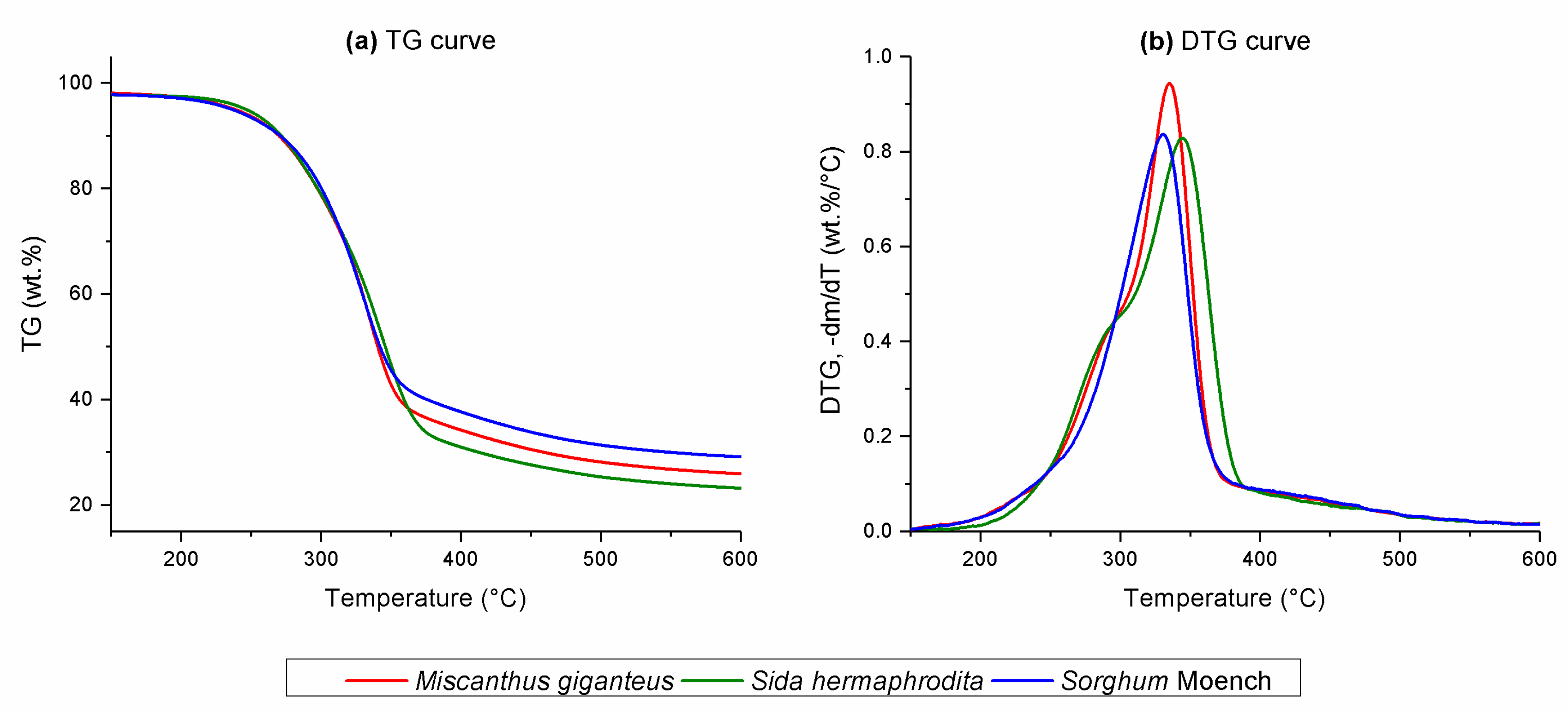
3.1. TG Results
3.2. Kinetic Analysis
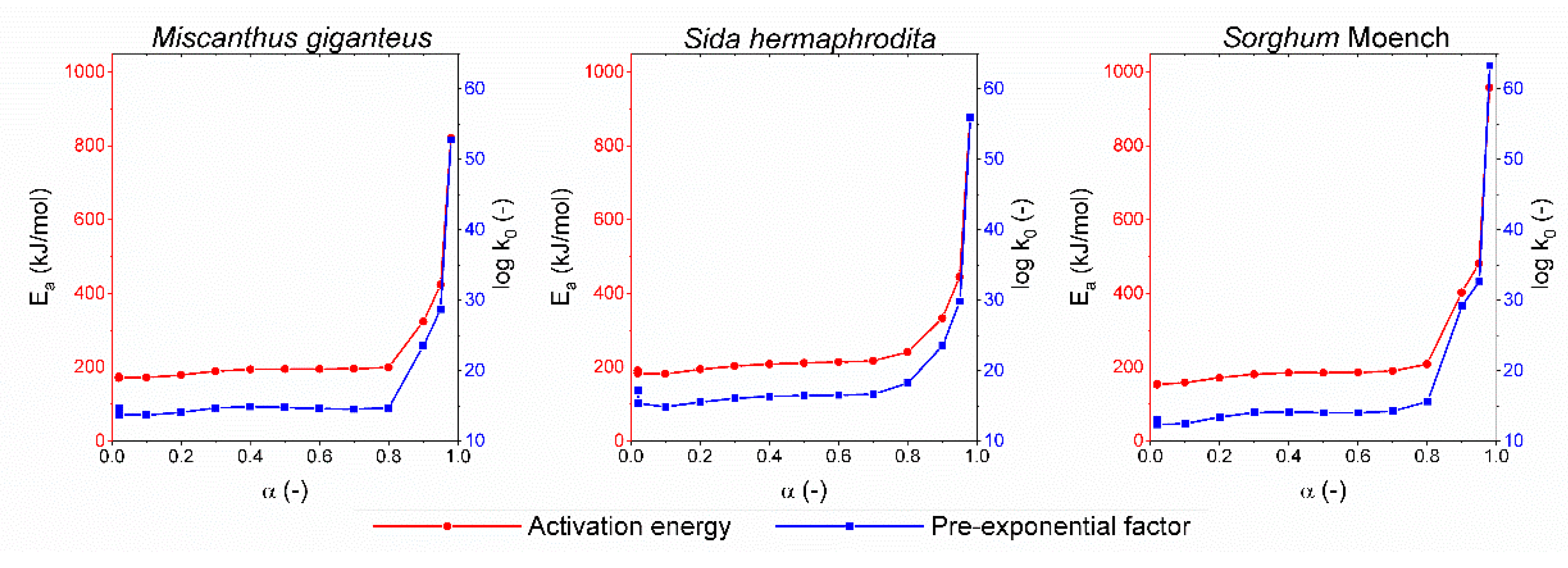
3.2.1. Isoconversional Kinetic Method
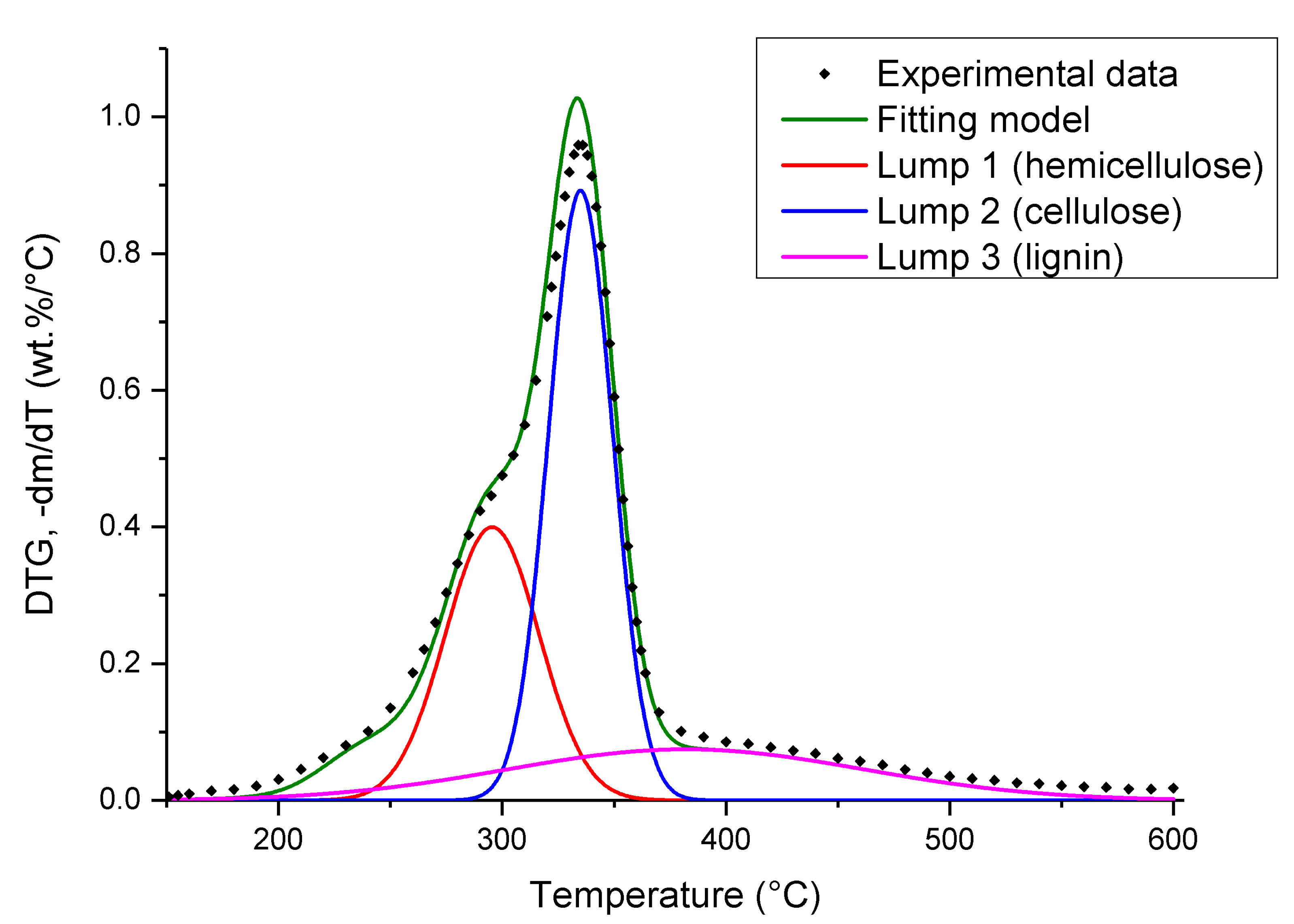
3.2.2. Nonlinear Regression
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bilgili, F.; Koçak, E.; Bulut, Ü.; Kuşkaya, S. Can biomass energy be an efficient policy tool for sustainable development? Renew. Sustain. Energy Rev. 2017, 71, 830–845. [Google Scholar] [CrossRef]
- Piwowar, A.; Dzikuć, M. Outline of the economic and technical problems associated with the co-combustion of biomass in Poland. Renew. Sustain. Energy Rev. 2016, 54, 415–420. [Google Scholar] [CrossRef]
- Mann, M.; Spath, P. A life cycle assessment of biomass cofiring in a coal-fired power plant. Clean Technol. Environ. Policy 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Mofijur, M.; Rasul, M.G.; Mahlia, T.; Rahman, S.A. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Collura, S.; Azambre, B.; Weber, J.W. Kinetic modelling of the pyrolysis of Miscanthus × Giganteus from the thermogravimetric analysis of its fractionated components. Environ. Chem. Lett. 2005, 3, 95–99. [Google Scholar] [CrossRef]
- Cortes, A.M.; Bridgwater, T. Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process. Technol. 2015, 138, 184–193. [Google Scholar] [CrossRef]
- Hu, S.-W.; Wu, L.-M.; Persson, S.; Peng, L.; Feng, S. Sweet sorghum and Miscanthus: Two potential dedicated bioenergy crops in China. J. Integr. Agric. 2017, 16, 1236–1243. [Google Scholar] [CrossRef]
- Nahm, M.; Morhart, C. Virginia mallow (Sida hermaphrodita (L.) Rusby) as perennial multipurpose crop: Biomass yields, energetic valorization, utilization potentials, and management perspectives. GCB Bioenergy 2018, 10, 393–404. [Google Scholar] [CrossRef]
- Kacprzak, A.; Michalska, K.; Romanowska-Duda, Z.; Grzesik, M. Energy crops as a valuable material for biogas production. Kosmos. Probl. Nauk. Biologicznych 2012, 61, 281–293. [Google Scholar]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Slezak, R.; Krzystek, L.; Ledakowicz, S. Thermogravimetric analysis coupled with mass spectrometry of spent mushroom substrate and its fractions. J. Anal. Appl. Pyrolysis 2018, 133, 1–8. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H.; Kirtay, E. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Nicodème, T.; Berchem, T.; Jacquet, N.; Richel, A. Thermochemical conversion of sugar industry by-products to biofuels. Renew. Sustain. Energy Rev. 2018, 88, 151–159. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.; Bridgwater, T. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Carvalho, W.; Oliveira, T.J.; Cardoso, C.; Ataíde, C.H. Thermogravimetric analysis and analytical pyrolysis of a variety of lignocellulosic sorghum. Chem. Eng. Res. Des. 2015, 95, 337–345. [Google Scholar] [CrossRef]
- Hu, M.; Chen, Z.; Wang, S.; Guo, D.; Ma, C.; Zhou, Y.; Chen, J.; Laghari, M.; Fazal, S.; Xiao, B.; et al. Thermogravimetric kinetics of lignocellulosic biomass slow pyrolysis using distributed activation energy model, Fraser–Suzuki deconvolution, and iso-conversional method. Energy Convers. Manag. 2016, 118, 1–11. [Google Scholar] [CrossRef]
- Nowicki, L.; Ledakowicz, S. Comprehensive characterization of thermal decomposition of sewage sludge by TG–MS. J. Anal. Appl. Pyrolysis 2014, 110, 220–228. [Google Scholar] [CrossRef]
- Özveren, U.; Özdoğan, Z.S. Investigation of the slow pyrolysis kinetics of olive oil pomace using thermo-gravimetric analysis coupled with mass spectrometry. Biomass Bioenergy 2013, 58, 168–179. [Google Scholar] [CrossRef]
- Ashraf, A.; Sattar, H.; Munir, S. A comparative applicability study of model-fitting and model-free kinetic analysis approaches to non-isothermal pyrolysis of coal and agricultural residues. Fuel 2019, 240, 326–333. [Google Scholar] [CrossRef]
- Ali, I.; Bahaitham, H.; Naebulharam, R.; Naibulharam, R. A comprehensive kinetics study of coconut shell waste pyrolysis. Bioresour. Technol. 2017, 235, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; McGaughy, K.; Hasan, R.; Reza, M.T. Pyrolysis and carbon dioxide gasification kinetics of hydrochar produced from cow manure. Environ. Prog. Sustain. Energy 2018, 38, 154–162. [Google Scholar] [CrossRef]
- Lu, C.; Song, W.; Lin, W. Kinetics of biomass catalytic pyrolysis. Biotechnol. Adv. 2009, 27, 583–587. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Qin, G.; Chen, M.; Wang, J. Comparative study on pyrolysis characteristics and kinetics of lignocellulosic biomass and seaweed. J. Therm. Anal. Calorim. 2018, 132, 1317–1323. [Google Scholar] [CrossRef]
- Rueda-Ordóñez, Y.; Tannous, K.; Olivares-Gómez, E. An empirical model to obtain the kinetic parameters of lignocellulosic biomass pyrolysis in an independent parallel reactions scheme. Fuel Process. Technol. 2015, 140, 222–230. [Google Scholar] [CrossRef]
- Chen, T.; Li, L.; Zhao, R.; Wu, J. Pyrolysis kinetic analysis of the three pseudocomponents of biomass–cellulose, hemicellulose and lignin. J. Therm. Anal. Calorim. 2016, 128, 1825–1832. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Liu, H.; Imran, M.; Snape, C.E. Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: A way forward to renewable energy sources. Energy Convers. Manag. 2020, 203, 112266. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gökalp, I. Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Pazara, E.; Panopoulos, K. Kinetic modeling of five sustainable energy crops as potential sources of bioenergy. Energy Sour. Part A Recover. Util. Environ. Eff. 2016, 38, 1812–1818. [Google Scholar] [CrossRef]
- Jeguirim, M.; Dorge, S.; Loth, A.; Trouvé, G. Devolatilization Kinetics of Miscanthus Straw from Thermogravimetric Analysis. Int. J. Green Energy 2010, 7, 164–173. [Google Scholar] [CrossRef]
- Chen, D.; Shuang, E.; Liu, L. Analysis of pyrolysis characteristics and kinetics of sweet sorghum bagasse and cotton stalk. J. Therm. Anal. Calorim. 2017, 131, 1899–1909. [Google Scholar] [CrossRef]
- Cardoso, C.; Miranda, M.; Santos, K.; Ataíde, C. Determination of kinetic parameters and analytical pyrolysis of tobacco waste and sorghum bagasse. J. Anal. Appl. Pyrolysis 2011, 92, 392–400. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass—Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z. Pyrolysis of Biomass; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Szwaja, S.; Magdziarz, A.; Zajemska, M.; Poskart, A. A torrefaction of Sida hermaphrodita to improve fuel properties. Advanced analysis of torrefied products. Renew. Energy 2019, 141, 894–902. [Google Scholar] [CrossRef]
- Michalska, K.; Ledakowicz, S. Alkali pre-treatment of Sorghum Moench for biogas production. Chem. Pap. 2013, 67, 1130–1137. [Google Scholar] [CrossRef]
- Michalska, K. Investigation of Hydrolysis and Methane Fermentation Process of Energy Crops. Ph.D. Thesis, Lodz Univeristy of Technology, Lodz, Poland, 28 November 2014. [Google Scholar]
- Stolarek, P.; Ledakowicz, S.; Ślęzak, R. Influence of Liming on Kinetics of Sewage Sludge Pyrolysis. Ecol. Chem. Eng. S 2019, 26, 175–188. [Google Scholar] [CrossRef]
- Renneboog, R. Activation Energy; Salem Press Encyclopedia of Science: Hackensack, NJ, USA, 2018; p. 5. [Google Scholar]
- Vyazovkin, S.; Lesnikovich, A. An approach to the solution of the inverse kinetic problem in the case of complex processes. Thermochim. Acta 1990, 165, 273–280. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, J.W.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevantagricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Silva, M.L.S.; López-González, D.; Villasenor, J.; Sanchez, P.; Valverde, J.L. Thermogravimetric–mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Ru, B.; Dai, G.; Wang, X.; Xiao, G.; Luo, Z. Kinetic modeling of biomass components pyrolysis using a sequential and coupling method. Fuel 2016, 185, 763–771. [Google Scholar] [CrossRef]
- Kreutter, W.; Liu, Z.; McNamara, P.J.; Singer, S. Kinetic Analysis of Dried Biosolid Pyrolysis. Energy Fuels 2019, 33, 8766–8776. [Google Scholar] [CrossRef]
- Aboyade, A.; Carrier, M.; Meyer, E.L.; Knoetze, J.H.; Görgens, J. Model fitting kinetic analysis and characterisation of the devolatilization of coal blends with corn and sugarcane residues. Thermochim. Acta 2012, 530, 95–106. [Google Scholar] [CrossRef]


| Biomass Characteristics | Substrate | ||
|---|---|---|---|
| Miscanthus giganteus | Sida hermaphrodita | Sorghum Moench | |
| Proximate analysis (wt%, dry basis) | |||
| Moisture | 1.8 ± 0.02 | 2.2 ± 0.04 | 2.2 ± 0.04 |
| Volatiles | 75.4 ± 0.72 | 78.4 ± 0.68 | 72.0 ± 0.57 |
| Fixed carbon | 19.4 ± 0.19 | 17.2 ± 0.26 | 19.0 ± 0.17 |
| Ash | 3.4 ± 0.06 | 2.2 ± 0.04 | 6.8 ± 0.13 |
| Ultimate analysis (wt%, dry basis) | |||
| C | 44.3 ± 0.12 | 43.8 ± 0.57 | 42.5 ± 0.14 |
| H | 5.9 ± 0.05 | 5.6 ± 0.13 | 5.6 ± 0.07 |
| N | 0.7 ± 0.02 | 0.4 ± 0.01 | 1.1 ± 0.02 |
| O 1 | 45.7 | 48.0 | 44.0 |
| S | 0 | 0 | 0 |
| HHV (MJ/kg dry basis) | 18.1 | 17.8 | 17.8 |
| Energy Crop | Miscanthus giganteus | Sida hermaphrodita | Sorghum Moench | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lump number | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Position (°C) | 296 | 335 | 382 | 297 | 343 | 384 | 288 | 330 | 386 |
| Amplitude (wt%/°C) | 0.396 | 0.88 | 0.076 | 0.32 | 0.712 | 0.064 | 0.276 | 0.072 | 0.096 |
| Full width at half maximum (°C) | 49.5 | 33.7 | 188 | 48.9 | 35.8 | 138.5 | 38 | 42.3 | 182.5 |
| Area (wt%) | 29.6 | 44.9 | 21.0 | 26.3 | 42.8 | 14.7 | 17.8 | 53.1 | 27.9 |
| Composition 1 (wt%) | 30.1 | 44.9 | 21.1 | 26.2 | 42.3 | 14.2 | 17.9 | 53.1 | 27.9 |
| R2 (-) | 0.996 | 0.988 | 0.990 | ||||||
| Lumps | Miscanthus giganteus | Sida hermaphrodita | Sorghum Moench | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| log k0 (-) | 7.02 | 14.61 | 12.12 | 5.99 | 14.8 | 15.76 | 4.73 | 14.48 | 15.36 |
| Ea (kJ/mol) | 92.9 | 190.1 | 170 | 97.7 | 192.5 | 171.9 | 93.2 | 190.1 | 175.2 |
| Reaction order (n) (-) | 3.99 | 1.38 | 3.99 | 3.97 | 1.93 | 3.97 | 3.35 | 1.66 | 3.97 |
| Lump fraction (wt fraction) | 0.31 | 0.47 | 0.22 | 0.32 | 0.51 | 0.17 | 0.18 | 0.54 | 0.28 |
| R2 (-) | 0.999 | 0.998 | 0.986 | ||||||
| Substrate | Kinetic Model | Ea (kJ/mol) | ||
|---|---|---|---|---|
| Hemicellulose | Cellulose | Lignin | ||
| Miscanthus giganteus | Nonlinear regression | 92.9 | 190.1 | 170.0 |
| Sida hermaphrodita | 97.7 | 192.5 | 171.9 | |
| Sorghum Moench | 93.2 | 190.1 | 175.2 | |
| Pine wood [19] | Distributed activation-energy model (DAEM) | 152.4 | 191.3 | 210.4 |
| Rice husk [19] | 159.3 | 179.3 | 199.9 | |
| Bamboo [19] | 167.1 | 188.4 | 203.6 | |
| Coconut shell waste [23] | Kissinger’s method (for hemicellulose and cellulose) and combined kinetics (for lignin) | 106.4 | 108.6 | 79.1–226.5 |
| Sugarcane straw [27] | Independent parallel-reaction scheme | 142.0 | 195.0212.0 | 40.0 |
| Pure component [28] | Sinusoidally modulated temperature method | 162.8 | 112.6 | 156.8 |
| Pure component [29] | Combined kinetics | 95.4 | 199.7 | 174.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusiak, M.; Ślęzak, R.; Ledakowicz, S. Thermogravimetric Kinetics of Selected Energy Crops Pyrolysis. Energies 2020, 13, 3977. https://doi.org/10.3390/en13153977
Matusiak M, Ślęzak R, Ledakowicz S. Thermogravimetric Kinetics of Selected Energy Crops Pyrolysis. Energies. 2020; 13(15):3977. https://doi.org/10.3390/en13153977
Chicago/Turabian StyleMatusiak, Magdalena, Radosław Ślęzak, and Stanisław Ledakowicz. 2020. "Thermogravimetric Kinetics of Selected Energy Crops Pyrolysis" Energies 13, no. 15: 3977. https://doi.org/10.3390/en13153977
APA StyleMatusiak, M., Ślęzak, R., & Ledakowicz, S. (2020). Thermogravimetric Kinetics of Selected Energy Crops Pyrolysis. Energies, 13(15), 3977. https://doi.org/10.3390/en13153977






