Dissolution Process Observation of Methane Bubbles in the Deep Ocean Simulator Facility
Abstract
1. Introduction
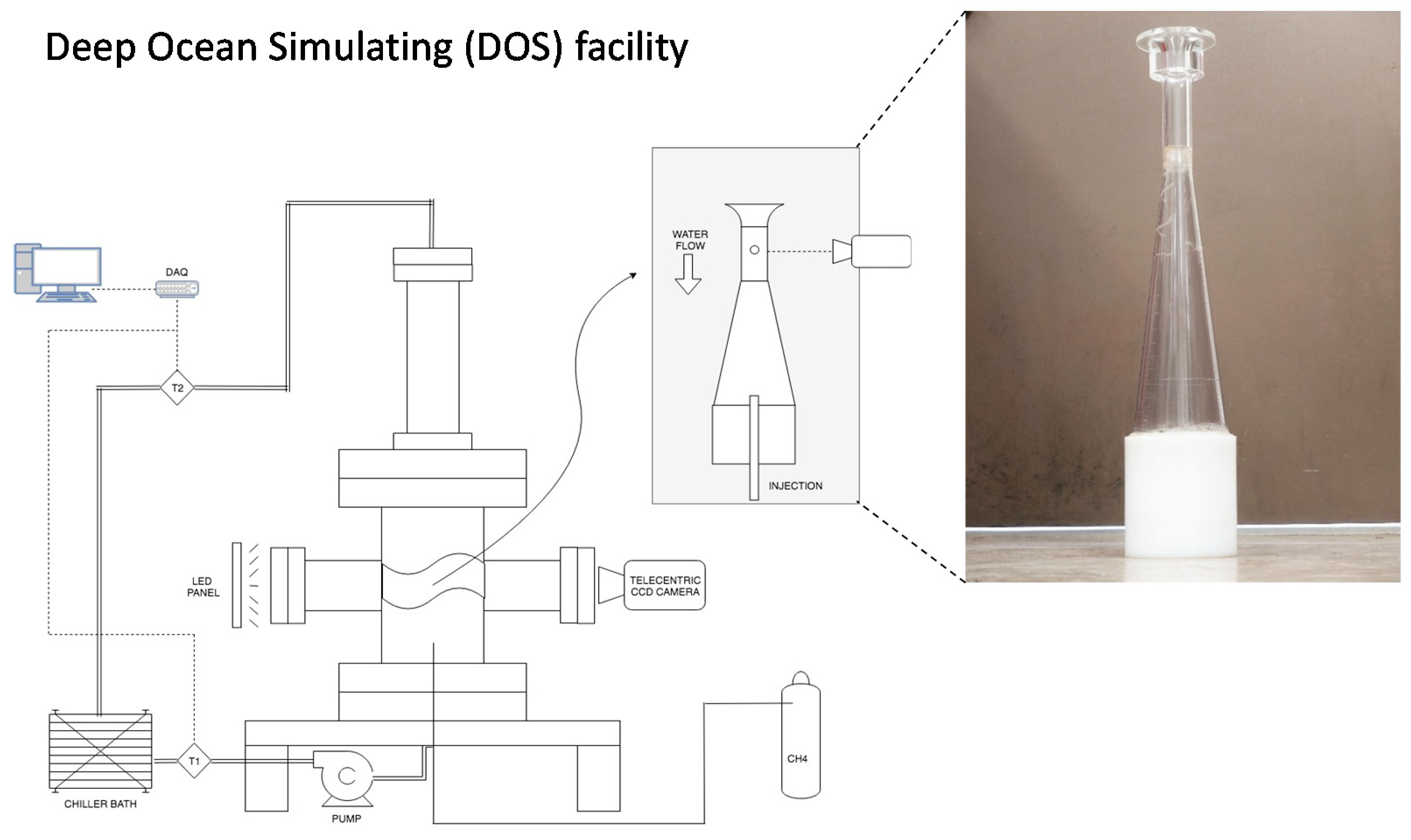
2. Materials and Methods
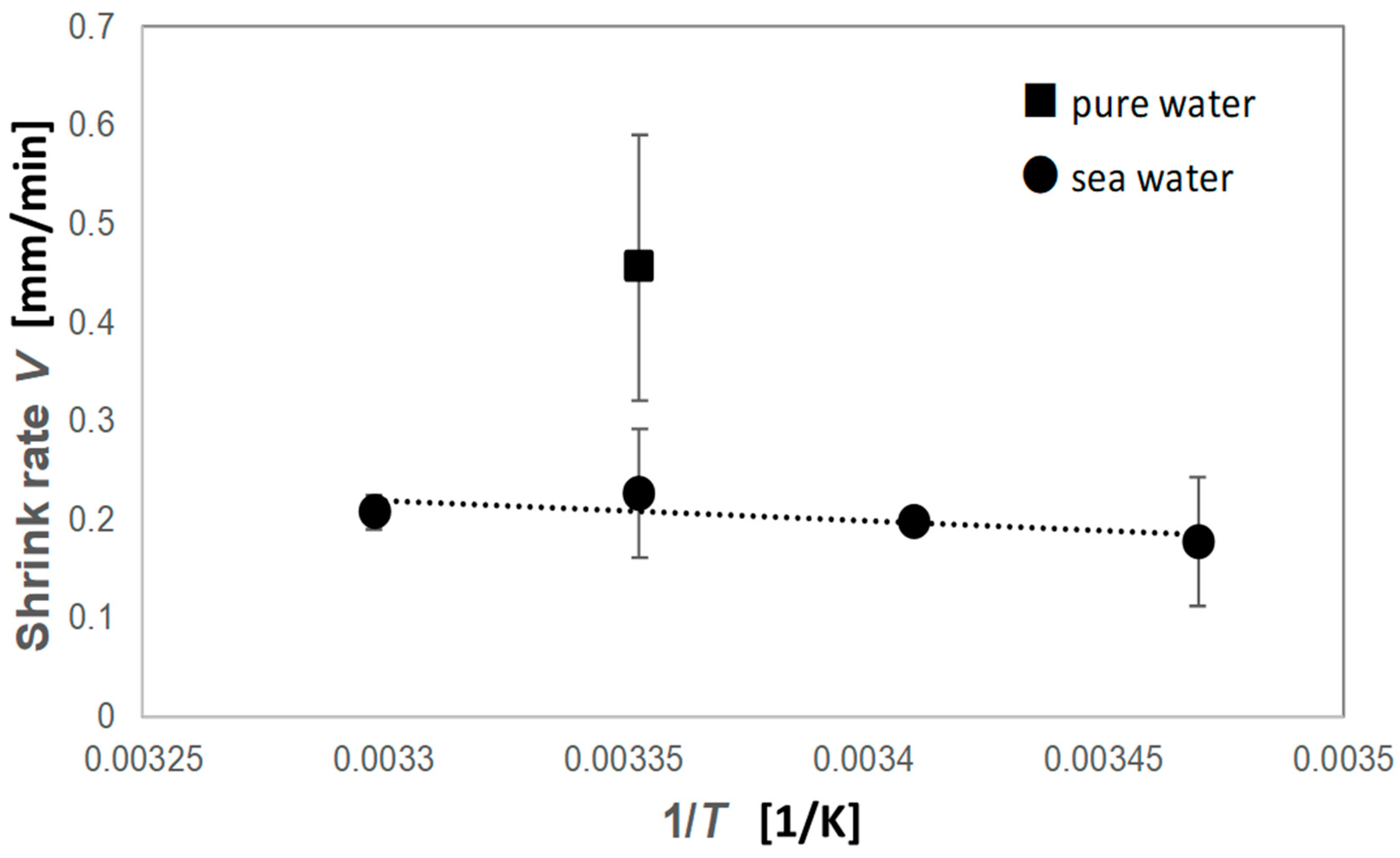
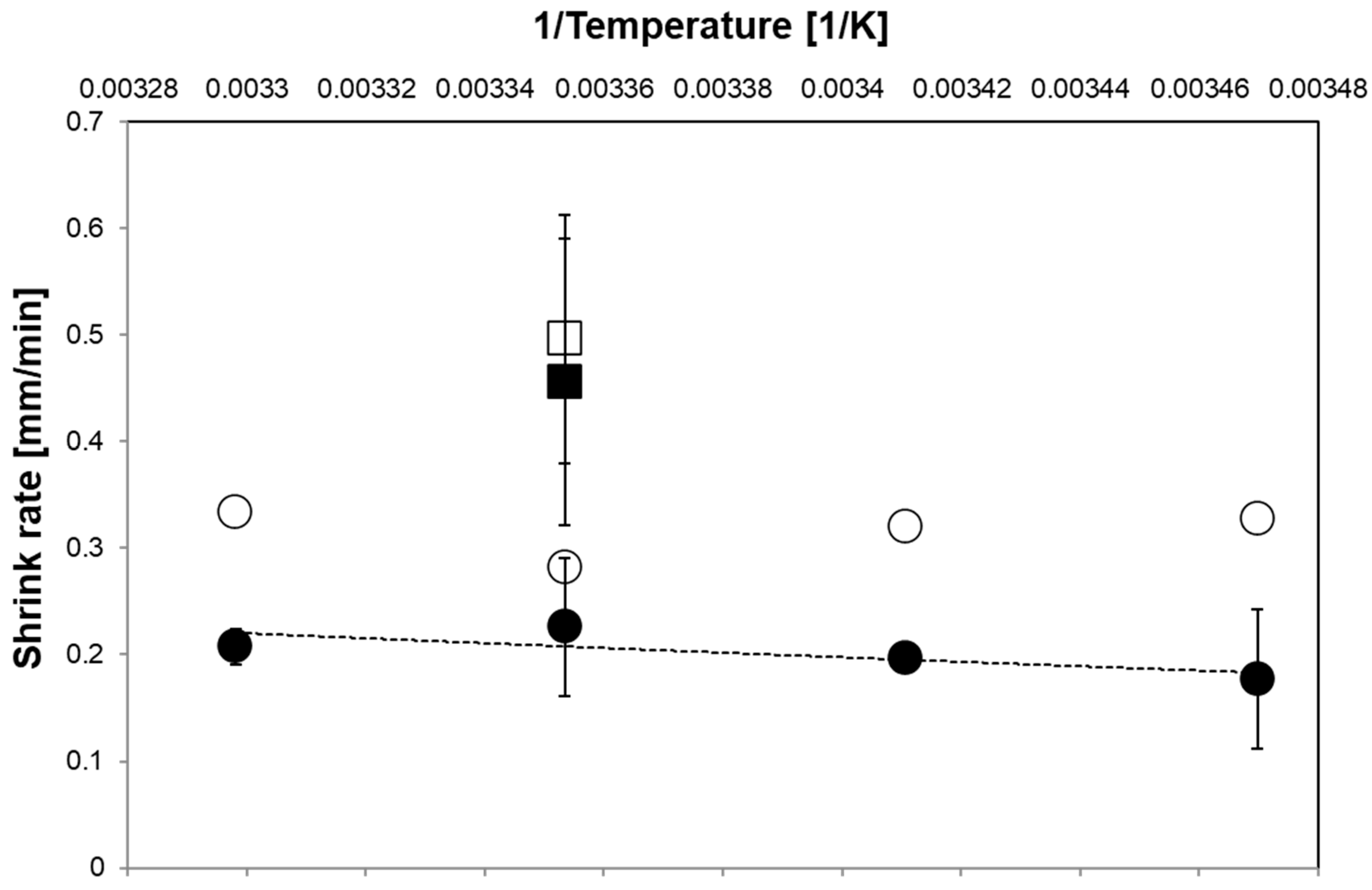
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kvenvolden, K. Methane hydrate–a major reservoir of carbon in the shallow geosphere? Chem. Geol. 1998, 71, 41–51. [Google Scholar] [CrossRef]
- Masuda, Y.; Uchida, T.; Nagakubo, S.; Satoh, M. Methane Hydrates. In Fossil Fuels World Scientific Series in Current Energy Issues; Crawley, G.M., Ed.; World Scientific Pub. Co. Pte. Ltd.: Singapore, 2016; Volume 1, Chapter 10; pp. 289–327. [Google Scholar]
- Argentino, C.; Conti, S.; Crutchley, G.J.; Fioroni, C.; Fontana, D.; Johnson, J.E. Methane-derived authigenic carbonates on accretionary ridges: Miocene case studies in the northern Apennines (Italy) compared with modern submarine counterparts. Mar. Pet. Geol. 2019, 102, 860–872. [Google Scholar] [CrossRef]
- Dickens, G.; O’Neil, J.; Rea, D.; Owen, R. Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the Paleocene. Paleoceanography 1995, 10, 965–971. [Google Scholar] [CrossRef]
- Kvenvolden, K. Methane hydrates and global climate. Glob. Biogeochem. Cycles 1988, 2, 221–229. [Google Scholar] [CrossRef]
- Sultan, N.; Cochonat, P.; Foucher, J.P.; Mienert, J. Effect of gas hydrates melting on seafloor slope instability. Mar. Geol. 2004, 213, 379–401. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 8 April 2020).
- Schmidt, G.A.; Shindell, D.T. Atmospheric composition, radiative forcing, and climate change as a consequence of a massive methane release from gas hydrates. Paleoceanography 2003, 18, 1004. [Google Scholar] [CrossRef]
- Carozza, D.A.; Mysak, L.A.; Schmidt, G.A. Methane and environmental change during the Paleocene-Eocene thermal maximum (PETM): Modeling the PETM onset as a two-stage event. Geophys. Res. Lett. 2011, 38, L05702. [Google Scholar] [CrossRef]
- Socolofsky, S.A.; Adams, E.E.; Sherwood, C.R. Formation dynamics of subsurface hydrocarbon intrusions following the Deepwater Horizon blowout. Geophys. Res. Lett. 2011, 38, L09602. [Google Scholar] [CrossRef]
- Maini, B.B.; Bishnoi, P.R. Experimental investigation of hydrate formation behavior of a natural gas bubble in a simulated deep sea environment. Chem. Eng. Sci. 1981, 36, 183–189. [Google Scholar] [CrossRef]
- Masutani, S.M.; Adams, E.E. Experimental Study of Multi-Phase Plumes with Application to Deep Ocean Oil Spills; Final Report, Contract 1435-01-98-CT-30964; U.S. Department of the Interior Minerals Management Service: Herndon, VA, USA, 2000. [Google Scholar]
- Bigalke, N.K.; Rehder, G.; Gust, G. Methane hydrate dissolution rates in undersaturated seawater under controlled hydrodynamic forcing. Mar. Chem. 2009, 115, 226–234. [Google Scholar] [CrossRef]
- Bigalke, N.K.; Enstad, L.I.; Rehder, G.; Alendal, G. Terminal velocities of pure and hydrate coated CO2 droplets and CH4 bubbles rising in a simulated oceanic environment. Deep Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 1102–1110. [Google Scholar] [CrossRef]
- Warzinski, R.P.; Lynn, R.; Haljasmaa, I.; Leifer, I.; Shaffer, F.; Anderson, B.J.; Levine, J.S. Dynamic morphology of gas hydrate on a methane bubble in water: Observations and new insights for hydrate film models. Geophys. Res. Lett. 2014, 41, 6841–6847. [Google Scholar] [CrossRef]
- Chen, L.; Levine, J.S.; Gilmer, M.W.; Sloan, E.D.; Koh, C.A.; Sum, A.K. Methane hydrate formation and dissociation on suspended gas bubbles in water. J. Chem. Eng. Data 2014, 59, 1045–1051. [Google Scholar] [CrossRef]
- Topham, D.R. Observations of the Formation of Hydrocarbon Gas Hydrates at Depth in Seawater; Catalogue No. IOS Note-4; Institute of Ocean Sciences: Patricia Bay, BC, Canada, 1978; p. 8. [Google Scholar]
- Rehder, G.; Brewer, P.W.; Peltzer, E.T.; Friederich, G. Enhanced lifetime of methane bubble streams within the deep ocean. Geophys. Res. Lett. 2002, 29, 21-1–21-4. [Google Scholar] [CrossRef]
- Rehder, G.; Kirby, S.H.; Durham, W.B.; Stern, L.A.; Peltzer, E.T.; Pinkston, J.; Brewer, P.G. Dissolution rates of pure methane hydrate and carbon-dioxide hydrate in undersaturated seawater at 1000-m depth. Geochim. Cosmochim. Acta 2004, 68, 285–292. [Google Scholar] [CrossRef]
- Rehder, G.; Leifer, I.; Brewer, P.G.; Friederich, G.; Peltzer, E.T. Controls on methane bubble dissolution inside and outside the hydrate stability field from open ocean field experiments and numerical modeling. Mar. Chem. 2009, 114, 19–30. [Google Scholar] [CrossRef]
- Johansen, Ø.; Rye, H.; Cooper, C. DeepSpill––Field Study of a Simulated Oil and Gas Blowout in Deep Water. Spill Sci. Technol. Bull. 2003, 8, 433–443. [Google Scholar] [CrossRef]
- Maksimov, A.O.; Sosedko, E.V. Dynamics of sea bubbles covered by a hydrate skin. In Proceedings of the. XVI Session of the Russian Acoustical Society, Moscow, Russia, 14–18 November 2005; pp. 459–462. [Google Scholar]
- Greinert, J.; Artemov, Y.; Egorov, V.; De Batist, M.; McGinnis, D. 1300-m-high rising bubbles from mud volcanoes at 2080m in the Black Sea: Hydroacoustic characteristics and temporal variability. Earth Planet. Sci. Lett. 2006, 244, 1–15. [Google Scholar] [CrossRef]
- Juanes, R. Fate of Methane Emitted from Dissociating Marine Hydrates: Modeling, Laboratory, and Field Constraints; DOE Project (No. DOE-MIT-0013999); Massachusetts Inst. of Technology (MIT): Cambridge, MA, USA, 2018. [Google Scholar]
- Wang, B.; Socolofsky, S.A.; Breier, J.A.; Seewald, J.S. Observations of bubbles in natural seep flares at MC 118 and GC 600 using in situ quantitative imaging. J. Geophys. Res. Ocean. 2014, 121, 2203–2230. [Google Scholar] [CrossRef]
- Johansen, C.; Todd, A.C.; MacDonald, I.R. Time series video analysis of bubble release processes at natural hydrocarbon seeps in the Northern Gulf of Mexico. Mar. Pet. Geol. 2017, 82, 21–34. [Google Scholar] [CrossRef]
- Johansen, O. DeepBlow—A Lagrangian Plume Model for Deep Water Blowouts. Spill Sci. Technol. Bulletin 2000, 6, 103–111. [Google Scholar] [CrossRef]
- Yapa, P.D.; Zheng, L.; Chen, F. A Model for Deepwater Oil/Gas Blowouts. Mar. Pollut. Bull. 2001, 43, 234–241. [Google Scholar] [CrossRef]
- Zheng, L.; Yapa, P.D.; Chen, F. A model for simulating deepwater oil and gas blowouts–Part I: Theory and model formulation. J. Hydraul. Res. 2002, 41, 339–351. [Google Scholar] [CrossRef]
- Zheng, L.; Yapa, P.D. Modeling gas dissolution in deepwater oil/gas spills. Geophys. Res. Lett. 2002, 29, 299–309. [Google Scholar] [CrossRef]
- Chen, F.; Yapa, P.D. A model for simulating deep water oil and gas blowouts–Part II: Comparison of numerical simulations with “Deepspill” field experiments. J. Hydraul. Res. 2003, 41, 353–365. [Google Scholar] [CrossRef]
- Yapa, P.D.; Chen, F. Behavior of oil and gas from deepwater blowouts. J. Hydraul. Eng. 2004, 130, 540–553. [Google Scholar] [CrossRef]
- McGinnis, D.F.; Greinert, J.; Artemov, Y.; Beaubien, S.E.; Wüest, A. Fate of rising methane bubbles in stratified waters: How much methane reaches the atmosphere? J. Geophys. Res. Ocean. 2006, 111(C9), C09007. [Google Scholar] [CrossRef]
- Yapa, P.D.; Dasanayaka, L.K.; Bandara, U.C.; Nakata, K. A model to simulate the transport and fate of gas and hydrates released in deepwater. J. Hydraul. Res. 2010, 48, 559–572. [Google Scholar] [CrossRef]
- Wimalaratne, M.R.; Yapa, P.D.; Nakata, K.; Premathilak, L.T. Transport of dissolved gas and its ecological impact after a gas release from deepwater. Mar. Pollut. Bull. 2015, 100, 279–288. [Google Scholar] [CrossRef]
- Nakata, K.; Arata, N.; Yapa, P.D. Development of a model to simulate methane gas and methane hydrates plumes in deepwater. J. Adv. Mar. Sci. Technol. Soc. 2015, 21, 37–57, (In Japanese with English Abstract). [Google Scholar]
- Premathilake, L.T.; Yapa, P.D.; Nissanka, I.D.; Kumarage, P. Impact on water surface due to deepwater gas blowouts. Mar. Pollut. Bull. 2016, 112, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Kawahara, S.; Okano, Y.; Kato, N. Numerical Simulation of Methane Seeping from the Seabed in the Japan Sea. J. Chem. Eng. Jpn. 2017, 50, 244–253. [Google Scholar] [CrossRef]
- Labs of Instant Ocean. Science behind synthetic sea salts. SeaScope 2008, 24, 3–4. Available online: http://www.instantocean.com/~/media/UPG/Files/Instant%20Ocean/SeaScope/Volume%2024%20Fall.ashx (accessed on 9 May 2020).
- Holder, E.L.; Conmy, R.N.; Venosa, A.D. Comparative laboratory scale testing of dispersant effectiveness of 23 crude oils using four different testing protocols. J. Environ. Prot. 2015, 6, 628–639. [Google Scholar] [CrossRef]
- Vascanselos, J.M.T.; Rodrigues, J.M.L.; Orvalho, S.C.P.; Alves, S.S.; Mendes, R.L.; Reis, A. Effect of contaminants on mass transfer coefficients in bubble column and airlift contactors. Chem. Eng. Sci. 2003, 58, 1431–1440. [Google Scholar] [CrossRef]
- Duan, Z.; Mao, S. A thermodynamic model for calculating methane solubility, density and gas phase composition of methane-bearing aqueous fluids from 273 to 523 K and from 1 to 2000 bar. Geochim. Cosmochim. Acta 2006, 70, 3369–3386. [Google Scholar] [CrossRef]
- Witherspoon, P.A.; Saraf, D.N. Diffusion of Methane, Ethane, Propane, and n-Butane. J. Phys. Chem. 1965, 69, 3752–3755. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, T.; Nagamine, I.; Yabe, I.; Fukumaki, T.; Oyama, A.; Yoza, B.; Tenma, N.; Masutani, S.M. Dissolution Process Observation of Methane Bubbles in the Deep Ocean Simulator Facility. Energies 2020, 13, 3938. https://doi.org/10.3390/en13153938
Uchida T, Nagamine I, Yabe I, Fukumaki T, Oyama A, Yoza B, Tenma N, Masutani SM. Dissolution Process Observation of Methane Bubbles in the Deep Ocean Simulator Facility. Energies. 2020; 13(15):3938. https://doi.org/10.3390/en13153938
Chicago/Turabian StyleUchida, Tsutomu, Ike Nagamine, Itsuka Yabe, Tatsunori Fukumaki, Ai Oyama, Brandon Yoza, Norio Tenma, and Stephen M. Masutani. 2020. "Dissolution Process Observation of Methane Bubbles in the Deep Ocean Simulator Facility" Energies 13, no. 15: 3938. https://doi.org/10.3390/en13153938
APA StyleUchida, T., Nagamine, I., Yabe, I., Fukumaki, T., Oyama, A., Yoza, B., Tenma, N., & Masutani, S. M. (2020). Dissolution Process Observation of Methane Bubbles in the Deep Ocean Simulator Facility. Energies, 13(15), 3938. https://doi.org/10.3390/en13153938





